Abstract
Background
Current guidelines do not recommend primary prophylactic anti-epileptic drug (AED) therapy for patients with brain metastases (BM). Yet, subgroups of patients at high seizure risk might still benefit from prophylaxis.
Methods
We identified 799 patients diagnosed with BM by retrospective screening of our electronic chart system. Candidate risk factors for the development of epilepsy were tested by univariate and multivariate Cox regression models.
Results
Epilepsy was diagnosed in 226 of 799 patients (28%). Risk factors for epilepsy in non-operated patients were single BM (P = 0.002, hazard ratio [HR] 3.2, 95% CI: 1.5–6.6) and detection of tumoral hemorrhage (P = 0.008, HR 2.5, 95% CI: 1.3–4.9). Preoperative seizures occurred predominantly in patients with supratentorial BM (P = 0.003, HR 20.78, 95% CI: 2.8–153.4) and lung cancer (P = 0.022; HR 2.0, 95% CI: 1.1–3.6). Postoperative seizures were associated with supratentorial localization (P = 0.017, HR 5.8, 95% CI: 1.4–24.3), incomplete resection (P = 0.005, HR 4.6, 95% CI: 1.6–13.1), and by trend for multiple brain surgeries (P = 0.095, HR 1.9, 95% CI: 0.9–4.0). These risk factors were integrated into a predictive score model for postoperative epilepsy (score sum 0–8). A gradual increase of seizure rates along with higher sum score was confirmed post hoc (score 0 = no seizures; score 8 = 48% seizures). Receiver operating characteristic analysis supported diagnostic accuracy (P = 0.00001, area under the curve = 0.75).
Conclusions
Here we have defined risk profiles for the development of BM-related epilepsy and derived a score which might help to estimate the risk of postoperative seizures and identify individuals at risk who might benefit from primary prophylactic AED therapy.
Keywords: CNS, prevention, prophylaxis, score, seizure
Importance of the Study.
Brain tumor–related epilepsy is a frequent and clinically highly relevant complication of brain metastasis. However, current guidelines do not recommend primary prophylactic AED treatment for seizure-free patients with BM, since no benefit from general prophylaxis has been demonstrated so far. Furthermore, AED treatment may have significant side effects, interfere with systemic cancer therapy, and generate additional cost. Conversely, seizure prevention is of high clinical importance, since seizures negatively impact quality of life, for patients and for caregivers, and possibly outcome, too. In clinical practice, AED treatment is started following a first seizure, or based on an individual decision of the treating physician. Here we provide risk profiles for the development of seizures in patients with and without tumor resection. We have developed a predictive score to support clinicians in the identification of patients at high risk for postoperative epileptic seizures who might benefit from primary prophylaxis with AED.
Key Points.
Epilepsy was diagnosed in 226 of 799 patients (28%) with brain metastases.
Postoperative seizures were associated with supratentorial brain metastases, residual tumor, or repeat brain surgery.
A predictive model for seizures was derived from patients’ risk profiles.
Brain tumor–related epilepsy (BTRE) is common in patients with brain metastases (BM) from systemic tumors and is thought to contribute to morbidity and mortality.1,2 Freedom from seizures is essential for favorable quality of life in brain tumor patients.3–5 BTRE lifetime risk in BM patients is estimated at 20–35% to 67%.6–8 Diagnosis of BTRE commonly necessitates the initiation of secondary prophylaxis, whereas primary prophylactic anti-epileptic drug (AED) treatment in response to a BM diagnosis is not indicated.9 This is because several retrospective studies have failed to demonstrate a general risk reduction for developing BTRE with primary prophylaxis.10–12 However, subgroups of patients with high risk of seizures might still benefit from primary prophylaxis.13
There are only limited data to estimate the risk of developing BTRE in BM patients, mostly from retrospective studies of patient cohorts that also include patients with primary brain tumors7,14 or patients with distinct tumor entities, such as melanoma.13
Although available data and current guidelines9 do not support the concept of primary prophylaxis with AED, although these are nevertheless often prescribed in BM patients without BTRE, such as prior to BM resection or on an individual basis if the patient is thought to be at increased seizure risk. Possible seizure prevention has to be weighed against risk of drug interactions and of relevant side effects associated with AED therapy. This is particularly true for an older AED like phenytoin or phenobarbital.15
A better understanding of risk factors for BTRE might help to define a role for primary prophylactic AED therapy in subgroups of BM patients. Gross total resection, radiotherapy, and chemotherapy are thought to contribute to seizure control in glioma patients.7,8,16 Conversely, pre- or postoperative hemorrhage and multiple lesions were associated with increased seizure risk in BM patients,13 and detection of cortical hemosiderin correlated with seizure risk in a retrospective study including 36 BM patients.14
The major goal of this study was to define subgroups of BM patients at high risk for BTRE who might benefit from primary AED therapy. To this end, we retrospectively studied a clinically well-annotated cohort of 811 patients with BM. Since surgery can either contribute to improved seizure control following resection or in turn result in postoperative onset of new seizures, separate analyses for preoperative BTRE, postoperative BTRE, and BTRE in non-operated patients were conducted, with the intent to build risk prediction models for BTRE in BM patients.
Materials and Methods
Patients
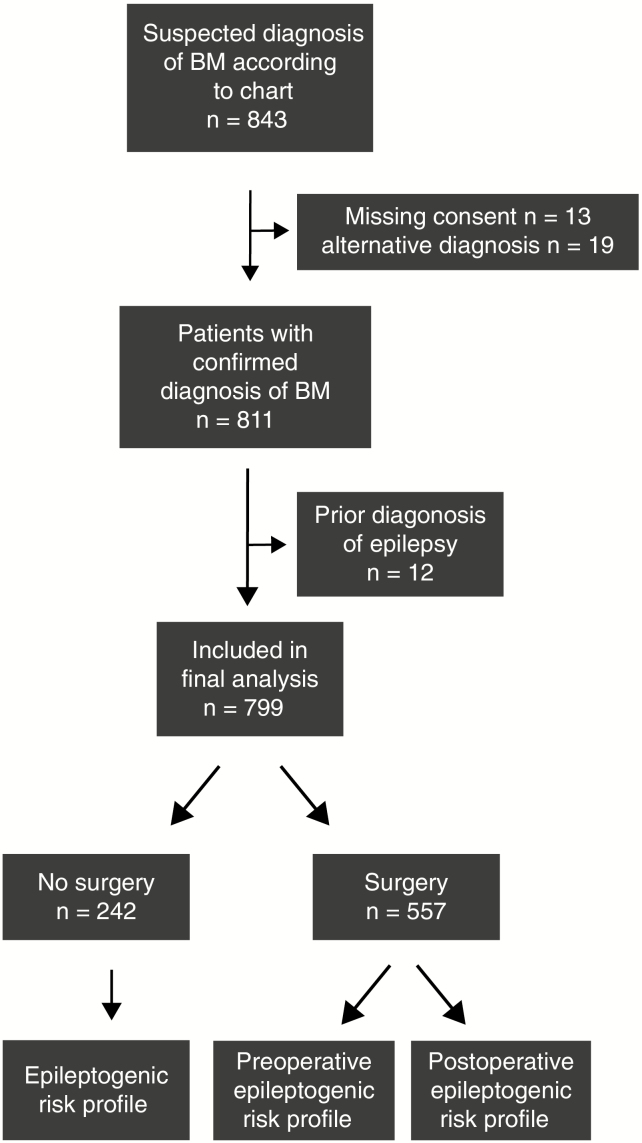
We screened the electronic chart system of the University Hospital Zurich for patients with BM diagnosed between January 2004 and December 2014, employing the search term “brain metastasis.” Of 843 adult patients, 13 were excluded because of missing informed consent and 19 because of alternative diagnoses. Of the remaining 811 patients, 568 had a brain biopsy or resection. Follow-up data until death were available for 628 patients (77%), whereas 183 patients (23%) were followed up for a median of 17.0 months (95% CI: 12.0–23.0 mo). This study was approved by the Cantonal Ethics Committee Zurich (KEK-ZH-Nr. 2018-00192).
Assessments
At least one unprovoked seizure in a patient with a diagnosis of BM was defined as BTRE according to the criteria of the International League Against Epilepsy (ILAE) that were valid when data were collected.17 A new classification of seizures has been established meanwhile.18 Postoperative epilepsy was diagnosed in patients with documented epileptic seizures during postoperative follow-up, except for seizures within 7 days of craniotomy. The latter are referred to as acute symptomatic postoperative and thus provoked seizures.19 All patients with provoked seizures only were assigned to the non-BTRE group.
Patient characteristics, histopathological data, and clinical data including neurological deficits, seizure history, postoperative course, complications, and medication were obtained by electronic chart reviews. Reports of cranial CT and MRI scans were reviewed to assess number, localization, and morphology of BM and to define presence of tumoral hemorrhage, tumor progression, and extent of resection. Absence of contrast-enhancing tumor on postoperative MRI scans was rated as gross total resection.
Statistical Methods
Analysis of nominal variables was performed employing the chi-square test, and analysis of linearly scaled variables was done with the Mann–Whitney U-test. Significant differences of paired nominal data were assessed employing McNemar’s test. Differences between ordinally coded data in unpaired samples were assessed by the Kruskal–Wallis test. Binary logistic regression analysis was performed to assess for factors independently associated with preoperative seizures. Variables which were associated with BTRE in univariate analyses were carried forward for multivariate testing using a Cox regression model including postoperative follow-up for seizures and seizure occurrence. Other variables previously reported to be associated with seizures were included in our model as possible confounders. Multivariate models for BTRE were derived separately for operated and non-operated patients, based on results from univariate analysis. We integrated the results in a predictive score model. Score values were derived from respective hazard ratios (HRs) for each item and confirmed using beta-coefficient values of regression. Receiver operating characteristic (ROC) curves were used to rate score validity. An area under the curve (AUC) of 0.5 reflects no discrimination between risk groups; an AUC of 1, a perfect discrimination between subjects at high and low risk; and an AUC >0.7 is widely accepted as cutoff for clinically relevant discrimination capacity of a clinical score.20
Statistical analysis was performed by SPSS v23 (IBM) and GraphPad Prism v7.0. For two-sided P-values, results with P < 0.05 were considered significant and with P < 0.01 highly significant.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request (fabian.wolpert@usz.ch).
Results
Patient Characteristics
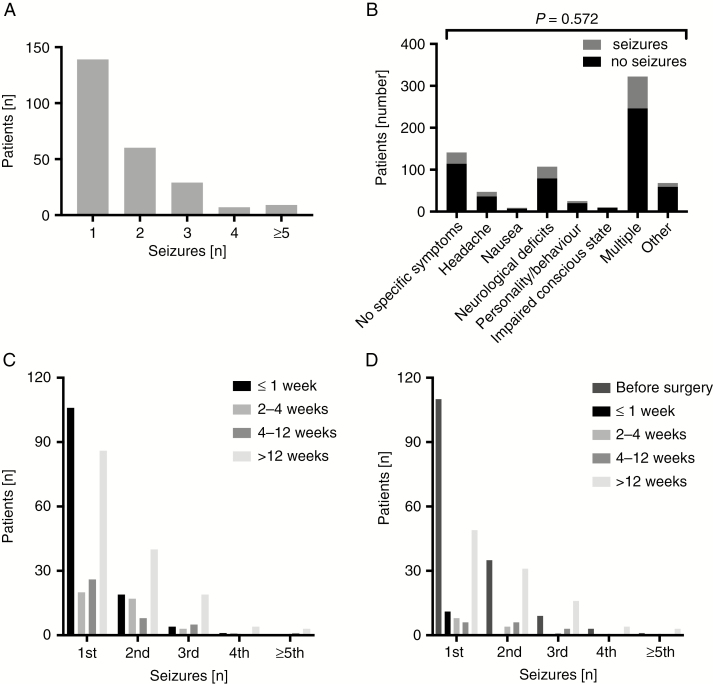
Interrogation of the electronic database allowed the identification of 811 BM patients. Twelve patients were excluded because of a history of prior epilepsy (Fig. 1, CONSORT [Consolidated Standards of Reporting Trials] chart). Of the final cohort of 799 patients, 237 (30%) had at least one documented seizure. In 5 patients, a provocation factor, such as more than moderate alcohol consumption, alcohol withdrawal, low serum sodium levels, or infection, was reported; 2 of these 5 patients later suffered from at least one unprovoked seizure and then fulfilled ILAE criteria for BTRE. Ten patients had a seizure within the first week after surgery, and the seizure was rated as provoked by surgery.17 Two of these patients had at least one or more unprovoked seizures in the further course of disease and were therefore included in the BTRE group. Altogether, 226 of 799 patients (28%) had at least one unprovoked epileptic seizure and thus fulfilled BTRE criteria (Table 1). Seizure rates varied from 1 to 13 seizures (Fig. 2A). There was no association between the first clinical symptom of BM and BTRE (Fig. 2B). Most seizures occurred within 1 week from diagnosis of BM, whereas other seizures occurred preferentially in the later course of disease beyond 12 weeks, potentially indicative of progressive disease (Fig. 2C). In operated patients the majority of the first and second seizures occurred before surgery, whereas few seizures occurred within the first 12 weeks after surgery (Fig. 2D), consistent with surgery-afforded seizure control.
Fig. 1.
CONSORT chart. The consort chart shows the selection path for patients to be included in this study. The upper part documents the preselection process to identify all BM patients and exclude patients with missing consent or alternative diagnosis. Next, patients with prior diagnosis of epilepsy were excluded from further assessment. The lower part shows separation of patients who underwent neurosurgery or no surgery. Pre- and postoperative BTRE risk profiles were assessed separately.
Table 1.
Patient characteristics stratified for absence versus presence of BTRE
| All Patients (n = 799) | No BTRE (n = 573, 71.7%) | Confirmed BTRE (n = 226, 28.3%) | P* | |
|---|---|---|---|---|
| Sex, m/f | 427/372 | 298/275 | 129/97 | 0.284a |
| Age, y, median (range) | 60.5 (20.5–90.1) | 60.9 (20.5–90.1) | 58.4 (26.6–84.9) | 0.004* b |
| Number of BM, median (range) | 2 (1–64) | 2 (1–64) | 1 (1–20) | 0.095b |
| KPS, median (range) | 80 (20–100) | 80 (30–100) | 80 (20–100) | 0.829b |
| Primary tumor, n (%) | ||||
| Unknown | 45 (5.6) | 34 (75.6) | 11 (24.4) | 0.556 a |
| Lung cancer | 332 (41.6) | 225 (67.8) | 107 (32.2) | 0.037 a |
| Melanoma | 143 (17.9) | 105 (73.4) | 38 (26.6) | 0.616 a |
| Breast cancer | 93 (11.6) | 76 (81.7) | 17 (18.3) | 0.023 a |
| Renal cell cancer | 28 (3.5) | 20 (71.4) | 8 (28.6) | 0.973 a |
| Gastrointestinal cancer | 63 (7.9) | 46 (73.0) | 17 (270) | 0.811 a |
| Other | 95 (11.9) | 66 (68.8) | 30 (31.3) | 0.784a |
| Alcohol, n (%) | ||||
| No abuse | 624 (85.5) | 444 (71.5) | 180 (28.5) | Ref. |
| Ongoing abuse (>30 g/d) | 84 (11.5) | 54 (64.3) | 30 (35.7) | 0.420a |
| Former abuse | 22 (3.0) | 16 (72.7) | 6 (27.3) | 0.812a |
| No information | 81 | |||
| First seizure, type, n (%) | ||||
| Focal seizures (intact awareness) | 83 (36.7) | |||
| Focal seizures (impaired awareness) | 21 (9.3) | |||
| Generalized tonic-clonic (onset unknown) | 92 (40.7) | |||
| Generalized non-motor (onset unknown) | 8 (3.5) | |||
| Status epilepticus | 12 (5.3) | |||
| Incomplete file | 10 | |||
| AED prophylaxis, n (%) | ||||
| No primary prophylaxis | 573 (72.7) | 395 (68.9) | 178 (31.1) | Ref. |
| Primary prophylaxis | 150 (19.0) | 104 (69.3) | 46 (30.7) | 0.582 a |
| Perioperative prophylaxis | 65 (8.2) | |||
| Localization of BM, n (%) | ||||
| Deep brain | 8 (2.6) | 8 (100.0) | 0 (0.0) | 0.942a |
| Cerebellum | 64 (21.1) | 57 (89.1) | 7 (10.9) | 0.899a |
| Brain stem | 6 (2.0) | 5 (83.3) | 1 (16.7) | 0.798a |
| Frontal | 110 (36.2) | 62 (56.4) | 48 (43.6) | 0.094a |
| Parietal | 49 (16.1) | 32 (65.3) | 17 (34.7) | 0.809a |
| Occipital | 27 (8.9) | 16 (59.3) | 11 (40.7) | 0.886a |
| Temporal | 39 (12.8) | 32 (82.1) | 7 (17.9) | 0.121a |
| Supratentorial | 226 (74.3) | 142 (62.8) | 84 (37.2) | <0.001 |
| Infratentorial | 78 (25.7) | 70 (89.7) | 8 (10.3) | Ref. |
| Missing information | 61 | |||
| Multiple BM | 434 | |||
| Surgery, n (%) | ||||
| No surgery | 242 (30.3) | 193 (79.8) | 49 (20.2) | Ref. |
| One or more surgeries | 557 (69.7) | 380 (68.2) | 177 (31.8) | 0.001 a |
| File incomplete | 2 | |||
| Extent of resection of supratentorial single BM | ||||
| No surgery | 28 (17.0) | |||
| Biopsy | 1 (0.6) | |||
| Partial resection | 87 (52.7) | |||
| Gross total resection | 49 (29.3) | |||
| No postoperative MRI | 61 | |||
| Treatments administered after BM diagnosis in non-operated patients, n (%) | ||||
| No chemotherapy or RT | 9 (3.8) | |||
| RT only | 103 (43.1) | |||
| Chemotherapy only | 5 (2.1) | |||
| Chemotherapy and radiotherapy | 122 (51.0) | |||
| Incomplete file | 4 | |||
| No radiotherapy | 14 (5.9) | 12 (85.7) | 2 (20.7) | Ref. |
| Radiotherapy | 224 (94.1) | 177 (79.0) | 47 (21.0) | 0.553a |
| No chemotherapy | 114 (47.1) | 91 (79.8) | 23 (20.2) | Ref. |
| Chemotherapy | 128 (52.9) | 101 (78.9) | 27 (21.1) | 0.860a |
| Treatments administered after surgery of BM, n (%) | ||||
| No chemotherapy or RT | 58 (11.0) | |||
| RT only | 216 (41.1) | |||
| Chemotherapy only | 12 (2.3) | |||
| Chemotherapy and radiotherapy | 239 (45.5) | |||
| Incomplete file | 32 | |||
| No radiotherapy | 72 (13.6) | 58 (80.6) | 14 (19.4) | Ref. |
| Radiotherapy | 456 (86.4) | 300 (65.8) | 156 (34.2) | 0.013 |
| No chemotherapy | 296 (53.5) | 218 (73.6) | 78 (26.4) | Ref. |
| Chemotherapy | 257 (46.5) | 158 (61.5) | 99 (38.5) | 0.002 |
+ The results of database screening are shown. The first column depicts the respective characteristics items, with main items in bold letters and subcharacters in normal letters. The second column shows overall values for all patients. Percentages for sub-items reflect their fraction compared with the whole entity of a main item. The third and fourth columns show the fraction of patients without and with seizures, marked with italic letters. Percentages refer to the fraction of patients with or without seizures for each item. RT indicates radiotherapy of the brain.
*Results of statistical testing, indicating P-values. Significant values are highlighted with bold letters; the respective statistical test is indicated with superscript letters: a = chi-square test; b = Mann–Whitney U-test; Ref. = group of patients that served as a reference.
Fig. 2.
Seizure characteristics in BM patients with BTRE. (A) The documented numbers of seizures per number of patients are shown as bar plots. (B) Stacked bar plots showing initial symptoms of patients with (gray) and without (black) seizures. Patients with a seizure as the first symptom of BM are excluded here. (C, D) The occurrence of the first 5 seizures per number of patients over time is shown from the diagnosis of BM (C) or from surgery (D) as grouped bar plots.
To further assess the role of tumor progression for BTRE in patients with known BM, we evaluated imaging results obtained within one week of the first seizure. This information was available for 76 previously seizure-free patients. Tumor progression was noted in 47 of these patients (62%).
AED Use in BTRE
AED treatment was assessed before and after the time of occurrence of a first seizure as well as one year after the diagnosis of BM. Information on AED prophylaxis after diagnosis of BM and prior to any documented seizure was available for 796 patients (Supplementary Table 1). BTRE risk did not differ between patients with (31%) versus without (31%) primary prophylaxis (Table 1). Justification of primary prophylaxis was documented for 138 of 153 patients: individual decisions of treating clinicians (52 patients, 38%), pathologic EEG findings (30 patients, 22%), continuation of perioperative prophylaxis (52 patients, 38%), other reasons like treatment of neuropathic pain (4 patients, 3%). Information on AED prophylaxis was available for 554 of 557 operated patients; in 115 of those, BTRE was diagnosed before surgery. In the remaining patients, BTRE was diagnosed in 14 of 236 patients (5.9%) with no AED prophylaxis, in 32 of 138 patients (23.2%) with primary prophylaxis, and in 1 of 65 patients (1.5%) with perioperative prophylaxis which was started prior to and stopped within 4 weeks after surgery (P = 0.0005).
Seizure Types
There was a trend toward association of generalized nonconvulsive seizures with lower median KPS (P = 0.053) and with higher number of BM (P = 0.073, Kruskal–Wallis test). There was no association of seizure type and sex (P = 0.187), extent of resection (P = 0.121), initial symptom (P = 0.405), lobe (P = 0.312), or hemisphere (P = 0.593) or detection of intracranial hemorrhage (P = 0.555, chi-square test). Focal seizures with retained awareness were more common in operated patients (75/182, 41.2% versus 10/47, 21.3% in non-operated patients), whereas focal seizures with impaired awareness occurred more frequently in non-operated patients (11/47, 23.4% versus 11/182, 6.0% in operated patients; P = 0.002, chi-square test).
Risk Factors for BTRE in Non-Operated Patients
Characteristics of 242 patients, who were not operated, are shown in Supplementary Table 2; 49 patients (20.2%) had BTRE. BTRE was associated with number of BM (P = 0.013, Mann–Whitney U-test) and was more common in patients with one brain metastasis (17/51, 33.3%) than in patients with multiple BM (31/189, 16.5%; P = 0.007, chi-square test). Patients with a single cortical supratentorial brain metastasis, defined as <1 cm distance from the cortex, or subcortical brain metastasis, defined as 1–2 cm from the cortex, showed no difference in BTRE rate, compared with BM in the deep white matter (>2 cm from the cortex; Supplementary Table 2). There was no association of BTRE with histology of the primary tumor (P = 0.816), chemotherapy after diagnosis of BM (P = 0.911, data not shown), or steroid intake at any time during the clinical course (P = 0.977, data not shown, all chi-square test). Supratentorial versus infratentorial tumor location of a single BM was not associated with BTRE either. This difference was still not significant for early occurrence of BTRE (<12 wk; supratentorial: 6/24 patients [25.0%] compared with infratentorial: 1/9 patients [11.1%] , P = 0.360). Hemorrhagic transformation of the tumor was found upon initial imaging in more than a third of the patients, with the highest rate in melanoma. Detection of tumoral hemorrhage was associated with the diagnosis of BTRE (P = 0.021). We used a Cox regression model with time to first seizure as an outcome measure for multivariate testing to exclude bias from diverging survival. Both single BM (P = 0.002, HR 3.2, 95% CI: 1.5–6.6) and detection of tumoral hemorrhage (P = 0.008, HR 2.5, 95% CI: 1.3–4.9) were retained as independent factors associated with BTRE. Post-hoc calculation revealed BTRE in 13 of 116 patients (11.2%) with neither factor, in 27 of 106 patients (25.5%) with one of the factors, and in 7 of 14 patients (50%) with both single BM and tumoral hemorrhage risk factors.
Risk Factors for Preoperative Seizures
Information on preoperative seizures was available for 554 of 557 operated patients. They were reported in 112 of these patients (19.2%) and were the first symptom of BM in 62 of these patients (11.2%). There was no association between preoperative BTRE and the number of metastases (P = 0.433), KPS (P = 0.319), age (P = 0.160; Supplementary Table 3), or steroid use (P = 0.187, not shown) or initial symptom (P = 0.572, both chi-square test). However, there was an association between primary tumor histology and preoperative seizures (P = 0.037): We noted an increased incidence of preoperative seizures in patients with lung cancer (P = 0.007) and a decreased incidence of preoperative seizures in patients with breast cancer (P = 0.005). Furthermore, patients with a single cortical or subcortical supratentorial BM showed a trend toward increased seizure risk compared with deep-seated BM (P = 0.075). The largest fraction of patients with preoperative seizures had frontal BM (33.7%, P = 0.007), followed by parietal single BM (24.4%, P = 0.911), occipital (16.0%, P = 0.297), and temporal (6.1%, P = 0.11) single BM (Supplementary Table 3).
Supratentorial versus infratentorial localization was the key risk factor for preoperative seizures on multivariate analysis (P = 0.003, HR 20.78, 95% CI: 2.8–153.4), followed by lung cancer as the primary tumor (P = 0.022, HR 2.0, 95% CI: 1.1–3.6). A sub-analysis for supratentorial tumors revealed localization in the frontal lobe (P = 0.001, HR 2.78, 95% CI: 1.5–5.2) as an independent risk factor.
Risk Factors for Postoperative Seizures
The associations of clinical disease characteristics with postoperative BTRE are summarized in Table 2. Supratentorial versus infratentorial (P = 0.012) and occipital versus other locations (P = 0.027) were associated with postoperative seizures. Furthermore, single versus more than one brain surgery in the disease course (P = 0.00001) and cerebral venous thrombosis (P = 0.030) were associated with postoperative BTRE. BTRE was less common in patients following gross total resection versus incomplete resection (P = 0.008). The development of postoperative BTRE was associated with brain irradiation (P = 0.013; Table 1) as well as chemotherapy (P = 0.002; Table 1).
Table 2.
Characteristics of operated BM patients and risk factors for postoperative seizures
| All Operated Patients (N = 557) | No Postoperative Seizures (N = 471, 84.6%) | One or More Postoperative Seizures (N = 86, 15.4%) | P | |
|---|---|---|---|---|
| Sex, m/f | 291/266 | 247/224 | 44/42 | 0.827a |
| Age, y, median (range) | 61.9 (22.9–90.1) | 62.2 (22.9–90.1) | 60.6 (31.7–84.9) | 0.073b |
| Number of BM, median (range) | 1 (1–36) | 1 (1–36) | 1 (1–20) | 0.854b |
| KPS, % (range) | 80 (20–100) | 80 (30–100) | 80 (20–100) | 0.988 |
| Primary tumor, n (%) | 0.479a | |||
| Unknown | 39 (7.4) | 35 (89.7) | 4 (10.3) | 0.342 a |
| Lung cancer | 224 (40.2) | 183 (81.7) | 41 (18.3) | 0.085 a |
| Melanoma | 90 (16.2) | 76 (84.4) | 14 (15.6) | 0.956 a |
| Breast cancer | 64 (11.5) | 56 (87.5) | 8 (12.5) | 0.307 a |
| Renal cell cancer | 23 (4.1) | 22 (95.7) | 1 (4.3) | 0.130 a |
| Gastrointestinal cancer | 53 (7.0) | 47 (88.7) | 6 (11.3) | 0.540 a |
| Other | 64 (11.5) | 52 (81.3) | 12 (18.8) | 0.585 a |
| Number of BM, n (%) | ||||
| Single BM | 239 (43.2) | 203 (84.9) | 36 (15.1) | Ref. |
| Multiple BM | 314 (56.8) | 265 (84.4) | 49 (15.6) | 0.861 a |
| Incomplete file | 1 | |||
| Localization of BM (supratentorial vs infratentorial), n (%) | ||||
| Single supratentorial BM | 198 (75.9) | 160 (80.8)) | 38 (19.2) | 0.012 |
| Single infratentorial BM | 66 (25.0) | 62 (93.9) | 4 (6.1) | Ref. |
| Localization of single supratentorial BM, n (%) | 0.020 a | |||
| Frontal | 94 (47.5) | 80 (85.1) | 14 (14.9) | 0.144a |
| Parietal | 44 (22.2) | 34 (77.3) | 10 (22.7) | 0.500a |
| Occipital | 25 (12.6) | 16 (64.0) | 9(36.0) | 0.027 a |
| Temporal | 33 (17.2) | 30 (88.2) | 4 (11.8) | 0.022a |
| Depth of single supratentorial BM, n (%) | ||||
| Cortical or subcortical | 175 (88.8) | 144 (82.3) | 31 (17.7) | 0.114a |
| Other | 22 (11.2) | 15 (68.2) | 7 (31.8) | Ref. |
| Number of surgeries, n (%) | 0.039 b | |||
| One brain surgery | 456 (81.9) | 401 (87.9) | 55 (12.1) | Ref. |
| Two or more brain surgeries | 101 (18.1) | 70 (69.3) | 31 (30.7) | <0.001 b |
| Cerebral venous thrombosis, n (%) | ||||
| No | 547 (98.3) | 465 (84.7)) | 82 (15.3) | Ref. |
| Yes | 10 (1.7) | 6 (60.0) | 4 (40.0) | 0.030 |
| Extent of resection of supratentorial single BM, n (%) | 0.011 | |||
| Biopsy | 2 (1.4) | 1 (100.0) | 0 (0) | Ref. |
| Partial resection | 87 (63.0) | 63 (72.4) | 24 (27.6) | |
| Gross total resection | 49 (35.5) | 45 (91.8) | 4 (8.2) | 0.008 |
+ The first column depicts the respective characteristics items, with main items in bold letters and sub-items in normal letters. The second column shows overall values for all patients. Percentages for sub-items reflect their fraction. The third and fourth columns show the fraction of patients without and with seizures. Percentages refer to the fraction of patients with or without seizures for each item.* Results of statistical testing, indicating P-values. Significant values are highlighted with bold letters; the respective statistical test is indicated with superscript letters. a = chi-square test; b = Mann–Whitney U-test; Ref. = group of patients that served as a reference.
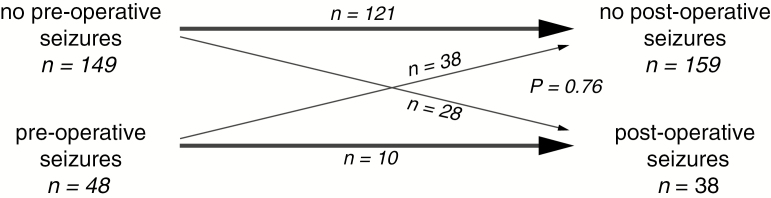
We then assessed the occurrence of seizures before and after surgery. Altogether, there was no significant association between pre- and postoperative seizure occurrence (P = 0.077, McNemar test). There was also no significant association of pre- with postoperative seizures in the subgroup of 197 patients with only single supratentorial metastasis with a median follow-up of 6 months (95% CI: 6.0–8.0 mo) (Fig. 3).
Fig. 3.
Pre- and postoperative seizures in operated patients. Schematic presentation of pre- and postoperative seizures in patients with a single supratentorial brain metastasis. Bold arrows indicate patients who had no diagnosis of BTRE and remained seizure free after surgery (upper bold arrow) or had a diagnosis of BTRE and ongoing seizures following surgery (lower bold arrow). Thin arrows indicate patients who were so far seizure free and developed postoperative BTRE (upper, down-going thin arrow) or patients with BTRE who became seizure free after surgery (lower up-going thin arrow). P-value as indicated (McNemar’s test).
Next, we evaluated the association of extent of resection determined by postoperative imaging obtained within one week with postoperative BTRE in patients with single supratentorial BM. Risk of postoperative seizures was significantly higher after biopsy or partial resection than after gross total resection (P = 0.008) (Table 2).
We then focused on patients with single supratentorial BM who had been seizure free prior to surgery and explored an association of new BTRE with extent of resection. Twenty of 66 patients (30.3%) who had a biopsy or partial resection developed postoperative BTRE, compared with only 2 of 38 patients (5.3%) with gross total resection (P = 0.003, chi-square test). In contrast to preoperative seizures that were associated with frontal localization of a single brain metastasis, new diagnosis of BTRE after surgery was associated with occipital tumor localization (P = 0.027; Table 2). In contrast to preoperative seizures, tumor histology was not associated with postoperative seizures (P = 0.479).
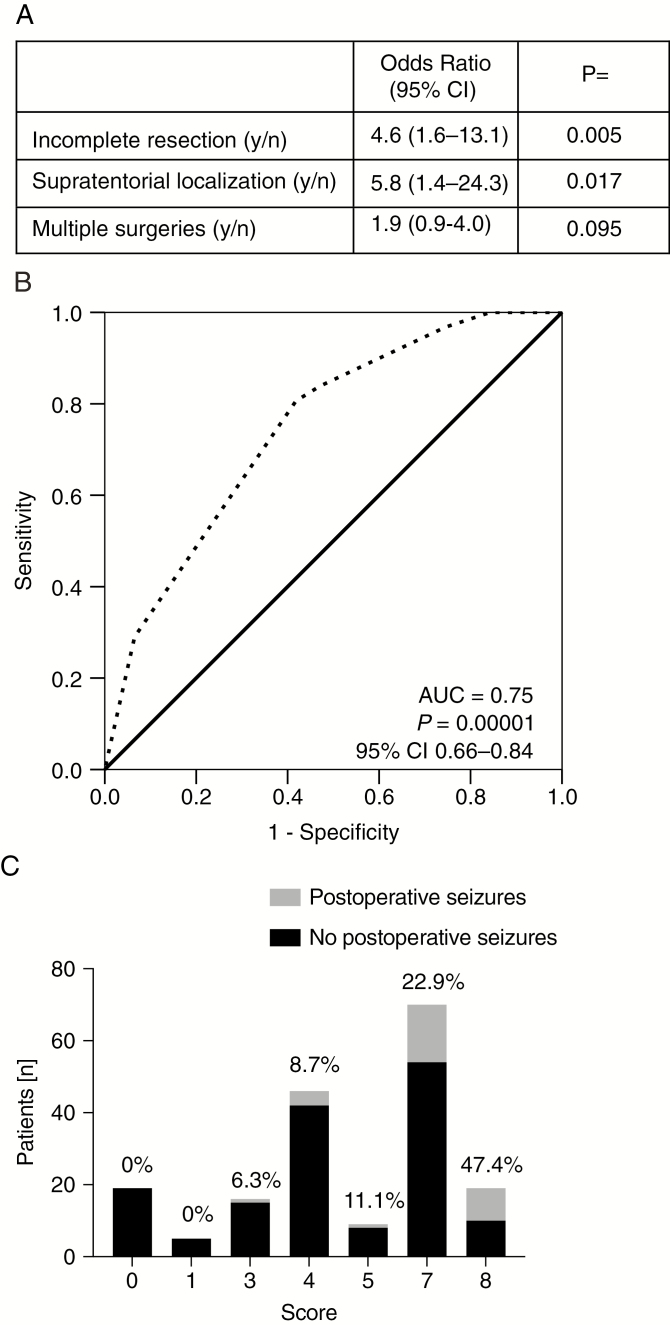
Factors that were associated with postoperative seizures in univariate analysis were then tested in multivariate analysis, using a Cox regression model. Median postoperative follow-up for seizures was 6.0 months (95% CI: 6.0–8.0). Supratentorial location (P = 0.017, HR 5.8, 95% CI: 1.4–24.3) and incomplete resection (P = 0.005, HR 4.6, 95% CI: 1.6–13.1) were independently associated with postoperative seizures. Multiple surgeries (P = 0.095, HR 1.9, 95% CI: 0.9–4.0) were associated with increased rate of postoperative seizures by trend (Fig. 4A).
Fig. 4.
Predictive score model for postoperative BTRE. (A) Results of multivariate testing are shown as a small table depicting risk factors, HR values with 95% CI, and two-sided P-values. (B) The ROC curve is shown for the predictive score model (dashed black line). AUC and P-values as well as the 95% CI are indicated. (C) The score sums and number of patients are shown as stacked bar plots. The black part of each bar represents the fraction of seizure-free patients, the gray part those with seizures. Percentages are indicated above each bar.
Score Models for Prediction of Postoperative Seizures
Finally we integrated the factors associated with postoperative seizures into a model where supratentorial localization accounts for a score value of 4, incomplete resection for a score of 3, and multiple surgeries for a score of 1, based on HR values from multivariate analysis. ROC analysis supported the diagnostic accuracy of the score (P = 0.000014, AUC = 0.75, 95% CI: 0.66–0.84) (Fig. 4B). Post-hoc calculation of this score revealed a gradual increase in seizure frequency (Fig. 4C).
Discussion
Although BTRE is a common complication in patients with BM, no general benefit from primary AED prophylaxis in BM patients has been demonstrated.9 However, subgroups of BM patients might be at higher risk for BTRE and therefore benefit from primary prophylactic AED treatment, such as patients with BM from melanoma.13
Here we define the risk profile for BTRE in a single institution cohort of 799 BM patients to identify subgroups of patients at increased risk of BTRE. We report a BTRE rate of 28%. The BTRE rate was lower in non-operated BM patients (20%) than in operated patients (32%) (Table 1). These data need to be interpreted with caution, since the decision for surgery was likely often biased—for example, to achieve seizure freedom or relief from intracranial pressure. Yet, because of this difference in BTRE frequency, we defined separate risk profiles for operated and non-operated patients. Since surgery has been reported to contribute to seizure control in patients with lower-grade gliomas,21 we also decided to define separately the risk profile for preoperative versus postoperative seizures. Non-operated patients with a single brain metastasis had a higher BTRE rate than patients with multiple BM (Supplementary Table 2). This finding was unexpected, since a higher tumor burden should result in increased seizure risk. Yet, BM in potentially epileptogenic regions like the precentral gyrus or mesiotemporal region might often be difficult to resect. This would result in a selection bias toward non-operated patients with single lesions in highly epileptogenic regions, whereas surgery is in general less frequently performed in patients with multiple BM (notable multiple small BM). We furthermore found an increased BTRE rate in non-operated patients with hemorrhagic lesions (P = 0.021)14 but no other clinical factors considered to be potentially associated with BTRE, including depth of BM location, histology of the primary tumor, type of cancer treatment, or steroid intake.
In operated patients, supratentorial versus infratentorial localization was the key factor associated with preoperative seizures on multivariate analysis (P = 0.003, HR 20.8), followed by lung cancer as a primary tumor (P = 0.022, HR 2.0). We next performed a subgroup analysis for patients with supratentorial tumors. Here, frontal localization was a risk factor for preoperative seizures (P = 0.001, HR 2.78). Primary AED prophylaxis was not associated with BTRE (P = 0.582); however, patients who received perioperative AED prophylaxis showed a significantly lower BTRE rate (P = 0.0005). However, the latter finding has to be regarded with caution, since decisions for or against primary or perioperative AED prophylaxis did not follow an algorithm, but were made on an individual basis.
A contribution of surgery to improved seizure control in BM patients with preoperative BTRE is assumed, but remains unproven. We found no significant postoperative decrease of seizure rates in patients with preoperative seizures, and inversely no increased rate of new seizures after surgery (Fig. 3). However, seizure freedom is not the primary goal of BM surgery, and neurosurgical interventions are probably not planned accordingly. Epilepsy surgery with intraoperative EEG mapping for BM patients might be an interesting concept for individuals refractory to AED treatment.
When defining the risk profile for postoperative seizures, supratentorial versus infratentorial localization of a single metastasis was again a very strong predictor of new postoperative BTRE. We thus refined the seizure risk profile of supratentorial tumors. Univariate testing revealed incomplete resection (P = 0.008), multiple surgeries (P = 0.0003), and occipital (P = 0.027) localization, but not tumor histology (P = 0.479) (Table 2) as possible predictors of higher seizure risk.
On multivariate analysis, supratentorial localization (P = 0.017, HR 5.8), incomplete resection (P = 0.005, HR 4.6), and, by trend, multiple surgeries (P = 0.095, HR 1.9) were retained as independent factors associated with postoperative seizures.
Our findings confirm and extend those from another recent cohort study.8 Here, headache, cognitive deficits, multiple BM, and localization in the temporal or occipital lobe were reported as risk factors for preoperative seizures, whereas absence of frontal lobe involvement and tumor size (diameter >5 cm) were associated with poor preoperative seizure control. We also found a lower frequency of postoperative seizures in patients with frontal BM, but no association with cognitive deficits or headache as first symptom from BM (Fig. 2). Furthermore, we found no association between preoperative seizures and the number of BM (Supplementary Table 3). We confirm incomplete resection as a strong predictor of postoperative seizures.8 In fact, we speculate that differential extent of resection may contribute to the relative association of postoperative BTRE with occipital rather than frontal BM location in our cohort. Alternatively, or in addition, there may be detection bias in preoperative patients where motor seizures from frontal areas are more often recognized as such than more subtle seizure types, with occipital or parietal lesions. BM in the latter location may be more often diagnosed because of focal neuro(psycho)logical deficits.
The retrospective character of our cohort study is its major limitation. There was a possible bias on underreporting of seizures. Sample sizes for some subgroup analyses were small. Causal links between BM and seizures are commonly considered compelling, but other seizure etiologies, including metabolic disturbances and side effects from cancer therapies, are difficult to rule out, notably in a retrospective setting and in this specific patient population.
A major strength is the overall large sample size, which allowed us to define risk factors across treatment modalities or primary tumor entities. This contrasts with previous cohort studies or controlled trials that were, for instance, restricted to tumor entities,13 single treatment modalities like neurosurgery,8,11 or included patients with primary brain tumors.7,14 The size of our cohort allowed us also to perform some subgroup analyses and assess risk factors for seizures in different clinical settings, namely non-operated patients and operated individuals in the pre- and postoperative phase. We finally developed a score model, by which groups with diverging postoperative seizure risk can be identified, ranging from 0% to almost 50%.
Although our score model is based on a retrospective analysis from a single center, it might provide a valuable clinical tool for clinical decision making. Patients who are not operated might not benefit from primary prophylaxis because of their low seizure risk, except for the subgroup with single and hemorrhagically transformed BM, which showed a seizure rate of about 50%. Patients who are planned for surgery of a single supratentorial BM are candidates for perioperative prophylaxis, which might be considered to be maintained in patients with incomplete resection of a supratentorial single brain metastasis. Further validation in independent cohorts and ideally in prospective controlled trials is required to refine the outlined predictive model and to further improve its prognostic accuracy.
Funding
None.
Conflict of interest statement. FW has received travel support from Roche. PR has received honoraria for advisory board participation and lectures from Bristol-Myers Squibb, Covagen, Medac, MSD, Novartis, Novocure, Roche, and Virometix. ELR has received research grants from Mundipharma and Amgen and honoraria for lectures or advisory board participation from Abbvie, Daiichi Sankyo, Mundipharma, and Novartis.
MW has received research grants from Acceleron, Actelion, Bayer, Isarna, MSD, Merck & Co, Novocure, PIQUR, and Roche and honoraria for lectures or advisory board participation or consulting from Celldex, Immunocellular Therapeutics, Isarna, Magforce, MSD, Merck & Co, Northwest Biotherapeutics, Novocure, Pfizer, Roche, and Teva.
Authorship statement. Conception and design of the study: MW, FW, ELR, LI. Acquisition and analysis of data: AL, RT, BG, FW, MCN, LI. Drafting a significant portion of the manuscript or figures: MW, FW, ELR, MCN, RN, LI, PR.
Supplementary Material
Acknowledgments
We thank our colleagues from the Cancer Registry Zurich and Zug for their support in completion of follow-up data.
References
- 1. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. [DOI] [PubMed] [Google Scholar]
- 2. Weller M, Stupp R, Wick W. Epilepsy meets cancer: when, why, and what to do about it? Lancet Oncol. 2012;13(9):e375–e382. [DOI] [PubMed] [Google Scholar]
- 3. Tanti MJ, Marson AG, Chavredakis E, Jenkinson MD. The impact of epilepsy on the quality of life of patients with meningioma: a systematic review. Br J Neurosurg. 2016;30(1):23–28. [DOI] [PubMed] [Google Scholar]
- 4. Avila EK, Chamberlain M, Schiff D, et al. Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro Oncol. 2017;19(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pace A, Dirven L, Koekkoek JAF, et al. ; European Association of Neuro-Oncology palliative care task force European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18(6):e330–e340. [DOI] [PubMed] [Google Scholar]
- 6. Oberndorfer S, Schmal T, Lahrmann H, Urbanits S, Lindner K, Grisold W. [The frequency of seizures in patients with primary brain tumors or cerebral metastases. An evaluation from the Ludwig Boltzmann Institute of Neuro-Oncology and the Department of Neurology, Kaiser Franz Josef Hospital, Vienna]. Wien Klin Wochenschr. 2002;114(21-22):911–916. [PubMed] [Google Scholar]
- 7. Skardelly M, Brendle E, Noell S, et al. Predictors of preoperative and early postoperative seizures in patients with intra-axial primary and metastatic brain tumors: A retrospective observational single center study. Ann Neurol. 2015;78(6):917–928. [DOI] [PubMed] [Google Scholar]
- 8. Wu A, Weingart JD, Gallia GL, et al. Risk factors for preoperative seizures and loss of seizure control in patients undergoing surgery for metastatic brain tumors. World Neurosurg. 2017;104:120–128. [DOI] [PubMed] [Google Scholar]
- 9. Soffietti R, Abacioglu U, Baumert B, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. 2017;19(2):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sirven JI, Wingerchuk DM, Drazkowski JF, Lyons MK, Zimmerman RS. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc. 2004;79(12):1489–1494. [DOI] [PubMed] [Google Scholar]
- 11. Ansari SF, Bohnstedt BN, Perkins SM, Althouse SK, Miller JC. Efficacy of postoperative seizure prophylaxis in intra-axial brain tumor resections. J Neurooncol. 2014;118(1):117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee MH, Kong DS, Seol HJ, Nam DH, Lee JI. Risk of seizure and its clinical implication in the patients with cerebral metastasis from lung cancer. Acta Neurochir (Wien). 2013;155(10):1833–1837. [DOI] [PubMed] [Google Scholar]
- 13. Goldlust SA, Hsu M, Lassman AB, Panageas KS, Avila EK. Seizure prophylaxis and melanoma brain metastases. J Neurooncol. 2012;108(1):109–114. [DOI] [PubMed] [Google Scholar]
- 14. Roelcke U, Boxheimer L, Fathi AR, et al. Cortical hemosiderin is associated with seizures in patients with newly diagnosed malignant brain tumors. J Neurooncol. 2013;115(3):463–468. [DOI] [PubMed] [Google Scholar]
- 15. Iivanainen M, Savolainen H. Side effects of phenobarbital and phenytoin during long-term treatment of epilepsy. Acta Neurol Scand Suppl. 1983;97:49–67. [DOI] [PubMed] [Google Scholar]
- 16. Wick W, Menn O, Meisner C, et al. Pharmacotherapy of epileptic seizures in glioma patients: who, when, why and how long? Onkologie. 2005;28(8-9):391–396. [DOI] [PubMed] [Google Scholar]
- 17. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. [DOI] [PubMed] [Google Scholar]
- 18. Fisher RS, Cross JH, D’Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58(4):531–542. [DOI] [PubMed] [Google Scholar]
- 19. Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671–675. [DOI] [PubMed] [Google Scholar]
- 20. Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013;4(2):627–635. [PMC free article] [PubMed] [Google Scholar]
- 21. Hirsch LJ. Seizures in patients undergoing resection of low-grade gliomas. Epilepsy Curr. 2009;9(4):98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request (fabian.wolpert@usz.ch).