Abstract
Background
VB-111 is a non-replicating adenovirus carrying a Fas-chimera transgene, leading to targeted apoptosis of tumor vascular endothelium and induction of a tumor-specific immune response. This phase I/II study evaluated the safety, tolerability, and efficacy of VB-111 with and without bevacizumab in recurrent glioblastoma (rGBM).
Methods
Patients with rGBM (n = 72) received VB-111 in 4 treatment groups: subtherapeutic (VB-111 dose escalation), limited exposure (LE; VB-111 monotherapy until progression), primed combination (VB-111 monotherapy continued upon progression with combination of bevacizumab), and unprimed combination (upfront combination of VB-111 and bevacizumab). The primary endpoint was median overall survival (OS). Secondary endpoints were safety, overall response rate, and progression-free survival (PFS).
Results
VB-111 was well tolerated. The most common adverse event was transient mild-moderate fever. Median OS time was significantly longer in the primed combination group compared with both LE (414 vs 223 days; hazard ratio [HR], 0.48; P = 0.043) and unprimed combination (414 vs 141.5 days; HR, 0.24; P = 0.0056). Patients in the combination phase of the primed combination group had a median PFS time of 90 days compared with 60 in the LE group (HR, 0.36; P = 0.032), and 63 in the unprimed combination group (P = 0.72). Radiographic responders to VB-111 exhibited characteristic, expansive areas of necrosis in the areas of initial enhancing disease.
Conclusions
Patients with rGBM who were primed with VB-111 monotherapy that continued after progression with the addition of bevacizumab showed significant survival and PFS advantage, as well as specific imaging characteristics related to VB-111 mechanism of action. These results warrant further assessment in a randomized controlled study.
Keywords: anti-angiogenesis, gene therapy, glioblastoma, VB-111, viral immuno-oncology
Key Points.
Patients with rGBM treated with VB-111 primed combination had a survival and PFS benefit.
VB-111 exhibited specific MRI characteristics related to its mechanism of action.
The encouraging data of this phase I/II study warrant further Investigation of VB-111 primed regimen in a controlled clinical trial.
Importance of the Study.
Glioblastoma has one of the highest unmet needs in oncology. Currently approved therapies have resulted in only limited incremental improvements in survival. This is the first clinical trial to evaluate the viral-based anticancer gene therapy VB-111 (ofranergene obadenovec) in rGBM. Results of this phase I/II study demonstrated that multiple doses of intravenous VB-111 were well tolerated. Notably, patients who were primed with VB-111 monotherapy, which continued after progression with the addition of bevacizumab, showed significant survival advantage compared with the limited exposure arm. Median OS time was 414 versus 223 days (HR, 0.48; P = 0.043). Survival advantage was also seen in comparison to historical controls. Although these encouraging results were not repeated in the following phase III GLOBE study, in which an unprimed combination treatment regimen was administered, further investigation of the VB-111 primed combination regimen in a randomized controlled study is planned.
Glioblastoma (GBM) is the most common and aggressive primary malignant brain tumor in adults and remains incurable, with median overall survival (OS) well below 2 years.1 Currently approved therapies (temozolomide and bevacizumab and one medical device) have resulted in only incremental improvements in survival,2–4 and no survival benefit was documented with bevacizumab in either newly diagnosed or recurrent settings.5–9
VB-111 (ofranergene obadenovec) is a non-replicating adenovirus 5 (Ad-5, E1-deleted) carrying a pro-apoptotic human Fas-chimera transgene (Fas and human tumor necrosis factor receptor 1) under the control of a modified murine pre-proendothelin promoter (PPE-1-3x). The semi-artificial tissue and condition-specific promoter targets the expression of the Fas-chimera cell-death receptor to angiogenic blood vessels, leading to targeted apoptosis of these vessels.10–12 VB-111 was thus designed to disrupt neovascularization independently of the pro-angiogenic signaling pathways utilized by tumors, and therefore is not susceptible to many of the resistance mechanisms inherent to other anti-angiogenic approaches which target a certain ligand/receptor. Moreover, VB-111 promotes specific intratumor activation of the immune system, thereby inducing antitumor immune response that includes tumor infiltration of cluster of differentiation (CD)4 and CD8 T cells, such as seen in viral immuno-oncology.10,13,14 The preclinical activity of VB-111 in orthotopic GBM models was sufficient to extend survival in nude rats bearing U87MG-luc2 or nude mice bearing U251-luc, as well as resulting in decreased vascular tumor density.15 Prior clinical studies have shown that VB-111 is safe and well tolerated in patients with advanced metastatic cancer at doses of up to 1 × 1013 viral particles (VP).14,16,17 We therefore initiated a phase I/II study to evaluate VB-111 in patients with recurrent glioblastoma (rGBM).
Methods
Study Design
This prospective, open-label, dose-escalating, multicenter, phase I/II study of VB-111 was designed to evaluate the safety, tolerability, and efficacy of single and multiple doses of intravenous infusion of VB-111 with and without bevacizumab in patients with rGBM (NCT01260506). The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice. OS was the primary efficacy endpoint, and secondary endpoints were safety, overall response rate, and progression-free survival (PFS).
Patient selection
Eligible patients were ≥18 years old with histologically confirmed GBM with measurable disease and progression or recurrence following standard-of-care treatment with temozolomide and radiation with measurable disease by Response Assessment in Neuro-Oncology (RANO) criteria. Patients had KPS ≥70. There were no restrictions based on tumor size or prior number of therapy lines and no requirement for prior debulking resection. For the dose escalation, subjects were excluded if imaging showed major mass tumor effect. Exclusion criteria for all cohorts prohibited prior anti-angiogenic exposure or stereotactic radiation, or an uncontrolled comorbidity.
Treatments administered and dose-escalation scheme
The study was launched early in the clinical development of VB-111 as a dose-escalation single dose study and was amended to allow multiple doses and combination with bevacizumab, based on the accumulating safety and efficacy data across the VB-111 program.16,17 The starting dose was 1 × 1012 VP, which represents a 2-dose level reduction from the maximum evaluated safe dose of 1 × 1013 VP in a previous phase I study.16 Monitoring for dose-limiting toxicities (DLTs) was performed to establish safety and to allow dose escalations to 3 × 1012 and 1 × 1013 VP. The maximum tolerated dose (MTD) was the highest dose at which <33% of patients experienced a DLT up to the maximum planned dose. Toxicity was graded per the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. Intrapatient dose escalation was allowed, and safety analysis was based on the highest dose administered, whereas efficacy analysis was based on initial cohort assignment.
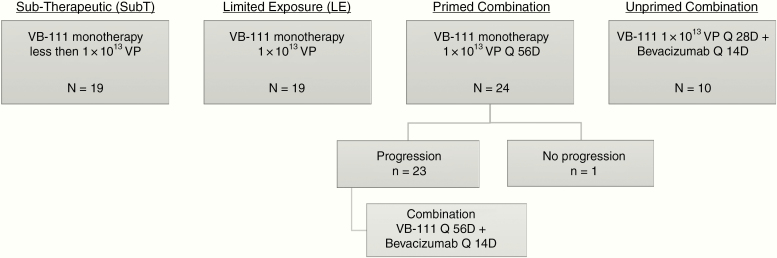
Analyses were performed according to 4 treatment groups (Fig. 1). The subtherapeutic (SubT) group included patients who received initial VB-111 doses lower than 1 × 1013 VP. All other treatment groups received VB-111 at the therapeutic dose of 1 × 1013 VP. The limited exposure (LE) group received VB-111 every 56 days until disease progression; afterward most patients received standard-of-care bevacizumab. The primed combination group received VB-111 monotherapy every 56 days until increased contrast consistent with disease progression (monotherapy priming phase), and beyond progression patients continued VB-111 combined with bevacizumab (10 mg/kg i.v.) every 2 weeks (combination phase). The unprimed combination group received upfront combination treatment with VB-111 every 28 days with bevacizumab every 2 weeks.
Fig. 1.
Study disposition diagram. Analyses were performed according to 4 treatment groups: SubT: initial VB-111 doses lower than 1 × 1013 VP. Limited exposure (LE): VB-111 1 × 1013 VP every 56 days until disease progression. Primed combination: VB-111 1 × 1013 VP monotherapy every 56 days until disease progression and after progression VB-111 every 56 days combined with bevacizumab (10 mg/kg i.v.) every 2 weeks. Unprimed combination: upfront combination treatment with VB-111 1 × 1013 VP every 28 days with bevacizumab every 2 weeks.
Concomitant medications included pre-dose acetaminophen (1 g) to mitigate posttreatment fever, and dexamethasone (4 mg orally b.i.d. for 14 days with the first infusion and for 3 days with subsequent infusions) to prevent possible vascular disruptive effects of VB-111 and cerebral edema.
Biodistribution analysis
Quantitative polymerase chain reaction (qPCR) detected adenovirus vector VB-111 in whole blood and urine. DNA was isolated and analyzed by validated qPCR for the presence of the adenovirus hexon gene. Each sample was analyzed in triplicate, and the resulting mean copy number from replicates was converted to copies/μg of DNA.
Magnetic resonance imaging and radiographic response evaluation
Patients were followed with MRI scans every 8 weeks. MRI acquisition parameters adhered to the international standardized brain tumor imaging protocol.18 Conventional radiographic response and disease progression using the standard RANO criteria19 were assessed by both local investigators and by central radiological assessment by Bioclinica (Princeton, New Jersey). This report presents only the central assessments. Additional post-hoc quantitative tumor volumetrics were performed by the UCLA Brain Tumor Imaging Laboratory using contrast-enhanced T1-weighted digital subtraction maps and segmentation techniques described previously.20–25
Statistical Analysis
OS time, in days (from enrollment or start of treatment to death), was assessed using Kaplan–Meier curves, and the log-rank test evaluated survival differences among groups. As a further comparison, a historical control group of 694 rGBM patients treated with bevacizumab was established based on 8 published trials and case series and was compared with the primed combination group.3,26–32 PFS time was examined based on investigator and independent central review assessments. Since the exact dates of progression are not known, a version of the log-rank test using interval censoring33 tested differences in PFS among groups.
For patients in the primed combination group, 2 progression endpoints were defined: after VB-111 monotherapy (measured from the start of monotherapy) and after VB-111 + bevacizumab combination therapy (measured from the start of combination therapy).
The initial percentage of change in contrast-enhancing tumor volume after first treatment was assessed for the 2 phases of the primed combination group and for the unprimed combination group. A one-sample t-test was applied to the mean percentage change in tumor volume.
Results
Between August 2011 and May 2015, seventy-two patients with rGBM were enrolled at 4 sites and treated in 4 consecutive treatment groups: 2 VB-111 monotherapy groups, SubT (n = 19) and LE (n = 19), and 2 VB-111 + bevacizumab combination groups, primed combination (n = 24) and unprimed combination (n = 10). Three patients initially started dosing with subtherapeutic VB-111 and underwent intrapatient dose escalation to 1 × 1013 VP. As of the data cutoff date (July 23, 2015, except for the unprimed combination group; June 26, 2016), 4 patients were alive (3 in the primed combination group, 1 in the SubT group), 2 were lost to follow-up, 3 had withdrawn consent, and 63 had died (Fig. 1). One patient in the primed combination group did not progress and received VB-111 monotherapy throughout the follow-up period until study data cutoff (censored at 581 days). By the time of manuscript submission, 1 patient was alive with complete remission and had voluntarily stopped VB-111 monotherapy after receiving 32 doses over a period of 5 years.
The baseline characteristics of the SubT, LE, and primed combination groups were similar; however, the unprimed combination group differed as patients were younger, with more advanced disease and larger tumors at baseline: 3036 (mm2) compared with 555, 693, and 1064 in the SubT, LE, and primed combination groups, respectively (Table 1).
Table 1 .
Baseline patient characteristics
| Characteristic | SubT (n = 19) | LE (n = 19) | Primed Combination (n = 24) | Unprimed Combination (n = 10) |
|---|---|---|---|---|
| Median age, y (range) | 56.1 (28–65) | 55.9 (27–76) | 60 (19–72) | 42 (24–64) |
| Sex, n (%) | ||||
| Male | 13 (68.4) | 11 (57.9) | 12 (50.0) | 6 (60.0) |
| Female | 6 (31.6) | 8 (42.1) | 12 (50.0) | 4 (40.0) |
| Race, n (%) | ||||
| White | 18 (94.7) | 19 (100) | 24 (100) | 10 (100) |
| Asian | 1 (5.3) | 0 | 0 | 0 |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 1 (5.3) | 3 (15.8) | 4 (16.7) | 4 (40.0) |
| Non-Hispanic or Latino | 18 (94.7) | 16 (84.2) | 20 (83.3) | 6 (60.0) |
| KPS, n (%) | ||||
| 90–100 | 15 (78.9) | 11 (57.9) | 9 (37.5) | 4 (40) |
| 70–80 | 4 (21.1) | 7 (36.8) | 14 (58.3) | 3 (30) |
| ≤60 | 0 | 1 (5.3) | 1 (4.2) | 0 |
| Unknown | 3 (30) | |||
| Initial surgery, n (%) | ||||
| Biopsy only | 2 (10.5) | 3 (15.8) | 5 (20.8) | 0 |
| Partial resection | 10 (52.6) | 10 (52.6) | 8 (33.3) | 5(50.0) |
| Complete resection | 6 (31.6) | 6 (31.6) | 9 (37.5) | 1 (10.0) |
| Other/unknown | 1 (5.3) | 0 | 2 (8.3) | 4 (40.0) |
| Recurrence, n (%) | ||||
| First | 13 (68.4) | 14 (73.7) | 13 (54.2) | 3 (30) |
| Second | 3 (15.8) | 3 (15.8) | 10 (41.7) | 3 (30) |
| >Second | 3 (15.8) | 2 (10.5) | 1 (4.2) | 4(40) |
| No. of target lesions, n (%) | ||||
| 1 | 17 (89.5) | 15 (78.9) | 22 (91.7) | 4 (40%) |
| >1 | 2 (10.5) | 4 (21.1) | 2 (8.3) | 6 (60%) |
| Tumor area (mm2) mean, mediana | 794, 555 | 1107, 693 | 1388, 1064 | 3205, 3036 |
| No. of prior lines of therapy | ||||
| 1, n (%) | 13 (68.4) | 14 (73.7) | 16 (66.7) | 6 (60.0) |
| 2, n (%) | 3 (15.8) | 3 (15.8) | 8 (33.3) | 3 (30.0) |
| >2, n (%) | 3 (15.8) | 2 (10.5) | 0 | 1 (10.0) |
| MGMT methylation status, n (%) | ||||
| Methylated | 4 (21.1) | 4 (21.1) | 9 (37.5) | |
| Unmethylated | 5 (26.3) | 10 (52.6) | 5 (20.8) | |
| Unknown | 10 (52.6) | 5 (26.3) | 10 (41.7) | 10 (100) |
a Sum of products of perpendicular diameters per central imaging assessment.
Differences between O6-methylguanine-DNA methyltransferase (MGMT) status between groups were not statistically significant (P = 0.18, Fisher’s exact test); however, the MGMT methylation status remained unknown for 50% of patients due to lack of testing at referring centers or available archival specimens.
By the date of data cutoff, patients had received up to 13 doses of VB-111. The median (mean) number of doses was 1 (2.2) in the LE group, 4 (4.7) Q56 day doses in the primed combination group, and 3.5 (4.8) Q28 day doses in the unprimed combination group.
Biodistribution
Biodistribution of the VB-111 Ad-5 vector was assessed for 35 subjects and showed a uniform peak of approximately 107 adenovirus DNA copies/μg DNA in the blood immediately following VB-111 infusions; no attenuation of peak levels was observed with repeated dosing. All patients had rapid clearance of viral DNA levels within several hours post-infusion, with a drop of 2–3 logs. Upon repeat dosing, in 60% of the patients, viral DNA levels dropped to zero after the treatment, while 40% of patients retained basal viral DNA levels between the first few initial doses, dropping to zero in-between doses after a few cycles (see Supplementary Figure S1). The elimination of viral DNA from the blood indicated no accumulation of the virus and supports the safety of bimonthly dosing.
Safety and Tolerability
In the phase I portion of the study, dose escalation proceeded to the maximum planned dose level of 1 × 1013 VP. No DLTs were observed, and MTD was not reached. Table 2 summarizes reported adverse events (AEs). Approximately one-half of patients treated with 1 × 1013 VP developed self-limiting fever and/or flu-like symptoms starting a few hours post-infusion, characteristic of infection with a viral vector; these events were generally grades 1–2 and responded to antipyretic treatment. The rate of grade ≥3 AEs ranged 13‒41% in the first 3 groups, and was higher (80%) in the unprimed combination group. As expected in this patient population, most of these events (up to 40%) were central nervous system related (ie, neurologic and psychiatric). Four patients in the VB-111 and bevacizumab combination groups reported grade 3 events in the Medical Dictionary for Regulatory Activities (MedDRA) Vascular Disorders System Organ Class: 3 events were in the primed combination group (hypertension: n = 2; deep vein thrombosis: n = 1) and 1 event of hypertensive urgency with acute kidney injury was reported in the unprimed combination group.
Table 2 .
Adverse events, n (%)
| Event | SubT n = 16 | LE-DE n = 22 | Primed Combination n = 24 | Unprimed Combination n = 10 |
|---|---|---|---|---|
| Any TEAE | 15 (93.8) | 21 (95.5) | 24 (100) | 10 (100.0) |
| AE leading to study drug discontinuation | 0 | 0 | 2 (8.3) | 3 (30.0) |
| Serious AE | 2 (12.5) | 9 (40.9) | 10 (41.7) | 8 (80.0) |
| Most Frequent AE a by PT | ||||
| Pyrexia | 3 (18.8) | 12 (54.5) | 14 (58.3) | 1 (10) |
| Chills | 0 | 7 (31.8) | 9 (37.5) | 3 (30.0) |
| Fatigue | 5 (31.3) | 9 (40.9) | 10 (41.7) | 1(10) |
| Headache | 4 (25.0) | 4 (18.2) | 7 (29.2) | 1(10) |
| Seizure | 0 (0.0) | 3 (13.6) | 8 (33.3) | 1(10) |
| Nausea | 1 (6.3) | 8 (36.4) | 6 (25.0) | 0 |
| Hypertension | 0 (0.0) | 2 (9.1) | 5 (20.8) | 3 (30.0) |
| Grade ≥3 AE by SOC b | 2 (12.5) | 9 (40.9) | 9 (37.5) | 8 (80.0) |
| Blood and lymphatic system | 0 | 1 (4.5) | 0 | 1 (10.0) |
| Eye disorders | 0 | 1 (4.5) | 1 (4.2) | 0 |
| Gastrointestinal | 1 (6.3) | 1 (4.5) | 1 (4.2) | 0 |
| General disorders and administration site conditions | 0 | 2 (9.1) | 0 | 3 (30.0) |
| Infections and infestations | 0 | 1 (4.5) | 1 (4.2) | 2 (20) |
| Investigations | 0 | 0 | 0 | 1 (10.0) |
| Musculoskeletal and connective tissue | 0 | 2 (9.1) | 1 (4.2) | 0 |
| Nervous system | 0 | 5 (22.7) | 6 (25.0) | 2 (20.0) |
| Psychiatric | 0 | 1 (4.5) | 1 (4.2) | 2 (20.0) |
| Respiratory | 2 (12.5) | 0 | 0 | 1 (10.0) |
| Vascular disorders | 0 | 0 | 3 (12.5) | 1 (10.0) |
Abbreviations: LE-DE, limited exposure‒dose escalation; TEAE, treatment-emergent adverse event; PT, preferred term; SOC, system organ class. Note: subjects are counted only once per cohort for each row.
a TEAEs reported in ≥25% of subjects in any group presented by MedDRA preferred term. b Presented by MedDRA system organ class.
AEs leading to treatment withdrawal were reported by 5 patients receiving combination treatment; 2 patients (8.3%) in the primed combination and 3 patients (30%) in the unprimed combination. The AEs reported by these patients were all considered by the investigators as unrelated to study medication and included 2 cases of death due to disease progression and 1 event each of port infection, general aches (continuing several months after drug discontinuation), and blurred vision and aphasia (both reported by a single patient who was considered to have disease progression).
Radiographic Response and Initial Tumor Volume Decrease
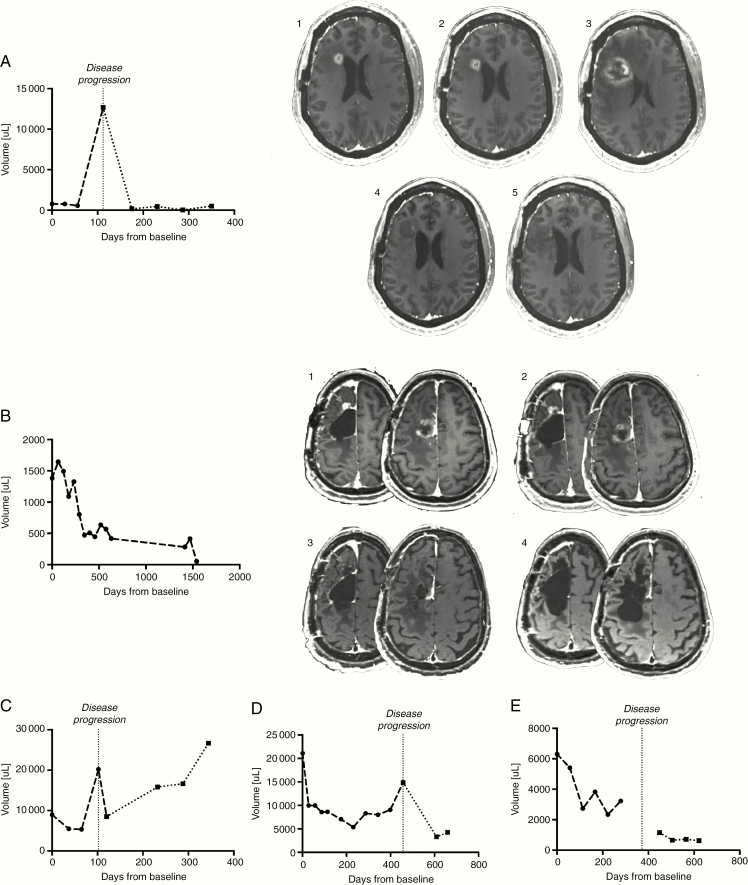
Tumor response according to RANO was documented in 5 patients (21%) treated with primed combination, and the responders exhibited characteristic, expansive areas of necrosis in the areas of initial enhancing disease (Fig. 2A–E). At least 1 patient presented initial pseudoprogression (Fig. 2A). Four of the responders had a partial response (PR), and 1 had a complete response (CR) that was observed during the monotherapy priming phase and maintained for >5 years; response was first observed as PR at study day 392 and later improved to CR on day 504 (Fig. 2B). Best response of PR was observed in 2 patients (20%) in the unprimed combination group. In the SubT and LE groups, CR/PR were not reported and the best response was stable disease in 12 patients (63%) and 10 patients (53%), respectively.
Fig. 2.
(A–E) Tumor volume change over time in radiographic responders from the primed combination group. Patients in the primed combination group exhibiting radiographic evidence of tumor shrinkage on VB-111 monotherapy prior to progression. Vertical line = time of noted disease progression. Dashed lines showing volumes to the left of the vertical line indicate time on VB-111 monotherapy, while dotted lines to the right of the vertical lines indicate time on combination VB-111 and bevacizumab. 2A, 2B: MRI series of 2 of the radiographic responders demonstrating specific radiographic changes: characteristic, expansive areas of necrosis in the areas of initial enhancing disease (2B), and pseudoprogression (2A).
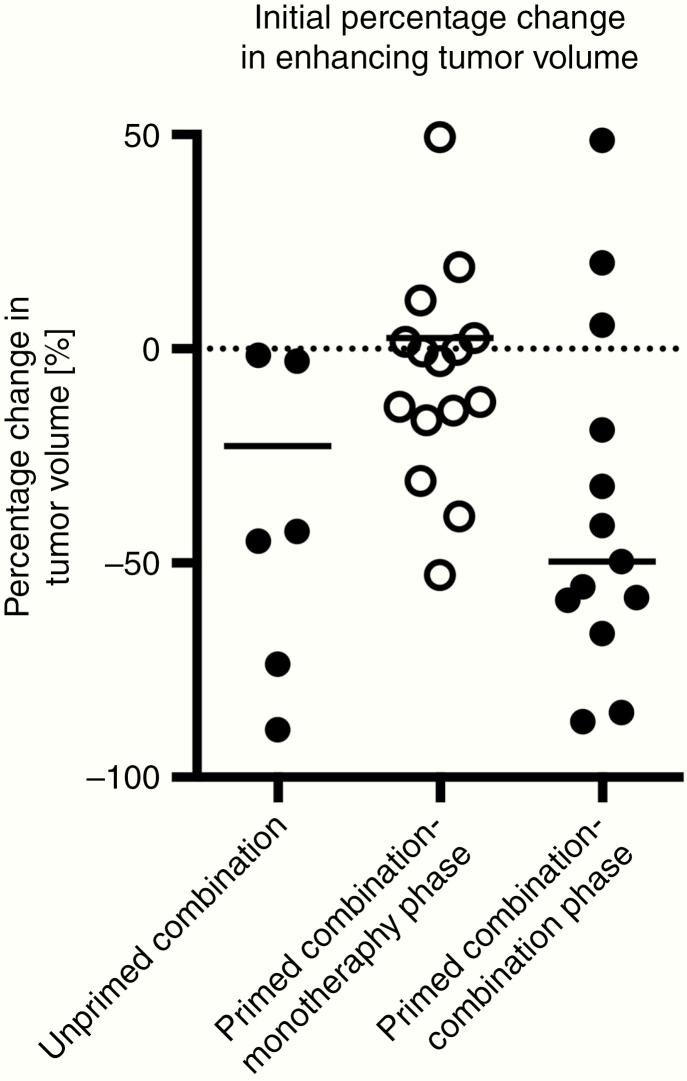
A similar tumor growth rate was observed in patients treated with VB-111 monotherapy in the LE and primed combination monotherapy phase (Supplementary Figure S2). We assessed whether the administration of bevacizumab after VB-111 priming results in a different response compared with the administration of bevacizumab without VB-111 priming. Interestingly, combination treatment with VB-111 + bevacizumab given after VB-111 priming resulted in a larger initial median decrease in tumor volume (49.7%) compared with the upfront combination treatment received by the unprimed group (22.7%), and a median decrease of 33% for bevacizumab monotherapy (UCLA institutional data). A one-sample t-test applied to the mean percentage change in tumor volume illustrated that the response in the combination phase of the primed combination group resulted in a statistically significant decrease in percentage tumor volume compared with baseline (Fig. 3) (t-test, P = 0.0068).
Fig. 3.
Initial percentage change in enhancing tumor volume. A significant decrease in tumor volume compared with baseline is seen after the first dose of bevacizumab in patients who received VB-111 priming (combination phase of the primed combination group) compared with patients who were not primed with VB-111, in the unprimed combination.
Progression-free and Overall Survival
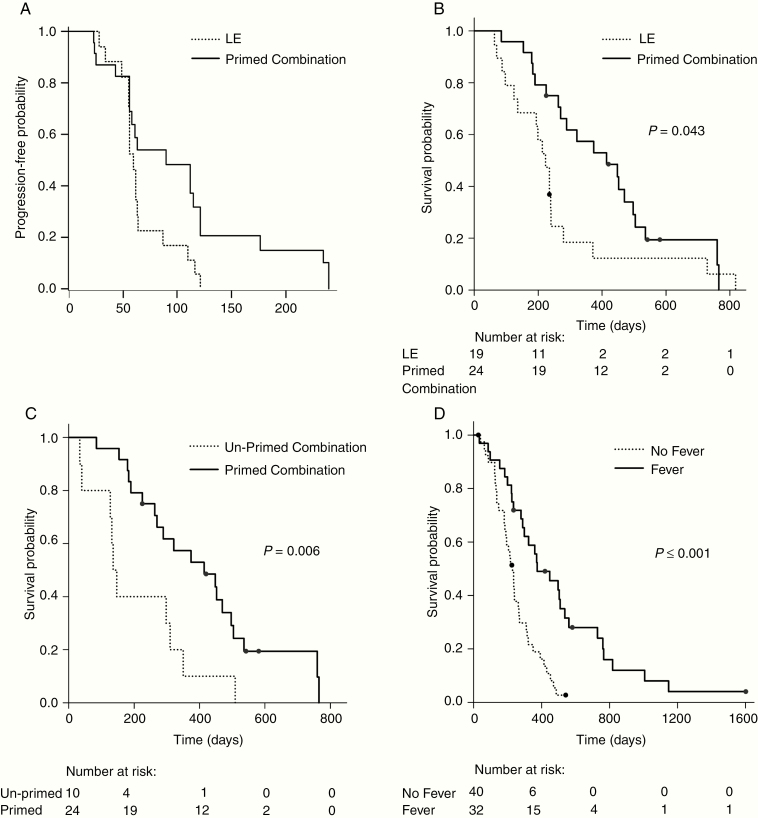
Median PFS times assessed by central imaging review were 55, 60, 61, 90, and 63 days for the SubT, LE, primed combination monotherapy phase, combination phase, and unprimed combination groups, respectively. The median PFS times on VB-111 monotherapy in primed combination and LE were similar (61 and 60 days, respectively). Nevertheless, the median PFS in the primed combination group at the combination phase (from start of combination therapy until further disease progression) was 90 days versus 60 days for the LE group (hazard ratio [HR], 0.36; 95% CI: 0.14–0.93; P = 0.032, log-rank) (Fig. 4A) and versus 63 days for the unprimed combination (HR, 1.24; 95% CI: 0.45–3.40).
Fig. 4.
Overall survival (OS) and progression-free survival (PFS). (A) PFS: LE vs primed combination, combined phase; P = 0.01 (log-rank). (B) OS: LE vs primed combination; P = 0.043 (log-rank). (C) OS: unprimed combination vs primed combination; P = 0.006 (log-rank). (D) OS: fever vs no fever; P < 0.001 (log-rank).
Median OS was 315 days, 223 days, 414 days, and 141.5 days in the SubT, LE, primed combination, and unprimed combination groups, respectively. OS was significantly longer in the primed combination compared with the LE group (HR, 0.48; 95% CI: 0.23–0.998; P = 0.043, log-rank; Fig. 4B) and compared with the unprimed combination group (HR, 0.24; 95% CI: 0.09–0.66; P = 0.0056, log-rank; Fig. 4C). Twelve-month survival rates were 39% for SubT, 18% for LE, 57% for primed combination, and 10% for the unprimed combination. The 12-month OS rate of the historical control group was 24% versus 57% in the primed combination (P = 0.03). Post hoc analysis demonstrated that within the primed combination group, patients with tumors smaller than 25 mL had improved OS compared with those with larger tumors. Development of a febrile reaction post-infusion occurred in 45% of patients in the first 3 groups (N = 62) and was associated with improved survival (Fig. 4D), with a median OS of 448 days versus 235 days in patients with and without fever (HR, 0.34; 95% CI: 0.18–0.62; P < 0.001).
Discussion
Recurrent GBM is a devastating disease with poor prognosis and a median OS of about 7 months. There is a great unmet need for novel therapies that will prolong patient survival, and accordingly survival was selected as the primary endpoint in this study. Our results demonstrate a survival benefit in patients with rGBM treated with VB-111 priming followed up on disease progression with combination of VB-111 and bevacizumab (median OS, 414 compared with 223 days in the LE group). Survival advantage was also seen in comparison to historic controls where percentage of patients living more than one year doubled from 24% to 57%.
The survival benefit was accompanied with a significant increase in PFS in the combination phase of the primed combination group. Nevertheless, analysis of drug activity based on PFS can be challenging with regard to vasculature-modifying agents. Anti-angiogenic or vascular disruptive agents may affect vascular permeability in a manner that increases contrast enhancement due to edema and may lead to misinterpretation of data. Bevacizumab, a vascular endothelial growth factor (VEGF) blocking antibody, “normalizes” blood vessels and leads to reduced edema and thus improves PFS; however, this effect is not translated to OS. On the other hand, VB-111 disrupts tumor vasculature and increases angiogenic/vascular permeability and inflammation in the tumor environment and therefore increases edema, which may be misinterpreted as progression. In fact, in patients receiving VB-111 monotherapy, the rate of initial presumable progression was rapid, which may have been representative of pseudoprogression in some cases, as evident by MRI (Fig. 2A) and the substantial decrease in tumor volume once repeating dose of VB-111 in combination treatment was added. Furthermore, unlike chemotherapy or bevacizumab, which may affect tumor volume quickly, the mechanism of VB-111 involves tumor starvation and immune response, which are slower processes. Thus in patients treated with VB-111, PFS may be misleading and OS is a preferred means to assess efficacy.
The similar tumor growth rate and PFS that were reported in the monotherapy phase of the primed combination group and the LE group are expected, since all patients were treated at this phase with VB-111 monotherapy. This similarity provides further validation that the significantly different OS seen between these 2 groups is not related to different prognostic characteristics. Also of interest are the characteristic radiographic changes among responders to VB-111 (monotherapy and combination) with expansive areas of necrosis in the areas of initial enhancing disease, which is not typical for other anti-angiogenic drugs such as bevacizumab. Previous work has shown a significant survival advantage in patients who exhibit a substantial decrease in their enhancing tumor.20,23 Indeed, this matches the observation of the significantly large decrease in tumor volume during combination therapy after VB-111 priming, which was followed by better survival outcome compared with the unprimed cohort.
Administration of dexamethasone was required due to the potential of the study drug to disrupt tumor vasculature and to increase vasogenic edema. It is possible that any VB-111 immune mediated effect would have been increased without concurrent steroids. Nevertheless, results were encouraging despite the administration of corticosteroids.
In the small group of patients in the unprimed combination group, radiographic responses were not associated with a survival advantage; in fact, survival was even less than expected with bevacizumab alone. The lack of survival benefit could be related to the poor prognostic baseline characteristics of this group, as well as to the different treatment regimen of upfront combination treatment with VB-111 and bevacizumab. It is plausible that priming with VB-111 monotherapy is essential to VB-111 activity and that the upfront addition of bevacizumab blocks VB-111 activity, possibly due to antagonistic mechanisms of action. The PPE-1 promoter is activated by VEGF, and lack of VEGF reduces PPE-1-3x promoter-regulated Fas-chimera expression and prevents VB-111 activity.12
Given the heavy burden of disease and morbidity associated with rGBM, the tolerability of combination therapy is a prominent concern. VB-111 was well tolerated. Dose escalation progressed as planned, MTD was not reached, and discontinuation rate due to AE toxicity was low. The rate of grade 3 or higher AEs in the LE and primed combination groups was ~40% and most commonly related to CNS AEs, as expected in this population. This rate compares favorably with single-arm studies in this indication and is lower than that reported for bevacizumab combined with either lomustine or irinotecan.3,9,27,34 A signal for increased rate of grade ≥3 AEs was noted in the unprimed combination group, but due to the small sample size of this treatment group as well as their having more advanced disease, it could not be confirmed.
The most common AE of any grade associated with VB-111 was a mild-moderate febrile response, which occurred in approximately 50% of patients treated with the therapeutic dose. Interestingly, the development of a febrile response was associated with improved survival, suggesting that fever may be a biomarker for better survival with VB-111, possibly related to the drug’s immunologic mechanism of action.
This study has several limitations that mandate cautious interpretation of the observed results. The enrolled population included a heterogeneous group of patients as any number of prior therapies and recurrences were allowed as long as the patients were bevacizumab naive. Allocation to treatment groups was not randomized but rather sequential, attributing to unbalanced unfavorable baseline characteristics of the unprimed combination group, which had a substantially higher tumor volume at baseline. The small sample size of the unprimed combination group and its different baseline characteristics confound the interpretation of the efficacy and safety results of this group. The lack of a bevacizumab-only control arm is another limitation; however, the comparable OS among patients in the LE group (which received a median of one VB-111 dose) and the survival reported for historical controls3,26–32 serve as internal references and argue against any potential bias that may have accounted for improved survival in the primed combination group. Another limitation is the lack of isocitrate dehydrogenase mutation and MGMT methylation status for a substantial proportion of patients, although these prognostic factors are not expected to mechanistically influence the activity of VB-111. Finally, there is an inherent challenge in the analysis due to the evolving design of the study, allowing intrapatient dose escalation, and introducing further dosing cohorts with bevacizumab combination; this was due to the nature of early stage and dynamic development of a novel viral-based therapy in a devastating disease condition. Due to the study’s sequential design, the survival results of the unprimed combination arm were not available during the design and conduct of the following controlled phase III GLOBE trial, which used an unprimed combination treatment regimen, and was a negative trial.35
In summary, our results propose that VB-111 monotherapy priming that is continued after progression with the addition of bevacizumab is associated with a significant OS and PFS benefit, with a favorable safety profile and a typical radiologic response. The observed radiologic response and survival benefit of the VB-111 primed combination regimen merit further investigation in a randomized controlled trial.
Supplementary Material
Acknowledgments
The authors would like to thank the participating patients and their families as well as the study site personnel. Data presented within this article were previously presented in part at the 2016 Annual Meeting of the American Society for Clinical Oncology.
Funding
This work was supported by VBL Therapeutics.
Conflict of interest statement. YCC, IM, NL-S, TRM, NS, and EB are employees of VBL Therapeutics. BO and LSF are statistical consultants to VBL Therapeutics, and their institute (Gertner Institute for Epidemiology and Health Policy Research) received payment for the statistical advice and analysis provided. AJB, TC, and PYW participated in the GLOBE Steering Committee for which they received honoraria from VBL Therapeutics. AJB received research funding from VBL Therapeutics.
Authorship statement. Experimental design: YC, NS, NL-S, IM, EB, AJB, PYW. Study implementation: AJB, KBP, JV, FB, DTB, SY-K, IP, PYW. Analysis and interpretation: AJB, LSF, BO, BME, TFC, NS, YCC, NL-S, TRM, NY. Writing support was provided by VBL Therapeutics. All authors contributed to manuscript draft writing and review.
References
- 1. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(Suppl 5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 5. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 6. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang SB, Gao KD, Jiang T, Cheng SJ, Li WB. Bevacizumab combined with chemotherapy for glioblastoma: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8(34):57337–57344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Field KM, Jordan JT, Wen PY, Rosenthal MA, Reardon DA. Bevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversies. Cancer. 2015;121(7):997–1007. [DOI] [PubMed] [Google Scholar]
- 9. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 10. Greenberger S, Shaish A, Varda-Bloom N, et al. Transcription-controlled gene therapy against tumor angiogenesis. J Clin Invest. 2004;113(7):1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harats D, Kurihara H, Belloni P, et al. Targeting gene expression to the vascular wall in transgenic mice using the murine preproendothelin-1 promoter. J Clin Invest. 1995;95(3):1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varda-Bloom N, Shaish A, Gonen A, et al. Tissue-specific gene therapy directed to tumor angiogenesis. Gene Ther. 2001;8(11):819–827. [DOI] [PubMed] [Google Scholar]
- 13. Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–567. [DOI] [PubMed] [Google Scholar]
- 14. Penson RT, Berlin ST, Hanbury AM, et al. [5551] Tumor responses and preliminary survival data in a phase II trial of ofranergene obadenovac (VB-111) combined with paclitaxel in patients with recurrent platinum resistant ovarian cancer [abstract]. J Clin Oncol. 2016;34:5551–5551. [Google Scholar]
- 15. Gruslova A, Cavazos DA, Miller JR, et al. VB-111: a novel anti-vascular therapeutic for glioblastoma multiforme. J Neurooncol. 2015;124(3):365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brenner AJ, Cohen YC, Breitbart E, et al. Phase I dose-escalation study of VB-111, an antiangiogenic virotherapy, in patients with advanced solid tumors. Clin Cancer Res. 2013;19(14):3996–4007. [DOI] [PubMed] [Google Scholar]
- 17. Sina J, Michael M, Smallridge R, et al. [poster 638] A multi-cohort phase II trial of VB-111 in advanced radioactive iodine-refractory differentiated thyroid cancer [abstract]. Presented at: the 15th International Thyroid Congress; October 18–23, 2015; Lake Buena Vista, FL.
- 18. Ellingson BM, Bendszus M, Boxerman J, et al. ; Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11): 1963–1972. [DOI] [PubMed] [Google Scholar]
- 20. Ellingson BM, Kim HJ, Woodworth DC, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellingson BM, Nguyen HN, Lai A, et al. Contrast-enhancing tumor growth dynamics of preoperative, treatment-naive human glioblastoma. Cancer. 2016;122(11):1718–1727. [DOI] [PubMed] [Google Scholar]
- 22. Ellingson BM, Harris RJ, Woodworth DC, et al. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol. 2017;19(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellingson BM, Aftab DT, Schwab GM, et al. Volumetric response quantified using T1 subtraction predicts long-term survival benefit from cabozantinib monotherapy in recurrent glioblastoma. Neuro Oncol. 2018;20(10):1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellingson BM, Abrey LE, Nelson SJ, et al. Validation of postoperative residual contrast-enhancing tumor volume as an independent prognostic factor for overall survival in newly diagnosed glioblastoma. Neuro Oncol. 2018;20(9):1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellingson BM, Abrey LE, Garcia J, et al. Post-chemoradiation volumetric response predicts survival in newly diagnosed glioblastoma treated with radiation, temozolomide, and bevacizumab or placebo. Neuro Oncol. 2018;20(11):1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duerinck J, Clement PM, Bouttens F, et al. Patient outcome in the Belgian medical need program on bevacizumab for recurrent glioblastoma. J Neurol. 2015;262(3):742–751. [DOI] [PubMed] [Google Scholar]
- 27. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 28. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chamberlain MC, Johnston SK. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol. 2010;96(2):259–269. [DOI] [PubMed] [Google Scholar]
- 30. Field KM, Simes J, Nowak AK, et al. ; CABARET/COGNO investigators Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17(11):1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagane M, Nishikawa R, Narita Y, et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol. 2012;42(10):887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen C, Ravelo A, Yu E, Dhanda R, Schnadig I. Clinical outcomes with bevacizumab-containing and non-bevacizumab-containing regimens in patients with recurrent glioblastoma from US community practices. J Neurooncol. 2015;122(3):595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fay MP, Shaw PA. Exact and asymptotic weighted logrank tests for interval censored data: the interval R package. J Stat Softw. 2010;36(2):i02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weathers SP, Han X, Liu DD, et al. A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma. J Neurooncol. 2016;129(3):487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cloughesy TF, Brenner A, de Groot JF. A randomized controlled phase III study of VB-111 combined with bevacizumab vs. bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro Oncol. 2020;22(5):705–717. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.