Abstract
Objective
Hyperleptinemia per se is sufficient to promote leptin resistance in the obese state. Leptin sensitivity can be restored by reducing circulating leptin levels within a physiologically healthy range and is a viable antiobesity and antidiabetic strategy. However, a previous study suggests that partial leptin deficiency favors diet-induced obesity and related metabolic disorders in mice, arguing that a lower leptin level may indeed promote diet-induced obesity and its associated metabolic disorders. Here, we aim to elucidate what the impact of partial leptin deficiency is on fat mass and insulin sensitivity.
Methods
We used two different mouse models of partial leptin deficiency: an adipocyte-specific congenital heterozygous leptin knockout mouse line (LepHZ) and the well-established whole body heterozygous leptin knockout mouse (OBHZ). The metabolic studies of OBHZ and LepHZ mice were performed both on normal carbohydrate-rich chow diet and on a high-fat diet (HFD). Male and female mice were included in the study to account for sex-specific differences. Body weight, food intake, glucose tolerance, and insulin tolerance were tested. Histology of adipose tissue and liver tissue allowed insights into adipose tissue inflammation and hepatic triglyceride content. Immunohistochemistry was paired with RT-PCR analysis for expression levels of inflammatory markers.
Results
Both OBHZ and LepHZ mice displayed reduced circulating leptin levels on the chow diet and HFD. On chow diet, male OBHZ and LepHZ mice showed elevated fat mass and body weight, while their glucose tolerance and insulin sensitivity remained unchanged. However, the inability in partially leptin-deficient mice to fully induce circulating leptin during the development of diet-induced obesity results in reduced food intake and leaner mice with lower body weight compared to their littermate controls. Importantly, a strong reduction of adipose tissue inflammation is observed along with improvements in insulin sensitivity and enhanced glucose tolerance. Additionally, partial leptin deficiency protects the mice from fatty liver and liver fibrosis. Chronically HFD-fed OBHZ and LepHZ mice remain more sensitive to exogenous leptin injection, as reflected by their reduced food intake upon an acute leptin treatment.
Conclusion
In response to HFD feeding, the inability to upregulate leptin levels due to partial leptin deficiency protects mice from diet-induced obesity and metabolic dysregulation. Thus, in an obesogenic environment, maintaining lower leptin levels is highly beneficial for both obesity and diabetes management. Chronic leptin reduction represents a viable preventive strategy whose efficacy awaits clinical testing.
Keywords: Leptin, Partial leptin deficiency, Leptin resistance, Adipose tissue inflammation, Fatty liver, Liver fibrosis, Obesity
Highlights
-
•
Partial leptin deficiency protects from diet-induced obesity.
-
•
Reduced leptin protects from diet-induced obesity independent of sex.
-
•
Reduction of circulating leptin levels inhibits HFD-induced adipose tissue inflammation.
-
•
Partial leptin deficiency confers resistance to HFD-induced liver fibrosis.
-
•
Partial leptin deficiency enhances leptin sensitivity.
1. Introduction
The overconsumption of calorically dense foods coupled to a sedentary lifestyle dramatically increases the risk for the development of obesity. Obesity positively correlates with a reduction in health span, i.e., the loss of disease-free years [1], because it puts an individual at high risk toward the development of many pathological sequelae, such as coronary heart disease, stroke, liver cirrhosis, type 2 diabetes (T2D), and a number of different cancer types [1,2]. The continuous global increase of obesity highlights the urgent need for the development of new preventive treatment modalities aimed at reducing the obesity incidence [2,3].
The identification and elucidation of the physiological roles of two key adipokines, leptin and adiponectin, changed the accepted view of adipose tissue from an energy storage organ to an active endocrine organ. Leptin specifically entered the limelight as a factor with the promise to be a potent antiobesity agent [4,5]. Leptin is predominately (though not exclusively) secreted from adipose tissue. Genetic inactivation of the lep gene in adipose tissue leads to undetectable levels of leptin in circulation [6]. The physiological importance of leptin is best highlighted by the classical leptin-null mouse model—the ob/ob mouse. These mice carry a homozygous nonsense mutation of lep, characterized by massive obesity, severe insulin resistance, and many other metabolic disorders [7]. The extraordinary ability of leptin to promote energy expenditure and reduce food intake in lean mice is well documented. Injection of recombinant leptin yields the expected results, increasing energy expenditure and reducing food intake [8,9]. Of special note, leptin can completely rescue the ob/ob phenotype and reverse severe insulin resistance and fatty liver in mice and humans with lipodystrophy [[10], [11], [12]]. However, in initial clinical trials, the long-term treatment of obese patients with supraphysiological doses of leptin casts doubt on the ability of recombinant leptin to act as an antiobesity factor [8]. Many studies reported that the treatment of diet-induced obesity with recombinant leptin is largely ineffective [[13], [14], [15]]. This can be explained by the fact that obese mice or humans do not lack leptin; instead, they display very high levels of circulating leptin (“hyperleptinemia”). This has led various investigators to suggest that hyperleptinemia per se may induce leptin resistance [[16], [17], [18]]. Furthermore, hyperleptinemia-associated leptin resistance may promote metabolic disorders under obesogenic conditions [[19], [20], [21]]. Similarly, chronic overexpression of leptin enhances obesity development and its associated metabolic disorders [22]. Consistent with these observations, we recently demonstrated that increasing leptin levels in obese mice, with genetically inducible overexpression of leptin in adipose tissue, further accelerates weight gain and metabolic disorders, even upon increasing circulating leptin levels by as little as 50% [23]. Conversely, we have shown that reducing circulating leptin, either by doxycycline-induced partial leptin deletion or with a neutralizing monoclonal leptin antibody, reduces body weight gain and greatly improves glucose tolerance and insulin sensitivity [23]. Based on these observations, we propose that limiting the rise in leptin levels under obesogenic conditions exerts beneficial effects on energy homeostasis [45].
In contrast to all the data above, an older study claims that partial leptin deficiency favors obesity, nonalcoholic steatohepatitis, and other metabolic abnormalities, particularly under conditions of high caloric intake [24]. This casts doubt on the beneficial effects of a lower leptin level under obesogenic conditions. Our study here aims to revisit this question as to whether congenital partial leptin deficiency impacts the development of diet-induced obesity and its associated metabolic abnormalities. To that end, we examined two mouse models with partial leptin deficiency: the classical ob heterozygous mice (OBHZ) and mice with only one copy of an intact lep locus, the other one eliminated by an adiponectin promoter-driven constitutively expressed Cre (LepHZ). Our results generated from partially leptin-deficient mice fully support the idea that the inability to effectively increase leptin levels during obesity due to a 50% reduction in gene dosage slows down diet-induced obesity, accompanied by enhanced glucose tolerance and insulin sensitivity.
2. Material and methods
2.1. Animals
All of the animal experimental protocols have been approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center at Dallas. The mice were housed under standard laboratory conditions (12 h on/off; lights on at 7:00 a.m.) and the temperature-controlled environment with food and water available ad libitum. Mice were fed a standard chow diet (number 5058, LabDiet, St. Louis, MO, USA) or high-fat diet (HFD) (60% energy from fat, made by BioServ, Frenchtown, NJ, USA) for various periods as indicated in the figures. All experiments were initiated at approximately 8 or 9 weeks of age, unless indicated otherwise. Mouse phenotyping studies were performed with littermate controls and a minimum of two independent cohorts with more than five mice in each group.
OBHZ mice (Jax no. 000632) were obtained from Jackson Labs (Bar Harbor, ME, USA). This mouse colony is on a pure C57BL6 background. Leptin floxed mice on a 129 background were kindly provided by the Dr. Childs lab at the University of Arkansas College of Medicine of [6]. These mice carrying a floxed leptin locus were extensively backcrossed to a C57BL6 background (Jax no. 000664) for more than eight generations [23]. Adiponectin-Cre mice (Jax no. 028020) were obtained from Jackson Labs and maintained on a pure C57BL/6 background.
2.2. Food intake and body weight
In order to measure food intake and body weight gain, OBHZ, LepHZ, and littermate controls were single-housed. Before each experiment, the mice were acclimated in the single cage for at least one week to reduce stress. For some of the studies, the food intake and body weights were measured on a daily basis, while in some chronic studies, these parameters were measured once a week, as indicated in the figure legends.
In order to test leptin sensitivity, OBHZ, LepHZ, and littermate control mice were starved overnight. The next day, mice were injected with leptin (National Hormone & Peptide Program, Harbor-UCLA Medical Center) at a dose of 0.5 mg per kg body weight. Subsequently, food intake was measured at the following time points: 0, 1 h, 2 h, 4 h, 8 h, 24 h, and 30 h.
2.3. Glucose and insulin tolerance test
Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed as previously described [25,26]. For GTTs, the mice were fasted for 4–6 h in the morning, and then the mice orally received 2 g of glucose per kg body weight dissolved in phosphate-buffered saline (Cat. 806,552, Sigma–Aldrich). Injection volume was calculated based on 10 μL/g body weight. Blood glucose concentrations were measured by glucose meters (Contour) at the indicated time points. For ITTs, mice were fasted for 6 h in the morning, and chow-fed animals were intraperitoneally injected with insulin at a dose of 0.25 U per kg body weight, while HFD-fed animals were injected with a dose of 0.5 U per kg body weight. Blood glucose concentrations were measured by glucose meter at the indicated time points. For some of the experiments, the area under the curve (AUC) was calculated.
2.4. Blood parameters
Blood was taken from fed animals in the morning and was centrifuged at 8000 g for 5 min, and then the supernatants were collected for multiple analyses. Leptin was measured using an ELISA kit from Crystal Chem (catalog number: 90,080). Adiponectin was measured using an ELISA kit from Invitrogen (catalog number: EZMADP-60K). Estradiol was measured using an ELISA kit from Calbiotech (catalog number: ES180S-100).
2.5. RT-qPCR and analysis
RNA was extracted from fresh or frozen tissues by homogenization in TRIzol reagent (Invitrogen) as previously described [27]. We used 1 μg RNA to transcribe cDNA with a reverse transcription kit (Bio-Rad). Most of RT-qPCR primers were from the Harvard PrimerBank (https://pga.mgh.harvard.edu/primerbank/). The relative expression levels were calculated using the comparative threshold cycle method, normalized to the housekeeping gene Rps16.
2.6. Histology
Histology was done as previously described [28]. In brief, adipose tissues and livers were excised and fixed overnight in 10% PBS-buffered formalin and were, thereafter, switched to 50% ethanol for long time storage. Tissues were processed at the UTSW Molecular Pathology Core.
2.7. Immunofluorescence
Immunofluorescence was done as previously described [29]. In brief, formalin-fixed, paraffin-embedded sections from white adipose and brown adipose tissues were blocked in PBST with 5% BSA. Primary antibodies used were against perilipin (1:500 dilution NB100-60554, Novus), UCP1 (1:250 dilution, ab10983, Abcam), or Mac2 (1:500 dilution, Cat#: 125,401, BioLegend). Secondary antibodies (1:250 dilution) used were Alexa Fluor 488 or 594 donkey anti-rabbit IgG (HCL) or Alexa Fluor 488 or 594 donkey anti-goat IgG (HCL) (Invitrogen). Slides were counterstained with DAPI. Fluorescent images were acquired using an AxioObserver Epifluorescence Microscope (Zeiss) or an FSX100 microscope (Olympus).
2.8. Statistical analysis
All values are expressed as the mean ± SEM. The significance between the mean values for each study was evaluated by Student's t-tests for the comparisons of the two groups. One-way or two-way ANOVA was used for the comparisons of more than two groups. P ≤ 0.05 is regarded as statistically significant.
3. Results
3.1. Partial leptin deficiency on chow diet slightly increases body weight gain
In order to assess the effects of partial leptin deficiency on body weight gain and glucose homeostasis, we applied two different mouse models that carry a heterozygous deletion of one of the lep alleles. The first model we used is the heterozygous ob mouse (OBHZ) that represents a congenital partial leptin deficiency. The second mouse model is the Cre-loxP-mediated heterozygous adipocyte-specific lep gene deletion mouse (LepHZ), in which Cre expression is limited to adipocytes, driven by the adiponectin promoter.
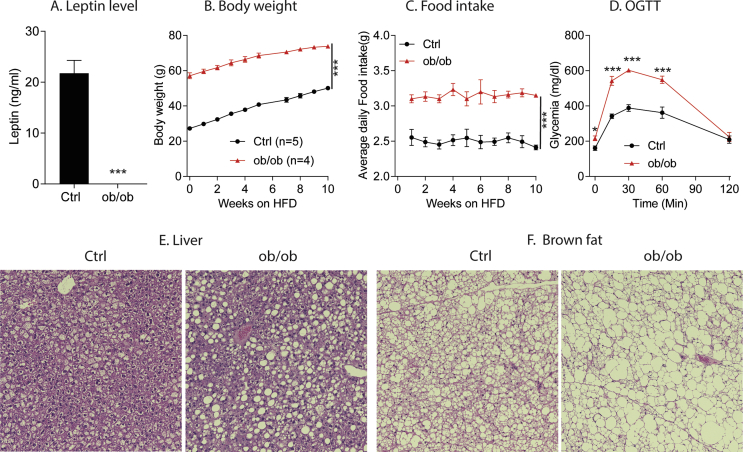
In order to make sure that the OBHZ mice used in this study represent a real partial leptin deficiency model, we first crossed male and female OBHZ mice to generate classical ob/ob mice and littermate controls (Ctrl). As expected, circulating levels of leptin in our ob/ob mice were completely undetectable with the specific ELISA kit used (Figure S1A). Also, ob/ob mice are massively obese with greatly increased food intake (Figure S1B and C) and greatly impaired glucose tolerance (Figure S1D). In addition, ob/ob mice display severe fatty liver and “whitening” of brown adipose tissue, compared to littermate Ctrl mice (Figure S1E and F). All these results suggest that our ob/ob mice are indeed fully deficient for leptin.
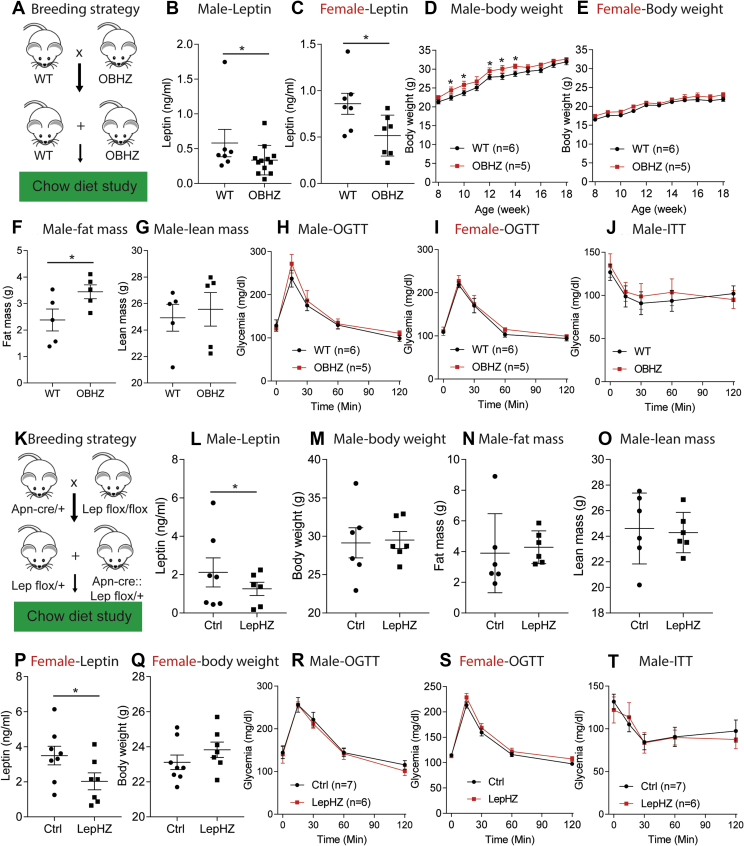
In order to study the effects of partial leptin deficiency on glucose homeostasis, we crossed OBHZ mice with wild type (WT) mice to obtain approximately 50% OBHZ and littermate control WT mice (Figure 1A). Circulating leptin levels were measured on 8-week-old chow-fed male and female mice. As both gene copies are transcriptionally active in a wildtype mouse, leptin serum concentrations were reduced by approximately half in both male and female OBHZ mice (Figure 1B,C). On chow diet, only male, but not female, OBHZ mice increased their body weights (Figure 1D,E), which can be attributed to an increase in fat mass (Figure 1F) without a change in lean mass (Figure 1G). Furthermore, GTTs and ITTs were performed on chow-fed OBHZ and WT mice. In both male and female mice, glucose and insulin tolerance remained unchanged (Figure 1H–J). As expected, chronic reduction of leptin leads to a weight gain, which did not, however, translate into the expected change in glucose homeostasis.
Figure 1.
Partially leptin-deficient mice on chow diet display a marginally increased body weight gain. (A) Breeding strategy used to generate OBHZ mice and littermate controlled WT mice. (B) Circulating leptin levels measured in 8-week-old male mice (n = 7 for WT mice; n = 10 for OBHZ mice). (C) Circulating leptin levels measured in 8-week-old female mice (n = 7 per group). (D) Body weight gain of male OBHZ and littermate ctrl mice (n = 6 for WT mice; n = 5 for OBHZ mice). (E) Body weights of female OBHZ mice (n = 6 for WT mice; n = 5 for OBHZ mice). (F) Fat mass measured by EchoMRI on 20-week-old male OBHZ and WT mice (n = 5 per group). (G) Lean mass of 20-week-old male OBHZ and WT mice (n = 5 per group). (H) OGTT on 16-week-old male OBHZ and WT mice (n = 6 for WT mice; n = 5 for OBHZ mice). (I) OGTT on 16-week-old female OBHZ and WT mice (n = 6 for WT mice; n = 5 for OBHZ mice). (J) ITT on male OBHZ and WT mice (n = 5 mice per group). (K) Breeding strategy used to generate LepHZ and Ctrl mice. (L) Circulating leptin levels measured on 8-week-old male LepHZ and Ctrl mice (n = 7 for Ctrl mice; n = 6 for OBHZ mice). (M) Body weights of 20-week-old LepHZ and Ctrl mice (n = 6 mice per group). (N) Fat mass of 20-week-old LepHZ and Ctrl mice (n = 6 mice per group). (O) Lean mass of 20-week-old LepHZ and Ctrl mice (n = 6 mice per group). (P) Circulating leptin levels measured on 12-week-old female LepHZ and Ctrl mice. (Q) Body weights of 20-week-old female LepHZ and Ctrl mice. (R) OGTT on 16-week-old male LepHZ and Ctrl mice (n = 7 for Ctrl mice; n = 6 for LepHZ mice). (S) OGTT on 16-week-old female LepHZ and Ctrl mice. (T) ITT on LepHZ and Ctrl mice (n = 7 for Ctrl mice; n = 6 for LepHZ mice). Data are mean ± SEM. Student's t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus OBHZ or Ctrl versus LepHZ.
To confirm the findings made in OBHZ mice using a second independent mouse model of partial leptin deficiency, LepHZ mice underwent the same metabolic characterization. We crossed adiponectin (Apn)-Cre/+ mice with lepflox/flox to obtain littermate control (Ctrl) mice (lepflox/+) and LepHZ (Apn-Cre::lepflox/+) mice (Figure 1K). At 8 weeks of age, male and female LepHZ mice showed significantly reduced circulating leptin levels (Figure 1L,P). Body weights of male LepHZ mice at 16 weeks were increased (Figure 1M), with a slight increase in fat mass and no change in lean mass (Figure 1N,O). Female LepHZ mice showed a trend toward increased body weights, but this did not reach statistical significance (Figure 1Q). Similar to OBHZ mice, chow-fed male and female LepHZ mice did not show any significant differences in glucose and insulin tolerance, compared to littermate Ctrl mice (Figure 1R–T). All these observations support the notion that chow-fed partially leptin-deficient mice reduce circulating leptin levels and slightly increase their body weights without any impact on glucose and insulin tolerance.
3.2. Chow-fed partially leptin-deficient mice do not show any functional differences in adipose tissue and liver
As partially leptin-deficient mice on chow diet display a slight increase in fat mass, we wondered whether adipose tissue and liver function are affected. We collected three major adipose tissue depots (inguinal white adipose tissue (ingWAT), epididymal white adipose tissue (epiWAT), and interscapular brown adipose tissue (iBAT)), as well as liver.
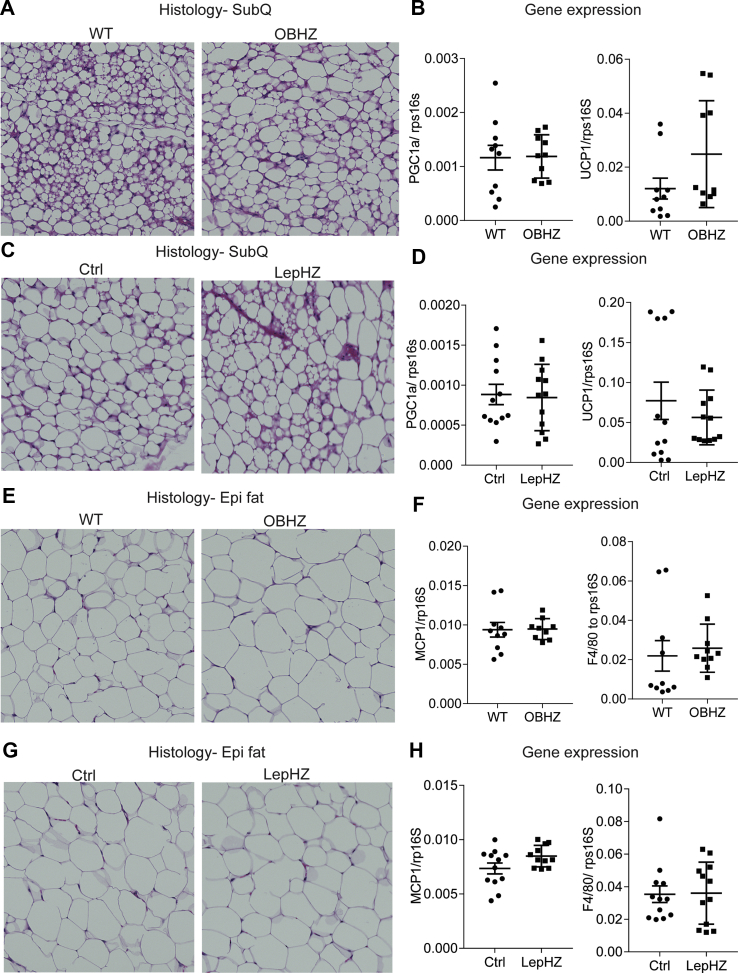
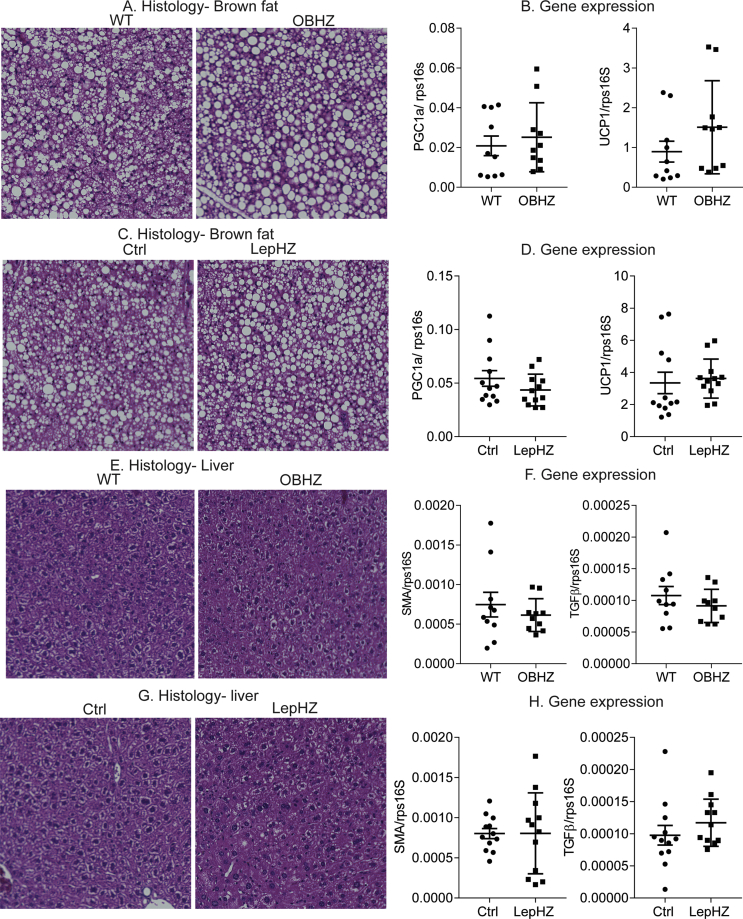
In the ingWAT depot, a higher degree of browning is positively associated with better glucose tolerance [30]. H&E staining indicates that both OBHZ and LepHZ mice showed a similar degree of browning and that their adipocyte size is comparable (Figure 2A,C). Furthermore, expression levels of the browning markers Pgc1α and Ucp1 were almost identical in both partially leptin-deficient mice and the Ctrl mice (Figure 2B,D).
Figure 2.
No differences observed in inguinal and epididymal white adipose tissues in chow-fed partially leptin-deficient mice. (A) H&E staining of a SubQ fat depot of a 20-week-old OBHZ and WT mice. (B) Expression of browning markers in a SubQ fat depot of 20-week-old OBHZ and WT mice. (C) H&E staining of a SubQ fat depot of 20-week-old LepHZ and Ctrl mice. (D) Expression of browning markers in a SubQ fat depot of 20-week-old LepHZ and Ctrl mice. (E) H&E staining of an Epi fat depot of 20-week-old OBHZ and WT mice. (F) Expression of inflammation markers in an Epi fat depot of 20-week-old OBHZ and WT mice. (G) H&E staining of an Epi fat depot of 20-week-old LepHZ and Ctrl mice. (H) Expression of browning markers in Epi fat depots of 20-week-old LepHZ and Ctrl mice.
EpiWAT hypertrophy and widespread macrophage infiltration are hallmarks of developing systemic insulin resistance [31]. H&E staining of epiWAT in both partially leptin-deficient mouse models shows no significant difference in the size of adipocytes and in macrophage infiltration (Figure 2E,G). In addition, the expression levels of classical markers of adipose tissue inflammation remain unchanged, which argues against the alteration of epiWAT functionality (Figure 2F,H).
Chow-fed partially leptin-deficient mice did not show any difference in brown fat and liver, as demonstrated by similar lipid droplet accumulation in brown fat and liver by H&E staining (Figure S2A, C, E, and G). Furthermore, the unaltered gene expression of brown marker genes in the iBAT depot points at an overall healthy state of the brown adipose tissue (Figure S2B and D). The livers of partially leptin-deficient mice on a chow diet show no sign of enhanced fibrosis as judged by fibrotic gene expression in the liver (Figure S2F and H).
3.3. Partial leptin deficiency causes resistance to HFD-induced obesity
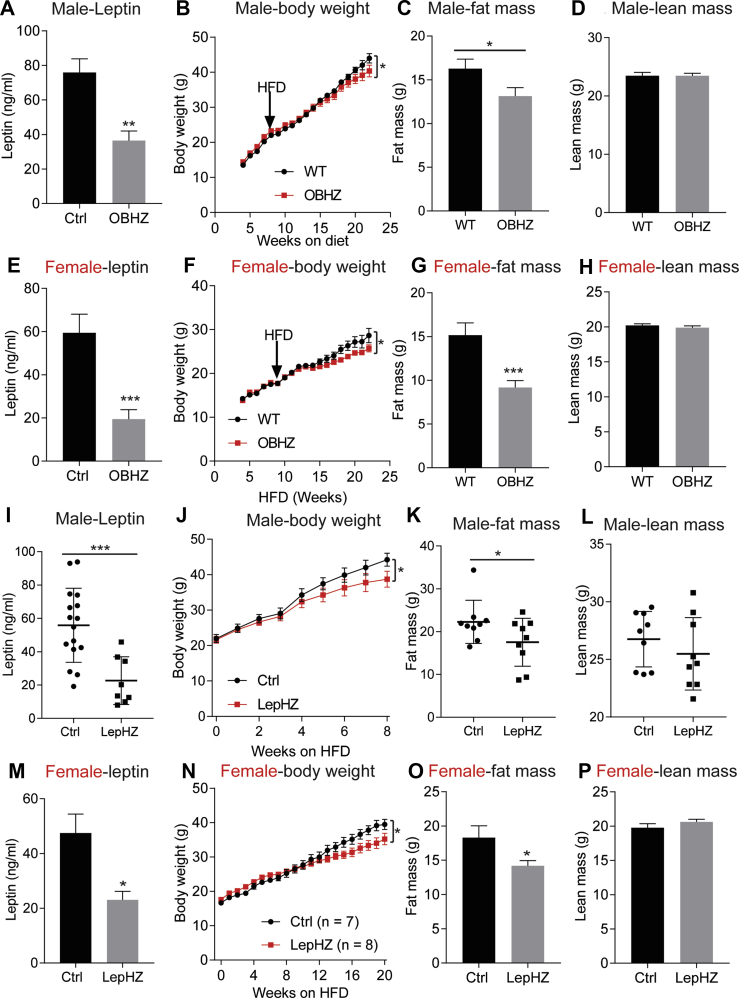
HFD is known to rapidly elevate circulating leptin levels and promote leptin resistance [32]. We, therefore, probed for the impact of chronic HFD feeding on partially leptin-deficient mice. Upon exposure to an HFD challenge, male OBHZ mice maintain lower circulating leptin levels compared to their littermate Ctrl mice (Figure 3A). Consistent with our recent findings [23], the lower leptin levels lead to reduced overall weight gain (Figure 3B), driven mostly by reduced fat mass expansion with no impact on lean mass (Figure 3C,D). To probe for any sexually dimorphic responses, we additionally performed the same HFD feeding experiment with female OBHZ and littermate Ctrl mice. Relative to male OBHZ mice, female OBHZ mice show an even higher degree of leptin reduction in circulation (Figure 3E). Similar to male mice, female OBHZ mice are resistant to diet-induced obesity, with a profound reduction in fat mass and no change in lean mass (Figure 3F,G). These observations contradict previously published data made with a similar mouse model [24].
Figure 3.
Partially leptin-deficient mice are resistant to diet-induced obesity. (A) Circulating leptin levels measured in 22-week-old male OBHZ and WT mice. (B) Body weights of male OBHZ and Ctrl mice measured during the diet study. (C) Fat mass of 22-week-old male OBHZ and WT mice. (D) Lean mass of 22-week-old male OBHZ and WT mice. (E) Circulating leptin levels measured in 22-week-old female OBHZ and WT mice. (F) Body weight of male OBHZ and Ctrl mice measured during the diet study. (G) Fat mass of 22-week-old female OBHZ and WT mice. (H) Lean mass of 22-week-old female OBHZ and WT mice. (I) Circulating leptin level measured in male LepHZ and Ctrl mice after 8-week HFD feeding. (J) Body weights of LepHZ and Ctrl mice during the HFD feeding period. (K) Fat mass of male LepHZ and Ctrl mice after 12-week HFD feeding. (L) Lean mass of male LepHZ and Ctrl mice after 12-week HFD feeding. (M) Circulating leptin levels measured in female LepHZ and Ctrl mice after 20-week HFD feeding. (N) Body weights of LepHZ and Ctrl mice during the HFD feeding period. (O) Fat mass of female OBHZ and WT mice after 20-week HFD feeding. (P) Lean mass of female LepHZ and Ctrl mice after 20-week HFD feeding. Data are mean ± SEM. Student's t-test or one-way ANOVA: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus OBHZ or Ctrl versus LepHZ.
In order to further confirm our findings made on OBHZ mice, we conducted similar experiments on the second partially leptin-deficient mouse model. This independent approach of congenitally eliminating one lep gene allele in adipocytes of LepHZ mice on an HFD reproduced the reduction of circulating leptin levels (Figure 3I). The partial reduction in circulating leptin also protects the LepHZ mice from diet-induced obesity (Figure 3J), which can be attributed to reduced fat mass growth, with no impact on lean mass (Figure 3K and L). Similarly, female LepHZ mice reduced circulating leptin levels as well, leading to resistance to diet-induced obesity, and greatly reduced fat mass without affecting lean mass (Figure 3M−P).
3.4. Partially leptin-deficient mice on HFD display improved glucose tolerance and insulin sensitivity
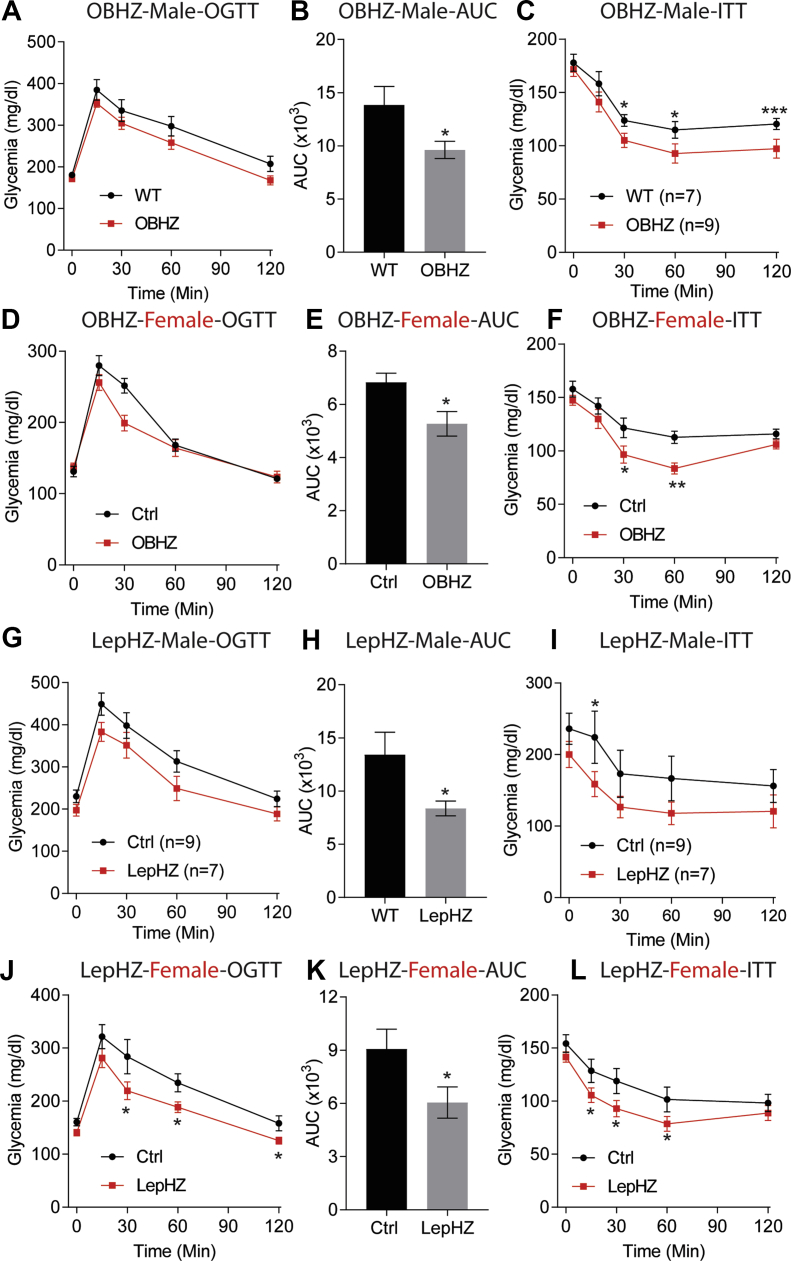
To assess whether the reduced body weight gain on HFD was associated with a better metabolic phenotype, GTTs and ITTs were performed on both male and female mice. Male and female OBHZ mice clearly displayed improved glucose tolerance and increased insulin sensitivity (Figure 4A–F). Likewise, improved glucose tolerance and higher insulin sensitivity were also observed in male and female LepHZ mice after chronic HFD feeding (Figure 4G–L).
Figure 4.
Improved glucose tolerance and insulin sensitivity in HFD-fed partially leptin-deficient mice. (A) OGTT performed on male OBHZ and WT mice after 8-week HFD feeding. (B) AUC calculated based on (A). (C) ITT on male OBHZ and WT mice after 10-week HFD feeding.(D) OGTT performed on HFD-fed female OBHZ and WT mice. (E) AUC calculated based on (D). (F) ITT on female OBHZ and WT mice. (G) OGTT on male LepHZ and Ctrl mice after 8-week HFD feeding. (H) AUC calculated based on (G). (I) ITT on male LepHZ and Ctrl mice after 10-week HFD feeding. (J) OGTT on female LepHZ and Ctrl mice. (K) AUC calculated based on (J). (L) ITT on female LepHZ and Ctrl mice. Data are mean ± SEM. Student's t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus OBHZ or Ctrl versus LepHZ.
3.5. Partial leptin deficiency reduces adipose tissue inflammation and protects the mice from fatty liver and liver fibrosis during HFD exposure
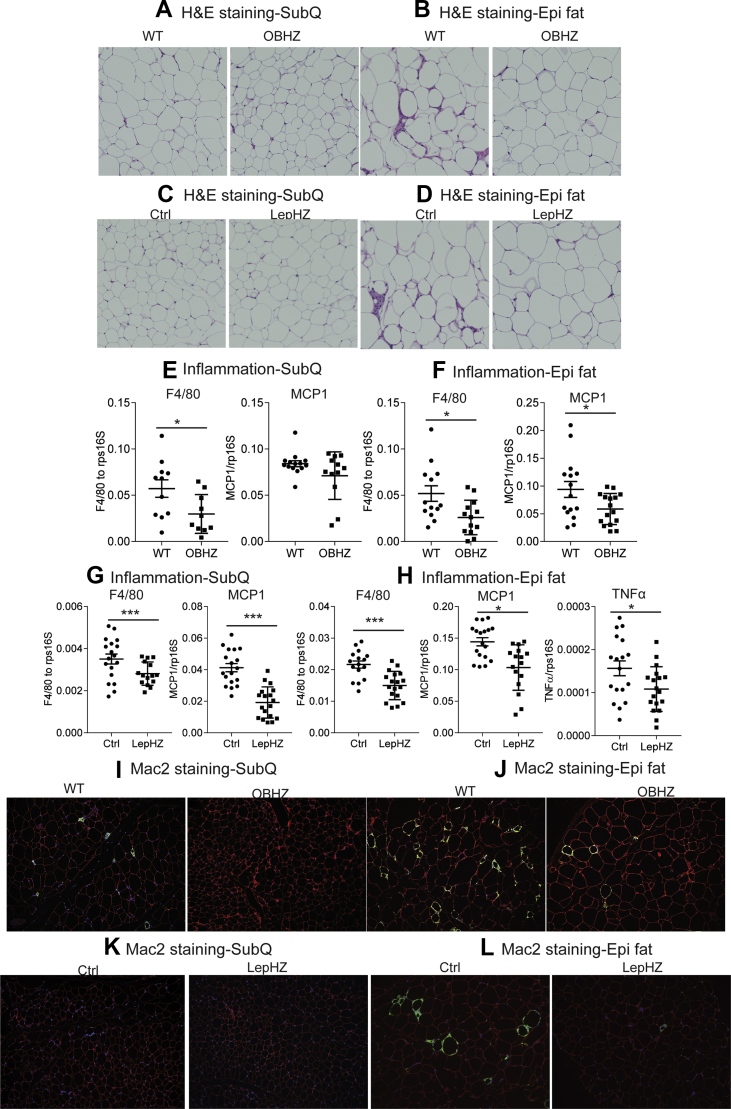
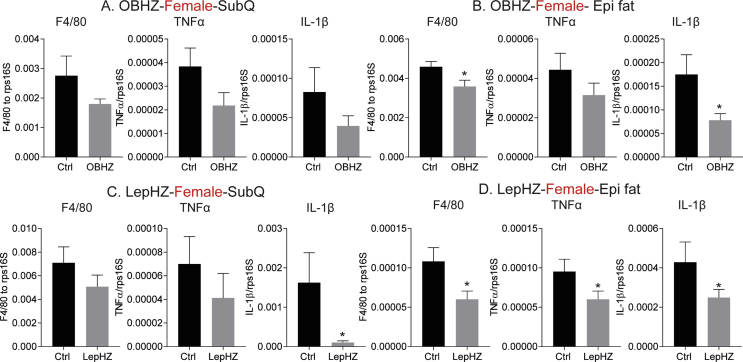
One of the hallmarks of diet-induced obesity is that HFD greatly promotes macrophage infiltration into adipose tissue. Infiltrating macrophages form “crown-like” structures and are a major source of adipose tissue inflammation. We performed histological studies to assess whether partial leptin deficiency protects the mice from adipose tissue inflammation induced by chronic HFD. Both the morphology and the size of adipocytes in the ingWAT depot of OBHZ, LepHZ, or Ctrl mice were unchanged after chronic HFD feeding (Figure 5A,C). However, in the epiWAT depot of OBHZ and LepHZ mice, macrophage infiltration into adipose tissue was greatly reduced (Figure 5B,D), suggesting a potential reduction in adipose tissue inflammation. To further assess whether the inability to fully induce leptin is associated with reduced adipose tissue inflammation, markers of adipose tissue inflammation, such as Mcp1, F4/80, and Tnfα, were assessed in both ingWAT and epiWAT, isolated from both male and female mice. Consistent with the histological appearance, the expression levels of inflammation markers were greatly reduced in adipose tissue depots of male and female OBHZ and LepHZ mice (Figure 5E–H and Figure S3A–D). Furthermore, immunohistochemical staining with Mac2 antibodies (a macrophage marker) highlighted that macrophage infiltration into adipose tissue is potently reduced in partially leptin-deficient mice (Figure 5I,L). All these observations clearly indicate that partial leptin deficiency on HFD reduces adipose tissue inflammation. This phenomenon correlates well with the improved metabolically healthy phenotype.
Figure 5.
Reduced adipose tissue inflammation in HFD-fed partially leptin-deficient mice. (A) H&E staining of a SubQ fat depot of OBHZ and WT mice after 20-week HFD feeding. (B) H&E staining of a SubQ fat depot of OBHZ and WT mice after 20-week HFD feeding. (C) H&E staining of a SubQ fat depot of LepHZ and Ctrl mice after 12-week HFD feeding. (D) H&E staining of an Epi fat depot of LepHZ and Ctrl mice after 12-week HFD feeding. (E) Expression of inflammatory markers in SubQ fat depots of OBHZ and WT mice after 20-week HFD feeding. (F) Expression of inflammatory markers in Epi fat depots of OBHZ and WT mice after 20-week HFD feeding. (G) Expression of inflammatory markers in SubQ fat depots of LepHZ and WT mice after 12-week HFD feeding. (H) Expression of inflammatory markers in Epi fat depots of LepHZ and WT mice after 12-week HFD feeding. (I) Mac2 staining of a SubQ fat depot of OBHZ and WT mice after 20-week HFD feeding. (J) Mac2 staining of an Epi fat depot of OBHZ and WT mice after 20-week HFD feeding. (K) Mac2 staining of a SubQ fat depot of LepHZ and Ctrl mice after 12-week HFD feeding. (L) Mac2 staining of an Epi fat depot of LepHZ and Ctrl mice after 12-week HFD feeding. Data are mean ± SEM. Student's t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus OBHZ or Ctrl versus LepHZ.
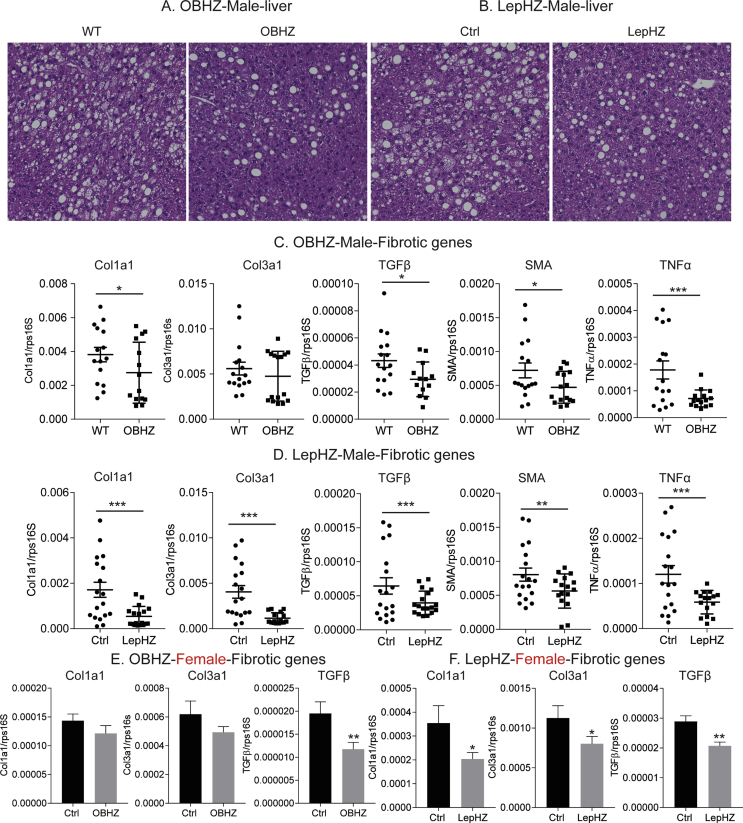
As clinical studies suggest that high circulating levels of leptin positively are associated with the severity of fatty liver [33], we explored whether partial leptin deficiency can protect mice from a fatty liver phenotype. HFD is a potent promoter of lipid droplet accumulation in the liver in wildtype mice. In contrast, partial leptin deficiency clearly results in reduced lipid droplet accumulation (Figure S4A and B), reflecting the protective role that leptin reduction exerts on a fatty liver phenotype. Strikingly, the expression levels of classical markers of liver fibrosis, such as Col1a1, Col3a1, Tgfβ, and Sma, are significantly reduced in both partially leptin-deficient male and female mice (Figure S4C–F). Additional inflammation markers, such as Tnfα, are also dramatically reduced (Figure S4C and D). These results indicate that partial leptin deficiency not only protects the mice from a fatty liver but also exerts potent anti-inflammatory and antifibrotic effects in the liver.
3.6. Partial leptin deficiency results in enhanced leptin sensitivity
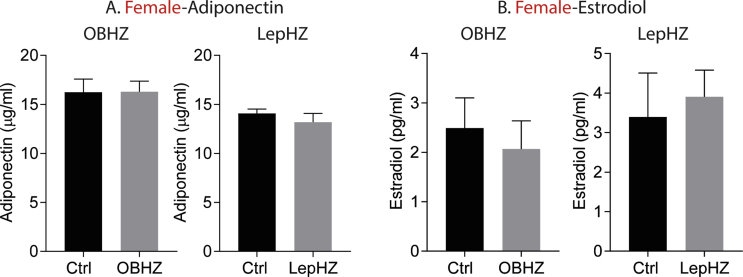
High levels of circulating adiponectin promote higher insulin sensitivity and better glucose tolerance, and we wondered whether a net increase in adiponectin levels can help explain the beneficial effects observed in partially leptin-deficient mice. We measured circulating adiponectin levels in partially leptin-deficient female mice and we did not observe an elevation adiponectin level in these mice (Figure S5A). However, with unaltered adiponectin levels and lower leptin levels, the adiponectin/leptin ratio significantly increases, which may contribute to the improved metabolic phenotype.
In addition, we have previously demonstrated that partial leptin reduction in obese mice is associated with restored leptin sensitivity in hypothalamic neurons. We, therefore, wondered whether partial leptin deficiency leads to a better leptin sensitivity during HFD exposure.
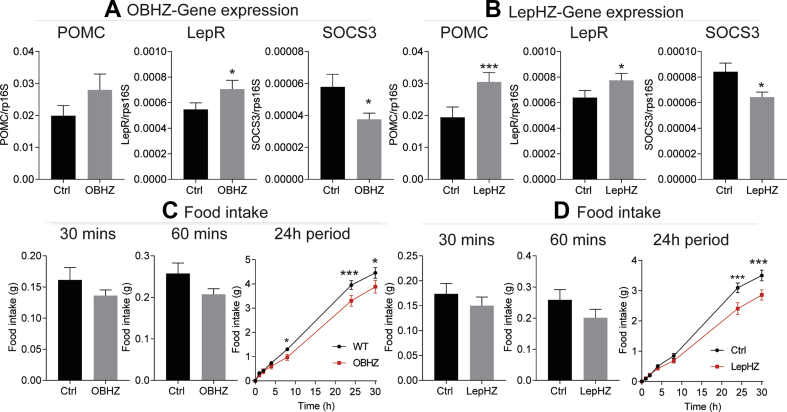
We observed an overall reduced food intake, in both HFD-fed OBHZ and LepHZ mice. This is consistent with a potential increase in leptin sensitivity. In order to directly assess whether increased hypothalamic leptin sensitivity contributes to the observed beneficial effects, including resistance to diet-induced obesity and improved glucose tolerance, we measured several important genes in the hypothalamic region of the brain. We found that the expression of POMC and LepR was significantly increased, while the expression of Socs3 was greatly reduced (Figure 6A,B), consistent with enhanced leptin action in the hypothalamic neurons. Furthermore, we injected exogenous leptin into overnight-fasting mice and measured food intake at various time points. We found that HFD-fed OBHZ and LepHZ mice show trends to reduce food intake at 30 min and 60 min followed by a significantly reduced food intake at 8 h, 24 h, and 30 h (Figure 6C,D), indicating that partially leptin-deficient mice display a much higher level of leptin sensitivity. The sustained enhanced leptin sensitivity is likely a key driver for the beneficial effects on body weight and glucose tolerance.
Figure 6.
HFD-fed partially leptin-deficient mice sustain higher leptin sensitivity. (A) Gene expression in the hypothalamic region of HFD-fed OBHZ and Ctrl mice. (B) Gene expression in the hypothalamic region of HFD-fed LepHZ and Ctrl. (C) Food intake measured at different time points in HFD-fed OBHZ and WT mice after acute leptin injection. (D) Food intake measured at different time points in HFD-fed LepHZ and Ctrl mice after acute leptin injection. Data are mean ± SEM. Student's t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus OBHZ or Ctrl versus LepHZ.
Partially leptin-deficient mice display higher leptin sensitivity. We wondered whether this affects fertility in female mice. While we did not assess this in depth, partially leptin-deficient female mice maintain normal menstrual cycles and reproductive function. We measured estradiol levels and found that female OBHZ and LepHZ mice display normal circulating levels (Figure S5B).
4. Discussion
With two partially leptin-deficient mouse models (OBHZ and LepHZ), we report the following observations: (1) Both OBHZ and LepHZ mice display reduced circulating leptin levels. (2) On chow diet, male OBHZ and LepHZ display slightly increased body weights, but despite the increased weight, they show no significant changes in glucose and insulin tolerance, adipose tissue, and liver function. (3) Upon a HFD challenge, both male and female OBHZ and LepHZ mice are resistant to diet-induced obesity. (4) Both OBHZ and LepHZ mice display better glucose tolerance and improved insulin sensitivity and are protected from adipose tissue inflammation, fatty liver, and hepatic fibrosis. (5) Both OBHZ and LepHZ maintain a higher level of leptin sensitivity. All these observations are in support of our model that the inability to increase leptin levels under obesogenic conditions is beneficial for the management of obesity and diabetes [23]. In addition, this observation lends further support to our previous findings that a reduction in leptin levels serves as a novel approach for weight loss and insulin sensitization [23].
The observations made on two mouse models of partial leptin deficiency are in contrast to a previous study on OBHZ mice, in which the authors claimed that partial leptin deficiency favors obesity, nonalcoholic steatohepatitis, and other metabolic abnormalities, particularly under conditions of high caloric intake [24]. We are at a loss to explain these disparate results. The OBHZ mice we are using in our study are truly partially leptin-deficient mice, as ob/ob mice generated by crossing our heterozygous OBHZ with OBHZ indeed display the classical ob/ob phenotype without any circulating leptin and have increased food intake, massive obesity, glucose intolerance, and fatty liver. In addition, OBHZ mice used in our study display an approximately 40%–50% reduction in circulating leptin. Importantly, we are exclusively comparing littermate controls in all of our experiments. In contrast, in the previous study, the absolute amount of circulating leptin measured in the “partially leptin-deficient” mice is equal to or even much higher than the control mice used, questioning the authors’ premise of partial leptin deficiency [24]. In addition, it is still unclear whether littermate controls were used in the previous study, an essential condition for all metabolic studies [34].
The effects of partial leptin deficiency on body weight can be viewed in two different settings: leptin-sensitive versus leptin-resistant states. In the context of a leptin-sensitive state, increasing leptin levels can indeed reduce food intake and body weight, while reducing leptin levels leads to increased food intake and body weight. Thus, in the chow-fed young OBHZ and LepHZ mice, the partial leptin deficiency creates a state of modest leptin insufficiency with a slightly increased food intake and body weight gain [35]. This observation is consistent with the classical physiological role attributed to leptin and supported by many additional observations. Injection of leptin into leptin sensitive young mice leads to a reduction in food intake and body weight. Leptin transgenic mice on chow diet lose 80%–90% fat and are highly insulin sensitive (even though these mice express pharmacological levels of leptin) [36]. In the states of leptin resistance with high circulating leptin, further increases in leptin levels do not produce any measurable additional effects on food intake and body weight. However, reducing leptin levels in this setting generated unexpected and dramatic effects on reducing food intake and body weight [23]. Based on these observations, we propose that a reduction of leptin levels in the obese state leads to a dramatic enhancement of leptin sensitivity. Our current observations on HFD-fed partially leptin-deficient mice fully support this notion. Circulating leptin levels in an HFD-fed partially leptin-deficient state are reduced by approximately 50%, and these lower leptin levels protect the mice from diet-induced obesity. In addition, a peripherally restricted CB1R reverse agonist, JD5037, reduces circulating leptin levels in an HFD setting, resulting in reduced food intake and reduced body weight gain, and it reverses insulin resistance [37]. Furthermore, the selective hypoxia-inducible factor 1α (HIF1α) inhibitor PX-478 reduces circulating leptin levels, leading to improved glucose tolerance, increased energy expenditure, and reduced liver steatosis [38].
As a positive correlation between lower body weight and better glucose tolerance has been well documented in many animal models and clinical studies, it is reasonable to speculate that the observed beneficial effects, such as improved glucose tolerance and reduced adipose tissue inflammation and fatty liver, are simply the result of the reduced body weight gain. However, a more detailed kinetic analysis will need to be performed in the cases of pharmacological or inducible genetic models, as we frequently find that the reduction in leptin levels precedes the reduction in weight and the improvements in insulin sensitivity.
Are some of these beneficial effects weight-independent for additional reasons? Recently, the role of leptin as an activator of immune cells is increasingly appreciated [39]. Leptin stimulates the proliferation and activation of circulating monocytes into macrophages in vitro [40]. Moreover, leptin can dose-dependently stimulate the production of proinflammatory cytokines [41]. Thus, HFD-induced high leptin levels may contribute to increased macrophage infiltration into epididymal fat, as reflected in the HFD-fed control mice [42]. In contrast, partially leptin-deficient mice reduce circulating leptin levels and display reduced monocyte activation, leading to reduced macrophage infiltration into adipose tissue and a healthy adipose tissue phenotype. As such, the negative impact of leptin, even at high levels, on peripheral cells is in contrast to the complete lack of response to elevated leptin in critical regions of the brain. This strongly supports the idea of “selective leptin resistance”.
Leptin mediates hepatic stellate cell activation and liver fibrosis through indirect effects on Kupffer cells [43,44]. No attempts have been made to reverse liver fibrosis by targeting circulating leptin levels [45]. Here, we provide evidence that reducing circulating leptin levels may be a powerful approach to greatly prevent and alleviate liver fibrosis. This beneficial effect may be the result of direct action on the liver, independent of any impact on body weight. Additional more detailed studies are warranted to assess the effects of partial leptin reduction in liver fibrosis.
Based on this and our previous studies, we conclude that, in leptin sensitive states, as observed in young and lean animals, reducing leptin level leads to modest increases in food intake and weight gain; in contrast, in states of leptin resistance, as observed in diet-induced obesity, reducing leptin levels restores leptin sensitivity, leading to reduced food intake and body weight gain, accompanied by increased glucose tolerance and insulin sensitivity [46]. Partial leptin reduction holds great promise in developing novel therapies for obesity and type 2 diabetes.
Authors’ contributions
Conceptualization is contributed by S. Z., J. K. E., and P. E. S.; methodology, S. Z. and N. A., investigation, S. Z., N. A., Y. Z., L. S., Z. Z., M. W., and Q. Z.; writing the original draft, S.Z.; reviewing and editing the manuscript, L. S., C. M. K., J. K. E, and P. E. S; supervision, J. K. E and P. E. S.
Acknowledgments
This work was supported by the US National Institutes of Health grants R01-DK55758, R01-DK099110, RC2-DK118620, and P01-DK088761 (to P. E. S.) and was also supported by the US National Institutes of Health grants R01-DK118725 and R01-DK088423 (to J. K. E.). S. Z. was supported by a Post-Doctoral Fellowship from FRQS. Y. Z. was supported by a US National Institutes of Health grant R00-DK114498. We would like to thank Angela K. Odle and Gwen V. Childs from the University of Arkansas for the mice carrying a floxed copy of the leptin gene.
Conflicts of Interest
None of the authors declare any conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.100995.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Verification of OBHZ as a true partially leptin-deficient model. (A) Circulating leptin levels in ob/ob and Ctrl mice. (B) Body weight gain of ob/ob and WT mice during HFD feeding. (C) Food intake of ob/ob and WT mice on HFD feeding. (D) OGTT on ob/ob and WT mice. (E) H&E staining of the liver. (F) H&E staining of brown fat. Data are mean ± SEM. Student's t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus ob/ob.
Supplementary Figure 2.
Partially leptin-deficient mice on chow diet do not show any difference in liver and brown fat. (A) H&E staining of brown fat of OBHZ and WT mice. (B) Gene expression of brown fat of OBHZ and WT mice. (C) H&E staining of brown fat of LepHZ and Ctrl mice. (D) Gene expression of brown fat of LepHZ and Ctrl mice. (E) H&E staining of the liver of OBHZ and WT mice. (F) Gene expression of the liver of OBHZ and WT mice. (G) H&E staining of the liver of LepHZ and Ctrl mice. (H) Gene expression of the liver of LepHZ and Ctrl mice. Data are mean ± SEM. Student's t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus OBHZ or Ctrl versus LepHZ.
Supplementary Figure 3.
HFD-fed partially leptin-deficient female mice reduce adipose tissue inflammation. (A) Expression of inflammation markers in SubQ fat depots of HFD-fed female OBHZ and Ctrl mice. (B) Expression of inflammation markers in Epi fat depots of HFD-fed female OBHZ and Ctrl mice. (C) Expression of inflammation gene markers in SubQ fat depots of HFD-fed female OBHZ and Ctrl mice. (D) Expression of inflammation gene markers in Epi fat depots of HFD-fed female LepHZ and Ctrl mice. Data are mean ± SEM. Student's t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus OBHZ or Ctrl versus LepHZ.
Supplementary Figure 4.
HFD-fed partially leptin-deficient mice are protected from liver liver steatosis and fibrosis. (A) H&E staining of liver from male OBHZ and WT mice. (B) H&E staining of liver from male LepHZ and WT mice. (C) Expression of fibrotic gene markers in the liver of HFD-fed male OBHZ and Ctrl mice. (D) Expression of fibrotic gene markers in the liver of HFD-fed male LepHZ and Ctrl mice. (E) Expression of fibrotic gene markers in the liver of HFD-fed female OBHZ and Ctrl mice. (F) Expression of fibrotic gene markers in the liver of HFD-fed female LepHZ and Ctrl mice. Data are mean ± SEM. Student's t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus OBHZ or Ctrl versus LepHZ.
Supplementary Figure 5.
No differences in circulating adiponectin and estradiol levels in partially leptin-deficient female mice. (A) Circulating adiponectin level in female OBHZ and LepHZ mice. (B) Circulating estradiol level in female OBHZ and LepHZ mice. Data are mean ± SEM. Student's t-test: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 for WT versus OBHZ or Ctrl versus LepHZ.
References
- 1.Nyberg S.T., Batty G.D., Pentti J., Virtanen M., Alfredsson L., Fransson E.I. Obesity and loss of disease-free years owing to major non-communicable diseases: a multicohort study. Lancet Public Health. 2018;3:e490–e497. doi: 10.1016/S2468-2667(18)30139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauby-Secretan B., Scoccianti C., Loomis D., Grosse Y., Bianchini F., Straif K., G. International Agency for Research on Cancer Handbook Working Body fatness and cancer--viewpoint of the IARC working group. New England Journal of Medicine. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusminski C.M., Bickel P.E., Scherer P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nature Reviews Drug Discovery. 2016;15:639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 4.Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 5.Ahima R.S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 6.Odle A.K., Haney A., Allensworth-James M., Akhter N., Childs G.V. Adipocyte versus pituitary leptin in the regulation of pituitary hormones: somatotropes develop normally in the absence of circulating leptin. Endocrinology. 2014;155:4316–4328. doi: 10.1210/en.2014-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelleymounter M.A., Cullen M.J., Baker M.B., Hecht R., Winters D., Boone T. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 8.Heymsfield S.B., Greenberg A.S., Fujioka K., Dixon R.M., Kushner R., Hunt T. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. Journal of the American Medical Association. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 9.Luheshi G.N., Gardner J.D., Rushforth D.A., Loudon A.S., Rothwell N.J. Leptin actions on food intake and body temperature are mediated by IL-1. Proceedings of the National Academy of Sciences of the U S A. 1999;96:7047–7052. doi: 10.1073/pnas.96.12.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimomura I., Hammer R.E., Ikemoto S., Brown M.S., Goldstein J.L. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi I.S., Jebb S.A., Langmack G., Lawrence E., Cheetham C.H., Prentice A.M. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. New England Journal of Medicine. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 12.Farooqi I.S., Matarese G., Lord G.M., Keogh J.M., Lawrence E., Agwu C. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. Journal of Clinical Investigation. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zelissen P.M., Stenlof K., Lean M.E., Fogteloo J., Keulen E.T., Wilding J. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism. 2005;7:755–761. doi: 10.1111/j.1463-1326.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 14.Korner J., Conroy R., Febres G., McMahon D.J., Conwell I., Karmally W. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity. 2013;21:951–956. doi: 10.1002/oby.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon H.S., Matarese G., Brennan A.M., Chamberland J.P., Liu X., Fiorenza C.G. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan W.W., Myers M.G., Jr. Leptin and the maintenance of elevated body weight. Nature Reviews Neuroscience. 2018;19:95–105. doi: 10.1038/nrn.2017.168. [DOI] [PubMed] [Google Scholar]
- 17.Allison M.B., Myers M.G., Jr. 20 years of leptin: connecting leptin signaling to biological function. Journal of Endocrinology. 2014;223:T25–T35. doi: 10.1530/JOE-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caron A., Lee S., Elmquist J.K., Gautron L. Leptin and brain-adipose crosstalks. Nature Reviews Neuroscience. 2018;19:153–165. doi: 10.1038/nrn.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight Z.A., Hannan K.S., Greenberg M.L., Friedman J.M. Hyperleptinemia is required for the development of leptin resistance. PloS One. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpace P.J., Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2009;296:R493–R500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balland E., Chen W., Tiganis T., Cowley M.A. Persistent leptin signaling in the arcuate nucleus impairs hypothalamic insulin signaling and glucose homeostasis in obese mice. Neuroendocrinology. 2019;109:374–390. doi: 10.1159/000500201. [DOI] [PubMed] [Google Scholar]
- 22.Ogus S., Ke Y., Qiu J., Wang B., Chehab F.F. Hyperleptinemia precipitates diet-induced obesity in transgenic mice overexpressing leptin. Endocrinology. 2003;144:2865–2869. doi: 10.1210/en.2002-0178. [DOI] [PubMed] [Google Scholar]
- 23.Zhao S., Zhu Y., Schultz R.D., Li N., He Z., Zhang Z. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metabolism. 2019;30:706–719 e6. doi: 10.1016/j.cmet.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begriche K., Letteron P., Abbey-Toby A., Vadrot N., Robin M.A., Bado A. Partial leptin deficiency favors diet-induced obesity and related metabolic disorders in mice. American Journal of Physiology. Endocrinology and Metabolism. 2008;294:E939–E951. doi: 10.1152/ajpendo.00379.2007. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S., Mugabo Y., Iglesias J., Xie L., Delghingaro-Augusto V., Lussier R. alpha/beta-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metabolism. 2014;19:993–1007. doi: 10.1016/j.cmet.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Zhao S., Mugabo Y., Ballentine G., Attane C., Iglesias J., Poursharifi P. Alpha/beta-hydrolase domain 6 deletion induces adipose browning and prevents obesity and type 2 diabetes. Cell Reports. 2016;14:2872–2888. doi: 10.1016/j.celrep.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y., Zhao S., Deng Y., Gordillo R., Ghaben A.L., Shao M. Hepatic GALE regulates whole-body glucose homeostasis by modulating Tff3 expression. Diabetes. 2017;66:2789–2799. doi: 10.2337/db17-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z., Shao M., Hepler C., Zi Z., Zhao S., An Y.A. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. Journal of Clinical Investigation. 2019;129:5327–5342. doi: 10.1172/JCI130239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y., Gao Y., Tao C., Shao M., Zhao S., Huang W. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metabolism. 2016;24:420–433. doi: 10.1016/j.cmet.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amato M.C., Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. The Internet Journal of Endocrinology. 2014;2014:730827. doi: 10.1155/2014/730827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin S., Thomas T.C., Storlien L.H., Huang X.F. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. International Journal of Obesity and Related Metabolic Disorders. 2000;24:639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 33.Polyzos S.A., Aronis K.N., Kountouras J., Raptis D.D., Vasiloglou M.F., Mantzoros C.S. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia. 2016;59:30–43. doi: 10.1007/s00125-015-3769-3. [DOI] [PubMed] [Google Scholar]
- 34.Skowronski A.A., LeDuc C.A., Foo K.S., Goffer Y., Burnett L.C., Egli D. Physiological consequences of transient hyperleptinemia during discrete developmental periods on body weight in mice. Science Translational Medicine. 2020;12 doi: 10.1126/scitranslmed.aax6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flatt P.R., Bailey C.J. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia. 1981;20:573–577. doi: 10.1007/BF00252768. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa Y., Masuzaki H., Hosoda K., Aizawa-Abe M., Suga J., Suda M. Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes. 1999;48:1822–1829. doi: 10.2337/diabetes.48.9.1822. [DOI] [PubMed] [Google Scholar]
- 37.Tam J., Cinar R., Liu J., Godlewski G., Wesley D., Jourdan T. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metabolism. 2012;16:167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun K., Halberg N., Khan M., Magalang U.J., Scherer P.E. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Molecular and Cellular Biology. 2013;33:904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann A., Ebert T., Kloting N., Kolb M., Gericke M., Jeromin F. Leptin decreases circulating inflammatory IL-6 and MCP-1 in mice. BioFactors. 2019;45:43–48. doi: 10.1002/biof.1457. [DOI] [PubMed] [Google Scholar]
- 40.Santos-Alvarez J., Goberna R., Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cellular Immunology. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed M., Shaban Z., Yamaji D., Okamatsu-Ogura Y., Soliman M., Abd Eldaim M. Induction of proinflammatory cytokines and caspase-1 by leptin in monocyte/macrophages from holstein cows. Journal of Veterinary Medical Science. 2007;69:509–514. doi: 10.1292/jvms.69.509. [DOI] [PubMed] [Google Scholar]
- 42.Coenen K.R., Gruen M.L., Chait A., Hasty A.H. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 43.Wang J., Leclercq I., Brymora J.M., Xu N., Ramezani-Moghadam M., London R.M. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713–723. doi: 10.1053/j.gastro.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honda H., Ikejima K., Hirose M., Yoshikawa M., Lang T., Enomoto N. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology. 2002;36:12–21. doi: 10.1053/jhep.2002.33684. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Q., Scherer P.E. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nature Reviews Nephrology. 2018;14:105–120. doi: 10.1038/nrneph.2017.157. [DOI] [PubMed] [Google Scholar]
- 46.Zhao S., Kusminski C.M., Elmquist J.K., Scherer P.E. Leptin: less is more. Diabetes. 2020;69:823–829. doi: 10.2337/dbi19-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]