Abstract
Background
Patients with pollinosis are often multi-sensitized to diverse pollen allergens. However, little is known about pollen allergy types among Chinese pollinosis patients. This study is aimed to characterize clinical manifestations of food allergy among patients with different types of pollen allergy.
Methods
We retrospectively analyzed 402 pollinosis patients from an outpatient allergy department of the Peking Union Medical College Hospital who had been diagnosed by experienced allergists. All included patients who answered a questionnaire regarding seasonal pollinosis and clinical symptoms after ingestion of food and underwent intradermal skin tests. Total IgE and specific IgE levels were quantified by ImmunoCAP, using 0.35 kUA/L as a threshold for positivity.
Results
The patients were divided into 3 groups, based on the season during which they experienced symptoms and the 2 peaks of Chinese airborne pollen: spring-tree pollen symptoms group (SG), autumn-weed pollen symptoms group (AG), and a combined spring and autumn pollen symptoms group (CG). Birch pollen (83%) and ash pollen (74%) were common allergens among patients with spring symptoms, while mugwort pollen (87%) was a common allergen among patients with autumn symptoms. In total, 30% of the study population experienced pollen-related food allergy. Pollen-related food allergies were more prevalent among the single-season symptom groups (68% and 50% for the SG and AG, respectively) than among the CG (20%). All patients with pollen-related food-induced anaphylaxis exhibited autumn weed pollen symptoms. Except for 2 patients, all patients with food-induced anaphylaxis were allergic to mugwort pollen. In the SG, all patients with food allergy were sensitive to birch pollen, with birch and Bet v 1-specific IgE levels higher in this group than in the group without food allergy (p < 0.001). In the AG, Art v 3 was more prevalent among patients with pollen-related food allergy than without food allergy (79% vs. 33%, p < 0.001), a proportion similar to the one in the CG (67% vs. 48%, p = 0.01). Meanwhile, the Art v 3-specific IgE levels among patients with pollen-related food allergy were higher than among tolerant patients in the AG (p < 0.001) and CG (p = 0.02). Unexpectedly, the Art v 3-specific IgE levels were higher in patients with food-induced anaphylaxis than with oral allergy syndrome only in the CG.
Conclusions
Bet v 1 (a Pathogenesis-related 10 protein) and Art v 3 (a non-specific lipid transfer protein; nsLTP) are candidate molecular biomarkers for the diagnosis of food allergies in patients with season-specific pollen-related allergies. Measuring pollen allergen component-specific IgE levels might be an effective tool for the management of pollinosis in clinical practice in China.
Keywords: Pollinosis, Food allergy, Anaphylaxis, Bet v 1, Art v 3
Introduction
Pollen is one of the most common causes of allergic rhinitis, allergic conjunctivitis, and bronchial asthma. Because there is known cross-reactivity of pollen and food allergens,1 patients with a pollen allergy may also experience food allergy after the ingestion of fruits, nuts, or vegetables, with clinical symptoms such as oral allergy syndrome, urticaria, angioedema, and anaphylaxis.2 The majority of patients suffer pollen-related food allergy in adulthood.3 Defense-related proteins in plant tissues that are considered major allergens, and which induce pollen-related food allergies, include members of the pathogenesis-related protein 10 family, profilins, non-specific lipid transfer proteins, and gibberellin-regulated proteins.2 In Western countries, birch pollen has historically been considered the most common allergen among patients with pollen-related food allergy,4 presenting mainly as oral allergy syndrome. Additionally, recent studies have shown lipid transfer protein (LTP) derived from mugwort pollen to be a major allergen that causes severe allergic symptoms in Europe.5,6 Anaphylaxis is the primary symptom of mugwort pollen-related food allergy in China; peach is the most common culprit.7 Birch pollen sensitization and pollen-food allergy syndrome are also believed to be common in China, especially in the north.8
China is characterized by significant regional variation in climate and vegetation; nevertheless, most regions have 2 peak seasons for airborne pollen allergens, spring and autumn.9 Most pollinosis patients show symptoms from March to May (which is a peak season for tree pollen) and August to September (which is a peak season for weed pollen). Although previous8,10,11 studies have examined pollen-related food allergy by component-resolved diagnosis in China, they have neglected to discern pollinosis patients that are multi-sensitized to diverse pollen allergens, which would manifest different characteristics of food allergy. The frequency of pollen-related food allergy also varies depending on pollen types.12 However, little is known about the impact of seasonal sensitization to specific pollen on pollen related-food allergy.
This study aimed to characterize clinical manifestations of food allergy among pollinosis patients allergic to different pollen types by correlating specific IgE levels and food allergy in seasonal groups.
Materials and methods
Patients
A total of 402 pollinosis patients were enrolled in an outpatient allergy department of the Peking Union Medical College Hospital (PUMCH) from March 2011 to April 2012, diagnosed by an experienced allergist, and recruited for this study. Seasonal pollinosis was defined as the presence of allergic rhinitis, allergic conjunctivitis, or bronchial asthma, and a positive response to one of the common Chinese pollen allergens. All patients had a convincing clinical history of pollinosis, a positive skin test to a pollen allergen extract, and the presence of pollen allergen-specific IgE. The diagnosis of a pollen-related food allergy was based on the clinical history of allergic reactions after ingestion of a culprit food and detection of food-specific IgE and/or a positive skin prick test. All participants were face-to-face interviewed to complete a questionnaire about food allergies, The standardized questionnaires included 3 questions: 1) Have you experienced any symptoms after ingesting any of the foods? 2) Which symptoms have you had? 3) Which kinds of food is it? The food list contained 6 categories of plant foods (fruits, legumes, grains, nuts, vegetables, and seeds). Based on the clinical manifestation, pollinosis patients were categorized into 2 groups: those who had exhibited oral allergy syndrome (OAS) and those who had experienced food-induced anaphylaxis (FIA), where anaphylaxis was defined based on guidelines from the National Institute of Allergy and Infectious Diseases.13
Written informed consent was obtained from each participant, or their parent or guardian, before study enrollment. The study protocol was reviewed and approved by the Ethical Committee of Peking Union Medical College (No. PUMCH1103).
Skin test
Based on data on airborne pollen allergens in China,14 participants underwent intradermal skin tests with extracts of the 8 most common pollens. These pollens included 4 types of spring tree pollen (birch, plane tree, ash, and sabina) and 4 types of autumn weed pollen (mugwort, Japanese hop, kochia, and ragweed). All extracts were from the Allergen Manufacturing and Research Center (PUMCH, Beijing, China). Histamine phosphate (0.01 mg/ml) was used as a positive control, and an allergen diluent was used as a negative control. The results were evaluated 15 min after application. A pollen allergen wheal size of at least 5 mm after subtraction of the negative control, alongside a positive control wheal size of at least 5 mm, was considered a positive skin response.15
Determination of total IgE and allergen-specific lgE
Total serum IgE and allergen-specific IgE antibody levels were detected using the ImmunoCAP system (ThermoFisher Scientific, Uppsala, Sweden) available at our clinical laboratory. All 402 participants were tested for IgE specific to the eight common pollens, in addition to 2 components of birch pollen (Bet v 1 and Bet v 2), 2 components of mugwort pollen (Art v 1 and Art v 3), and 2 components of peach (Pru p 1 and Pru p 3). Values above the threshold of 0.35 kUA/L were considered positive.
Statistical analysis
SPSS 20.0 (IBM Inc., Chicago, IL, USA) and Prism 8.0 (GraphPad, California, USA) were used to analyze the data. Pearson's chi-squared test was used to compare frequencies of categorical variables. The Wilcoxon rank-sum test was used to compare specific IgE levels between the groups. Receiver operating characteristics (ROC) curves were used to assess the diagnostic power of the specific IgE types and levels. P-values<0.05 were considered statistically significant.
Results
Clinical characteristics of patients
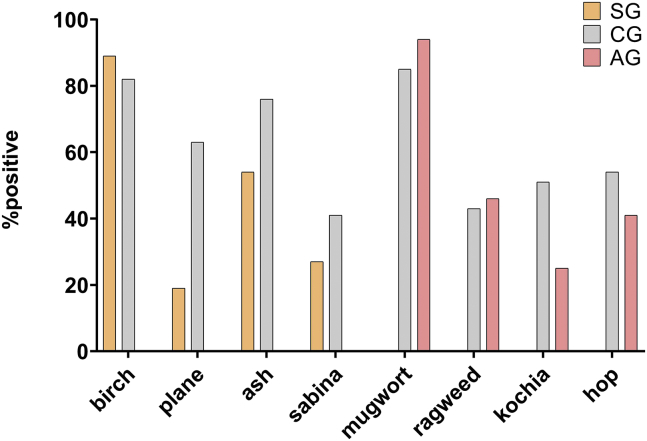
A total of 402 pollinosis patients (189 males; mean age, 30.4 ± 14.1 years) were categorized into 3 groups (Table 1). The spring (SG, n = 37) and autumn symptom groups (AG, n = 63) were both smaller than the combined seasons groups (CG, n = 302). There were between-group differences in pollen-specific sensitivity (Fig. 1), with birch pollen (89%) and ash pollen (54%) emerging as the major pollen allergens among SG, mugwort (94%) as the major source of allergy among AG, and birch (82%), ash pollen(76%), mugwort (85%) as the major source of allergy among CG.
Table 1.
Clinical and demographic characteristics of individuals with pollen allergies, per symptoms season.
| Spring symptoms (n = 37) | Autumn symptoms (n = 63) | Combined seasons (n = 302) | Total (n = 402) | |
|---|---|---|---|---|
| Male, n (%) | 14 (38%) | 27 (43%) | 148 (49%) | 189 (47%) |
| Age, mean (range) | 37.8 (13–58) | 30.7 (8–68) | 29.5 (8–84) | 30.4 (8–84) |
| Drug allergy, n (%) | 4 (10%) | 6 (10%) | 29 (10%) | 39 (10%) |
| Food allergy, n (%) | 25 (68%) | 33 (52%) | 61 (20%) | 119 (30%) |
| -OAS, n (%) | 25 (100%) | 21 (64%) | 40 (66%) | 86 (71%) |
| -SR, n (%) | 0 (0%) | 12 (36%) | 21 (34%) | 33 (28%) |
| Total IgE, KU/L, median, (range) | 202.0 (28.2–1565) | 229 (27.4–1465) | 325 (23.3–5000) | 280.5 (23.3–5000) |
OAS, oral allergy syndrome; SR, systemic reaction
Fig. 1.
Frequencies of specific pollen allergies by seasonal symptoms group. A wheal size of at least 5 mm and detection of specific IgE >0.35 kUA/L were considered positive indicators of allergy. SG, spring symptoms group; AG, autumn symptoms group; CG, combined seasons group
In addition, 119 of 402 (30%) participants reported having had a pollen-related allergic reaction to food, with fruits being the most common trigger (n = 86, 72%), followed by legumes (n = 28, 24%), grains (n = 24, 20%), nuts (n = 24, 20%), vegetables (n = 23,19%), and seeds (n = 9, 8%). Pollen-related food allergy were more prevalent in the single-season symptoms groups (68% and 52% for spring and autumn, respectively) than for the combined seasons symptoms group (20%). Among the 119 patients, 33 (28%) had experienced food-induced anaphylaxis at least once; no patient in the spring symptoms group had experienced anaphylaxis.
Bet v 1 and Art v 3 as predictive biomarkers of pollen-related food allergy
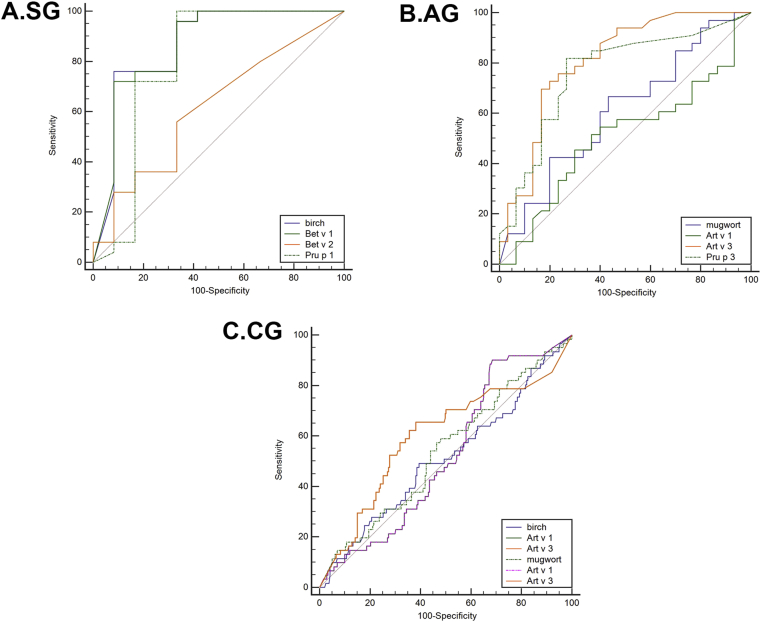
Birch and mugwort pollen were triggers for all patients except 2 with pollen-related food allergy. There were no between-group differences in Bet v 2-, mugwort pollen-, or Art v 1-specific IgE levels. The ROC curves were used to assess the diagnostic power of the specific IgE types and levels which are shown in Fig. 3.
Fig. 3.
The results of the receiver operating curve analysis for pollen allergen components as predictors of pollen-related food allergy in different seasonal symptoms groups: SG, spring symptom group; AG, autumn symptom group; CG, combined seasons group
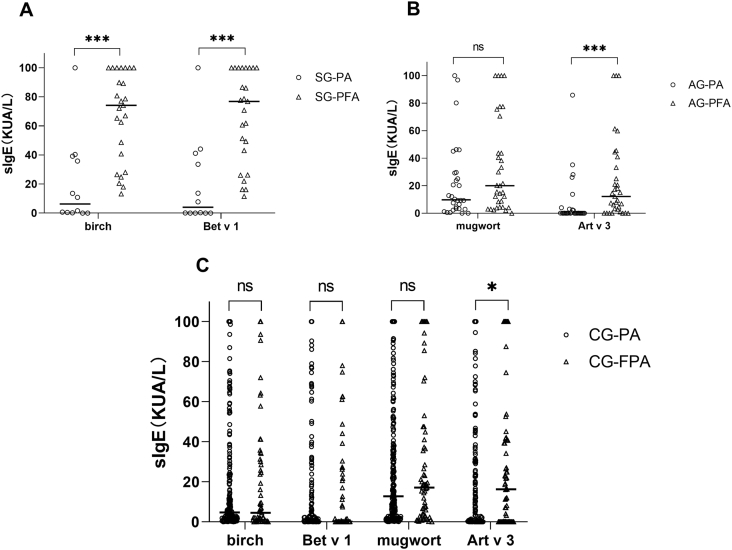
In the SG, birch pollen-specific IgE levels measured in patients with a food allergy (median, 74.2 kUA/L; range, 34.4–100 kUA/L) were significantly higher than those of tolerant patients (median, 6.27 kUA/L; range, 0.26–38.4 kUA/L). Likewise, Bet v 1-specific IgE levels were significantly higher in patients with a food allergy (median, 76.9 kUA/L; range, 34.7–100 kUA/L) than in tolerant patients (median, 4.05 kUA/L; range, 0.02–39.3 kUA/L) (Fig. 2A). All participants with a food allergy were sensitized to birch and Bet v 1. This was in contrast to tolerant participants (66%, p < 0.01 and 50%, p < 0.001, respectively). ROC analysis showed that birch pollen- and Bet v 1-specific IgE had uniform areas under the curve (AUC, 0.865; 95% confidence interval [CI]: 0.71–1 and AUC, 0.863; 95% CI: 0.718–1, respectively). The optimum cutoff value for birch pollen-specific IgE was 40.6 kUA/L, with 76% sensitivity and 92% specificity, and for Bet v 1-specific IgE, it was 46.8 kUA/L, with 72% sensitivity and 92% specificity (Fig. 3A). There were no differences in birch pollen- and Bet v 1-specific IgE levels between the CG participants with and without food allergy.
Fig. 2.
Specific IgE levels in participants with pollen-related food allergy, stratified by symptom season: A) spring symptom group, B) autumn symptom group, and C) combined seasons group. ∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001. sIgE, specific IgE; SG, spring symptom group; AG, autumn symptom group; CG, combined seasons group; PA, pollen allergy; PFA, pollen-related food allergy
For mugwort allergens, Art v 3-specific IgE levels measured in patients with food allergy (median, 12.2 kUA/L; range, 1.95–37.2 kUA/L) were significantly higher than those of tolerant patients (median, 0.07 kUA/L; range, 0.03–2.59 kUA/L) (Fig. 2B) in the AG. Similarly, Art v 3-specific IgE levels were higher in patients with food allergy (median, 16.3 UA/L; range, 0.05–40.9 kUA/L) than in patients without food allergy (median, 0.24 kUA/L; range, 0.02–22.7 kUA/L) (Fig. 2C) in the CG. Meanwhile, Art v 3-specific IgE levels were higher in individuals with food allergy than in tolerant individuals in the AG (79% vs. 33%, p < 0.001) and the CG (67% vs. 48%, p = 0.01). ROC analysis showed the largest AUC was for Art v 3-specific IgE in the AG (AUC, 0.808; 95% CI, 0.698–0.918) (Fig. 3B) and that the Art v 3-specific IgE cutoff value (4.35 kUA/L) had a high predictive power for diagnosing food allergy. Art v 3-specific IgE also had the largest AUC in the CG (AUC, 0.598; 95% CI, 0.512–0.684) (Fig. 3C).
Art v 3 in pollen-related food-induced anaphylaxis
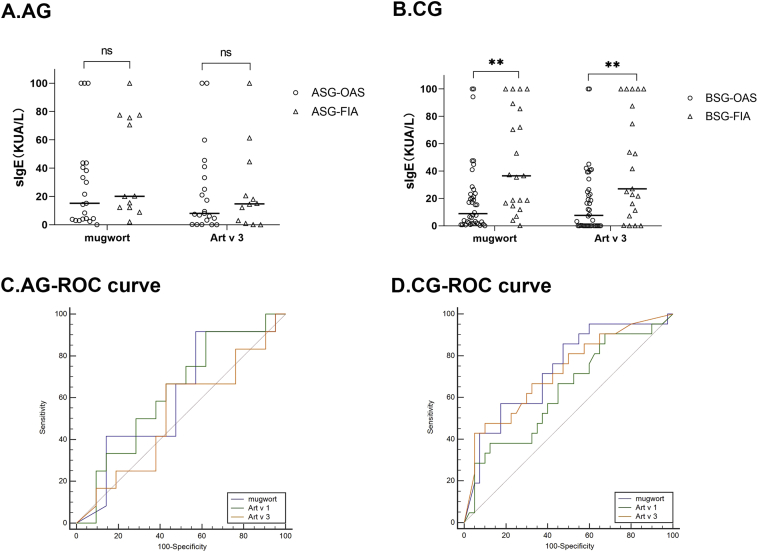
In the AG, allergen component-specific IgE levels were higher in patients with FIA (median, 14.8 kUA/L; range, 1.44–38.5 kUA/L) than in patients with OAS (median, 8.03 kUA/L; range, 1.74–37.2 kUA/L) (Fig. 4A), although there was no statistical significance. Meanwhile, in the CG, mugwort pollen- and Art v 3-specific IgE levels were significantly higher in patients with FIA (median, 36.5 kUA/L; range, 15.6–87.4 kUA/L and median, 27 kUA/L; range, 9.24–93.8 kUA/L, respectively) than OAS (median, 8.84 kUA/L; range, 1.76–23.5 kUA/L and median, 7.64 kUA/L; range, 0.01–26.1 kUA/L, respectively). The ROC analysis showed that the mugwort pollen- (AUC, 0.732; 95% CI, 0.599–0.865) (Fig. 4C) and Art v 3-specific IgE (AUC, 0.723; 95% CI, 0.585–0.860) could better predict an FIA diagnosis in the CG.
Fig. 4.
Specific gE levels of mugwort and Art v 3 were compared among patients with oral allergy syndrome and food-induced anaphylaxis. The diagnostic power of predicting systemic reactions to pollen-related food in different groups. ∗∗, p < 0.01. sIgE, specific IgE; AG, autumn symptom group; CG, combined seasons group; OAS, oral allergy syndrome; FIA, food-induced anaphylaxis
The correlation between pollen and food allergen components
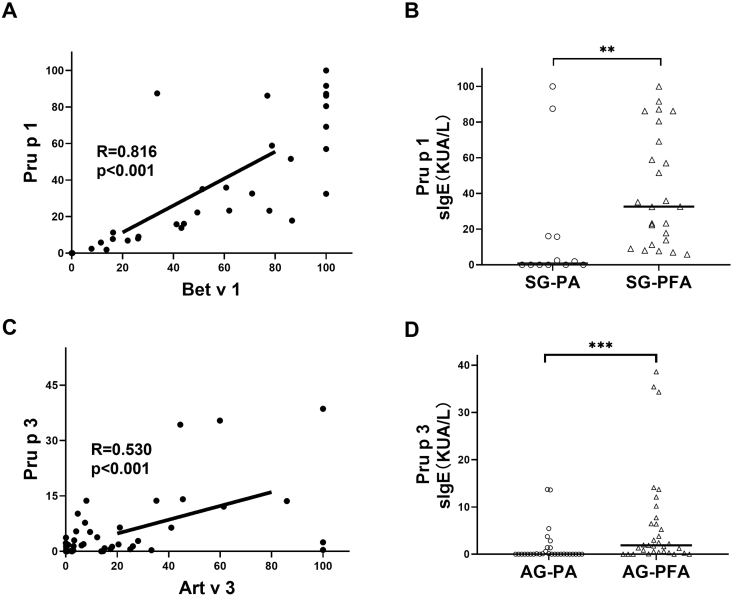
There was a strong correlation between Bet v 1- and Pru p 1-specific IgE levels (r = 0.816, p < 0.001) in the SG, while there was a moderate correlation between Art v 3- levels and Pru p 3-specific IgE (r = 0.530, p < 0.001) in the AG (Fig. 5). Pru p 1-specific IgE levels in participants with food allergy (median, 32.6 kUA/L; range, 12.6–74.9 kUA/L) were significantly higher than in participants without food allergy (median, 0.97 kUA/L; range, 0–16.0 kUA/L) in the SG. In the AG, Pru p 3-specific IgE levels were significantly higher in participants with food allergy (median, 1.89 kUA/L; range, 0.35–7.1 kUA/L) than in in participants without food allergy (median, 0.02 kUA/L; range, 0.01–0.8 kUA/L). The food allergy diagnostic power of Pru p 1 (AUC, 0.795; 95% CI, 0.594–0.996) and Pru p 3-specific IgE levels (AUC, 0.763; 95% CI, 0.640–0.885) were similar to those of Bet v 1- and Art v 3-specific IgE levels.
Fig. 5.
Results of the correlation analysis between pollen allergen components (Bet v 1 and Artv 3) and food allergen components (Pru p 1 and Pru p 3) in patients with and without food allergy. sIgE, specific IgE; SG, spring symptoms group; AG, autumn symptoms group; PA, pollen allergy; PFA, pollen-related food allergy
Discussion
This is first study of the prevalence and characteristics of season-dependent symptoms of pollinosis among patients with food allergies in China. We assessed the systemic symptoms of patients with food allergy and found that Art v 3-specific IgE levels were higher among autumn symptom patients who exhibited FIA rather than OAS. This is consistent with a previous study that reported a correlation between LTP sensitization and severity of allergic reactions in the subgroup of the Chinese population with mugwort and without birch pollen allergy.11
In our study, we found 30% of pollinosis patients reported symptoms of food allergies, which is a rate comparable to that reported for Europe11,16, 17, 18 and Korea,19 but higher than the rate reported for Japan.20 Moreover, 68% of participants in the spring symptoms group had a pollen-related food allergy, which is similar to a previous study that reported 73% of patients with birch pollen allergy experienced food allergy.21 However, the prevalence of patients with mugwort pollen-related food allergy in the present study was lower than a previous study that analyzed food allergy prevalence in Chinese patients allergic to mugwort.11
Bet v 1, a 17 kD protein that belongs to the pathogenesis-related protein class 10 family, is a major allergen in birch pollen. Many plant foods contain homologs cross-reactive in vitro to Bet v 1; examples of such foods include apple, peach, alder, hornbeam, hazel, oak, chestnut, and beech.4 This suggests that the majority of patients with birch pollen allergy are likely to experience pollen-related food allergy. A European study22 has shown that 53–95% of people with birch pollen allergy are sensitized to Bet v 1, depending on their region of residence. Indeed, in the present study, 85% (31/37) of participants with birch pollen allergy, and 100% (25/25) of participants with food allergy, in the SG tested positive for Bet v 1-specific IgE. However, 51% (99/248) of participants with birch pollen allergy in the CG, and only 60% (27/45) of participants with food allergy, were sensitized to Bet v 1. The results suggest that the prevalence of Bet v 1 sensitivity might depend on different pollen allergy types.
Members of the non-specific lipid transfer protein (nLTP) family are present in many plant foods. Originally discovered in apple23 and peach,24 subsequent studies have reported their cross-reactivity with the mugwort pollen allergen component Art v 3.25 Although Bet v 1 is a major culprit in pollen-related food allergy in Europe (except in the Mediterranean region26), a China-based study has indicated that Art v 3 might also be a major trigger among the Chinese population. In addition, Art v 3-specific IgE has recently been discovered in European populations.5,7 In our population, the prevalence of Art v 3-specific IgE was higher in patients with food allergy than without food allergy both in the AG and CG. Most of the participants in the present study were allergic to birch and mugwort pollens; these results are similar to a recent China-based study involving participants with mugwort pollen allergy only.11
Because nLTPs can resist pepsin and heat degradation, most studies suggest that nLTPs correlate to plant food-induced anaphylaxis.7,23,25,27 At present, more than 35 nLTP species have been identified as associated with food allergy,6 most of them within the nLTPs 1 subfamily. In the present study, we found that Art v 3-specific IgE levels in the AG and CG were higher in patients with OAS than in patients with food-induced anaphylaxis; however, the difference was only statistically significant in the CG. This is in line with a previous study,11 which suggested that nLTPs were correlated with the severity of allergic reactions in a Chinese population. This implies that Art v 3 might be a major biomarker for plant food allergy in Chinese individuals with autumn pollinosis.
Considering cross-reactivity of pollen and food allergens,28 we assessed a correlation between a food allergen component and a pollen allergen component. The results suggested that the diagnostic value of the pollen allergen component was similar to the diagnostic value of the food allergen component. So, we can measure one of homologous allergen components instead of a chip to reduce the medical expenditure in clinical practice.
This study has several limitations. First, we ascertained food allergy based on clinical history, questionnaire answers, and tests for allergen-specific IgE, instead of a double-blind placebo-controlled food challenge, which is considered the gold standard in the diagnosis of food allergy; so there is some risk of the recollection bias, and the food allergic reactions and symptoms might be exaggerated. Second, this study might have been subject to a selection bias as the included participants were patients treated at an outpatient department of our hospital, resulting in participants in this study having severe pollinosis and sensitivity to multiple allergens,. In addition, considering the limitations of clinical data, seasonality, and trigger dose of food allergy and how it relates to pollen seasonality were not explored; further studies are needed to resolve this.
In conclusion, the overall prevalence of pollen-related food allergy in our Chinese pollinosis patients was 30%, distributed differently depending on the season during which pollen allergy symptoms presented. Among the participants whose symptoms occurred in the spring, nearly 70% had a pollen-related food allergy. Concurrently, no patient with only spring symptoms suffered food-induced anaphylaxis. According to component-resolved diagnosis, Bet v 1 and Art v 3 are candidate molecular biomarkers for the diagnosis of pollen-related food allergies in patients whose pollen-related allergy symptoms occur in different seasons. In addition, measuring allergen component-specific IgE levels might be an effective tool for management of pollinosis in clinical practice in China.
Ethics approval and consent to participate
All the patients signed a written informed consent form before they were recruited. This study was approved by the ethics committee of Peking Union Medical College Hospital.
Authors contributions
Jun-Da Li analyzed and interpreted the data and drafted the article.
Zhi-Rong Du made substantial contributions to acquisition of the cases.
Juan Liu made substantial contributions to acquisition of the cases.
Ying-Yang Xu made substantial contributions to acquisition of the cases.
Rui-Qi Wang made substantial contributions to acquisition of the datas.
Jia Yin made substantial contributions to conception and design and gave final approval of the version to be published.
Funding
This study was supported by two foundations: CAMS Innovation Fund for Medical Sciences (CIFMS, NO. 2016-I2M-1–003), National Natural Science Foundation of China (NO. 81273277).
Declaration of competing Interest
All of authors report no competing interests or financial disclosure.
Acknowledgement
We are appreciate all the patients and investigators who participated in this study.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Yagami A., Ebisawa M. New findings, pathophysiology, and antigen analysis in pollen-food allergy syndrome. Curr Opin Allergy Clin Immunol. 2019;19:218–223. doi: 10.1097/ACI.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 2.Popescu F.D. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015;5:31–50. doi: 10.5662/wjm.v5.i2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahali Y., Dadar M. Plant food allergy: influence of chemicals on plant allergens. Food Chem Toxicol. 2018;115:365–374. doi: 10.1016/j.fct.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 4.Biedermann T., Winther L., Till S.J., Panzner P., Knulst A., Valovirta E. Birch pollen allergy in Europe. Allergy. 2019;74:1237–1248. doi: 10.1111/all.13758. [DOI] [PubMed] [Google Scholar]
- 5.Mothes-Luksch N., Raith M., Stingl G. Pru p 3, a marker allergen for lipid transfer protein sensitization also in Central Europe. Allergy. 2017;72:1415–1418. doi: 10.1111/all.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheurer S., Schulke S. Interaction of non-specific lipid-transfer proteins with plant-derived lipids and its impact on allergic sensitization. Front Immunol. 2018;9:1389. doi: 10.3389/fimmu.2018.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scala E., Till S.J., Asero R. Lipid transfer protein sensitization: reactivity profiles and clinical risk assessment in an Italian cohort. Allergy. 2015;70:933–943. doi: 10.1111/all.12635. [DOI] [PubMed] [Google Scholar]
- 8.Hao G.D., Zheng Y.W., Wang Z.X. High correlation of specific IgE sensitization between birch pollen, soy and apple allergens indicates pollen-food allergy syndrome among birch pollen allergic patients in northern China. J Zhejiang Univ - Sci B. 2016;17:399–404. doi: 10.1631/jzus.B1500279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qing yu Lq-sJS xLX-zZx-mW Seasonal and geographical dispersal regularity of airborne pollens in China. Med J Chin Peoples Lib Army. 2017;42:951–955. [Google Scholar]
- 10.Ma S.K., Wang R.Q., Nie L., Yin J. Pollen-food allergy syndrome in China. Food Agric Immunol. 2018;29:281–293. [Google Scholar]
- 11.Deng S., Yin J. Mugwort pollen-related food allergy: lipid transfer protein sensitization and correlation with the severity of allergic reactions in a Chinese population. Allergy Asthma Immunol Res. 2019;11:116–128. doi: 10.4168/aair.2019.11.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieths S., Scheurer S., Ballmer-Weber B. Current understanding of cross-reactivity of food allergens and pollen. Ann N Y Acad Sci. 2002;964:47–68. doi: 10.1111/j.1749-6632.2002.tb04132.x. [DOI] [PubMed] [Google Scholar]
- 13.Sampson H.A., Munoz-Furlong A., Campbell R.L. Second symposium on the definition and management of anaphylaxis: summary report-second national Institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. Ann Emerg Med. 2006;47:373–380. doi: 10.1016/j.annemergmed.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Ye S., Zhang J., Qiao B. Science Publishing House.; Beijing: 1988. Investigation of Airborne Allergenic Pollens in Different Regions of China. [Google Scholar]
- 15.Bousquet J., Heinzerling L., Bachert C. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 16.Amlot P.L., Kemeny D.M., Zachary C., Parkes P., Lessof M.H. Oral allergy syndrome (OAS): symptoms of IgE-mediated hypersensitivity to foods. Clin Allergy. 1987;17:33–42. doi: 10.1111/j.1365-2222.1987.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 17.Flores E., Cervera L., Sanz M.L., Diaz-Perales A., Fernandez J. Plant food allergy in patients with pollinosis from the Mediterranean area. Int Arch Allergy Immunol. 2012;159:346–354. doi: 10.1159/000338282. [DOI] [PubMed] [Google Scholar]
- 18.Bircher A.J., Van Melle G., Haller E., Curty B., Frei P.C. IgE to food allergens are highly prevalent in patients allergic to pollens, with and without symptoms of food allergy. Clin Exp Allergy. 1994;24:367–374. doi: 10.1111/j.1365-2222.1994.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim M.A., Kim D.K., Yang H.J. Pollen-food allergy syndrome in Korean pollinosis patients: a nationwide survey (vol 10, pg 648, 2018) Aller Asth Immunol Res. 2019;11:441–442. doi: 10.4168/aair.2019.11.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda N., Inomata N., Morita A., Kirino M., Ikezawa Z. Correlation of oral allergy syndrome due to plant-derived foods with pollen sensitization in Japan. Ann Allergy Asthma Immunol. 2010;104:205–210. doi: 10.1016/j.anai.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 21.Geroldinger-Simic M., Zelniker T., Aberer W. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG(4) antibodies. J Allergy Clin Immunol. 2011;127 doi: 10.1016/j.jaci.2010.10.027. 616-U124. [DOI] [PubMed] [Google Scholar]
- 22.Ciprandi G., Comite P., Mussap M. Profiles of birch sensitization (Bet v 1, Bet v 2, and Bet v 4) and oral allergy syndrome across Italy. J Investig Allergol Clin Immunol. 2016;26:244–248. doi: 10.18176/jiaci.0041. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Monge R., Lombardero M., Garcia-Selles F.J., Barber D., Salcedo G. Lipid-transfer proteins are relevant allergens in fruit allergy. J Allergy Clin Immunol. 1999;103:514–519. doi: 10.1016/s0091-6749(99)70479-3. [DOI] [PubMed] [Google Scholar]
- 24.Pastorello E.A., Farioli L., Pravettoni V. The major allergen of peach (Prunus persica) is a lipid transfer protein. J Allergy Clin Immunol. 1999;103:520–526. doi: 10.1016/s0091-6749(99)70480-x. [DOI] [PubMed] [Google Scholar]
- 25.DÍaz-Perales A., Lombardero M., SÁnchez-Monge R. Lipid-transfer proteins as potential plant panallergens: cross-reactivity among proteins of Artemisia pollen, Castanea nut and Rosaceae fruits, with different IgE-binding capacities. Clin Exp Allergy. 2000;30:1403–1410. doi: 10.1046/j.1365-2222.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 26.Cuesta-Herranz J., Lazaro M., Figueredo E., Igea J.M., Umpierrez A., De-Las-Heras M. Allergy to plant-derived fresh foods in a birch- and ragweed-free area. Clin Exp Allergy. 2000;30:1411–1416. doi: 10.1046/j.1365-2222.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 27.Scheurer S., Lauer I., Foetisch K. Strong allergenicity of Pru av 3, the lipid transfer protein from cherry, is related to high stability against thermal processing and digestion. J Allergy Clin Immunol. 2004;114:900–907. doi: 10.1016/j.jaci.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Pablos I., Wildner S., Asam C., Wallner M., Gadermaier G. Pollen allergens for molecular diagnosis. Curr Allergy Asthma Rep. 2016;16:31. doi: 10.1007/s11882-016-0603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]