Abstract
Objective
Central artery stiffness is a confirmed predictor of cardiovascular health status that has been consistently associated with cognitive dysfunction and dementia. The European Society of Hypertension has established a threshold of arterial stiffness above which a cardiovascular event is likely to occur. However, the threshold at which arterial stiffness alters brain integrity has never been established.
Methods
The aim of this study is to determine the arterial stiffness cut-off value at which there is an impact on the white matter microstructure. This study has been conducted with 53 cognitively elderly without dementia. The integrity of the white matter was assessed using diffusion tensor metrics. Central artery stiffness was evaluated by measuring the carotid-femoral pulse wave velocity (cfPWV). The statistical analyses included 4 regions previously denoted vulnerable to increased central arterial stiffness (the corpus callosum, the internal capsule, the corona radiata and the superior longitudinal fasciculus).
Results
The results of this study call into question the threshold value of 10 m/s cfPWV established by the European Society of Hypertension to classify patients in neuro-cardiovascular risk groups. Our results suggest that the cfPWV threshold value would be approximately 8.5 m/s when the microstructure of the white matter is taken as a basis for comparison.
Conclusions
Adjustment of the cfPWV value may be necessary for a more accurate distinction between lower and higher risk group of patients for white matter microstructural injury related to arterial stiffness. Targeting the highest risk group for prevention methods may, in turn, help preserve brain health and cognitive functions.
Keywords: Arterial stiffness, Hypertension, Aging, White matter, Cognition, Diffusion, MRI, Cut-off
Highlights
-
•
DTI (FA, RD) analysis of white matter microstructure reveals that the cfPWV cut-off value (10 m/s) may be too high
-
•
This study rather suggests a value of cfPWV cut-off of 8.5 m/s to separate lower and higher neurovascular risk groups
-
•
Better executive function performance is correlated with higher FA and lower RD in participants with a cfPWV above 8.5 m/s.
1. Introduction
Age-related cognitive decline is of increasing concern among the elderly (Deary et al., 2009; Sala et al., 2015). Thus, uncovering the complex mechanisms underpinning cognitive decline in the elderly may help tailor future interventions in order to preserve cognitive performance throughout the lifespan. Normal aging is associated with an increase in central artery stiffness resulting in accelerated pulse wave velocity (PWV) and downstream microvascular dysfunctions that could contribute to damage end-organs such as the brain (Hansen and Taylor, 2016). Although the association between arterial stiffness and cognitive decline is still poorly understood (Laurent et al., 2001), arterial stiffness has been consistently associated with white matter (WM) lesions, in particular WM hyperintensities. Whether these WM lesions are reversible or irreversible during aging is still a matter of debate, however, they may be preceded by microstructural changes that can be detected with diffusion tensor imaging (DTI). Indeed, in a study looking at DTI changes within WMHs (white matter hyperintensities) over a three year period in cognitively unimpaired elderly, Maillard et al. showed significant DTI changes over time in these WMHs, but no change in T2w Flair images (Maillard et al., 2014). By allowing a more detailed characterization of the underlying tissue microstructure compared to conventional MRI techniques, DTI may allow the identification of early microstructural changes before the appearance of more pronounced WM damage and offer a potential tool for early prediction of cognitive dysfunction (Badji et al., 2019; Jones, 2011; Maillard et al., 2014).
Few studies have employed DTI to assess WM microstructural changes in relation to arterial stiffness (Badji et al., 2018; de Groot et al., 2013; Maillard et al., 2017, Maillard et al., 2016; Tarumi et al., 2015). Findings from these studies have identified 4 WM regions as being vulnerable to increased carotid-femoral pulse wave velocity (cfPWV) which is the gold standard for measurement of arterial stiffness (Laurent et al., 2006): the corpus callosum, the internal capsule, the corona radiata and the superior longitudinal fasciculus (Badji et al., 2018; Maillard et al., 2017, Maillard et al., 2016; Tarumi et al., 2015).
Recently, a critical cut-off value of cfPWV of 10 m/s has been suggested by a study group on behalf of the Artery Society, the European Society of Hypertension Working Group on Vascular Structure and Function, and the European Network for Noninvasive Investigation of Large Arteries (Butlin and Qasem, 2017; Van Bortel et al., 2012). The same cut-off value has also been adopted by the European Society of Hypertension guidelines for hypertension management (Williams et al., 2018). This cut-off value has been estimated while considering the additive value of arterial stiffness beyond traditional risk factors in order to predict the occurence of a cardiovascular event. However, the threshold at which arterial stiffness alters brain integrity has never been established. The aim of this study is to use WM sensitive techniques to estimate the cut-off value at which arterial stiffness impacts the microstructure of WM and ultimately cognition in healthy elderly. Considering the fact that DTI metrics are tailored to detect the first subtle WM microstructural changes associated with arterial stiffness, we hypothesized that 1) the microstructural information from the vulnerable regions to arterial stiffness would allow the estimation of the cfPWV cut-off value that best identify individuals at higher risk for structural changes and cognitive decline and 2) that microstructural changes in the WM regions previously shown to be vulnerable to central artery stiffness would translate into a decrease in cognitive flexibility performance in participants above the cfPWV cut-off.
2. Material and methods
2.1. Study participants
Seventy-three healthy elderly between 65 and 75 years of age were recruited at the Centre de Recherche de l'Institut Universitaire de Gériatrie de Montréal (CRIUGM) via a telephone-based screening interview aiming to assess their eligibility. Participants underwent an exhaustive medical assessment conducted by a physician including blood analysis and neuropsychological assessment. Individuals with a history of major psychiatric or neurological disorders, uncontrolled hypertension, diabetes mellitus, heart failure (level III-IV), myocardial infarction (in the previous 3 months), cardiac arrhythmia, rheumatic mitral valve disease, liver failure, renal failure (creatinine clearance of <30 mL/min), stroke, non-compensated thyroid disorder, respiratory problems (i.e. asthma, emphysema), metallic implants and/or pacemaker claustrophobia, current or history of alcohol or drug abuse as well as current smoking were excluded from participation. Participants with a Mini-Mental State Examination score below 26 were also excluded. Ethics approval was obtained from the ethical review board of the CRIUGM and the Montreal Clinical Research Institute. An informed consent approved by the local institutional review board was signed by each participant.
From all participants that were recruited, twelve participants voluntarily withdrew themselves from the study for personal reasons, two were excluded and one didn't complete the last part of the study including the MRI. Among the 58 participants from which we obtained the MRI images, one was excluded due to an incidental finding, and four were excluded from this study due to missing data (either cfPWV or blood pressure measurements). Therefore, in this study, we only considered the remaining 53 participants. Based on previous imaging studies in which MRI-DTI techniques have been used to detect differences in WM integrity associated with psychiatric disorders, we estimate the Cohen's effect size for a biologically relevant change (15%) to be 1.2. An a priori power analysis, assuming this effect size, for a Type I error rate of α = 0.01 and a power (1-ˇβ) of 0.95 indicates that 27 samples per condition are required. A sample size of 27 patients per group should allow detection of <15% difference in WM integrity between groups. A similar study with a group of 54 subjects showed a correlation between cfPWV, DTI metrics and cognitive measures (Tarumi et al., 2015).
Among our participants, 14 were on antihypertensive medications, 7 were on angiotensin converting-enzyme inhibitors, 3 were on calcium channel blockers, 2 were on angiotensin converting-enzyme inhibitors and calcium channel blockers, 1 was on an angiotensin converting-enzyme inhibitor and hydrochlorothiazide, and 1 was on a calcium channel blocker and hydrochlorothiazide. Sample characteristics of all participants can be found in Table 1.
Table 1.
Sample characteristics of all participants including a comparison of drug-naive participants and those on anti hypertensive (Anti-HT) Treatment.
| All | On Anti-HT treatment | Drug naîve | P-value | |
|---|---|---|---|---|
| Women/men (N) | 39/14 (53) | 7/7 (14) | 30/9 (39) | 0.066 |
| Age (years) | 69.95 ± 3.33 | 70.10 ± 3.08 | 69.79 ± 3.44 | 0.880 |
| Education (years) | 16.42 ± 3.43 | 17.07 ± 3.03 | 15.97 ± 3.56 | 0.089 |
| Height (m) | 1.62 ± 0.08 | 1.61 ± 0.072 | 1.63 ± 0.087 | 0.486 |
| Weight (kg) | 70.04 ± 13.39 | 71.66 ± 12.97 | 69.53 ± 13.82 | 0.029 |
| Neurocognitive measures | ||||
| MMSE score | 29.35 ± 0.83 | 29.50 ± 0.94 | 29.28 ± 0.81 | 0.325 |
| MoCA score | 27.89 ± 2.17 | 27.73 ± 1.75 | 28.22 ± 1.94 | 0.115 |
| TMTB-A (s) | 36.35 ± 17.17 | 37.73 ± 16.08 | 35.78 ± 17.79 | 0.246 |
| Cardiovascular measures | ||||
| 24 h SBP (mmHg) | 125.87 ± 11.67 | 128.20 ± 10.63 | 125.00 ± 12.17 | 0.657 |
| 24 h DBP (mmHg) | 73.49 ± 7.45 | 76.00 ± 7.10 | 72.53 ± 7.64 | 0.275 |
| 24 h HR (bpm) | 69.81 ± 7.69 | 68.53 ± 7.18 | 70.47 ± 8.07 | 0.777 |
| cfPWV (m/s) [range] | 9.14 ± 2.19 [5.2–15.1] | 9.26 ± 2.64 | 9.10 ± 2.07 | 1.000 |
| White matter measures | ||||
| FA_t | 0.53 ± 0.02 | 0.45 ± 0.01 | 0.44 ± 0.01 | 0.183 |
| RD_t (10−4 m2/s) | 4.78 ± 1.88 | 4.80 ± 1.28 | 4.77 ± 1.96 | 0.190 |
| FA_ROI | 0.53 ± 0.02 | 0.52 ± 0.02 | 0.53 ± 0.01 | 0.323 |
| RD_ROI (10−4 m2/s) | 4.61 ± 2.40 | 4.61 ± 1.43 | 4.60 ± 2.55 | 0.267 |
Values are mean ± standard deviation. MMSE = Mini-Mental State Exam, MoCA = Montreal cognitive assessment, TMTB-A = Trail Making test Part B-A, SBP = systolic blood pressure, DBP = Diastolic blood pressure, HR = Heart rate, cfPWV = carotid-femoral pulse wave velocity, FA = Fractional Anisotropy, RD = Radial diffusivity, FA_t = FA in total white matter, RD_t = RD in total white matter, FA_ROI = FA in our 4 regions of interest, RD_ROI = RD in our 4 regions of interest, ROI are the corpus callosum, the internal capsule, the corona radiata and the superior longitudinal fasciculus. Bold: P-value<.05.
2.2. Cardiovascular measurements
The cfPWV and the 24 h SBP measurements were performed by the same trained expert (Adrian Noriega, MD) using an established reproducible protocol. The cfPWV was measured using arterial applanation tonometry following the Van Bortel protocol (Van Bortel et al., 2012). The SphygmoCor system (AtCor Medical Pty Ltd) was used to compute the cfPWV as described by Laurent et al. (2006). The cfPWV was measured twice by dividing arterial pulse traveling distance by the transit time, and expressed in meters per second. The arterial pulse traveling distance was measured as the straight distance between the carotid and femoral measurement sites using a tape ruler, whereas the transit time was determined from the time delay between the proximal and distal “foot” waveforms. The cfPWV was then calculated by taking the mean of the two measurements. However, when the difference between the two measurements was >0.5 m/s, a third measurement was taken and the median value of all 3 measures was computed. The cfPWV measurements were performed during the same time of the day across subjects (between 09:00–12:00 pm) to minimize the effects of diurnal variations following the Van Bortel protocol (Van Bortel et al., 2012).
In addition, a 24 h Ambulatory Blood Pressure Monitoring 90,207-3Q model (Spacelabs Healthcare®) was installed in the patient's non-dominant arm following the recommendation of Hypertension Canada (Nerenberg et al., 2018), in order to get blood pressure measurements every 30 min during 24 h. To calibrate the 24 h Ambulatory Blood Pressure Monitoring device, conventional blood pressure was measured manually by a sphygmomanometer (Korotkoff phases I and IV) after the subjects rested in supine position for at least 15 min. All measurements were completed after at least 3 h of abstinence from alcohol, caffeinated beverages, and intense physical activity as suggested by the Van Bortel protocol (Van Bortel et al., 2012).
2.3. Neurocognitive assessments
The Trail Making Test parts A (TMTA) and B (TMTB) (Tombaugh, 2004) were administered and scored independently by the same trained expert (Adrián Noriega, MD). During the TMTA, participants had to connect a series of numbers in the correct order as fast as possible (i.e. 1–2-3, etc.) while during the TMTB, participants had to connect numbers and letters by matching them in ascending order (i.e. 1-A-2-B, etc.). In this study, we considered the difference between the time spent in completing the TMTB and TMTA in seconds as a measure of cognitive flexibility (Corrigan and Hinkeldey, 1987; Reitan, 1958).
2.4. Brain MRI analysis
We used DTI measures of Fractional anisotropy (FA) and Radial diffusivity (RD) as measures of WM microstructure.
MRI data was acquired at 3 T (MAGNETOM Prisma Fit, Siemens Healthineers, Erlangen, Germany) using a 32-channel head coil. Diffusion weighted-imaging images were acquired using echo planar imaging with factor = 3 simultaneous multislice acceleration (Setsompop et al., 2012). Axial slices of 2.0 mm isotropic were acquired parallel to the posterior-anterior commissure line, encoded with 3 b-value shells: 300/1 k/2 k s/mm2, along: 7/29/64 directions, 9 b = 0, and a scan duration of 4.37 min. Other acquisitions parameters were: imaging matrix = 110 × 110, FOV = 220 × 220 mm2, Echo Time (TE) = 63 ms and Repetition Time (TR) = 2200 ms.
Processing of diffusion data was performed using the Toolkit for Analysis in Diffusion MRI (TOAD) (http://unf-montreal.ca/toad) and included: motion and eddy current distortion correction, denoising and calculation of FA and RD metrics by fitting a diffusion tensor model to the pre-processed diffusion data as previously reported (Badji et al., 2018). To minimize partial volume effects from gray matter and cerebrospinal fluid, an FA threshold of 0.20 was set for all voxels.
The FA and RD maps were registered to the Johns Hopkins International Consortium for Brain Mapping (ICBM) FA template using the Ants non-linear registration tools (Avants et al., 2011). The registration procedure is well described in a previous publication (Badji et al., 2018). The four regions of interest (the corpus callosum, the internal capsule, the corona radiata and the superior longitudinal fasciculus) were obtained from the ICBM-DTI-81 WM atlas (Mori et al., 2008) and were composed of 3, 6, 6 and 2 tracts respectively (Table 2), for a total of 17 tracts.
Table 2.
White matter regions and white matter tracts of interest extracted from the ICBM-DTI-81 WM atlas.
| White matter regions | White matter tracts | Tract ID | |
|---|---|---|---|
| Corpus callosum | Genu | 1 | |
| Body | 2 | ||
| Splenium | 3 | ||
| Internal capsule | Anterior | Right | 4 |
| Left | 5 | ||
| Posterior | Right | 6 | |
| Left | 7 | ||
| Retrolenticular part | Right | 8 | |
| Left | 9 | ||
| Corona radiata | Anterior | Right | 10 |
| Left | 11 | ||
| Superior | Right | 12 | |
| Left | 13 | ||
| Posterior | Right | 14 | |
| Left | 15 | ||
| Superior longitudinal fasciculus | Right | 16 | |
| Left | 17 |
2.5. Statistical analysis
The goal of the statistical analysis was to use WM metrics such as FA and RD to determine the optimal cfPWV cut-off value that best identifies individuals at higher risk for structural changes and cognitive decline following arterial stiffness.
2.5.1. Estimation of the cfPWV optical cut-off value
Firstly, a non parametric test was used to compare the demographic, cardiovascular, neuropsychological and MRI measurements between the groups below and above the 10 m/s cfPWV cut-off value suggested by the literature.
Secondly, a maximally selected rank statistics (Lausen and Schumacher, 1992), implemented in the maxstat R package (http://cran.r-project.org/web/packages/maxstat/index.html), was used to estimate an approximate cfPWV cut-off value that yields a good separation of observations in two groups with respect to the WM microstructural information of each region of interest. For every reasonable cut-off in cfPWV data value, participants are separated into two groups, then a standardized Wilcoxon rank test is computed to compare the DTI metrics of the two groups. The Wilcoxon test is a rank based non-parametric test for comparing two groups of observations without assuming a particular distribution (Wilcoxon, 1945). The value of the statistics of Wilcoxon rank statistic was recorded and plotted against the cfPWV data value. The higher the statistics, the more significant is the cut-off. The data value corresponding to the highest Wilcoxon rank statistic is identified as the optimal cut-off. For sensitivity estimation, the conditional Monte-Carlo method was used as the p-value approximation method.
Thirdly, in the light of the findings from this analysis, a cfPWV cut-off value was iterated from 10 to 7.5 m/s with a step size of 0.5 m/s, yielding six different grouping conditions. Next, a Wilcoxon rank sum test was used for groupwise comparisons of all tracts of interest (Table 2) using FA and RD. Here we used all 17 tracts, as opposed to the 4 regions of aggregated tracts, to highlight subtle differences between tracts within a large region. These differences could vanish after aggregating all other tracts. In addition, TMTB-A, age, cfPWV and SBP were compared for each grouping condition.
2.5.2. Association between cognitive performance and white matter integrity
Finally, we also wanted to test the presence of an association between cognitive flexibility performance and the microstructural integrity of the WM regions shown to be vulnerable to arterial stiffness, namely the corpus callosum, the internal capsule, the corona radiata and the superior longitudinal fasciculus. Therefore, we used a partial correlation between TMTB-A and both FA and RD measurements with the following covariates: age, sex, educational level (years of schooling). For the sake of clarity, in this analysis mean values of FA and RD were extracted from the 4 WM regions of interest encompassing the 17 tracts analyzed in the previous section. This partial correlation analysis was carried out 1) in the whole cohort, 2) in individuals below and above a cfPWV of 8.5 m/s independently and 3) in individuals below and above a cfPWV of 10 m/s independently. We also performed a linear regression with TMTB-A as the dependent variable and either measure of FA or RD as the independent variable, adjusting for age, sex and educational level (years of schooling).
2.5.3. Precision about the statistical analysis
All tests except from maximally selected rank statistics were implemented on SPSS (IBM SPSS 25 Statistics, Chicago, IL).
Maximally selected rank statistics were implemented in R (R version 3.5.1 MA, USA). Correction for multiple comparisons was achieved using the false discovery rate procedure described by Benjamini and Hochberg (1995). The bound on the number of expected false discovery rate for each MRI metrics was set to 0.05. As the thresholds under the Benjamini-Hochberg procedure rely not only on the number of tests (as with Bonferroni), but also on the calculated p-value for each test (all p-values are ranked and then assigned a specific correction factor), we decided for the sake of clarity to show original (non-adjusted) p-values and only highlight the ones that were found significant after correction for all statistical analysis. In addition, the critical adjusted p-value for each case (called “crit”) is shown in the figure legend.
3. Results
3.1. Assessment of cfPWV cut-off value
Table 3 permits us to assess if the suggested cfPWV cut-off value of 10 m/s allow for a good separation of observations in two groups with regard to the earliest differences in brain structure. Compared to the participants with cfPWV above the cut-off, the participants below the cut-off demonstrated no significant difference in cardiovascular outcomes (other than cfPWV with p < .001). In addition, no differences were found in neuropsychological measurements or the measures of DTI metrics (FA and RD), which assess the WM microstructure. A slight significant difference (p = .043) was observed for the parameter “Education”, which could be explained by our relatively small sample size.
Table 3.
Sample characteristics of below and above carotid-femoral pulse wave velocity participants.
| Below 10 m/s | Above 10 m/s | p value | |
|---|---|---|---|
| Women/men (n) | 27/11 | 10/5 | 0.751 |
| Age (years) | 70.20 ± 3.40 | 69.33 ± 3.17 | 0.429 |
| Education (years) | 17.03 ± 3.40 | 14.87 ± 3.11 | 0.043 |
| Height (m) | 1.63 ± 0.08 | 1.63 ± 0.10 | 0.112 |
| Weight (kg) | 68.21 ± 13.40 | 74.70 ± 12.73 | 0.693 |
| Neurocognitive measures | |||
| MMSE | 29.42 ± 0.76 | 11.90 ± 1.48 | 0.437 |
| MoCA | 28.11 ± 1.92 | 27.33 ± 2.69 | 0.539 |
| TMTB-A (s) | 43.21 ± 31.56 | 33.87 ± 16.20 | 0.396 |
| Cardiovascular measures | |||
| 24 h SBP (mmHg) | 124.16 ± 10.73 | 130.20 ± 13.19 | 0.066 |
| 24 h DBP (mmHg) | 72.97 ± 8.11 | 74.80 ± 5.50 | 0.318 |
| 24 h HR (bpm) | 68.79 ± 7.87 | 72.40 ± 6.79 | 0.097 |
| cfPWV (m/s) | 8.06 ± 1.30 | 11.90 ± 1.50 | 0.000 |
| White matter measures | |||
| FA-t | 0.45 ± 0.01 | 0.44 ± 0.02 | 0.782 |
| RD-t (10−4 m2/s) | 4.77 ± 1.62 | 4.59 ± 1.92 | 0.921 |
| FA-ROI | 0.53 ± 0.02 | 0.54 ± 0.02 | 0.228 |
| RD-ROI (10−4 m2/s) | 4.78 ± 2.16 | 4.80 ± 2.16 | 0.797 |
Values are mean ± standard deviation. MMSE = Mini-Mental State Exam, MoCA = Montreal cognitive assessment, TMTB-A = Trail Making test Part B-A, SBP = systolic blood pressure, DBP = Diastolic blood pressure, HR = Heart rate, cfPWV = carotid-femoral pulse wave velocity, FA = Fractional Anisotropy, RD = Radial diffusivity. Bold: P-value<.05.
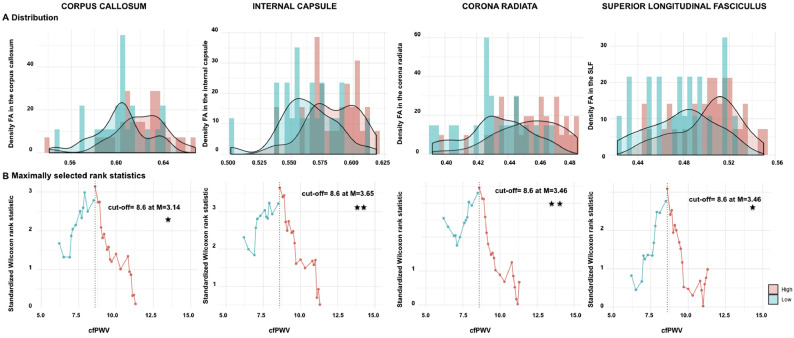
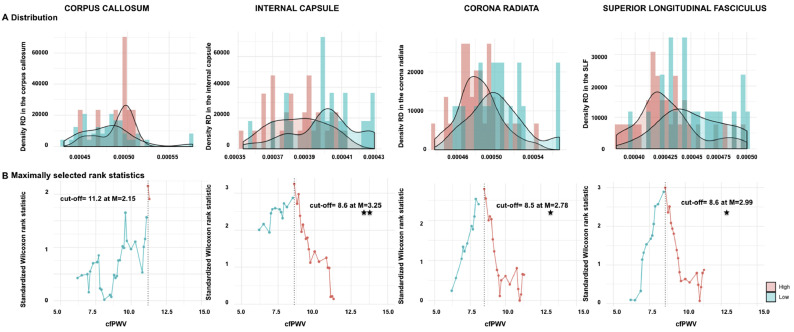
To assess the threshold at which cfPWV predicts WM alterations, a maximally selected rank statistics was first adopted and identified a cfPWV cut-off value for each of our regions of interest using FA and RD (Fig. 1, Fig. 2).
Fig. 1.
Estimation of carotid-femoral pulse velocity (cfPWV) cut-off value using fractional anisotropy (FA).
cfPWV (m/s) was estimated with maximally selected log-rank statistic and conditional Monte-Carlo as the p-value approximation method across white matter microstructure and 4 regions of interests. A. Density histogram of (FA) values per regions, B. Standardized log-rank statistics of all possible cut-off values, indicating the optimal cut-off as determined by the maximum standardized Wilcoxon rank statistic [M]. SLF: Superior longitudinal fasciculus. Significant p-values are corrected for multiple comparisons (adjusted threshold with FDR was 0.05).
Fig. 2.
Estimation of carotid-femoral pulse velocity (cfPWV) cut-off value using radial diffusivity (RD).
cfPWV (m/s) was estimated with maximally selected log-rank statistics and conditional Monte-Carlo as the p-value approximation method across white matter microstructure and 4 regions of interests. A. Density histogram of Radial diffusivity (RD) values per regions, B. Standardized log-rank statistics of all possible cut-off values, indicating the optimal cut-off as determined by the maximum standardized Wilcoxon rank statistic [M]. SLF: Superior longitudinal fasciculus. Significant p-values are corrected for multiple comparisons (adjusted threshold with FDR was 0.025).
Using FA, a minimum of 8.6 m/s cfPWV cut-off value was observed in all 4 regions of interest (Fig. 1B: The corpus callosum (p = .011), the internal capsule (p = .002), the corona radiata (p = .004) and the superior longitudinal fasciculus (p = .019). However, it is noteworthy to notice that the highest Wilcoxon rank statistic (M = 3.65) is identified when taking into account the FA values in the internal capsule.
Fig. 2B shows that using RD, a minimum of 8.5 m/s cfPWV cut-off value was observed for the corona radiata (p = .050, M = 2.78). However, the highest Wilcoxon rank statistic (M = 3.25) is identified for a 8.6 m/s cut-off value when taking into account the RD values in the internal capsule (p = .013).
Taken together, these findings indicate that the cut-off value of 10 m/s may be higher than the desired value to classify patients into neurocardiovascular risk groups (Table 3, Fig. 1, Fig. 2). Specifically, our results suggest that a cfPWV cut-off value of around 8.5 m/s is more promising for classification of risk groups for individuals with targeted brain damage and cognitive dysfunction.
Moreover, we compared our suggested cfPWV cut-off of 8.5 m/s with the previously recommended cut-off of 10 m/s through the comparison of the leave-one-out cross-validation root mean square error (RMSE) of the univariate regression of the outcomes over the cfPWV variable dichotomized according to these two thresholds. For this comparison, we used the FA and RD values in the internal capsule because these measures were associated with the highest Wilcoxon rank statistic (Fig. 1, Fig. 2). Our results indicate that the suggested cfPWV cut-off of 8.5 m/s yields to a 9.79% and 8.56% decrease of the RMSE compared to the 10 m/s cut-off indicated in the literature for FA and RD respectively, which further strengthens our findings (Table 3, Fig. 1, Fig. 2).
3.2. Groupwise comparison of WM metrics
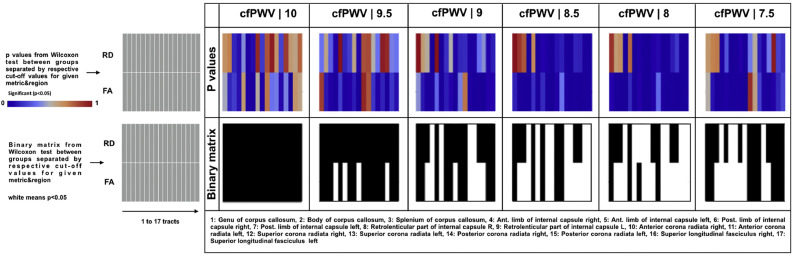
Wilcoxon rank sum results for testing the difference between WM metrics of subjects grouped by the varying values of cfPWV from 10 to 7.5 m/s (with the step size of 0.5) are shown in Fig. 3. Here, we used all 17 tracts (as opposed to the 4 regions of aggregated tracts) as mentioned in the statistical analysis section. Neither WM metrics showed significant differences between resultant groups for the cfPWV cut-off value of 10 m/s suggested by the current literature (Williams et al., 2018). Few significant differences occurred as the cut-off value gradually decreased from 10 to 9.5 m/s. Interestingly, the number of significantly different FA and RD regions were remarkably increased for the cut-off values of 8 and 8.5 m/s, emphasizing their promise in classification of subjects into neurocardiovascular risk groups. These findings are in agreement with those obtained from maximally selected rank statistics (Fig. 1, Fig. 2). Moreover, the same groupwise comparisons across the 4 regions of interest (Fig. S1) highlights similar results, meaning that the number of significantly different FA and RD regions were increased for the cut-off value of 8 and 8.5 m/s.
Fig. 3.
Groupwise comparison of white matter microstructure metrics between subjects with varying carotid-femoral pulse wave velocity (cfPWV).
Heatmaps showing p-values (top panel) and categorical value of significance in red if p < .05 (bottom panel), from GroupWise comparison between subjects separated by varying (with a step size of 0.5) cfPWV cut-off values from 10 to 7.5 m/s, for given regions. For this analysis, all 17 tracts (as opposed to the 4 regions of aggregated tracts) were included.
Groupwise comparison was also performed to test if any of these grouping thresholds entails a risk of biasing the statistics by separating significantly different means for covariates and tests scores. Findings from this inspection did not reveal any significant differences for age, sex and cognitive tests (Table 4). However, SBP was significantly different between resultant groups for the cfPWV cut-off value gradually decreasing from 9.5 to 8.5 m/s as well as for the cfPWV cut-off value of 7.5 m/s.
Table 4.
Sample characteristics of all groups. p-value between lower vs higher cfPWV subjects for each measurement.
| cfPWV cut-off value | Lower cfPWV (n) | Higher cfPWV (n) | p value | |
|---|---|---|---|---|
| 10 | All | 38 | 15 | |
| Age (years) | 70.20 ± 3.40 | 69.95 ± 3.33 | 0.43 | |
| cfPWV (m/s) | 8.06 ± 1.30 | 11.90 ± 1.50 | <0.01 | |
| 24 h SBP (mmHg) | 124.16 ± 10.73 | 130.20 ± 13.19 | 0.07 | |
| TMTB-A (s) | 43.21 ± 31.56 | 33.87 ± 16.20 | 0.40 | |
| 9.5 | All | 34 | 19 | |
| Age (years) | 70.13 ± 3.33 | 69.63 ± 3.40 | 0.63 | |
| cfPWV (m/s) | 7.86 ± 1.20 | 11.40 ± 1.60 | <0.01 | |
| 24 h SBP (mmHg) | 123.82 ± 9.96 | 129.53 ± 13.77 | 0.06 | |
| TMTB-A (s) | 42.88 ± 33.85 | 36.42 ± 17.33 | 0.79 | |
| 9 | All | 28 | 25 | |
| Age (years) | 70.00 ± 3.22 | 69.90 ± 3.51 | 0.94 | |
| cfPWV (m/s) | 7.54 ± 1.08 | 10.93 ± 1.66 | <0.01 | |
| 24 h SBP (mmHg) | 123.07 ± 9.73 | 129.00 ± 13.01 | <0.05 | |
| TMTB-A (s) | 46.96 ± 34.51 | 33.40 ± 16.92 | 0.12 | |
| 8.5 | All | 21 | 32 | |
| Age (years) | 69.64 ± 3.26 | 70.16 ± 3.41 | 0.54 | |
| cfPWV (m/s) | 7.10 ± 0.88 | 10.48 ± 1.70 | <0.01 | |
| 24 h SBP (mmHg) | 122.14 ± 10.23 | 128.31 ± 12.06 | <0.05 | |
| TMTB-A (s) | 40.86 ± 18.31 | 40.38 ± 33.49 | 0.29 | |
| 8 | All | 18 | 35 | |
| Age (years) | 69.47 ± 3.16 | 70.20 ± 3.43 | 0.45 | |
| cfPWV (m/s) | 6.91 ± 0.80 | 10.29 ± 1.75 | <0.01 | |
| 24 h SBP (mmHg) | 122.00 ± 10.67 | 127.86 ± 11.81 | 0.06 | |
| TMTB-A (s) | 41.83 ± 18.56 | 39.91 ± 32.35 | 0.28 | |
| All | 12 | 41 | ||
| 7.5 | Age (years) | 68.87 ± 2.72 | 70.27 ± 3.45 | 0.22 |
| cfPWV (m/s) | 6.5 ± 0.64 | 9.92 ± 1.85 | <0.01 | |
| 24 h SBP (mmHg) | 118.92 ± 9.80 | 127.90 ± 11.49 | <0.05 | |
| TMTB-A (s) | 42.00 ± 20.92 | 40.15 ± 30.26 | 0.45 |
cfPWV (m/s) = carotid-femoral pulse wave velocity, SBP (mmHg) = systolic blood pressure, TMTB-A = Trail Making test Part B-A. Significant p-values are in bold.
3.3. The relation between WM microstructure integrity and cognitive flexibility
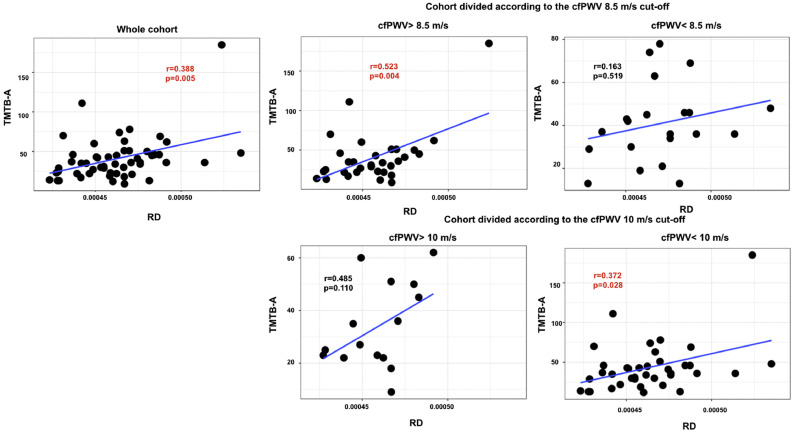
The results of the partial correlation between the cognitive flexibility performance and the microstructural WM integrity across the whole cohort (Fig. 4, Fig. 5) shows that there is a significant association between cognitive flexibility (Trail Making Test B-A) and RD in the brain regions vulnerable to arterial stiffness after correction for multiple comparisons (r = 0.388, p = .005).
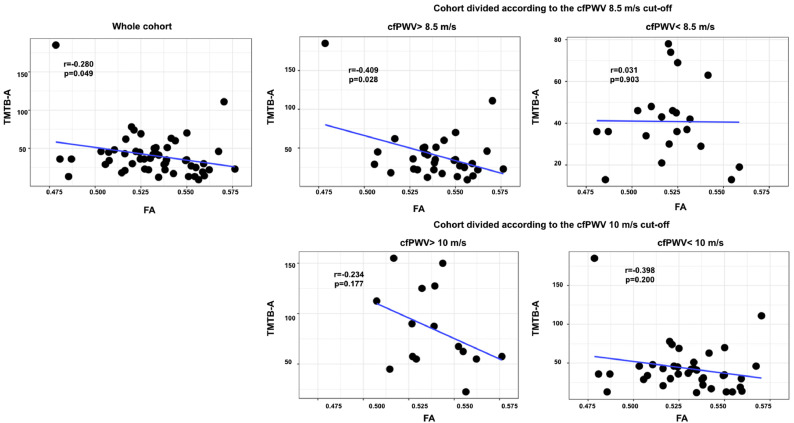
Fig. 4.
Scatter plots showing simple 8.5 m/s carotid-femoral pulse wave velocity (cfPWV) cut-off value and 3) the group below and above the 10 m/s cfPWV cut-off value.
Mean values of FA and RD were extracted from the white matter regions that are associated with carotid-femoral pulse wave velocity. Significant p-values (corrected for multiple comparisons) are in red bold font (adjusted threshold with FDR was 0.01).
Fig. 5.
Scatter plots showing simple correlations of radial diffusivity (RD) with the scores from the Trail Making test B-A for 1) the whole cohort, 2) the group below and above the 8.5 m/s carotid-femoral pulse wave velocity (cfPWV) cut-off value and 3) the group below and above the 10 m/s cfPWV cut-off value.
Mean values of FA and RD were extracted from the white matter regions that are associated with carotid-femoral pulse wave velocity. Significant p-values (corrected for multiple comparisons) are in red bold font (adjusted threshold with FDR was 0.03).
When dividing the cohort into two groups: participants with a cfPWV above or below our estimated 8.5 m/s cut-off, we can see that a longer time to complete the Trail Making Test part B-A was associated with a lower level of FA (r = −0.409, p = .028) for participants with a cfPWV above 8.5 m/s (Fig. 4). However, this association did not remain significant after correction for multiple comparisons. In addition, Fig. 5 shows that a longer time to complete the Trail Making Test B-A was associated with a higher level of RD (r = 0.523, p = .004) for participants above 8.5 m/s cut-off value after correction for multiple comparisons. In contrast, no association was found between RD and cognitive flexibility in individuals with a cfPWV below 8.5 m/s.
On the contrary, when dividing the cohort into two groups based on the 10 m/s cfPWV cut-off value adopted by the European Society of Hypertension, Fig. 4 shows no association between cognitive flexibility and either FA and RD in any grouping condition. However, Fig. 5 shows a significant correlation between cognitive flexibility and RD in individuals with a cfPWV below 10 m/s.
It should be noted that two outliers were found based on boxplot examination with SPSS of the Trail Making Test B-A variable. Although we do not have a reason to exclude those participants from the current study considering our set of inclusion and exclusion criteria, correlation analysis was also performed after removing these outliers. The (r = 0.353, p = .071).
Moreover, Table 5 shows that FA and RD measures in the corpus callosum were significant predictors of TMTB-A performances (p = .008 and p = .003 respectively) in participants with cfPWV value above 8.5 m/s while no region of interest was found to be a predictor of TMTB-A in participants with cfPWV below 8.5 m/s (Table 5). In addition, RD measure in the corona radiata was found to be a significant predictor of TMTB-A performance (p = .004) (Table 5). In contrast, cognitive flexibility was not found to be associated with any FA and RD measures in individuals with a cfPWV above 10 m/s or below 10 m/s (Table 6).
Table 5.
Multivariate adjusted relations of TMTB-A and white matter microstructural integrity as assessed by Fractional anisotropy (FA) and radial diffusivity (RD) in participants with a cfPWV value above and below 8.5 m/s.
| Participants with a cfPWV value above 8.5 m/s | ||||
|---|---|---|---|---|
| DTI Metrics | ROI | B | β | p-value |
| FA | corpus callosum | −704.110 | −0.510 | 0.008 |
| internal capsule | −505.337 | −0.309 | 0.122 | |
| corona radiata | −653.102 | −0.404 | 0.040 | |
| superior longitudinal fasciculus | −216.783 | −0.180 | 0.363 | |
| RD | corpus callosum | 6.28 105 | 0.560 | 0.003 |
| internal capsule | 5.45 105 | 0.260 | 0.180 | |
| corona radiata | 8.12 105 | 0.520 | 0.004 | |
| superior longitudinal fasciculus | 3.89 105 | 0.322 | 0.092 | |
| Participants with a cfPWV value below 8.5 m/s | ||||
|---|---|---|---|---|
| DTI Metrics | ROI | B | β | p-value |
| FA | corpus callosum | −28.923 | −0.037 | 0.888 |
| internal capsule | −0.976 | 0.001 | 0.996 | |
| corona radiata | 70.943 | 0.082 | 0.742 | |
| superior longitudinal fasciculus | 73.245 | 0.119 | 0.648 | |
| RD | corpus callosum | 9.40 105 | 0.172 | 0.551 |
| internal capsule | 2.21 105 | 0.232 | 0.356 | |
| corona radiata | 9.90 105 | 0.158 | 0.565 | |
| superior longitudinal fasciculus | 3.79 105 | 0.060 | 0.823 | |
Significant p-values (corrected for multiple comparisons) are in bold fonts (adjusted threshold is 0.006 and 0.018 for FA and RD respectively).
Table 6.
Multivariate adjusted relations of TMTB-A and white matter microstructural integrity as assessed by Fractional anisotropy (FA) and radial diffusivity (RD) in participants with a cfPWV value above and below 10 m/s.
| Participants with a cfPWV value above 10 m/s | ||||
|---|---|---|---|---|
| DTI Metrics | ROI | B | β | p-value |
| FA | corpus callosum | −388.324 | −0.501 | 0.106 |
| internal capsule | −315.331 | −0.358 | 0.256 | |
| corona radiata | −220.656 | −0.290 | 0.390 | |
| superior longitudinal fasciculus | −198.375 | −0.340 | 0.292 | |
| RD | corpus callosum | 2.37 105 | 0.363 | 0.277 |
| internal capsule | 3.94 105 | 0.405 | 0.203 | |
| corona radiata | 4.74 105 | 0.549 | 0.084 | |
| superior longitudinal fasciculus | 3.30 105 | 0.580 | 0.067 | |
| Participants with a cfPWV value below 10 m/s | ||||
|---|---|---|---|---|
| DTI Metrics | ROI | B | β | p-value |
| FA | corpus callosum | −380.274 | −0.317 | 0.073 |
| internal capsule | −201.212 | −0.159 | 0.365 | |
| corona radiata | −291.883 | −0.218 | 0.229 | |
| superior longitudinal fasciculus | −66.524 | −0.067 | 0.704 | |
| RD | corpus callosum | 4.55 105 | 0.483 | 0.007 |
| internal capsule | 3.16 105 | 0.193 | 0.267 | |
| corona radiata | 3.44 105 | 0.320 | 0.060 | |
| superior longitudinal fasciculus | 2.03 105 | 0.202 | 0.241 | |
Significant p-values (corrected for multiple comparisons) as indicated (adjusted treshold is 0.006 for FA and RD).
4. Discussion
While previous studies estimated a cfPWV cut-off value of 10 m/s while considering the additive effects of arterial stiffness above traditional risk factors in the incidence of cardiovascular events, the present study represents the first to estimate the cfPWV cut-off value at which arterial stiffness impacts the WM microstructure and ultimately cognition in healthy elderly without dementia.
The stiffening of large arteries (e.g. the aorta) increases flow pulsations through the carotid and vertebral arteries which eventually extend deep into the brain microvasculature. This often leads to vascular rupture, micro-hemorrhages, endothelial denudation and/or thrombotic obstruction resulting in lower cerebral blood flow (Henskens et al., 2008; O'rourke and Hashimoto, 2007). One can note that lower cerebral blood flow as a result of arterial stiffening can affect the deep WM tracts. This impact on the WM tracts is due to their geographic localization within areas perfused by long medullary arterioles arising from the anterior and middle cerebral arteries where arterial pulsatility may be at the highest (Brown and Thore, 2011; Rosano et al., 2013). Thus, in this study, we used DTI metrics, which are sensitive to microstructural changes in the WM, to allow an early detection of the relationship between central artery stiffness and brain integrity.
The major findings from this study are as follows: first, our findings reveal that the suggested 10 m/s cfPWV cut-off value may not be the optimal threshold to split individuals into high and low neurovascular risk groups. Instead, our findings suggest that this cut-off value is more likely to fall around 8.5 m/s when downstream effects of hemodynamic alterations to overall WM integrity are taken as the ground for comparison. Second, an altered microstructural integrity of the vulnerable regions to arterial stiffness seems to be associated with a decline in cognitive flexibility performance (longer time to complete the TMTB-A) in participants with a cfPWV value above 8.5 m/s, suggesting that preserving the white matter microstructure in vulnerable individuals may be used to help preserve brain integrity and ultimately cognitive function.
4.1. The estimation of a cfPWV cut-off value
Several longitudinal and epidemiological studies have focused on the predictive value of aortic stiffness in cardiovascular risk management (Laurent et al., 2007, Laurent et al., 2006; Vlachopoulos et al., 2010). In 2007, a PWV of 12m/s was suggested as the critical upper limit for occurrence of cardiovascular events by the European Society of Hypertension/European Society of Cardiology (Mancia et al., 2007). This cut-off value of 12 m/s was based on the 100% direct common carotid artery and femoral artery distance measurement Laurent et al. (2006). After standardization of the arterial pulse traveling distance measurement between the carotid and femoral measurements sites, this limit was then adjusted to 10 m/s by an expert consensus (Van Bortel et al., 2012) using the 80% of the direct carotid-femoral distance (common carotid artery - common femoral artery x 0.8) (Laurent et al., 2006). This latest cut-off value was established according to its cardiovascular consequences and risk of mortality and was not estimated by focusing on its effect in the brain. In fact, the cut-off value for the effect of arterial stiffness in the brain has not been previously defined (Badji et al., 2019; Suzuki et al., 2017). However, this relationship is important considering that the brain is one of the most sensitive organ to hemodynamic changes (O'Rourke and Safar, 2005).
Our results suggest that the cfPWV cut-off value of 10 m/s may be too high given that the WM microstructural alterations are more significantly associated with the cfPWV around 8.5 m/s in our cohort of participants between 65 and 75 years old with a set of inclusion and exclusion criteria. Considering DTI metrics can allow the characterization of microstructural changes that antedate the appearance of irreversible WM damage in the elderly (Maillard et al., 2014), the newly proposed cut-off may have a chance to accurately identify individuals that are at high risk of structural changes and cognitive dysfunction.
It is worth noting that vascular disease and eventual end-organ damage manifest at the end of a pathological spectrum which is extending from a prolonged latent phase (Tsao et al., 2016). Among preclinical neurologic deficits, WM alterations (e.g. WMHs, lacunar infarcts, cerebral microbleeds) have been found in individuals with chronic hypertension and arterial stiffness (Makin et al., 2013; Miwa et al., 2014; Suzuki et al., 2017; Tarumi et al., 2015; Tsao et al., 2016). Such deficits may ultimately lead to Alzheimer type dementia (Au et al., 2006; Kalaria et al., 2012) by strongly influencing beta amyloid deposition (Hughes et al., 2013; Roman, 1997; Shah et al., 2012). For instance, Iturria-Medina et al. recently suggested that vascular dysregulations are the earliest pathological alterations in Alzheimer's disease, followed by beta amyloid deposition by means of a multifactorial data driven analysis comparing 7700 brain images and different plasma and cerebrospinal fluid biomarkers from 1711 healthy controls (Iturria-Medina et al., 2016). This means that measuring cfPWV could be used to identify people at risk for neurovascular dysregulation in the elderly (Badji et al., 2019). These individuals could then be treated to reduce arterial stiffness, prevent WM alterations and ultimately cognitive decline.
4.2. Impact of WM neuronal fiber integrity in cognitive function
A slower executive function performance (longer time to complete the TMTB-A) was correlated with lower FA and higher RD from the vulnerable white matter regions to arterial stiffness in participants with a cfPWV above 8.5 m/s.
Interestingly, various studies have provided evidence for the contribution of microstructural WM reductions in executive skills performance (Badji et al., 2019; Gunning-Dixon et al., 2009). For instance, Sasson et al. showed that performance in executive functions is correlated with DTI measures in the superior longitudinal fasciculus (Sasson et al., 2013). In addition, Madden et al. (Madden et al., 2004) observed that a lower level of integrity of the cerebral WM (as assessed by FA) in specific brain regions was associated with slower responses in the visual task. In particular, Madden et al. (Madden et al., 2004) observed that the best predictor of response time for younger adults was a higher FA in the splenium of the corpus callosum, whereas for older adults the best predictor was a higher FA in the anterior limb of the internal capsule. Results from another study suggest that age-related reductions in FA in the pericallosal frontal region and in the genu of the corpus callosum mediate the relationship between processing speed and episodic retrieval (Bucur et al., 2008). Our findings for participants with a cfPWV value above 8.5 m/s shows that FA and RD measures in the corpus callosum and corona radiata are a significant predictor of TMTB-A performance (p < .05 after correction for multiple comparisons) are in agreement with these previous findings. Taken together, these findings highlight that WM tracts are crucial for information transfer between brain regions, and in the regions vulnerable to increased arterial stiffness, the loss of WM microstructural integrity may contribute to poorer cognitive performances. Thus, it is not surprising that the relationship between arterial stiffness, vascular dementia and Alzheimer's disease is consistent in the literature. For instance, a meta-analysis of six studies showed that arterial stiffness is significantly higher in subjects with vascular dementia compared to controls without dementia (Rabkin and Jarvie, 2011) and another study demonstrated that arterial stiffness is significantly higher in subjects with Alzheimer's disease than in subjects with mild cognitive impairment or normal cognitive functions (Hanon et al., 2005).
4.3. Strengths and limitations
The strength of this study lies in the inclusion of quantitative MRI metrics (DTI) that are sensitive to the WM neuronal tissue to investigate the impact of arterial stiffness on the WM microstructure as opposed to most previous studies using fluid attenuated inversion recovery (FLAIR). Conventional MRI techniques, such as FLAIR, only dichotomize tissue into abnormal and normal tissue. In contrast, FA and RD provide a quantitative evaluation of the WM and thus, a much more detailed evaluation of the underlying tissue microstructure.
In a longitudinal study that investigates DTI changes within WMHs over a 3 years period, Maillard et al. showed that while WMHs had not changed on FLAIR imaging, DTI metrics showed a significant change over time in these WMHs (Maillard et al., 2014). Using DTI metrics, this study is the first one to attempt to objectively estimate the cfPWV cut-off value for better classifying elderly populations with respect to their brain WM microstructure.
We found evidence that further adjustments of the cfPWV cut-off value may be needed to properly make the distinction between lower and higher risk group of patients for WM microstructural injury in the elderly. This can give a conceivable opportunity for early detection of Alzheimer's disease and initiation of disease-modifying treatments if and/or when those treatments will become available.
To reach this objective, we used a maximally selected rank statistics as opposed to more classic approaches, such as the receiver operating characteristic (ROC) curve. Indeed, when an investigator wants to evaluate the prognostic impact of a quantitative variable for screening purposes, the classic approach is to use the ROC curve, and then choose the cut-off value that is closest to the point of perfect classification (100% sensitivity and 100% specificity). However, with this analysis the outcome variable has to be transformed into a binary classification that is clinically relevant. Unfortunately, the ROC curve method cannot be used in this study because the normal values of DTI metrics (FA and RD) in WM are still unknown. Thus, our outcome variables (FA and RD) can't be used for a binary classification. On the contrary, maximally selected rank statistics is an interesting alternative with the advantage that it is not necessary to transform the outcome variable into a binary endpoint. Maximally selected rank sum has been previously used in several studies, especially studies in the field of cancer genomics aiming to know whether the expression profile of one gene is associated with the patient's prognosis. To answer this question, biologists have used maximally selected rank statistics to calculate an exact cut-off point allowing them to accurately split patients into two groups: high and low expression of that specific gene (Delgado et al., 2014; Hassen et al., 2015; Seckinger et al., 2012).
Moreover, although both of our statistical approaches used to estimate the cfPWV cut-off value yield consistent findings, it is worth noting that iterating cfPWV cut-off values by varying (with step size of 0.5) its value from 10 to 7.5 m/s, varies the number of samples assigned to resultant groups (supplementary material 1). Further studies with larger cohorts will be needed not only to confirm our findings but also to determine whether additional factors may contribute and/or modify this estimated cfPWV cut-off, such as the sex, the presence of hypertension, the use of antihypertensive medication, diabetes, etc.
In addition, one may consider the potential nonlinearity of the signal across the range of b values which could influence the interpretation of the findings (Kochunov et al., 2013). Whether or not it is the case is still a matter of debate (Baumann et al., 2012; Dudink et al., 2008; Hui et al., 2010).
Finally, the challenges of translating our findings into clinical practice should not be underestimated. Indeed, it took many years for blood pressure monitoring to be considered a public health necessity, despite the strong evidence between cardiovascular mortality and hypertension. As further studies and additional evidence will become available, we can envision a future where therapeutic correction of high arterial stiffness will be a priority for protecting both the heart and the brain.
5. Conclusion
Findings from this study shows that the suggested 10 m/s cfPWV cut-off value may not be the cfPWV threshold that best splits individuals into high and low neurocardiovascular risk groups. Instead, this cut-off value is more likely to fall around 8.5 m/s when downstream effects of hemodynamic alteration to overall WM integrity are taken as the ground for comparison. Adjustment of the cfPWV value may be needed to make a more accurate distinction between lower and higher risk group of patients for white matter microstructural injury related to arterial stiffness which may help preserve brain health and prevent cognitive decline.
Source of funding
This study was supported by the Centre de Recherche de l'Institut Universitaire de Gériatrie de Montréal (CRIUGM) and the MerckSharp & Dohme Corp Program of the Faculty of Medicine of the Université de Montréal, the Canada Research Chair in Quantitative Magnetic Resonance Imaging [950-230815], the Canadian Institute of Health Research [CIHR FDN-143263], the Canada Foundation for Innovation [32454, 34824], the Fonds de Recherche du Québec - Santé [28826], the Fonds de Recherche du Québec - Nature et Technologies [2015-PR-182754], the Natural Sciences and Engineering Research Council of Canada [435897-2013], the Canada First Research Excellence Fund (IVADO and TransMedTech), the Quebec BioImaging Network [5886]. Hélène Girouard was the holder of a new investigator award from the Fonds de Recherche du Québec-Santé (FRSQ) and the Heart and Stroke Foundation of Canada (HSFC). Atef Badji and Agah Karakuzu were supported by a TransMedTech excellence scholarship. Adrián Noriega de la Colina was supported by a Doctoral Fellowship from the Société Québécoise d'Hypertension Artérielle (SQHA).
Declaration of Competing Interest
The authors declare no competing financial interests.
Acknowledgments
The authors would also like to acknowledge all NeuroPoly Lab members (Polytechnique Montreal) for helpful discussions. Carollyn Hurst and André Cyr from the Functional Neuroimaging Unit (CRIUGM, Université de Montréal) are acknowledged for helping with data acquisitions. Ernesto L. Schiffrin, Pierre Paradis and Julio Fraulob-Aquino (Lady Davis Institute for Medical Research, McGill University) are acknowledged for their help in the acquisition of pulse wave velocity measurements. Marie-Christine Robitaille-Grou is acknowledged for helping with statistics and Arnaud Boré is acknowledged for helping with the processing of diffusion data. Alexa Mousley (Polytechnique Montreal) is acknowledged for language editing.
Footnotes
References
- Au R., Massaro J.M., Wolf P.A., Young M.E., Beiser A., Seshadri S., D’Agostino R.B., DeCarli C. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham heart study. Arch. Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badji A., Noriega de la Colina A., Karakuzu A., Duval T., Desjardins-Crépeau L., Joubert S., Bherer L., Lamarre-Cliche M., Stikov N., Girouard H., Cohen-Adad J. Arterial stiffness and white matter integrity in the elderly: a diffusion tensor and magnetization transfer imaging study. Neuroimage. 2018 doi: 10.1016/j.neuroimage.2018.11.015. [DOI] [PubMed] [Google Scholar]
- Badji A., Sabra D., Bherer L., Cohen-Adad J., Girouard H., Gauthier C.J. Arterial stiffness and brain integrity: a review of MRI findings. Ageing Res. Rev. 2019;53 doi: 10.1016/j.arr.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Baumann P.S., Cammoun L., Conus P., Do K.Q., Marquet P., Meskaldji D., Meuli R., Thiran J.-P., Hagmann P. High b-value diffusion-weighted imaging: a sensitive method to reveal white matter differences in schizophrenia. Psychiatry Res. 2012;201:144–151. doi: 10.1016/j.pscychresns.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. 1995. Controling the False Discovery Rate: A Practical and. Parmigiani et al 39. [Google Scholar]
- Brown W.R., Thore C.R. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucur B., Madden D.J., Spaniol J., Provenzale J.M., Cabeza R., White L.E., Huettel S.A. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol. Aging. 2008;29:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin M., Qasem A. Large artery stiffness assessment using SphygmoCor technology. Pulse. 2017;4:180–192. doi: 10.1159/000452448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan J.D., Hinkeldey N.S. Relationships between parts a and B of the trail making test. J. Clin. Psychol. 1987;43:402–409. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- de Groot M., Verhaaren B.F.J., de Boer R., Klein S., Hofman A., van der Lugt A., Ikram M.A., Niessen W.J., Vernooij M.W. Changes in normal-appearing white matter precede development of white matter lesions. Stroke. 2013;44:1037–1042. doi: 10.1161/STROKEAHA.112.680223. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Corley J., Gow A.J., Harris S.E., Houlihan L.M., Marioni R.E., Penke L., Rafnsson S.B., Starr J.M. Age-associated cognitive decline. Br. Med. Bull. 2009;92:135–152. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- Delgado J., Pereira A., Villamor N., López-Guillermo A., Rozman C. Survival analysis in hematologic malignancies: recommendations for clinicians. Haematologica. 2014;99:1410–1420. doi: 10.3324/haematol.2013.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudink J., Larkman D.J., Kapellou O., Boardman J.P., Allsop J.M., Cowan F.M., Hajnal J.V., Edwards A.D., Rutherford M.A., Counsell S.J. High b-value diffusion tensor imaging of the neonatal brain at 3T. AJNR Am. J. Neuroradiol. 2008;29:1966–1972. doi: 10.3174/ajnr.A1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Brickman A.M., Cheng J.C., Alexopoulos G.S. Aging of cerebral white matter: a review of MRI findings. Int. J. Geriatr. Psychiatr.: J. Psychiatr. Late Life Allied Sci. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanon O., Haulon S., Lenoir H., Seux M.-L., Rigaud A.-S., Safar M., Girerd X., Forette F. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- Hansen L., Taylor W.R. Is increased arterial stiffness a cause or consequence of atherosclerosis? Atherosclerosis. 2016;249:226–227. doi: 10.1016/j.atherosclerosis.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassen W., Kassambara A., Reme T., Sahota S., Seckinger A., Vincent L., Cartron G., Moreaux J., Hose D., Klein B. Drug metabolism and clearance system in tumor cells of patients with multiple myeloma. Oncotarget. 2015;6:6431–6447. doi: 10.18632/oncotarget.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henskens L.H.G., Kroon A.A., van Oostenbrugge R.J., Gronenschild E.H.B.M., Fuss-Lejeune M.M.J.J., Hofman P.A.M., Lodder J., de Leeuw P.W. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- Hughes T.M., Kuller L.H., Barinas-Mitchell E.J.M., Mackey R.H., McDade E.M., Klunk W.E., Aizenstein H.J., Cohen A.D., Snitz B.E., Mathis C.A., Dekosky S.T., Lopez O.L. Pulse wave velocity is associated with β-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81:1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E.S., Cheung M.M., Chan K.C., Wu E.X. B-value dependence of DTI quantitation and sensitivity in detecting neural tissue changes. Neuroimage. 2010;49:2366–2374. doi: 10.1016/j.neuroimage.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y., Sotero R.C., Toussaint P.J., Mateos-Pérez J.M., Evans A.C., Alzheimer’s Disease Neuroimaging Initiative Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016;7 doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K. OUP; USA: 2011. Diffusion MRI: Theory, Methods, and Applications. [Google Scholar]
- Kalaria R.N., Akinyemi R., Ihara M. Does vascular pathology contribute to Alzheimer changes? J. Neurol. Sci. 2012;322:141–147. doi: 10.1016/j.jns.2012.07.032. [DOI] [PubMed] [Google Scholar]
- Kochunov P., Chiappelli J., Hong L.E. Permeability–diffusivity modeling vs. fractional anisotropy on white matter integrity assessment and application in schizophrenia. NeuroImage: Clinical. 2013;3:18–26. doi: 10.1016/j.nicl.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S., Boutouyrie P., Asmar R., Gautier I., Laloux B., Guize L., Ducimetiere P., Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D., Pannier B., Vlachopoulos C., Wilkinson I., Struijker-Boudier H., European Network for Non-invasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D., Pannier B., Vlachopoulos C., Wilkinson I., Struijker-Boudier H. Abridged version of the expert consensus document on arterial stiffness. Artery Res. 2007;1:2–12. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Lausen B., Schumacher M. Maximally selected rank statistics. Biometrics. 1992;48:73–85. [Google Scholar]
- Madden D.J., Whiting W.L., Huettel S.A., White L.E., MacFall J.R., Provenzale J.M. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Maillard P., Fletcher E., Lockhart S.N., Roach A.E., Reed B., Mungas D., DeCarli C., Carmichael O.T. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke. 2014;45:1721–1726. doi: 10.1161/STROKEAHA.113.004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P., Mitchell G.F., Himali J.J., Beiser A., Tsao C.W., Pase M.P., Satizabal C.L., Vasan R.S., Seshadri S., DeCarli C. Effects of arterial stiffness on brain integrity in young adults from the Framingham heart study. Stroke. 2016;47:1030–1036. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P., Mitchell G.F., Himali J.J., Beiser A., Fletcher E., Tsao C.W., Pase M.P., Satizabal C.L., Vasan R.S., Seshadri S., DeCarli C. Aortic stiffness, increased white matter free water, and altered microstructural integrity: a continuum of injury. Stroke. 2017;48:1567–1573. doi: 10.1161/STROKEAHA.116.016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin S.D.J., Turpin S., Dennis M.S., Wardlaw J.M. Cognitive impairment after lacunar stroke: systematic review and meta-analysis of incidence, prevalence and comparison with other stroke subtypes. J. Neurol. Neurosurg. Psychiatry. 2013;84:893–900. doi: 10.1136/jnnp-2012-303645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G., De Backer G., Dominiczak A., Cifkova R., Fagard R., Germano G., Grassi G., Heagerty A.M., Kjeldsen S.E., Laurent S., Narkiewicz K., Ruilope L., Rynkiewicz A., Schmieder R.E., Boudier H.A.S., Zanchetti A., ESH-ESC Task Force on the Management of Arterial Hypertension 2007 ESH-ESC practice guidelines for the Management of Arterial Hypertension: ESH-ESC task force on the management of arterial hypertension. J. Hypertens. 2007;25:1751–1762. doi: 10.1097/HJH.0b013e3282f0580f. [DOI] [PubMed] [Google Scholar]
- Miwa K., Tanaka M., Okazaki S., Yagita Y., Sakaguchi M., Mochizuki H., Kitagawa K. Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology. 2014;83:646–653. doi: 10.1212/WNL.0000000000000692. [DOI] [PubMed] [Google Scholar]
- Mori S., Oishi K., Jiang H., Jiang L., Li X., Akhter K., Hua K., Faria A.V., Mahmood A., Woods R., Toga A.W., Pike G.B., Neto P.R., Evans A., Zhang J., Huang H., Miller M.I., van Zijl P., Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerenberg K.A., Zarnke K.B., Leung A.A., Dasgupta K., Butalia S., McBrien K., Harris K.C., Nakhla M., Cloutier L., Gelfer M., Lamarre-Cliche M., Milot A., Bolli P., Tremblay G., McLean D., Padwal R.S., Tran K.C., Grover S., Rabkin S.W., Moe G.W., Howlett J.G., Lindsay P., Hill M.D., Sharma M., Field T., Wein T.H., Shoamanesh A., Dresser G.K., Hamet P., Herman R.J., Burgess E., Gryn S.E., Grégoire J.C., Lewanczuk R., Poirier L., Campbell T.S., Feldman R.D., Lavoie K.L., Tsuyuki R.T., Honos G., Prebtani A.P.H., Kline G., Schiffrin E.L., Don-Wauchope A., Tobe S.W., Gilbert R.E., Leiter L.A., Jones C., Woo V., Hegele R.A., Selby P., Pipe A., McFarlane P.A., Oh P., Gupta M., Bacon S.L., Kaczorowski J., Trudeau L., Campbell N.R.C., Hiremath S., Roerecke M., Arcand J., Ruzicka M., Prasad G.V.R., Vallée M., Edwards C., Sivapalan P., Penner S.B., Fournier A., Benoit G., Feber J., Dionne J., Magee L.A., Logan A.G., Côté A.-M., Rey E., Firoz T., Kuyper L.M., Gabor J.Y., Townsend R.R., Rabi D.M., Daskalopoulou S.S., Hypertension Canada Hypertension Canada's 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can. J. Cardiol. 2018;34:506–525. doi: 10.1016/j.cjca.2018.02.022. [DOI] [PubMed] [Google Scholar]
- O’rourke M.F., Hashimoto J. 2007. Mechanical Factors in Arterial Aging: A Clinical Perspective. Of the American College of Cardiology. [DOI] [PubMed] [Google Scholar]
- O’Rourke M.F., Safar M.E. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- Rabkin S.W., Jarvie G. Comparison of vascular stiffness in vascular dementia, Alzheimer dementia and cognitive impairment. Blood Press. 2011;20:274–283. doi: 10.3109/08037051.2011.566246. [DOI] [PubMed] [Google Scholar]
- Reitan R.M. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Skills. 1958;8:271–276. [Google Scholar]
- Roman G.C. Brain infarction and the clinical expression of Alzheimer disease. JAMA. 1997;278:113–114. doi: 10.1001/jama.1997.03550020045023. [DOI] [PubMed] [Google Scholar]
- Rosano C., Watson N., Chang Y., Newman A.B., Aizenstein H.J., Du Y., Venkatraman V., Harris T.B., Barinas-Mitchell E., Sutton-Tyrrell K. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension. 2013;61:160–165. doi: 10.1161/HYPERTENSIONAHA.112.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M., de Roos A., Blauw G.J., Middelkoop H.A.M., Jukema J.W., Mooijaart S.P., van Buchem M.A., de Craen A.J.M., van der Grond J. Association between changes in brain microstructure and cognition in older subjects at increased risk for vascular disease. BMC Neurol. 2015;15:133. doi: 10.1186/s12883-015-0396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson E., Doniger G.M., Pasternak O., Tarrasch R., Assaf Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front. Neurosci. 2013;7:32. doi: 10.3389/fnins.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckinger A., Meissner T., Moreaux J., Depeweg D., Hillengass J., Hose K., Rème T., Rösen-Wolff A., Jauch A., Schnettler R., Ewerbeck V., Goldschmidt H., Klein B., Hose D. Clinical and prognostic role of annexin A2 in multiple myeloma. Blood. 2012;120:1087–1094. doi: 10.1182/blood-2012-03-415588. [DOI] [PubMed] [Google Scholar]
- Setsompop K., Cohen-Adad J., Gagoski B.A., Raij T., Yendiki A., Keil B., Wedeen V.J., Wald L.L. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage. 2012;63:569–580. doi: 10.1016/j.neuroimage.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N.S., Vidal J.-S., Masaki K., Petrovitch H., Ross G.W., Tilley C., DeMattos R.B., Tracy R.P., White L.R., Launer L.J. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: the Honolulu Asia aging study. Hypertension. 2012;59:780–786. doi: 10.1161/HYPERTENSIONAHA.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Gao H., Bai W., Evangelou E., Glocker B., O’Regan D.P., Elliott P., Matthews P.M. Abnormal brain white matter microstructure is associated with both pre-hypertension and hypertension. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T., de Jong D.L.K., Zhu D.C., Tseng B.Y., Liu J., Hill C., Riley J., Womack K.B., Kerwin D.R., Lu H., Munro Cullum C., Zhang R. Central artery stiffness, baroreflex sensitivity, and brain white matter neuronal fiber integrity in older adults. Neuroimage. 2015;110:162–170. doi: 10.1016/j.neuroimage.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh T. Trail making test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tsao C.W., Himali J.J., Beiser A.S., Larson M.G., DeCarli C., Vasan R.S., Mitchell G.F., Seshadri S. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology. 2016;86:619–626. doi: 10.1212/WNL.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bortel L.M., Laurent S., Boutouyrie P., Chowienczyk P., Cruickshank J.K., De Backer T., Filipovsky J., Huybrechts S., Mattace-Raso F.U.S., Protogerou A.D., Schillaci G., Segers P., Vermeersch S., Weber T., Artery Society, European Society of Hypertension Working Group on Vascular Structure and Function, European Network for Noninvasive Investigation of Large Arteries Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Aznaouridis K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Wilcoxon F. Individual comparisons by ranking methods. Biom. Bull. 1945;1:80–83. [Google Scholar]
- Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D., Coca A., De Simone G., Dominiczak A., Kahan T., Mahfoud F., Redon J., Ruilope L., Zanchetti A., Kerins M., Kjeldsen S., Kreutz R., Laurent S., Lip G.Y.H., McManus R., Narkiewicz K., Ruschitzka F., Schmieder R., Shlyakhto E., Tsioufis K., Aboyans V., Desormais I. Vol. 27. Blood Press; 2018. 2018 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) pp. 314–340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material