Abstract
Renin-angiotensin system exerted deleterious effects on learning and cognitive functions through different mechanisms. The present study has been designed to evaluate the protective effect of perindopril and azilsartan as monotherapy or in combination on aluminum chloride (AlCl3) induced neurobehavioral and pathological changes in Alzheimeric rats. Male Wistar rats were divided into nine groups (n = 6); negative control, AlCl3 treated, vehicle, AlCl3 and Azilsartan (3.5 mg/kg, 7 mg/kg) co-treated, AlCl3 and perindopril (0.5 mg/kg, 1 mg/kg) co-treated, AlCl3 and (Azilsartan 3.5 mg/kg + perindopril 0.5 mg/kg), and AlCl3 and (Azilsartan 7 mg/kg + perindopril 1 mg/kg), all groups were treated for consecutive 60 days. Then, memory function was evaluated by the Y- maze test. Amyloid Peptide − 42 (Aβ-42), Acetylcholinesterase (AChE), Malondialdehyde (MDA), Tumor necrosis factor (TNF-α) and Nitric Oxide (NO) levels in the hippocampus were assessed with (ELISA) kits. The histopathological studies of the hippocampal dentate gyrus (DG) and Cornu Ammonis-3 (CA3) were also performed. Oral administration of either azilsartan and perindopril alone or in combined for 60 days have shown; improvement of cognitive function, significant reduction in the hippocampal levels of Aβ-42, Acetylcholinesterase, Malondialdehyde (MDA), Tumor necrosis factor (TNF-α) and reserved most of histopathological changes in dentate gyrus (DG) and Cornu Ammonis-3 (CA3) that mediated by Alcl3. Our behavioral, biochemical, and histopathological studies indicate that perindopril and azilsartan have neuroprotective effects on the AD model of rats induced by AlCl3, suggesting that perindopril and azilsartan may be a candidate drugs for the treatment of AD.
Keywords: Azilsartan, Dementia, Memory, Perindopril, Renin-angiotensin system
1. Introduction
Dementia is a broad term for multiple brain disorders caused by neurodegenerative and vascular diseases (WHO, 2018, Prince et al., 2013). It is characterized by gradual, progressive impairment in the cognitive and behavioral functions such as learning skills, daily social or living activities, language, and memory (Prince et al., 2013). In 2015 approximately 50 million people globally were diagnosed with dementia and this number is expected to rise (Prince et al., 2016). Regionally, the prevalence of dementia in the kingdom of Saudi Arabia (KSA) is uncertain due to the paucity in the epidemiological studies (Brookmeyer et al., 2018). However, based on the Saudi Alzheimer's Disease Association there are about 130,000 cases of dementia in KSA.
Renin-Angiotensin System (RAS) is a hormonal system that has crucial regulatory and homeostatic actions through the body involving blood pressure and fluid balance; apart from peripheral functions, it has been also documented to be found in the central nervous system (CNS) and play an important role in various neurodegenerative diseases. In the RAS, both centrally and locally, the angiotensinogen is cleaved by renin enzyme to form angiotensin I (Ang I). After that, the Ang I is converted to angiotensin II (Ang II), a potent vasoconstrictor, by the action of the angiotensin‐converting enzyme (ACE). Ang II has two receptors, including the angiotensin type 1 receptor (AT1R) and angiotensin type 2 receptor (AT2R) (Miller et al., 2019). Many studies have revealed that RAS exerted deleterious effects on learning and cognitive functions through different mechanisms including a deposition of amyloid β, aggregation of tau protein, inflicting of oxidative stress, neurotransmitters abnormalities, and neuroinflammation (Mogi et al., 2012). Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptors blockers (ARBs) are the main drug classes that interfering with RAS.
Besides the roles of ACEIs and ARBs on treating different cardiovascular diseases (CVS), Findings from many previous studies provide convincing evidence on the functions of ACEIs and ARBs in different body system rather than cardiovascular system, involving nervous system (O’Caoimh et al., 2014).
ACEIs can be classified according to their ability to cross the BBB. Captopril, Ramipril, and perindopril penetrate the BBB and are referred to as centrally acting ACEI (CACE-Is), while Imidapril, Lisinopril, and Enalapril do not and are regarded as non-CACE Is (Fazal et al., 2017). Treatment with CACE-Is are associated with a reduced incidence of dementia compared to those taking non-CACEIs (Solfrizzi et al., 2013). Also, CACEIs show slower rates of cognitive decline in patients with established AD compared with patients not currently prescribed CACEIs (O’Caoimh et al., 2014b). Also, the inhibition of RAS by ARBs (valsartan, losartan and candesartan) has been suggested to prevent the onset of AD by attenuate oligomerization of Aβ peptides and AT1-initiated oxidative stress. Also, ARBs restore Cerebral blood flow (CBF), Ach level, decreased AChE activity and MDA level (Gebre et al., 2018). It is worth mentioning that the impact of azilsartan on cognitive function has not yet been evaluated.
To date, the management of dementia is limited to offer symptomatic effects rather than curative, and there are only a few drugs in clinical use which comprised of choline esterase inhibitors (ChEIs) and N-methyl-d-aspartate receptor antagonist (NMDA), so there is an urgent requirement to find alternative approaches that conferring radical cure for such syndromes (Ulep et al., 2018).
Recently, the focus on ACE-Is and ARBs has been intensified to target dementia as well as other neurodegenerative disorders and reported promising results (Rygiel, 2016).
The present study was aimed to examine whether perindopril and azilsartan as monotherapy or in combination have a protective role against Aluminum chloride-induced neurotoxicity in rats. Moreover, possible underlying mechanisms were also studied.
2. Materials and methods
2.1. Statement
All procedures conducted according to the ethical guidelines of the medical ethics committee of King Abdul-Aziz University (KAU). The research ethics committee approved the animal Protocol with Approval number (306-18).
2.2. Chemicals and reagents
Perindopril and azilsartan were procured from Sigma Aldrich, (CO., Saint Louis, MO, USA). Sodium- Carboxymethyl cellulose (Na-CMC) was obtained from the faculty of pharmacy at King Abdulaziz University. Aluminum chloride (AlCl3) was obtained from KFCMR at King Abdulaziz University. Rat ELISA kits for the determination of beta-amyloid Peptide - 42 (Aβ-42) and Nitric Oxide (NO) were bought from (SunLong Biotech Co. China). Tumor necrosis factor (TNF-α), Malondialdehyde (MDA) and Acetylcholinesterase (AChE) were purchased from (Elabscience Co. USA)
2.3. Drugs doses and preparations
Perindopril was prepared daily in sterile water and orally administered by gavage at doses of (0.5 and 1 mg/kg/day). Azilsartan was freshly prepared in suspension form and given orally by gavage at doses (3.5 and 7 mg/kg/day). The doses of perindopril and azilsartan were obtained based on the human therapeutic dose using the formula of converting human dose to rat dose (Nair et al., 2016). AlCl3 was daily prepared and dissolved in normal saline, and given at the dose of (100 mg/kg); the dose was selected based on previous reports (Lakshmi et al., 2015, Thenmozhi et al., 2015).
2.4. Animal and housing
Fifty- four adult male Wister rats, aged 6–8 weeks, weighing (180–220 g) were procured from the faculty of Pharmacy - King Saud University. Animals were housed in groups of six animals per cage and kept at a controlled environment at a temperature of 23.2 °C, 12:12-h light/dark cycle, relative humidity between 50% 70% and free access to water and food.
2.5. Experimental design
Fifty-four rats were randomly divided into nine groups (n = 6) as follows: Group I: rats treated with p.o saline and used as negative control, group II: rats treated with oral Na-CMC; vehicle group , group III: rats given AlCl3 (100 mg/kg, p.o) which considered as positive control, group IV: as group III + Azilsartan dose 1 (3.5 mg/kg, p.o) ,group V: as group III + Azilsartan dose 2 (7 mg/kg, p.o), group VI: as group III + perindopril dose 1 (0.5 mg/kg, p.o), group VII: as group III + perindopril dose 2 (1 mg/kg, p.o), group VIII: as group III + (Azilsartan dose 1 + Perindopril dose 1) and group IX: as group III + (Azilsartan dose 2 + Perindopril dose 2). Saline, Na-CMC, AlCl3, Azilsartan, and perindopril were administrated once daily for consecutive 60 days. Groups IV, V, VI, VII, VIII, and IX were pretreated with azilsartan and perindopril in three hours interval from the administration of ALCL3.
At the end of the treatment period, the Y-Maze test was performed, and then all rats were deprived of food for 12 h then sacrificed by decapitation under humane conditions.
2.6. Assessment of cognitive function
2.6.1. Y-Maze test
Short-term memory was assessed by spontaneous alternation behavior (SAB) in the Y-maze task. The Y-maze used in the present study has consisted of three arms (35 cm long, 25 cm high and 10 cm wide) and an equilateral triangular central area. The rat was placed at the end of one arm and allowed to move freely through the maze for 8 min. An arm entry was counted when all four limbs of the rat were completely within the arm. Also, the maze was cleaned with alcohol-free disinfectant wipes between each trial. SAB was defined as the entry into all three arms on consecutive choices. The number of maximum SAB was calculated manually as the total number of arms entered minus 2 and percent spontaneous alternation was calculated as (actual alternations/maximum alternations) × 100 (Kraeuter et al., 2019).
2.7. Tissues preparation and biochemical analyses
After the Y-maze task was finished, the whole brain of each rat was rapidly extracted, washed with isotonic saline, dried, and sagittally divided into two halves; the hippocampus of the left side was dissected and kept in-80 °C for homogenate preparation and the right-sided cerebral hemisphere for histopathological examination.
For preparing of hippocampus homogenate, tissue samples with ice-cold 0.3 M phosphate buffer (pH 7.4) were homogenized using a TisssuLyser II homogenizer (Qiagen, Germany) at oscillation frequencies (180 – 1800 oscillations per minute). The homogenates were then centrifuged at 15 000 rpm at 4 °C for 15 min using a refrigerated centrifuge. The clear supernatants obtained were used to estimate the hippocampal levels of; Aβ-42, NO, TNF-α, MDA and AchE and were determined by quantification ELISA kits following the company's recommended procedures (Konstantinou, 2017).
2.8. Histopathological examination
The second portion of each brain was fixed in phosphate buffer formalin (10%) for 24 h. The brains were processed and embedded in paraffin blocks. Sections (4 μm thick) were stained with hematoxylin and eosin stains stain (Amin et al., 2013). Sections were examined and photographed using an Olympus light microscope (model: BX51TF- Japan). Photomicrograph was done at a magnification of (x4, x 200 and x600).
2.9. Data analysis
The experimental results were expressed as means ± SEM. The intergroup variation between various groups were analyzed statistically using one-way analysis of variance (ANOVA) using Statistical Package for the Social Science software package (SPSS) version 23.0, followed by Tukey’s post hoc test. Results were considered statistically significant when P < 0.05.
3. Results
3.1. Effects of azilsartan and perindopril on the cognitive dysfunction
As shown in (Table 1) the existence of cognitive decline in rats was evidenced by a significant decrease (p < 0.001) of spontaneous alternation percentage (SAP) in the AlCl3-treated group compared to the negative control and vehicle groups. Multiple comparisons of the present results showed a significant increase (p < 0.001) of SAP in each of perindopril, azilsartan either alone or in combination groups compared to the positive control group.
Table 1.
Effects of azilsartan, perindopril and combined groups on Y-maze test in AlCl3 -induced Alzheimer’s disease in rats.
| Groups | Spontaneous Alternation Percentage % |
|---|---|
| Negative Control | 72 ± 0.85* |
| Vehicle | 72.16 ± 0.87* |
| AlCl3 (100 mg/kg) - Positive control | 45 ± 0.68 |
| Azilsartan 1 (3.5 mg/kg) | 71.33 ± 0.49* |
| Azilsartan 2 (7 mg/kg) | 72.66 ± 0.84* |
| Perindopril 1 (0.5 mg/kg) | 73.16 ± 0.70* |
| Perindopril 2 (1 mg/kg) | 72.16 ± 0.94* |
| Combined 1 (Azilsartan 1 + Perindopril 1) | 69.83 ± 0.60* |
| Combined 2 (Azilsartan 2 + Perindopril 2) | 70.66 ± 1.20** |
Values are expressed as means ± SEM, n = 6 rats.
P < 0.001 compared with the corresponding AlCl3 group values; by one way ANOVA and Tukey HSD post hoc test.
3.2. Effects of azilsartan and perindopril on the hippocampal level of Aβ-42
Table 2 illustrated that Aβ-42 was significantly high in the AlCl3 treated group (p < 0.001) compared with negative and vehicle groups and this elevation was significantly mitigated (p < 0.001) in perindopril and azilsartan groups either alone or in combination compared to the positive control group. However, no significant difference of the Aβ-42 level was detected between perindopril and azilsartan groups either alone or in combination and control, vehicle groups (p > 0.05). While combined groups have shown the lowest reduction in the Aβ-42 level.
Table 2.
Effects of azilsartan, perindopril and combined groups on the hippocampal level of Aβ-42 in AlCl3 -induced Alzheimer’s disease in rats.
| Groups | Aβ-42 (pg/mg of protein) |
|---|---|
| Negative Control | 74 ± 2.04* |
| Vehicle | 70.58 ± 1.21* |
| AlCl3 (100 mg/kg) - Positive control | 118 ± 5.01 |
| Azilsartan 1 (3.5 mg/kg) | 70 ± 2.30* |
| Azilsartan 2 (7 mg/kg) | 72 ± 1.12* |
| Perindopril 1 (0.5 mg/kg) | 70 ± 3.10* |
| Perindopril 2 (1 mg/kg) | 68 ± 2.33* |
| Combined 1 (Azilsartan 1 + Perindopril 1) | 66.40 ± 1.17* |
| Combined 2 (Azilsartan 2 + Perindopril 2) | 63 ± 1.94* |
Values are expressed as means ± SEM, n = 6 rats.
P < 0.001 compared with the corresponding AlCl3 group values; by one way ANOVA and Tukey HSD post hoc test.
3.3. Effect of perindopril and azilsartan on the hippocampal level of AChE
As showed in Table 3, AlCl3 correlated with a significant increase (p < 0.05) in the level of AchE versus negative control and vehicle groups. This overexpression of AchE level was significantly reduced (p < 0.05) in perindopril and azilsartan either monotherapy or in combination. However, no significant difference of the AchE level was observed between perindopril and azilsartan either monotherapy or in combination and control, vehicle groups (p > 0.05).
Table 3.
Effects of azilsartan, perindopril and combined groups on the hippocampal level of AChE in AlCl3 -induced Alzheimer’s disease in rats.
| Groups | AchE (ng/mg of protein) |
|---|---|
| Negative Control | 0.62 ± 0.08* |
| Vehicle | 0.66 ± 0.01* |
| AlCl3 (100 mg/kg) - Positive control | 1.39 ± 0.14 |
| Azilsartan 1 (3.5 mg/kg) | 0.73 ± 0.07* |
| Azilsartan 2 (7 mg/kg) | 0.68 ± 0.09* |
| Perindopril 1 (0.5 mg/kg) | 0.72 ± 0.06* |
| Perindopril 2 (1 mg/kg) | 0.75 ± 0.08* |
| Combined 1 (Azilsartan 1 + Perindopril 1) | 0.80 ± 0.07* |
| Combined 2 (Azilsartan 2 + Perindopril 2) | 0.73 ± 0.05* |
Values are expressed as means ± SEM, n = 6 rats.
P < 0.001 compared with the corresponding AlCl3 group values; by one way ANOVA and Tukey HSD post hoc test.
3.4. Effect of perindopril and azilsartan on hippocampal level of MDA
In the present work, as shown in Table 4, a significantly high level of MDA was detected (p < 0.001) in the AlCl3 group versus negative control and vehicle groups. This elevation was significantly reserved (p < 0.001) in perindopril and azilsartan either alone or in combination compared with the positive control group. However, no significant difference of the MDA level was observed in perindopril and azilsartan either alone or in combination, negative control & vehicle groups (p > 0.05).
Table 4.
Effects of azilsartan, perindopril and combined groups on the hippocampal level of MDA in AlCl3 -induced Alzheimer’s disease in rats.
| Group | MDA (ug/mg of protein) |
|---|---|
| Negative Control | 1.08 ± 0.12* |
| Vehicle | 1.11 ± 0.51* |
| AlCl3 (100 mg/kg) - Positive control | 2 ± 0.12 |
| Azilsartan 1 (3.5 mg/kg) | 1.17 ± 0.02** |
| Azilsartan 2 (7 mg/kg) | 1.15 ± 0.04* |
| Perindopril 1 (0.5 mg/kg) | 1.23 ± 0.10* |
| Perindopril 2 (1 mg/kg) | 1.16 ± 0.03* |
| Combined 1 (Azilsartan 1 + Perindopril 1) | 1.18 ± 0.07* |
| Combined 2 (Azilsartan 2 + Perindopril 2) | 1.18 ± 0.03* |
Values are expressed as means ± SEM, n = 6 rats.
P < 0.001 compared with the corresponding AlCl3 group values; by one way ANOVA and Tukey HSD post hoc test.
3.5. Effect of perindopril and azilsartan on the hippocampal level of TNF-α
Table 5 clarified that the level of the TNF-α was significantly elevated (p < 0.001) in the AlCl3 group versus negative control and vehicle groups. This overexpression of the level of TNF-α was significantly decreased (p < 0.001) in the perindopril and azilsartan as monotherapy or in combination compared with a positive control group. Also, post hoc analysis showed that perindopril groups (0.5 mg/kg and 1 mg/kg), produced the lowest reduction in the TNF-α level.
Table 5.
Effects of azilsartan, perindopril and combined groups on the hippocampal level of TNF-α in AlCl3 -induced Alzheimer’s disease in rats.
| Group | TNF-α (pg/mg of protein) |
|---|---|
| Negative Control | 20.22 ± 0.66* |
| Vehicle | 19.19 ± 1.19* |
| AlCl3 (100 mg/kg) - Positive control | 30.54 ± 1.7 |
| Azilsartan 1 (3.5 mg/kg) | 19.87 ± 1.2* |
| Azilsartan 2 (7 mg/kg) | 21.21 ± 1.5* |
| Perindopril 1 (0.5 mg/kg) | 18.33 ± 0.97* |
| Perindopril 2 (1 mg/kg) | 16.45 ± 0.62* |
| Combined 1 (Azilsartan 1 + Perindopril 1) | 20.01 ± 1.7* |
| Combined 2 (Azilsartan 2 + Perindopril 2) | 21 ± 1.4* |
Values are expressed as means ± SEM, n = 6 rats.
P < 0.001 compared with the corresponding AlCl3 group values; by one way ANOVA and Tukey HSD post hoc test.
3.6. Effect of perindopril and azilsartan on hippocampal level of NO
Table 6 showed no significant change (p > 0.05) in the level of NO in the AlCl3 group versus negative control and vehicle groups. Moreover, no significant group difference was observed (p > 0.05) in perindopril and azilsartan either alone or in combination groups versus the AlCl3-treated group.
Table 6.
Effects of azilsartan, perindopril and combined groups on the hippocampal level of NO in AlCl3 -induced Alzheimer’s disease in rats.
| Group | NO (μmol /mg of protein) |
|---|---|
| Negative Control | 42.40 ± 1.03 |
| Vehicle | 41.66 ± 1.26 |
| AlCl3 (100 mg/kg) - Positive control | 37 ± 1.06 |
| Azilsartan 1 (3.5 mg/kg) | 39.80 ± 2.13 |
| Azilsartan 2 (7 mg/kg) | 39.73 ± 1.51 |
| Perindopril 1 (0.5 mg/kg) | 34.43 ± 2.60 |
| Perindopril 2 (1 mg/kg) | 37.03 ± 3.74 |
| Combined 1 (Azilsartan 1 + Perindopril 1) | 30.46 ± 0.98 |
| Combined 2 (Azilsartan 2 + Perindopril 2) | 35.34 ± 0.83 |
Values are expressed as means ± SEM, n = 6 rats.
The intergroup variation between various groups was performed using one-way ANOVA.
3.7. Effect of azilsartan and perindopril on hippocampal histology
In the present work, two major components of the hippocampus were studied, dentate gurus (DG) and CA3.
Examination of DG of the hippocampus region in the negative control group exhibited three layers; molecular, granular cell, and polymorphic layers. The polyhedral granular cell layer the major constituent and had vesicular nuclei as illustrated in (Fig. 1a, b). While, in the positive control group, granular cells were disorganized, with marked vacuolation. Others were shrunk with pyknotic nuclei. The pathological hallmarks of AD were also evident; neurofibrillary tangles (NFTs) and amyloid plaques (AP) as presented in (Fig. 1c, d).
Fig. 1.
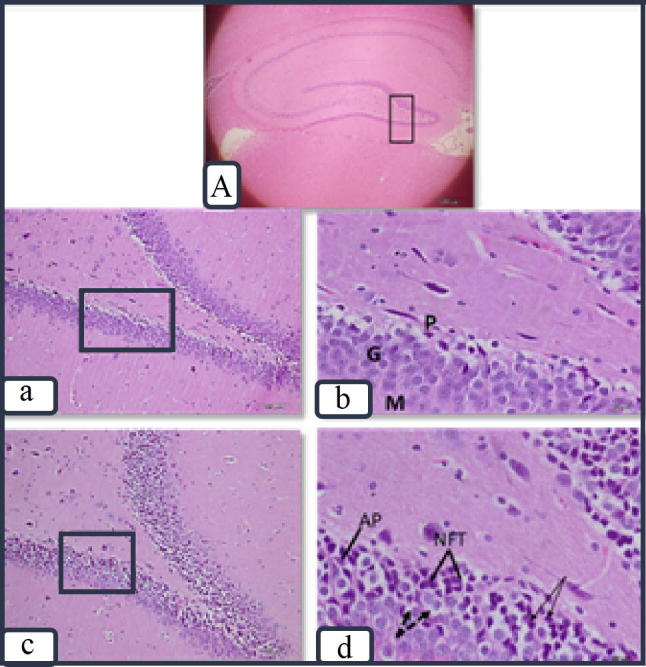
Photomicrographs showing the hippocampus at the DG in the negative control group (a & b) with its layers; molecular (M) Granular (G) & Polymorphic (P). The granular cells are polyhedral with vesicular nuclei. In the positive control group (c & d), granular cells are vacuolated (dashed arrows), or shrunken with pyknotic nuclei (arrows). Neurofibrillary tangles (NFTs) and amyloid plaques (AP) are noted. Figure (A) shows low magnification of the hippocampus with DG marked (×4) (H&E stain. a & c ×200) (b &d × 600).
In groups treated with perindopril doses 1&2 (0.5 mg/kg and 1 mg/kg, respectively), the granular cells retained normal criteria and normal vesicular nuclei were prominent as depicted (Fig. 2a, b,c.,d) simultaneously, compared to the positive control (Fig. 2e, f). In treated groups with azilsartan dose 1 (3.5 mg/kg), most of the granular cells retained normal structure but still scattered NFTs were evident as shown in (Fig. 3a, b). While on administration of azilsartan dose2, (7 mg/kg), vacuolated granular cells were only evident as observed in (Fig. 3c, d). Moreover, the perindopril and azilsartan combined group retained most of the normal histopathological changes compared to the positive control group as illustrated in (Fig. 4a, b, c, d).
Fig. 2.

Photomicrographs of sections of the hippocampus at DG showing a normal histological structure, and normal granular cells with vesicular nuclei in groups treated with Perindopril dose 1 (0.5 mg/kg) (a & b) and perindopril dose 2 (1 mg/kg) (c & d) compared to sections hippocampus from the positive control group (e&f). (H &E stain . a,c &e × 200) (b,d &f × 600).
Fig. 3.
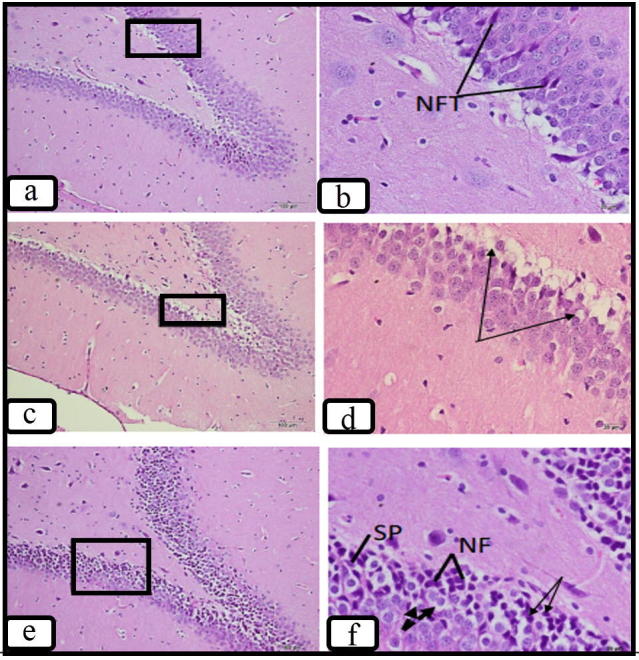
Photomicrographs of sections of the hippocampus at DG showing few scattered NFTs in groups treated with azilsartan dose 1 (3.5 mg/kg) (a &b) and only vacuolated cells (arrows) in group treated with azilsartan dose 2 (7 mg/kg) (c& d) compared to sections hippocampus from the positive control group (e&f). (H&E stain. a,c &e × 200) (b,d &f ×600).
Fig. 4.

Photomicrographs of sections of the hippocampus at DG showing scattered NFTs and vacuolated cells (dashed arrows) among the normal granular cells in combined Group 1 (azilsartan 3.5 mg/kg + perindopril 0.5 mg/kg) (a &b), on administrating (azilsartan 7 mg/kg + perindopril 1 mg/kg) in combined group 2 multiple NFTs were noted (c&d). This was in comparison to sections hippocampus from the positive control group (e&f). (H &E stain. a,c &e ×200) (b,d &f ×600).
The CA3 part of the hippocampus in the negative control group was composed of 3 layers; polymorphic, pyramidal and molecular cell layers. The pyramidal cell layer packed in 4–5 layers, held the principal cell bodies. They have large vesicular nuclei and basophilic cytoplasm as showed in (Fig. 5a, b). While, in sections of the positive control group, the hippocampus at CA3 revealed the NFTs and AP. Some pyramidal cells were vacuolated while others were shrunken with pyknotic nuclei as shown in (Fig. 5c, d). Moreover, In groups treated with perindopril 1&2 (0.5 mg/kg and 1 mg/kg, respectively), the pyramidal cells were comparable to that of the negative control group as depicted in (Fig. 6a,b,c,d). Also, groups treated with azilsartan dose 1 (3.5 mg/kg) still showed scanty NFTs and SP (Fig. 7a, b) but those treated with azilsartan dose 2 (7 mg/ kg) (Fig. 7c, d) almost retained the normal structure of pyramidal cells compared to positive control (Fig. 7e,f). Furthermore, perindopril and azilsartan combined group maintained most of the normal histopathological changes compared to the positive control group as depicted (Fig. 8a, b, c, d).
Fig. 5.

Photomicrographs showing hippocampus at the CA3 in the negative control group (a&b) with its layers; molecular (M), pyramidal (py) & Polymorphic (p). The main polyhedral pyramidal cells show vesicular nuclei. In the positive control group (c&d), pyramidal cells are vacuolated (dashed arrows), or shrunken with pyknotic nuclei (arrows). NFTs and AP are noted. Figure (A) shows low magnification of the hippocampus with CA3 marked (H&E stain. a&c ×200) (b&d ×600).
Fig. 6.

Photomicrographs of sections of the hippocampus at CA3 showing a normal histological structure in groups treated with Perindopril dose 1 (0.5 mg/kg) (a &b) and perindopril dose 2 (1 mg/kg) (c & d) compared to sections hippocampus from the positive control group (e&f). (H &E stain . a,c &e ×200) (b,d &f ×600).
Fig. 7.

Photomicrographs of sections of the hippocampus at CA3 showing few scattered NFTs in groups treated with azilsartan dose 1 (3.5 mg/kg) (a &b). While, in group treated with azilsartan dose 2 (7 mg/kg) (c& d) pyramidal cells retained vesicular nuclei compared to sections hippocampus from the positive control group (e&f). (H &E stain. a,c &e ×200) (b,d &f ×600).
Fig. 8.

Photomicrographs sections of the hippocampus at CA3 in the combined group 1 (azilsartan 3.5 mg/kg + perindopril 0.5 mg/kg) (a &b) and combined group 2 (azilsartan 7 mg/kg + perindopril 1 mg/kg) (c&d) showing few NFT (arrows) among normal pyramidal cells .This is in comparison to sections of hippocampus in the positive control group (e&f). (H&E stain. a,c &e × 200) (b,d &f × 600).
4. Discussion
The main findings of this work were as follows: (A) AlCl3 produced significant cognitive impairment, with an increased hippocampal level of Aβ-42, also, significant elevation of AChE, MDA, TNF-α level in the hippocampus, while no change was found on the hippocampal level of NO. (B) Chronic treatment with perindopril and azilsartan for 60 days produced a significant improvement of cognitive decline which was evaluated by the Y-Maze test. (C) Perindopril and azilsartan administration alone or in combination for 60 days significantly decreased each of Aβ-42, AChE, MDA, TNF-α level in the hippocampus, with no change detected in the hippocampal NO level. It is worth noting that no significant change was obtained between either perindopril & azilsartan groups and combined groups. (D) Perindopril and azilsartan-received groups retained most of the histopathological changes in different regions of the hippocampus irrespective of doses compared to the Alcl3 group.
In the current study, the existence of cognitive dysfunction in rats received AlCl3 was confirmed by a significant decrease (p < 0.001) in spontaneous alteration percentage (SAP) compared to the negative control group. In agreement with the present study, Zghari et al.(2018), stated that SAP was significantly lowered in AlCl3 -treated group versus the negative control group (p < 0.001).
Moreover, multiple comparisons of the present results showed a significant increase (p < 0.001) of SAP in each of perindopril, azilsartan either alone or in combination groups compared to the positive control group which suggested the essential role of perindopril and azilsartan in the improvement of hippocampus-dependent learning and memory. In accordance with the results of the present work, the previous study documented that perindopril was able to enhance memory and learning function in behavioral tests in colchicine-induced memory impairment in animals (Awasthi et al., 2012). In addition, perindopril exhibited an improvement in the Y-Maze through the increase in the SAP in the transgenic animal model of AD (Dong et al., 2011). Moreover, valsartan was reported to improve cognitive impairment in the animal models of AD evaluated by WMT (Yang et al., 2014).
The amyloid-beta deposition is one of the most important hallmarks of AD, in the present work, the level of Aβ-42 in the hippocampus was significantly increased in the positive control group (p < 0.001) versus a negative control group. In agreement with the current finding, the previous study reported that AlCl3-induced oxidative stress and resulted in Aβ formation and accumulation in the transgenic animal model of AD (Praticò et al., 2002). While in perindopril and azilsartan groups the level of Aβ was significantly reduced (p < 0.001) compared to the positive control group. This goes hand with the previous report which documented that perindopril exerted an inhibitory effect on Aβ deposition in lipopolysaccharide (LPS)-induced cognition impairment and amyloidogenesis in mice (Ali et al., 2016). In addition, Captopril provided a neuroprotective action via preventing amyloidogenic pathways of APP consequently, inhibited the deposition of Aβ (AbdAlla et al., 2013). Furthermore, telmisartan has enhanced cognitive functions in STZ-induced AD in mice through decreasing the level Aβ −42, APP and beta-secretase 1 (BACE1) (Du et al., 2014).
In AD patients, the cholinergic neurons severely degenerated and the activity of acetylcholine neurotransmitter is diminished, the present investigation showed significant AChE increase (p < 0.001) in the positive control group compared with the negative control group. In consistence with the current study, Auti et al. (2019), posited that chronic administration of AlCl3 increases the activity of AChE . In contrast, in the present work, treatment with perindopril and azilsartan whether alone or in combination significantly decreased the level of AChE, this was supported by previous research determined that perindopril improved cholinergic transmission by decreasing AChE level and consequently, enhancing Ach action in scopolamine-induced memory impairment in mice (Tota et al., 2012). In addition, lisinopril and telmisartan were found to reduce the activity of AChE in STZ-induced AD in mice and enhanced memory functions (Singh et al., 2013).
Oxidative stress has been hypothesized to be involved in the pathogenesis of many CNS diseases such as AD, and it has been associated with amyloidosis and tauopathy(Gandhi et al., 2012). In the present study, the MDA level was significantly enhanced (p < 0.001) in the AlCl3 group compared to the negative control group, this finding was in agreement with previous literature which reported that the administration of AlCl3 resulted in significant high level of MDA compared to the treated group (Gol et al., 2019). While perindopril and azilsartan groups whether alone or in combination the MDA significantly lowered compared to the positive group. This is in accordance with the previous study which documented that perindopril exhibited antioxidant effect by significantly increased the activities of SOD and GSH-Px, which decreased the MDA level in the mice model of AD induced (Yang et al., 2013). In addition, captopril administration in animals showed a significant decrease in MDA and increase in SOD and GSH (Bild et al., 2013). Furthermore, treatment with telmisartan in animals produced antioxidant effects via decreasing MDA, total oxidant status (TOS) (Erdi et al., 2016).
Several experimental and clinical evidence has reported the presence of neuroinflammation in the brain of AD due to the activation of microglia as well as other CNS immune cells (Kinney et al., 2018), In the present investigation, TNF-α was significantly elevated (p < 0.001) in the positive control group versus the negative control. Further confirmatory evidence was obtained by Tsunoda and Sharma (1999), who reported a significant increase in the mRNA expression of TNF-α in mice brains that exposed to AlCl3. This was further supported by another researcher who documented that a higher level of TNF-α was observed after 10 weeks of exposure to AlCl3 in drinking water in the brain of mice (Campbell et al., 2004). In the current work, the level of TNF-α significantly reduced in perindopril and azilsartan either alone or in combination compared to the positive control group, this finding suggested the crucial role of perindopril and azilsartan as anti-inflammatory agents. In accordance with the present data, Ali et al., (2016), have demonstrated that perindopril showed an anti-inflammatory effect by decreasing the level of TNF-α significantly in an animal model of AD. Moreover, Candesartan showed strong anti-inflammatory actions evidenced by attenuating the release of different proinflammatory markers in the brains of animal models (Goel et al., 2018).
In AD, the roles of NO are controversial either neuroprotective or neurotoxic (Balez et al., 2016). In the present work, the hippocampal level of NO was insignificantly changed (p > 0.05) in all treated groups versus positive control. This finding has consisted with a previous study reported that the level of NO was insignificantly changed after treatment with perindopril in scopolamine-induced memory impairment in mice (Tota et al., 2012).
Regarding the histopathological studies, two major components of the hippocampus were studied, dentate gyrus (DG) and cornu ammonis 3 (CA3). These areas in the hippocampus exert essential roles in learning, memory, and spatial coding (Jonas et al., 2014). In the present study, histopathological changes in different areas of the hippocampus including DG and CA3 regions were noted in the Alcl3 group compared to the negative control group, which were indicated by the presence of disorganized granular and pyramidal cells with marked vacuolation. Others were shrunk with pyknotic nuclei. Moreover, the pathological hallmarks of AD were also evident; NFT and AP. These findings are consistent with previous research, Chronic administration of Al via drinking water produced injurious effects on pyramidal neurons manifested by cellular shrinking and disorganized pyramidal cells in the CA3 region of the hippocampus in rats (Liaquat et al., 2019, Sethi et al., 2008). While, treatment with perindopril and azilsartan retained most of the histopathological alternations induced by Alcl3 and showed normal criteria and morphology, in accordance with present results, administration of Lisinopril and telmisartan reserved histopathological damage by decreasing Aβ deposits in the brain of an animal model of AD induced by STZ (Singh et al., 2013). Treatment with captopril and valsartan mitigated histological changes in the C1A region of the hippocampus (Abbassi et al., 2016).
5. Conclusion
In the present study, either azilsartan and perindopril alone or in combined have shown significant ameliorative effects on different aspects of AD pathogenesis, producing improvement of cognitive function, significant reduction in the hippocampal levels of Aβ-42 and AChE, antioxidant activity by decreasing the hippocampal level of MDA, anti-inflammatory effect evidenced by significant reduction in the level of TNF-α in the hippocampus and reserved most of histopathological changes that mediated by Alcl3. It is worth mentioning that in this study we found no significant effect in the combination of perindopril and azilsartan compared to mono-therapy, also, different doses have been used from both perindopril and azilsartan produced insignificant effects (i.e. there effects were non-dose dependent). Lack of combined effect could be explained by the need for prolonged treatment since the AD is a chronic disease. Moreover, higher doses may be needed to detect the difference between combined and monotherapy if present.
5.1. Recommendations
Further evidence from well-designed and adequately powered clinical trials is required to establish the roles of perindopril and azilsartan in preventing or treating AD. Moreover, Due to the prevalence of CVD in the KSA which are considered to be established risk factors for AD, it is recommended that better control of these diseases could consequently lower or prevent AD.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The present study funded by a grant from King Abdulaziz City for Science and Technology, Saudi Arabia (Ref. No. 1-18-03-009-0052).The authors, therefore, acknowledge with thanks the KACST and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbassi Y.A., Mohammadi M.T., Foroshani M.S., Sarshoori J.R. Captopril and valsartan may improve cognitive function through potentiation of the brain antioxidant defense system and attenuation of oxidative/nitrosative damage in STZ-induced dementia in rat. Adv. Pharmaceut. Bull. 2016;6:531–539. doi: 10.15171/apb.2016.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdAlla S., Langer A., Fu X., Quitterer U. ACE inhibition with captopril retards the development of signs of neurodegeneration in an animal model of Alzheimer’s disease. Int. J. Mol. Sci. 2013;14:16917–16942. doi: 10.3390/ijms140816917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.R.A.A., Abo-Youssef A.M.H., Messiha B.A.S., Khattab M.M. Tempol and perindopril protect against lipopolysaccharide-induced cognition impairment and amyloidogenesis by modulating brain-derived neurotropic factor, neuroinflammation and oxido-nitrosative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016;389:637–656. doi: 10.1007/s00210-016-1234-6. [DOI] [PubMed] [Google Scholar]
- Amin, S.N., Younan, S.M., Youssef, M.F., Rashed, L.A., Mohamady, I., 2013. A histological and functional study on hippocampal formation of normal and diabetic rats. F1000Research, 2. [DOI] [PMC free article] [PubMed]
- Auti, S.T., Kulkarni, Y.A., 2019: Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front. Neurol., 10. [DOI] [PMC free article] [PubMed]
- Awasthi H., Kaushal D., Siddiqui H.H. Chronic inhibition of central angiotensin-converting enzyme ameliorates colchicine-induced memory impairment in mice. Sci. Pharm. 2012;80:647–662. doi: 10.3797/scipharm.1203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balez, R., Ooi, L., 2016. Getting to NO Alzheimer’s disease: Neuroprotection versus neurotoxicity mediated by nitric oxide. Oxid. Med. Cell. Long. [DOI] [PMC free article] [PubMed]
- Bild W., Hritcu L., Stefanescu C., Ciobica A. Inhibition of central angiotensin II enhances memory function and reduces oxidative stress status in rat hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;43:79–88. doi: 10.1016/j.pnpbp.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R., Abdalla N., Kawas C.H., Corrada M.M. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s Dementia. 2018;14:121–129. doi: 10.1016/j.jalz.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A., Becaria A., Lahiri D.K., Sharman K., Bondy S.C. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J. Neurosci. Res. 2004;75:565–572. doi: 10.1002/jnr.10877. [DOI] [PubMed] [Google Scholar]
- Dong Y.F., Kataoka K., Tokutomi Y., Nako H., Nakamura T., Toyama K., Sueta D., Koibuchi N., Yamamoto E., Ogawa H., Kim-Mitsuyama S. Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB J. 2011;25:2911–2920. doi: 10.1096/fj.11-182873. [DOI] [PubMed] [Google Scholar]
- Du G.T., Hu M., Mei Z.L., Wang C., Liu G.J., Hu M., Long Y., Miao M.X., Li J.C., Hong H. Telmisartan treatment ameliorates memory deficits in streptozotocin-induced diabetic mice via attenuating cerebral amyloidosis. J. Pharmacol. Sci. 2014;124:418–426. doi: 10.1254/jphs.13157fp. [DOI] [PubMed] [Google Scholar]
- Erdi F., Keskin F., Esen H., Kaya B., Feyzioglu B., Kilinc I., Karatas Y., Cuce G., Kalkan E. Telmisartan ameliorates oxidative stress and subarachnoid haemorrhage-induced cerebral vasospasm. Neurol. Res. 2016;38:224–231. doi: 10.1080/01616412.2015.1105626. [DOI] [PubMed] [Google Scholar]
- Fazal K., Perera G., Khondoker M., Howard R., Stewart R. Associations of centrally acting ACE inhibitors with cognitive decline and survival in Alzheimer’s disease. BJPsych Open. 2017;3:158–164. doi: 10.1192/bjpo.bp.116.004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S., Abramov A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longevity. 2012 doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebre, A.K., Altaye, B.M., Atey, T.M., Tuem, K.B., Berhe, D.F., 2018, April 30: Targeting Renin-Angiotensin System Against Alzheimer’s disease. Front. Pharmacol. [DOI] [PMC free article] [PubMed]
- Goel R., Bhat S.A., Hanif K., Nath C., Shukla R. Angiotensin II Receptor Blockers Attenuate Lipopolysaccharide-Induced Memory Impairment by Modulation of NF-κB-Mediated BDNF/CREB Expression and Apoptosis in Spontaneously Hypertensive Rats. Mol. Neurobiol. 2018;55:1725–1739. doi: 10.1007/s12035-017-0450-5. [DOI] [PubMed] [Google Scholar]
- Gol M., Ghorbanian D., Soltanpour N., Faraji J., Pourghasem M. Protective effect of raisin (currant) against spatial memory impairment and oxidative stress in Alzheimer disease model. Nutr. Neurosci. 2019;22:110–118. doi: 10.1080/1028415X.2017.1354959. [DOI] [PubMed] [Google Scholar]
- Jonas, P., Lisman, J., 2014. Structure, function, and plasticity of hippocampal dentate gyrus microcircuits. Front. Neural Circ. [DOI] [PMC free article] [PubMed]
- Kinney, J.W., Bemiller, S.M., Murtishaw, A.S., Leisgang, A.M., Salazar, A.M., Lamb, B.T., 2018: Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s and Dementia: Transl. Res. Clin. Intervent.. [DOI] [PMC free article] [PubMed]
- Konstantinou G.N. Enzyme-linked immunosorbent assay (ELISA) Methods Mol. Biol. 2017;1592:79–94. doi: 10.1007/978-1-4939-6925-8_7. [DOI] [PubMed] [Google Scholar]
- Kraeuter, A.K., Guest, P.C., Sarnyai, Z., 2019. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods in Molecular Biology. Humana Press Inc., Vol. 1916, pp. 105–111. [DOI] [PubMed]
- Lakshmi B.V.S., Sudhakar M., Prakash K.S. Protective Effect of Selenium Against Aluminum Chloride-Induced Alzheimer’s Disease: behavioral and biochemical alterations in rats. Biol. Trace Elem. Res. 2015;165:67–74. doi: 10.1007/s12011-015-0229-3. [DOI] [PubMed] [Google Scholar]
- Liaquat L., Sadir S., Batool Z., Tabassum S., Shahzad S., Afzal A., Haider S. Acute aluminum chloride toxicity revisited: Study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci. 2019;217:202–211. doi: 10.1016/j.lfs.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Miller A.J., Arnold A.C. April 1: The renin–angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin. Auton. Res. 2019 doi: 10.1007/s10286-018-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M., Iwanami J., Horiuchi M. Roles of brain angiotensin II in cognitive function and dementia. Int. J. Hypertens. 2012 doi: 10.1155/2012/169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7:27. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Caoimh R., Kehoe P.G., Molloy D.W. Renin angiotensin aldosterone system inhibition in controlling dementia-related cognitive decline. J. Alzheimer’s Disease. 2014 doi: 10.3233/JAD-141284. [DOI] [PubMed] [Google Scholar]
- Praticò D., Uryu K., Sung S., Tang S., Trojanowski J.Q., Lee V.M.Y. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J.: Official Publicat. Feder. Am. Soc. Exp. Biol. 2002;16:1138–1140. doi: 10.1096/fj.02-0012fje. [DOI] [PubMed] [Google Scholar]
- Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., Ferri, C.P., 2013. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s and Dementia. [DOI] [PubMed]
- Prince, M., Comas-Herrera, A., Knapp, M., Guerchet, M., Karagiannidou, M., 2016. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia. Alzheimer’s Disease International.
- Rygiel K. Can angiotensin-converting enzyme inhibitors impact cognitive decline in early stages of Alzheimer’s disease? An overview of research evidence in the elderly patient population. J. Postgrad. Med. 2016;62:242–248. doi: 10.4103/0022-3859.188553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi P., Jyoti A., Singh R., Hussain E., Sharma D. Aluminium-induced electrophysiological, biochemical and cognitive modifications in the hippocampus of aging rats. NeuroToxicology. 2008;29:1069–1079. doi: 10.1016/j.neuro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Singh B., Sharma B., Jaggi A.S., Singh N. Attenuating effect of lisinopril and telmisartan in intracerebroventricular streptozotocin induced experimental dementia of Alzheimer’s disease type: Possible involvement of PPAR-γ agonistic property. JRAAS – J. Renin-Angiotensin-Aldosterone Syst. 2013;14:124–136. doi: 10.1177/1470320312459977. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V., Scafato E., Frisardi V., Seripa D., Logroscino G., Kehoe P.G., Imbimbo B.P., Baldereschi M., Crepaldi G., Di Carlo A., Galluzzo L., Gandin C., Inzitari D., Maggi S., Pilotto A., Panza F. Angiotensin-converting enzyme inhibitors and incidence of mild cognitive impairment. The Italian Longitudinal Study on Aging. Age. 2013;35:441–453. doi: 10.1007/s11357-011-9360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thenmozhi A.J., Raja T.R.W., Janakiraman U., Manivasagam T. Neuroprotective Effect of Hesperidin on Aluminium Chloride Induced Alzheimer’s Disease in Wistar Rats. Neurochem. Res. 2015;40:767–776. doi: 10.1007/s11064-015-1525-1. [DOI] [PubMed] [Google Scholar]
- Tota S., Nath C., Najmi A.K., Shukla R., Hanif K. Inhibition of central angiotensin converting enzyme ameliorates scopolamine induced memory impairment in mice: role of cholinergic neurotransmission, cerebral blood flow and brain energy metabolism. Behav. Brain Res. 2012;232:66–76. doi: 10.1016/j.bbr.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Tsunoda M., Sharma R.P. Modulation of tumor necrosis factor α expression in mouse brain after exposure to aluminum in drinking water. Arch. Toxicol. 1999;73:419–426. doi: 10.1007/s002040050630. [DOI] [PubMed] [Google Scholar]
- Ulep M.G., Saraon S.K., McLea S. Alzheimer disease. J. Nurse Pract. 2018;14:129–135. [Google Scholar]
- WHO, 2018. WHO | 10 facts on dementia. WHO.
- Yang W.N., Han H., Hu X.D., Feng G.F., Qian Y.H. The effects of perindopril on cognitive impairment induced by d-galactose and aluminum trichloride via inhibition of acetylcholinesterase activity and oxidative stress. Pharmacol. Biochem. Behav. 2013;114–115:31–36. doi: 10.1016/j.pbb.2013.10.027. [DOI] [PubMed] [Google Scholar]
- Yang W.N., Hu X.D., Han H., Shi L.L., Feng G.F., Liu Y., Qian Y.H. The effects of valsartan on cognitive deficits induced by aluminum trichloride and d-galactose in mice. Neurol. Res. 2014;36:651–658. doi: 10.1179/1743132813Y.0000000295. [DOI] [PubMed] [Google Scholar]
- Zghari O., Rezqaoui A., Ouakki S., Lamtai M., Chaibat J., Mesfioui A., El Hessni A., Rifi E.H., Essamri A., Ouichou A. Effect of chronic aluminum administration on affective and cognitive behavior in male and female rats. J. Behav. Brain Sci. 2018;08:179–196. doi: 10.3390/brainsci8080141. [DOI] [PMC free article] [PubMed] [Google Scholar]


