Abstract
Research studies have indicated that the comorbidity burden of mood disorders and obesity is reasonably high. Insulin signaling has been shown to modulate multiple physiological functions in the brain, indicating its association with neuropsychiatric diseases, including mood disorders. Leptin is a hormone responsible for regulating body weight and insulin homeostasis. Previous studies on db/db mice (a mouse model that carries a spontaneous genetic mutation in leptin receptor Leprdb) have shown that they exhibit inflammation as well as neurobehavioral traits associated with mood. Therefore, targeting inflammatory pathways such as TNF-α may be an effective strategy in the treatment of obesity-linked mood disorders. The objective of this study was to investigate the effect of long-term administration of etanercept (a TNF-α blocker) on anxiety and depressive-like behaviors in db/db mice. This was performed using light/dark box, forced swim, and open field tests with lean littermate wild type (WT) mice serving as a control group. Using flow cytometry in peripheral blood, we further examined the molecular effects of etanercept on NF-κB p65, TNF-α, IL-17A, and TLR-4 expressing CD4+, CD8+, and CD14+ cells in the peripheral blood. Our data show that peripheral administration of etanercept decreased these cells in db/db mice. Furthermore, our results indicated that peripheral administration of etanercept reduced anxiety and depressive-like behaviors. Therefore, targeting TNF-α signaling might be an effective strategy for modulating obesity-associated depression and anxiety.
Keywords: db/db mice, TNF-α, Etanercept, Inflammation, Obesity, Depression, Anxiety-like behavior, Depressive-like behavior
1. Introduction:
There is a high prevalence of comorbidities associated with obesity, including neuropsychiatric disorders such as depression and anxiety (Brumpton et al., 2013, Dawes et al., 2016). Type-2 diabetes mellitus (T2DM) or non-insulin dependent diabetes mellitus is a disease characterized by chronic hyperglycemia, insulin resistance, and obesity. These symptoms can result in microvascular complications that impact multiple organs and tissues, including the eyes, kidneys, and peripheral nerves (Malik, Tesfaye & Ziegler, 2013). Persistent hyperglycemia causes peripheral oxidative stress and inflammation, both of which are associated with cardiovascular complications, a decline in cognitive function, anxiety, and depression (Vincent et al., 2011, O'Brien et al., 2015, Wang et al., 2014). Over the past few decades, considerable resources have been devoted to T2DM research using different animal models, such as the leptin receptor-deficient db/db mouse model (Leprdb/db mice) (Martinez-Botas et al., 2000, O'Brien et al., 2015).
Previous research employing forced swim and open field tests with db/db mice has shown that they exhibit anxiety and depressive-like behaviors. However, the long-term pharmacological administration of rosiglitazone, an antidiabetic agent, altered these phenotypes. This suggests that the management of blood glucose in a T2DM animal model may reduce the co-occurrence of anxiety and depressive-like behaviors (Sharma, Elased & Lucot, 2012).
Recent studies have indicated an essential role for innate and adaptive immune cells such as macrophages and T cells in the release of inflammatory mediators located within adipose tissue (Weisberg et al., 2003); most notably, tumor necrosis factor-α (TNF-α) level (Mantzoros et al., 1997). TNF-α plays a vital role in the progression of cognitive decline, as well as depressive and anxiety disorders (Bai, Chiou, Su, Li & Chen, 2014). Similarly, IL-17A has been reported to be crucial in mediating inflammatory processes associated with CNS disorders, including anxiety and depression (Beurel et al., 2013, Waisman et al., 2015).
Neuroinflammation is associated with obesity as well as depression (Wang, Xu, Liu, Li & Li, 2018). The toll-like receptor 4 (TLR-4) is expressed on circumventricular organs and the choroid plexus, and is linked with NF-κB activation and subsequent production of TNF-α (Nadeau & Rivest, 2000). Augmented TLR-4/NF-κB signaling has been reported in stressful conditions, indicating a possible functional association with the pathophysiology of depression (Rethorst, Bernstein & Trivedi, 2014). NF-κB activation induces cytokine expression and regulates the inflammatory cascade (Li et al., 2013). It was also found to be associated with increased TNF-α expression in the hippocampus and frontal cortex in a chronic mild stress animal model (Jiang et al., 2013, Wang et al., 2018).
Etanercept is one of the most widely used anti-TNF-α agents and has been approved for the treatment of a variety of inflammatory diseases including rheumatoid arthritis, ankylosing spondylitis, psoriasis, and psoriatic arthritis (Caporali et al., 2009). Recent studies have shown that the administration of etanercept reduces anxiety and depressive-like behaviors in rodents (Bayramgürler et al., 2013, Camara et al., 2014). Inflammatory cytokines have the potential to induce sickness behavior that may later develop into depression-like symptoms (Adzic et al., 2018, Dantzer et al., 2008). Furthermore, inflammatory cytokines such as IL-6, IL-1β, and TNF-α have been found to be elevated in patients with depression. This suggests there is bidirectional communication between the peripheral inflammation and the central nervous system (Adzic et al., 2018, Dowlati et al., 2010).
In the present study, we examined whether in vivo blocking of peripheral TNF-α signaling would improve anxiety/depression-like behavior in db/db mice. Our data show that the peripheral administration of etanercept reduced anxiety and depressive-like behaviors, which was associated with a concomitant reduction in peripheral inflammation. This suggests that the peripheral administration of an anti-inflammatory agent could be useful in the modulation of obesity-associated anxiety and depressive-like behavior.
2. Materials and methods
2.1. Chemicals and antibodies
Etanercept was purchased from Enbrel (Enbrel®, MyClic-Germany). Heparin was purchased from Sigma-Aldrich, USA. Fluoroisothiocyanate (FITC), Phycoerythrin (PE), Allophycocyanin (APC) labeled and PE/Dazzle-labeled CD4, CD8, CD14, TLR-4, IL-17A, NF-κB, and TNF-α anti-mouse monoclonal antibodies, FcR blocking reagent, RBC’s lysing, fixation, and permeabilizing buffers were purchased from Miltenyi Biotech, Germany; BioLegend, Santa Cruz, Dallas; and BD Biosciences.
2.2. Animals
Adult male db/db mice (six-eight weeks old; Strain B6.BKS (D)-Leprdb/J, Stock No. 000697 B6 db) and age-matched non-diabetic lean control mice (littermate wild type) were included in the study. They were bred in the KSU animal care facility by mating heterozygous (Leprdb/+) males and females. Mice genotypes were confirmed by tail tissue and PCR analysis conducted by Transnetyx, Inc (Cordova, TN, USA). The mice were maintained at a room temperature of 22 °C ± 2 °C with a 12:12-h light/dark cycle and 40 to 60% humidity. They were housed in a specific pathogen-free environment, fed with standard rodent chow, and provided with water ad libitum. All experimental procedures were performed in accordance with the guidelines of the Institutional Research Ethics Committee (REC), King Saud University. To confirm the presence of diabetes in the models, the mice were made to fast for 6 h. Their blood glucose levels were then measured using 1–2 drops of blood collected from their tails on a glucometer chip (Accu-Check Advantage Blood Glucose Monitor, Roche Diagnostics Corp., Indianapolis, IN, USA). All db/db mice with a fasting blood glucose level >200 mg/dl were considered diabetic and included in the study (data not shown).
2.3. Experimental design and drug administration
The mice were acclimatized for two weeks and divided randomly into the following four groups: (1) control lean mice (littermate WT) given normal saline intraperitoneally (i.p.); (2) db/db mice + normal saline; (3) littermate WT mice + etanercept; and (4) db/db mice + etanercept. In line with Chio et al. (2013), a 5 mg/kg (i.p.) dose of etanercept was administered once every other day for 21 days. Each group consisted of six mice, and the amount administered was based on the body weight of each mouse. Following treatment, behavioral studies were conducted, after which the mice were anesthetized using a mixture of ketamine (50 mg/ml) (Tekam) and xylazine (20 mg/ml) (Seton) (dose 0.1 ml/10 gm i.p.). Mice blood was collected from the retro-orbital sinus.
2.4. Locomotor activity
Locomotor activity was determined using a Supermex apparatus, comprising a floor area 26 × 26 cm2 in size and a sensor monitor mounted above the chamber (Muromachi Kikai, Tokyo, Japan). As described in previous research (York, Blevins, McNeil & Freund, 2013), each mouse was kept in the Supermex apparatus for 10 min prior to the locomotor activity test to habituate them to the environment. They were then kept for another 10 min in the apparatus to record their locomotor activity. This was measured using activity-monitoring software (Any-Maze). All locomotor activity (distance traveled) was automatically scored and summed and the cut-off time for the test session was 1 min.
2.5. Open field test
An open field test was used to measure the anxiety-like behavior and locomotion of the mice, as described in previous research (Pietropaolo, 2010). All mice were carried to the test room in their home cages. Each mouse was housed in the apparatus 10 min prior to the open field test to habituate them to the environment. They were then placed in the center of the open field test apparatus and allowed to explore for 5 min, after which time they were returned to their cages. The cumulative time spent in corners by each mouse was calculated during the test period to assess anxiogenic behavior. The open field was then cleaned with 70% ethyl alcohol and permitted to dry between tests.
2.6. Light/dark exploration test
Light/dark (L/D) was used to assess anxiety-like behavior. The test apparatus consisted of a 42 × 21 × 25 cm3 box that was equally divided into light and dark compartments. The opening between these compartments was 4 cm wide and 3 cm high. As described in previous research (Bourin & Hascoët, 2003), each mouse was placed in the light compartment at the start of the experiment and its behavior examined for a period of 5 min. The cumulative time spent in the dark compartment by each mouse was calculated during the test period to assess anxiogenic behavior.
2.7. Forced swim test
In the forced swim test (FST), cylindrical Plexiglas water tanks 24 cm × 18 cm in size were used. Each tank contained sensors, a mercury thermometer, a paper towel, and heating pads. We used the Forced swimming test system (CompACT FSS ver. 2) to record data for a six-minute test session. The time spent immobile within the final 4 min was used to assess the depressive-like behavior of mice (Yankelevitch-Yahav, Franko, Huly & Doron, 2015).
2.8. Flow cytometric analysis
Blood was collected in heparinized tubes from the retro-orbital plexus to assess CD4, CD8, CD14, and TLR-4 surface markers. FITC- or PE-labeled CD4, PE- or FITC-labeled CD8, APC-labeled TLR-4, and PE/Dazzle-labeled CD14 (BioLegend, USA) monoclonal antibodies were used to determine the percentage of CD4+, CD8+, TLR-4+, and CD14+cell surface receptors, respectively. These were added to 100 µL of blood lysed with a lysing buffer (BioLegend, USA). Approximately 20 µL of the fluorescently labeled monoclonal antibodies were added to the suspension of blood leucocytes (CD4 FITC, CD8 PE, CD14 PE/Dazzle-labeled, and TLR-4 APC, BioLegend, USA). These were then incubated at room temperature for 30 min. To stain the intracellular markers IL-17A, NF-κB p65, and TNF-α, the cells were permeabilized, subjected to fixation by standard buffers (BioLegend, USA), and stained with intracellular APC-IL-17A, FITC-NF-κB p65, and PE-TNF-α antibodies (BioLegend, USA; Santa Cruz, Dallas, USA), (Ahmad, Attia, Zoheir, Ashour & Bakheet, 2014). After centrifugation at 300g for 10 min, the samples were kept at 4 °C until flow cytometry analysis was conducted. Immunostained leukocytes in each sample were acquired using an FC 500 flow cytometer; 10,000 events/sample were then analyzed using CXP software (Beckman Coulter, USA).
2.9. Statistical analysis
Data are presented as mean ± SEM. Data were analyzed using a one-way ANOVA with Dunnett’s post hoc test for multiple comparisons on GraphPad Prism 5 (GraphPad Software, San Diego, USA). The level of statistical significance was set at p < 0.05.
3. Results
3.1. Etanercept reversed anxiety-like behavior in db/db mice
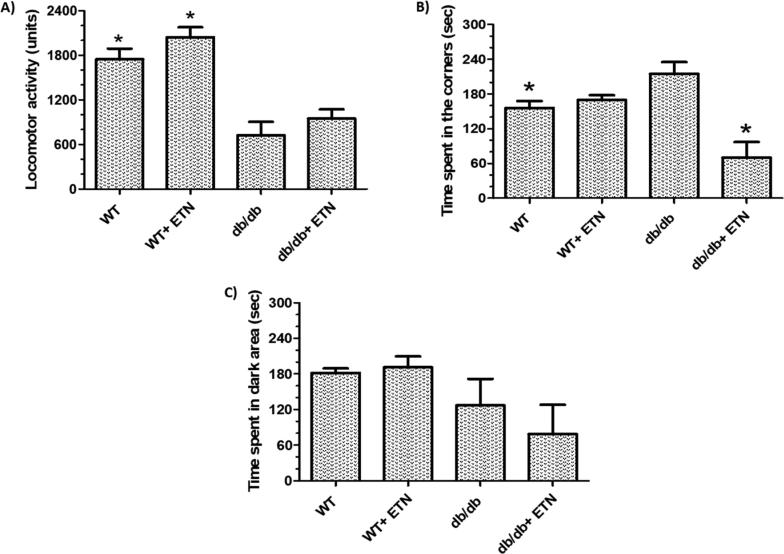
Because obesity is linked with anxiety and depression, we examined whether the reduction in obesity-associated inflammation would ameliorate anxiety like-behavior in db/db mice. Our data show there was an increase in anxiety-like behavior in these mice, as reflected by a significant decrease in locomotor activity (Fig. 1A). Furthermore, time spent in the corner by db/db mice significantly increased during the open field test (Fig. 1B). However, there was no significant difference between db/db and littermate WT mice in their exploration of the dark area in the light-dark box (Fig. 1C). These observations reversed significantly in the open field test following etanercept treatment in db/db mice, and there was an upward trend in the locomotor activity test. Notably, the administration of etanercept did not affect the performance of treated littermate WT mice compared with the control group (normal saline group; Fig. 1). These results suggest that the inhibition of TNF-α signaling could be responsible for the reversal of obesity-induced behavioral changes. This indicates that a functional link may exist between obesity, inflammation, and anxiety-like phenotypes.
Fig. 1.
Anxiety-related behavioral tests in db/db, littermate WT mice, and Etanercept-treated mice. (A) Locomotor activity test, (B) Open field test, and (C) Light/dark box test. The four groups of mice were: littermate WT + saline, db/db + saline, littermate WT + 5 mg/kg ETN, and db/db mice + 5 mg/kg ETN; i.p. an injection every other day for 21 days. *P < 0.05 compared with db/db saline-treated mice. Data are shown as mean ± SEM (n = 6). Littermate WT = lean control mice, ETN = etanercept treated mice.
3.2. Etanercept reduced depressive-like behavior in db/db mice
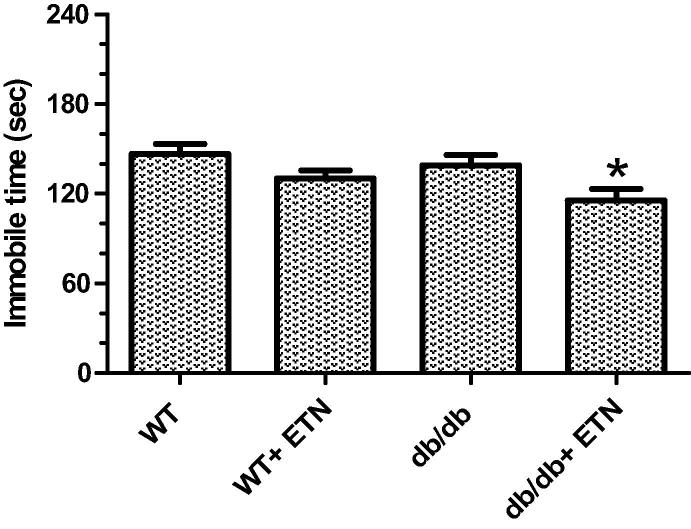
We also examined depressive-like behavior using the forced swim test. The results indicate that the administration of etanercept reduced immobility time in etanercept-treated db/db mice (Fig. 2).
Fig. 2.
Results of a forced swim-test to examine depressive-like behavior in four groups of mice: littermate WT + saline, db/db + saline, littermate WT + 5 mg/kg ETN, and db/db mice + 5 mg/kg ETN; i.p. an injection every other day for 21 days. *P < 0.05 compared with littermate WT saline-treated mice. Data are shown as mean ± SEM (n = 6). Littermate WT = lean control mice, ETN = etanercept treated mice.
3.3. Etanercept reversed the increase in IL-17A+ and IL-17A-producing CD4+ and CD8+T cells peripherally in db/db mice
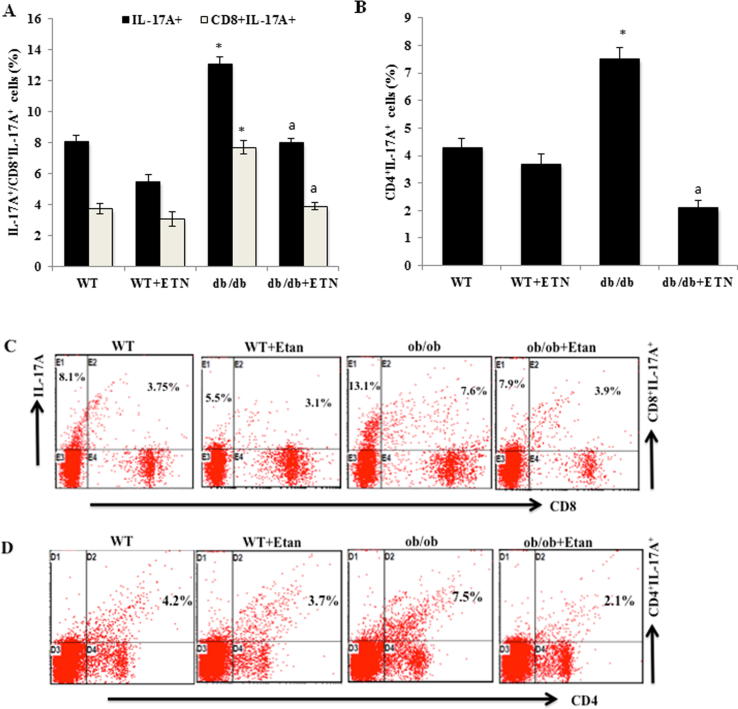
Because IL-17A has been linked with both anxiety and depressive disorders, we evaluated the effect of TNF blockade on IL-17A expression in CD4+ and CD8+ T cells of peripheral circulation. Our data showed significantly elevated IL-17A expression in both CD4+ and CD8+ T cells of untreated db/db mice compared with untreated littermate WT mice (Fig. 3A and B). However, there was significant attenuation of IL-17A expression in CD4+ and CD8+ T cells in etanercept-treated db/db mice compared with untreated db/db mice (Fig. 3A and B), as reflected by dot plots in Fig. 3C and D, respectively.
Fig. 3.
Etanercept reversed the increase in IL-17A + and IL-17A-producing CD4 + and CD8 + T cells peripherally in db/db mice. A and B. The effects of ETN on IL-17A+ and IL-17A- expressing CD4+ and CD8+ T cells in the peripheral blood (analyzed using flow cytometry). C. Representative dot plots for one mouse from each group. *P < 0.05 compared with db/db saline-treated mice. Data are shown as mean ± SEM (n = 4).
3.4. Etanercept reduced NF-κB p65 in db/db mice
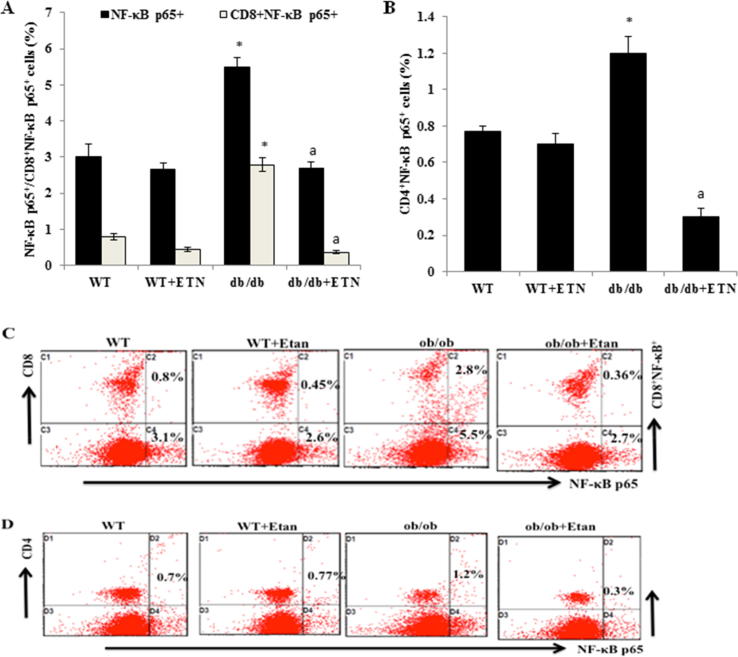
We further explored the effect of etanercept on NF-κB p65 in db/db and littermate WT mice. We found higher levels of NF-κB p65+ and NF-κB p65- expressing CD4+ and CD8+ T cells in untreated db/db mice than in littermate WT mice (Fig. 4A and B). By contrast, when db/db mice were treated with etanercept, there was a significant decrease in NF-κB p65+ and NF-κB p65-expressing CD4+ and CD8+ T cells compared with untreated db/db mice (Fig. 4A and B), as reflected by dot plots in Fig. 4C and D respectively. Etanercept-treated db/db mice also exhibited significantly decreased NF-κB p65 expression compared with untreated db/db mice (Fig. 4A).
Fig. 4.
Etanercept reduced NF-κBp65 expressing CD4 + and CD8 + T cells peripherally in db/db mice. A and B. The effects of ETN on NF-κBp65+ and NF-κBp65+-expressing CD4+ and CD8+ T cells in the peripheral blood (analyzed using flow cytometry). C. Representative dot plots for one mouse from each group using flow-cytometric studies. *P < 0.05 compared with db/db saline-treated mice. Data are shown as mean ± SEM (n = 4).
3.5. Etanercept normalized TNF-α in db/db mice
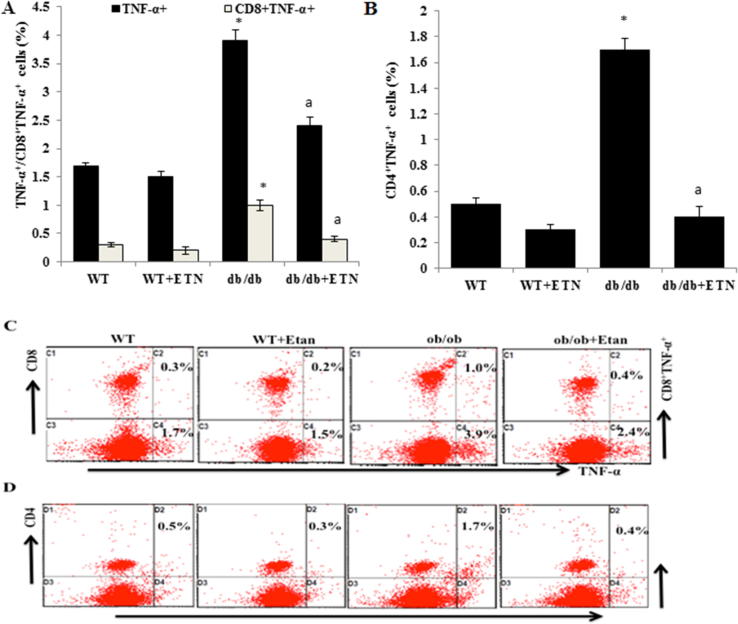
We further assessed the effect of etanercept on the expression of TNF-α in peripheral blood. As shown in Fig. 5A, there was a higher level of TNF-α -producing CD4+ and CD8+ T cells in untreated db/db mice than in littermate WT mice. Moreover, db/db mice treated with etanercept exhibited a significant decrease in TNF-α -producing CD4+ and CD8+ T cells compared with untreated db/db mice (Fig. 5A and B), as reflected by dot plots in Fig. 5C and D, respectively. Together, these results suggest that inflammatory signaling was increased peripherally in db/db mice. This could be a factor contributing to the anxiety/depressive-like behaviors observed in db/db mice and can be attenuated by treatments with anti-inflammatory agents.
Fig. 5.
Etanercept reduced TNF-α+ and TNF-α- expressing CD4 + and CD8 + T cells peripherally in db/db mice. A and B. The effects of ETN on TNF-α+ and TNF-α- expressing CD4+ and CD8+ T cells in the peripheral blood (analyzed using flow cytometry). 6C. Representative dot plots for one mouse from each group. *P < 0.05 compared with db/db saline-treated mice. Data are shown as mean ± SEM (n = 4).
3.6. Etanercept mitigated TLR-4 signaling
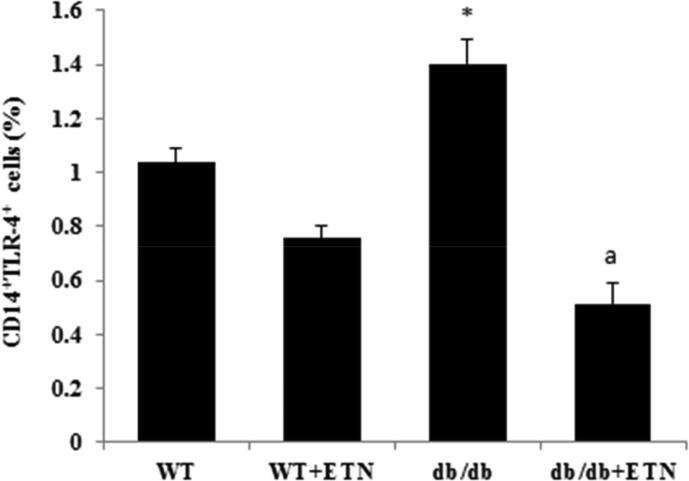
We further investigated the effect of etanercept on TLR-4-expressing CD14+ cells. Untreated db/db mice exhibited an increase in TLR-4-expressing CD14+ cells in the peripheral blood compared with untreated littermate WT; this was significantly mitigated by the administration of etanercept (Fig. 6A). These results suggest that obesity-linked inflammation plays an essential role in the upregulation of TLR-4 in the periphery of db/db mice. Blocking TNF-α signaling could therefore be responsible for the amelioration of anxiety/depressive-like behavior as it reduces TLR-4 signaling.
Fig. 6.
The effects of ETN on TLR-4-producing CD14+ cells in the peripheral blood (analyzed using flow cytometry). *P < 0.05 compared with db/db saline-treated mice. Data are shown as mean ± SEM (n = 4).
4. Discussion
The main objective of this study was to examine whether a reduction in systemic inflammation using etanercept (a TNF-α inhibitor) has the potential to improve obesity-associated mood dysregulations such as anxiety/depression. Our results showed that systemic treatment with etanercept reduced both anxiety and depressive-like behavior as well as peripheral inflammation in db/db mice. This suggests there is a strong link between obesity, inflammation, and mood disorders.
Stress could be a significant contributory factor in the development of diabetes and metabolic disorders (Castaneda et al., 2011, Trento et al., 2010). A preclinical post-traumatic stress disorder study indicated a significant impairment in glucose tolerance (Castaneda et al., 2011, Lin et al., 2004). The prevalence of depression in diabetic patients is approximately double that of non-diabetic individuals (Anderson, Freedland, Clouse & Lustman, 2001). Previous studies have established a link between chronic systemic inflammation, diabetes, insulin resistance, and major depressive disorders (Steckhan et al., 2016). Furthermore, systemic leptin treatment has been found to lower immobility in rodents in FST and tail suspension tests, reflecting the antidepressant-like effect of leptin (Lu, Kim, Frazer & Zhang, 2006). Asakawa et al. (2003) also reported that leptin treatment could ameliorate anxiety-like behavior. In our study, we investigated anxiety and depressive-like behavior in db/db mice using FST, light-dark exploration, and open field tests. Increased immobility in FST, as well as a decrease in locomotor activity, have been previously reported in obese mice (Collin, Hakansson-Ovesjo, Misane, Ogren & Meister, 2000). During the open field test, db/db mice exhibited a decrease in basic movements compared with littermate WT controls. Similar changes in the locomotor behavior of db/db mice have been reported in previous studies (Hesse et al., 2010). Furthermore, hypo-locomotion activity has been observed in association with psychiatric disorders (Collin et al., 2000, Crawley et al., 1997). Previous studies have shown that db/db mice exhibit depressive and anxiety phenotypes (Asakawa et al., 2003, Dinel et al., 2011, Guo and Lu, 2014, Sharma et al., 2010). In the present study there was no increase in immobility time in the FST among db/db mice; however, this could be because six animals were used which, in behavioral tests, may be a confounding factor.
Etanercept is a TNF-α inhibitor that, due to its molecular weight, does not cross the blood-brain barrier (Chang et al., 2017). Nevertheless, the peripheral administration of etanercept has been shown to reduce the symptoms of depression (Tyring et al., 2006). In line with this finding, studies have indicated that etanercept modulates neuronal inflammation (Kerfoot et al., 2006) and normalizes corticosterone induced synaptic changes (Brymer, Fenton, Kalynchuk & Caruncho, 2018). These central effects can be achieved indirectly by changing the level of circulating cytokines and inflammatory mediators. Research conducted by Campbell and his colleagues (2007) showed that the peripheral administration of etanercept significantly reduced neuronal inflammation in a rat model of brain trauma. They also identified a correlation between these reductions and the hepatic synthesis of circulatory chemokines and neutrophil. In the current study, etanercept decreased immobility time in FST and reduced the time spent in corners in the open field test. These results suggest a reversal of depression/anxiety-like behavior in db/db mice as a result of administering etanercept.
Obesity is characterized by metabolic dysfunctions and is associated with peripheral as well as neuronal inflammation (Capuron, Lasselin & Castanon, 2017). Previous studies have shown that increased systemic or neuronal inflammation is linked to neurobehavioral deficits, including anxiety-related symptoms in chronic inflammatory conditions (Chen et al., 2015). Similarly, secreted pro-inflammatory cytokines induce neuroinflammation by impacting neurocircuitry, thereby leading to emotional/mood dysregulations (Miller, Haroon, Raison & Felger, 2013). Furthermore, the administration of anti-inflammatory agents appears to exert beneficial effects on depression and anxiety as evidenced by a reduction in anxiety-like behaviors in animal models of inflammatory diseases (Lee et al., 2018). Although the neuropsychological consequences of diabetes have been studied in considerable detail in clinical settings, there is a dearth of preclinical research identifying correlations between neuropsychological consequences and diabetes.
Previous studies have confirmed that stress/depression is associated with elevated systemic and neuronal inflammation, suggesting a crucial role for inflammation in the etiology of depression (Slavich & Irwin, 2014). IL-17A has also been found to play an essential role in neuroinflammatory diseases (Jadidi-Niaragh & Mirshafiey, 2011). Several studies have found that inflammation in the periphery could affect brain function, thereby causing the pathological changes associated with depression (Beurel et al., 2013, Waisman et al., 2015). Moreover, IL-17A has been found to be strongly associated with obesity-related systemic and adipose tissue inflammation (Chehimi, Vidal & Eljaafari, 2017). Therefore, IL-17A may be a common link between obesity-induced forms of depression. Based on these observations, we examined the effect of etanercept on IL-17A expression in db/db mice. We recorded a significant downregulation of IL-17A-producing CD4+ and CD8+ T cells following etanercept administration. The results support our hypothesis that etanercept may be a beneficial therapy for the anxiety/depression symptoms associated with obesity.
We further observed that the db/db mice exhibited a substantial increase in TNF-α-producing CD4+ and CD8+ T cells in the blood, and that this effect was reduced following etanercept treatment. TNF-α induces the development of anxiety-like behavior in normal and obese mice (Andre et al., 2014, Jia et al., 2007, Takeuchi et al., 2006). In line with this finding, inhibition of TNF-α signaling by fluoxetine has been shown to improve depression-like symptoms (Todorovic & Filipovic, 2017). The TNF-α antagonist has also been found to reduce anxiety-like behavior in mice (Chen et al., 2013, Jia et al., 2007). Our results provide valuable evidence to suggest that a blockade of TNF-α signaling reduces obesity-associated inflammation as well as depression in db/db mice.
A previous study by Kassed and Herkenham (2004) showed that a deficiency in NF-κB signaling reduces anxiety-like behaviors in a mouse model. Another study by Bingham (2002) demonstrated that therapeutic targeting of NF-κB signaling reduces the production of pro-inflammatory mediators. In the current study, we found that NF-κB expression in CD4+/CD8+ T cells significantly increases in db/db mice but can be downregulated by treatment with etanercept in the periphery. These results provide evidence to show that a blockade of TNF-α by etanercept may reduce systemic NF-κB activation. This might explain the potent anti-inflammatory effects of etanercept.
Recent data have reported that TLRs perform a critical function in learning and memory processes (Femenia et al., 2018). TLR-4 is involved in a multitude of physiological processes within the CNS that regulate stress/depression, including neuroinflammation and hippocampal neurogenesis (Liu et al., 2014, Potter et al., 2019). Previous studies have investigated the role played by TLR-4 in the regulation of anxiety-like behavior and sociability (Femenia et al., 2018). Another study by Shannonhouse et al. (2014) found that elevated TLR-4 expression in the hypothalamus was associated with an increase in anxiety-like behavior and stress responses. These findings indicate that dysfunction in TLR-4 signaling may contribute to systemic/neuronal inflammation as well as anxiety-like behavior in db/db mice. Therefore, etanercept-mediated reduction in TLR-4 signaling could be one of the factors that ameliorate anxiety/depression-like symptoms. Antidepressant treatment in patients with major depressive disorder has also been shown to normalize the expression of different TLRs, including TLR-4 (Hung, Huang, Kang, Huang & Huang, 2016).
5. Conclusion
The current study showed that increased systemic inflammation (as reflected by increased TNF-α/TLR-4 /NF-κB/IL-17A signaling) is associated with anxiety-like behavior in db/db mice. The blockade of TNF-α signaling by etanercept improved anxiety and depression-like behavior in db/db mice through the suppression of multiple inflammatory pathways. Therefore, our data suggest that a systemic blockade of TNF-α signaling by etanercept may be a promising approach to the treatment of obesity-associated anxiety/depression.
Funding
This research project was supported by a Research Initiative from the Prince Naif bin AbdulAziz Health Research Center, King Saud University Medical City.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors acknowledge the help of the staff and use of the facilities of the Molecular and Cell Biology (MCB) Laboratory, a core research facility of the King Saud University (College of Dentistry) in collaboration with Prince Naif bin AbdulAziz Health Research Center.
Author contributions
M.A.A., M.R.K., S.F.A., A. N., and T.K.A: contributed to the design of the work; the acquisition, analysis, and interpretation of the data; and drafted the manuscript. M.A.A. and M.R.K: prepared mouse samples and supervised and maintained the animal colony and animal genotyping in the laboratory. A.O.A: maintained the animal colony and animal genotyping in the laboratory. M. A. A., H. M. M., and M. R. K: contributed to behavioral experiments and behavioral data analysis. S.D.A: allowed behavioral experiments to be performed in his laboratory and provided intellectual support. S.F.A, A. N: Performed Flow cytometry. F.F.A., F. M. A., A. F. A., A.A: provided intellectual support and contributed to editing the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adzic M., Brkic Z., Mitic M., Francija E., Jovicic M.J., Radulovic J., Maric N.P. Therapeutic strategies for treatment of inflammation-related depression. Curr. Neuropharmacol. 2018;16(2):176–209. doi: 10.2174/1570159X15666170828163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S.F., Attia S.M., Zoheir K.M.A., Ashour A.E., Bakheet S.A. Attenuation of the progression of adjuvant-induced arthritis by 3-aminobenzamide treatment. Int. Immunopharmacol. 2014;19(1):52–59. doi: 10.1016/j.intimp.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Anderson R.J., Freedland K.E., Clouse R.E., Lustman P.J. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Andre C., Dinel A.L., Ferreira G., Laye S., Castanon N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain Behav. Immun. 2014;41:10–21. doi: 10.1016/j.bbi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Asakawa, A., Inui, A., Inui, T., Katsuura, G., Fujino, M. A., Kasuga, M., 2003. Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diabetes Complications, 17(2), 105-107. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12614977. [DOI] [PubMed]
- Bai Y.-M., Chiou W.-F., Su T.-P., Li C.-T., Chen M.-H. Pro-inflammatory cytokine associated with somatic and pain symptoms in depression. J. Affect. Disord. 2014;155:28–34. doi: 10.1016/j.jad.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Bayramgürler D., Karson A., Özer C., Utkan T. Effects of long-term etanercept treatment on anxiety- and depression-like neurobehaviors in rats. Physiol. Behav. 2013;119:145–148. doi: 10.1016/j.physbeh.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Beurel E., Harrington L.E., Jope R.S. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol. Psychiatry. 2013;73(7):622–630. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham, C. O., 3rd. (2002). The pathogenesis of rheumatoid arthritis: pivotal cytokines involved in bone degradation and inflammation. J. Rheumatol. Suppl., 65, 3–9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12236620. [PubMed]
- Bourin M., Hascoet M. The mouse light/dark box test. Eur. J. Pharmacol. 2003;463:55–65. doi: 10.1016/S0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Brumpton B., Langhammer A., Romundstad P., Chen Y., Mai X.M. The associations of anxiety and depression symptoms with weight change and incident obesity: the HUNT study. Int. J. Obes. (Lond) 2013;37(9):1268–1274. doi: 10.1038/ijo.2012.204. [DOI] [PubMed] [Google Scholar]
- Brymer K.J., Fenton E.Y., Kalynchuk L.E., Caruncho H.J. Peripheral etanercept administration normalizes behavior, hippocampal neurogenesis, and hippocampal reelin and GABAA receptor expression in a preclinical model of depression. Front. Pharmacol. 2018;9(121) doi: 10.3389/fphar.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara, M.l., Corrigan, F., Jaehne, E. J., Jawahar, M.C., Anscomb, H., Baune, B.T., 2014. Effects of centrally administered etanercept on behavior, microglia, and astrocytes in mice following a peripheral immune challenge. Neuropsychopharmacology, 40(2), 502–512. doi: 10.1038/npp.2014.199. [DOI] [PMC free article] [PubMed]
- Campbell S.J., Jiang Y., Davis A.E.M., Farrands R., Holbrook J., Leppert D., Anthony D.C. Immunomodulatory effects of etanercept in a model of brain injury act through attenuation of the acute-phase response. J. Neurochem. 2007;103(6):2245–2255. doi: 10.1111/j.1471-4159.2007.04928.x. [DOI] [PubMed] [Google Scholar]
- Caporali R., Pallavicini F.B., Filippini M., Gorla R., Marchesoni A., Favalli E.G., Montecucco C. Treatment of rheumatoid arthritis with anti-TNF-alpha agents: a reappraisal. Autoimmun. Rev. 2009;8(3):274–280. doi: 10.1016/j.autrev.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Capuron L., Lasselin J., Castanon N. Role of adiposity-driven inflammation in depressive morbidity. Neuropsychopharmacology. 2017;42(1):115–128. doi: 10.1038/npp.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda T.R., Nogueiras R., Muller T.D., Krishna R., Grant E., Jones A., Tschop M.H. Decreased glucose tolerance and plasma adiponectin:resistin ratio in a mouse model of post-traumatic stress disorder. Diabetologia. 2011;54(4):900–909. doi: 10.1007/s00125-010-2019-y. [DOI] [PubMed] [Google Scholar]
- Chang R., Knox J., Chang J., Derbedrossian A., Vasilevko V., Cribbs D., Sumbria R.K. Blood-brain barrier penetrating biologic TNF-alpha inhibitor for Alzheimer's disease. Mol Pharm. 2017;14(7):2340–2349. doi: 10.1021/acs.molpharmaceut.7b00200. [DOI] [PubMed] [Google Scholar]
- Chehimi M., Vidal H., Eljaafari A. Pathogenic role of IL-17-producing immune cells in obesity, and related inflammatory diseases. J. Clin. Med. 2017;6(7) doi: 10.3390/jcm6070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Song Y., Yang J., Zhang Y., Zhao P., Zhu X.J., Su H.C. The contribution of TNF-alpha in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci. Lett. 2013;541:275–280. doi: 10.1016/j.neulet.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Chen J., Winston J.H., Fu Y., Guptarak J., Jensen K.L., Shi X.Z., Sarna S.K. Genesis of anxiety, depression, and ongoing abdominal discomfort in ulcerative colitis-like colon inflammation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308(1):R18–R27. doi: 10.1152/ajpregu.00298.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio C.C., Chang C.H., Wang C.C., Cheong C.U., Chao C.M., Cheng B.C., Chang C.P. Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-alpha. BMC Neurosci. 2013;14:33. doi: 10.1186/1471-2202-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, M., Hakansson-Ovesjo, M. L., Misane, I., Ogren, S. O., Meister, B., 2000. Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Brain Res. Mol. Brain Res., 81(1–2), 51-61. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11000478. [DOI] [PubMed]
- Crawley, J. N., Belknap, J. K., Collins, A., Crabbe, J. C., Frankel, W., Henderson, N., . . . Paylor, R. (1997). Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl), 132(2), 107–124. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9266608. [DOI] [PubMed]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes A.J., Maggard-Gibbons M., Maher A.R., Booth M.J., Miake-Lye I., Beroes J.M., Shekelle P.G. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysismental health conditions among bariatric surgery PatientsMental health conditions among bariatric surgery patients. JAMA. 2016;315(2):150–163. doi: 10.1001/jama.2015.18118. [DOI] [PubMed] [Google Scholar]
- Dinel A.L., Andre C., Aubert A., Ferreira G., Laye S., Castanon N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS ONE. 2011;6(9):e24325. doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctot K.L. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Femenia T., Qian Y., Arentsen T., Forssberg H., Diaz Heijtz R. Toll-like receptor-4 regulates anxiety-like behavior and DARPP-32 phosphorylation. Brain Behav. Immun. 2018;69:273–282. doi: 10.1016/j.bbi.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Guo M., Lu X.Y. Leptin receptor deficiency confers resistance to behavioral effects of fluoxetine and desipramine via separable substrates. Transl. Psychiatry. 2014;4:e486. doi: 10.1038/tp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse D., Dunn M., Heldmaier G., Klingenspor M., Rozman J. Behavioural mechanisms affecting energy regulation in mice prone or resistant to diet- induced obesity. Physiol. Behav. 2010;99(3):370–380. doi: 10.1016/j.physbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Hung Y.Y., Huang K.W., Kang H.Y., Huang G.Y., Huang T.L. Antidepressants normalize elevated Toll-like receptor profile in major depressive disorder. Psychopharmacology. 2016;233(9):1707–1714. doi: 10.1007/s00213-015-4087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadidi-Niaragh F., Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand. J. Immunol. 2011;74(1):1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- Jia D., Gao G.D., Liu Y., He S.M., Zhang X.N., Zhang Y.F., Zhao M.G. TNF-alpha involves in altered prefrontal synaptic transmission in mice with persistent inflammatory pain. Neurosci. Lett. 2007;415(1):1–5. doi: 10.1016/j.neulet.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Jiang H., Wang Z., Wang Y., Xie K., Zhang Q., Luan Q., Liu D. Antidepressant-like effects of curcumin in chronic mild stress of rats: Involvement of its anti-inflammatory action. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;47:33–39. doi: 10.1016/j.pnpbp.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Kassed C.A., Herkenham M. NF-kappaB p50-deficient mice show reduced anxiety-like behaviors in tests of exploratory drive and anxiety. Behav. Brain Res. 2004;154(2):577–584. doi: 10.1016/j.bbr.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Kerfoot S.M., D'Mello C., Nguyen H., Ajuebor M.N., Kubes P., Le T., Swain M.G. TNF-alpha-secreting monocytes are recruited into the brain of cholestatic mice. Hepatology. 2006;43(1):154–162. doi: 10.1002/hep.21003. [DOI] [PubMed] [Google Scholar]
- Lee V.K., Hosking B.M., Holeniewska J., Kubala E.C., Lundh von Leithner P., Gardner P.J., Shima D.T. BTBR ob/ob mouse model of type 2 diabetes exhibits early loss of retinal function and retinal inflammation followed by late vascular changes. Diabetologia. 2018;61(11):2422–2432. doi: 10.1007/s00125-018-4696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Zhang N., Cao Y., Zhang W., Su G., Sun Y., Yang Z. Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-κB and MAPKs signal pathways. Eur. J. Pharmacol. 2013;705(1–3):79–85. doi: 10.1016/j.ejphar.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Lin E.H., Katon W., Von Korff M., Rutter C., Simon G.E., Oliver M., Young B. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- Liu J., Buisman-Pijlman F., Hutchinson M.R. Toll-like receptor 4: innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Front Neurosci. 2014;8:309. doi: 10.3389/fnins.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X.Y., Kim C.S., Frazer A., Zhang W. Leptin: a potential novel antidepressant. Proc. Natl. Acad. Sci. USA. 2006;103(5):1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R.A., Tesfaye S., Ziegler D. Medical strategies to reduce amputation in patients with Type 2 diabetes. Diabet. Med. 2013;30(8):893–900. doi: 10.1111/dme.12169. [DOI] [PubMed] [Google Scholar]
- Mantzoros C.S., Moschos S., Avramopoulos I., Kaklamani V., Liolios A., Doulgerakis D.E., Flier J.S. Leptin concentrations in relation to body mass index and the tumor necrosis factor-α system in humans1. J Clin Endocrinol Metabol. 1997;82(10):3408–3413. doi: 10.1210/jcem.82.10.4323. [DOI] [PubMed] [Google Scholar]
- Martinez-Botas J., Anderson J.B., Tessier D., Lapillonne A., Chang B.H.-J., Quast M.J., Chan L. Absence of perilipin results in leanness and reverses obesity in Leprdb/db mice. Nat. Genet. 2000;26(4):474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- Miller A.H., Haroon E., Raison C.L., Felger J.C. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30(4):297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S., Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor κ B activity in the brain during endotoxemia. J. Neurosci. 2000;20(9):3456–3468. doi: 10.1523/jneurosci.20-09-03456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien P.D., Hur J., Hayes J.M., Backus C., Sakowski S.A., Feldman E.L. BTBR ob/ob mice as a novel diabetic neuropathy model: Neurological characterization and gene expression analyses. Neurobiol. Disease. 2015;73:348–355. doi: 10.1016/j.nbd.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaolo, S., 2010. Mood and anxiety-related phenotypes in mice: characterization using behavioral tests – Edited by T. D. Gould. Genes, Brain and Behavior, 9(5), 544–544. doi: 10.1111/j.1601-183X.2010.00592.x.
- Potter O.V., Giedraitis M.E., Johnson C.D., Cox M.N., Kohman R.A. Young and aged TLR4 deficient mice show sex-dependent enhancements in spatial memory and alterations in interleukin-1 related genes. Brain Behav. Immun. 2019;76:37–47. doi: 10.1016/j.bbi.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethorst C.D., Bernstein I., Trivedi M.H. Inflammation, obesity, and metabolic syndrome in depression. J. Clin. Psychiatry. 2014;75(12):e1428–e1432. doi: 10.4088/jcp.14m09009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannonhouse J.L., Fong L.A., Clossen B.L., Hairgrove R.E., York D.C., Walker B.B., Morgan C. Female-biased anorexia and anxiety in the Syrian hamster. Physiol. Behav. 2014;133:141–151. doi: 10.1016/j.physbeh.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Sharma A.N., Elased K.M., Garrett T.L., Lucot J.B. Neurobehavioral deficits in db/db diabetic mice. Physiol. Behav. 2010;101(3):381–388. doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.N., Elased K.M., Lucot J.B. Rosiglitazone treatment reversed depression- but not psychosis-like behavior of db/db diabetic mice. J. Psychopharmacol. 2012;26(5):724–732. doi: 10.1177/0269881111434620. [DOI] [PubMed] [Google Scholar]
- Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 2014;140(3):774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckhan N., Hohmann C.D., Kessler C., Dobos G., Michalsen A., Cramer H. Effects of different dietary approaches on inflammatory markers in patients with metabolic syndrome: a systematic review and meta-analysis. Nutrition. 2016;32(3):338–348. doi: 10.1016/j.nut.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Jin S., Wang J., Zhang G., Kawanokuchi J., Kuno R., Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006;281(30):21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- Todorovic N., Filipovic D. The antidepressant- and anxiolytic-like effects of fluoxetine and clozapine in chronically isolated rats involve inhibition of hippocampal TNF-alpha. Pharmacol. Biochem. Behav. 2017;163:57–65. doi: 10.1016/j.pbb.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Trento M., Kucich C., Tibaldi P., Gennari S., Tedesco S., Balbo M., Porta M. A study of central serotoninergic activity in healthy subjects and patients with Type 2 diabetes treated by traditional one-to-one care or Group Care. J. Endocrinol. Invest. 2010;33(9):624–628. doi: 10.3275/681510.1007/BF03346660. [DOI] [PubMed] [Google Scholar]
- Tyring S., Gottlieb A., Papp K., Gordon K., Leonardi C., Wang A., Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. The Lancet. 2006;367(9504):29–35. doi: 10.1016/s0140-6736(05)67763-x. [DOI] [PubMed] [Google Scholar]
- Vincent A.M., Callaghan B.C., Smith A.L., Feldman E.L. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nature Reviews Neurology. 2011;7(10):573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- Waisman A., Hauptmann J., Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015;129(5):625–637. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]
- Wang B., Chandrasekera P., Pippin J. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr. Diabet. Rev. 2014;10(2):131–145. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu J., Liu Y., Li Z., Li X. TLR4-NF-κB signal involved in depressive-like behaviors and cytokine expression of frontal cortex and hippocampus in stressed C57BL/6 and ob/ob Mice. Neural Plasticity. 2018;2018:1–12. doi: 10.1155/2018/7254016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112(12):1796–1808. doi: 10.1172/jci19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankelevitch-Yahav, R., Franko, M., Huly, A., Doron, R., 2015. The Forced Swim Test as a Model of Depressive-like Behavior, J. Visualized Experiments (97). doi: 10.3791/52587. [DOI] [PMC free article] [PubMed]
- York, J.M., Blevins, N.A., McNeil, L.K., Freund, G.G., 2013. Mouse short- and long-term locomotor activity analyzed by video tracking software, J Visual Exp (76). doi: 10.3791/50252. [DOI] [PMC free article] [PubMed]