Abstract
Herein, we designed a novel gastroretentive drug delivery system as floating matrix tablets based on a polysaccharide material from linseeds (Linum usitatissimum L.) for fluoroquinolone antibiotics. A number of formulations were designed with a combination of linseed hydrogel (LSH) and different excipients to obtain a desired sustained release profile of moxifloxacin. The drug release study was performed basically at pH 1.2. However, the tablet may pass through the stomach to intestine due to certain reasons then it also offered sustained drug release at intestinal pH 4.5, 6.8 and 7.4, as well. Results indicated that sustained moxifloxacin release was directly proportional to the concentration of LSH and the release of drug followed non-Fickian diffusion. SEM of the tablets indicated porous nature of LSH with elongated channels which contributed to the swelling of the tablet and then facilitated the discharge of moxifloxacin from the core of the tablet. In vivo X-ray study was performed to assess disintegration and real-time floating of tablet that confirmed its presence for 6 h in the stomach. These findings indicated that LSH can be used to develop novel gastroretentive sustained release drug delivery systems with the double advantage of sustained drug release at all pH of GIT.
Keywords: Linseed hydrogel, Sustained release, Gastroretentive, Oral drug delivery, Moxifloxacin
1. Introduction
In the orally administered dosage forms, the pH of the gastrointestinal tract (GIT) has played a major role in the absorption of the drugs. Most of the drugs are absorbed from the upper part of the GIT (stomach, duodenum and jejunum), therefore, the retention time of the dosage form in these parts plays a vital role in the therapeutic efficacy of the drugs (Awasthi and Kulkarni, 2016). One approach to increase the absorption and bioavailability of the drug is to increase the retention time in the upper part of the GIT. Different techniques are employed to retain the dosage forms in the stomach (Waterman, 2007), i.e., floating or low-density dosage forms (Rao et al., 2013, Singh and Kim, 2000), mucoadhesive (Mansuri et al., 2016), high-density forms (Mandal et al., 2016), expansion of dosage forms by swelling or unfolding of the excipients (Klausner et al., 2003) and modified shape systems (Urguhart et al., 1994), etc. These gastro-retentive drug delivery systems have shown many pharmacodynamics and pharmacokinetics advantages (Hwang et al., 1998, Hoffman and Stepensky, 1999).
Naturally occurring polysaccharides exhibited high swelling indices at various physiological pH and stimuli-responsive swelling/deswelling against different solvent systems (Haseeb et al., 2016, Muhammad et al., 2016, Ashraf et al., 2017), hence, drawn the attention of the researcher to use these polysaccharides in various biomedical fields (Lodhi et al., 2019, Ashraf et al., 2018, Shelke et al., 2014, Imre and Pukanszky, 2015). Flax is one of the oldest crops and its seeds (flaxseeds/linseeds) are the rich source of carbohydrates (15–20%) (Barbary et al., 2009, Kaewmanee et al., 2014). Upon soaking in hot water, linseeds excreted mucilage which is mainly composed of rhamnogalacturonans (higher molecular weight fraction (1510 kDa) and lower molecular weight fraction (341 kDa)) and arabinoxylan (Cui et al., 1994, Qian et al., 2012a, Qian et al., 2012b). Hot water extracted linseed hydrogel/mucilage (LSH) is considered a safe excipient (Haseeb et al., 2018) as well as exhibiting diverse biomedical applications such as reducing and capping agent for the synthesis of silver nanoparticles and incorporated in wound healing dressing (Haseeb et al., 2017a), stimuli-responsive oral sustained release drug delivery system (Haseeb et al., 2017b), development of nanoparticle carrier system for anticancer drug (Hseeb et al., 2019), mucoadhesive beads (Hasnain et al., 2018), nasal gel (Basu et al., 2007) and microspheres (Nerkar et al., 2011).
Literature (Haseeb et al., 2016, Haseeb et al., 2017) indicates that LSH has shown negligible swelling at pH 1.2, therefore, our aim was to design LSH-based sustained release gastroretentive drug delivery system for fluoroquinolone antibiotics with the help of a channeling agent (β-cyclodextrin) and swelling agent (HPMC). Fluoroquinolones are soluble at acidic pH and may precipitate at neutral or slightly basic pH of the intestine which adversely affects the absorption and bioavailability of these drugs. To overcome such problems associated with acidic soluble drugs, we selected moxifloxacin as a model drug for the development of gastroretentive controlled/sustained release drug delivery system with a main focus to release the active ingredient in the acidic environment (upper part of the GIT, i.e., stomach) for a maximum period of time. Furthermore, our focus was also on the evaluation of floating and swelling properties of the formulations and effect of the concentrations of excipients on drug release from the formulations. In vivo behavior of the tablet formulation in the stomach was also the focus of this research. In some cases, floating tablets may pump to the intestine, therefore, we are reporting a novel and precise gastro-retentive formulation with the double advantage of sustained release at all pH of GIT.
2. Materials and methods
2.1. Materials
Linseeds were procured from Pansari inn, Pakistan. Linseeds were de-dusted and cleaned from physical impurities, and stored in an airtight container at ambient temperature. Microcrystalline cellulose (Avicel® PH 102) and HPMC K100M were procured from Merck, Germany. Moxifloxacin used in this research was according to the specification of United States Pharmacopoeia (USP). The KCl, NaOH, n-hexane, potassium dihydrogen phosphate and HCl were purchased from Riedel-de Haën, Germany. Magnesium stearate, polyvinylpyrrolidone K30 (PVP K30), talcum, sodium bicarbonate and β-cyclodextrin were procured from Sigma-Aldrich, USA. Linseeds hydrogel (LSH) was separated from linseeds using a nylon mesh. Deionized water was used for the entire study.
2.2. Isolation of LSH
LSH was isolated as per a previously reported method (Haseeb et al., 2016). Briefly, linseeds (200 g) were washed thoroughly with deionized water and then soaked in 1000 mL deionized water. After 48 h, soaked seeds were heated at 80 °C for 30 min. Mucilage extruded from seeds were separated by nylon mesh and washed thoroughly with n-hexane to remove lipophilic components and then deionized water. To isolate LSH, mucilage was centrifuged at 4000 rpm for 30 min and dried at 60 °C in a vacuum oven for 24 h. Finally, dried LSH was milled, passed through mesh no. 40 and stored at ambient temperature in an airtight container.
2.3. Drug-excipient compatibility study
In order to characterize LSH and find any potential incompatibility with other ingredients of tablet formulation, Fourier transform infrared (FT-IR) spectroscopy was used. KBr pellet technique was employed for FTIR analysis and spectra were recorded on IR Prestige-21 (Shimadzu, Japan) from 4000 to 400 cm−1.
2.4. Preparation of tablets
For each tablet formulation (Table 1), all ingredients except talcum and magnesium stearate were passed through sieve no. 40 and mixed thoroughly before kneading with 5% (w/v) solution of PVP K30 in isopropyl alcohol. Damp mass was granulated by passing through sieve no. 10 and dried at 40 °C for 8 h. Dried granules were further passed through sieve no. 20 and lubricated with talcum and magnesium stearate. Lubricated granules were evaluated through pre-compression parameters. Granules were then compressed using 15 mm flat surface punch with the hardness of 7.5–9.0 kg/cm2. Prepared tablets were evaluated through post-compression parameters.
Table 1.
Composition of formulations to study the effect of ingredients on the release of moxifloxacin from LSH based tablets.
| Formulations composition (mg) | F0 | FF | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|---|---|
| Moxifloxacin | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 | 400 |
| Linseed hydrogel (LSH) | – | 30 | 20 | 40 | 30 | 30 | 30 | 30 | 30 | 30 |
| β-cyclodextrin | 130 | 130 | 130 | 130 | 100 | 160 | 130 | 130 | 130 | 130 |
| Sodium bicarbonate | 100 | 100 | 100 | 100 | 100 | 100 | 70 | 130 | 100 | 100 |
| HPMC K100M | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 10 | 30 |
| Avicel® PH 102 | 105 | 75 | 85 | 65 | 105 | 45 | 105 | 45 | 85 | 65 |
| PVP K30 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Magnesium stearate | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Talcum | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Weight of each tablet | 800 | 800 | 800 | 800 | 800 | 800 | 800 | 800 | 800 | 800 |
2.4.1. Pre-compression evaluation
Before feeding into tablet compression machine, lubricated granules of each formulation were evaluated for flow properties and compressibility by determining the angle of repose, loose and tapped bulk density, Hausner ratio and compressibility index (Lachman et al., 1987).
The angle of repose (θ) was determined by the fixed funnel and cone method. The funnel was adjusted at a specific height from a flat plane surface. Granules were allowed to flow freely through the funnel until the top of the heap formed just touched the lower tip of the funnel. The angle of repose was calculated using Eq. (1):
| (1) |
where θ is the angle of repose, h is the height of heap (cm) and r is the radius of the heap base (cm).
Weighed lubricated granules (W) were taken in a graduated cylinder and volume (Vi) was noted. Loose bulk density (DLB) was determined using Eq. (2):
| (2) |
Tapped bulk density (DTB) was calculated by tapping the graduated cylinder filled with a measured amount of lubricated granules (W) until no further change in the volume of these granules was observed. The final volume of granules (Vf) was noted and tapped bulk density was calculated using Eq. (3):
| (3) |
Values of loose bulk density (DLB) and tapped bulk density (DTB) were used to determine the compressibility index (Ci) and Hausner ratio (Hr) as shown in Eqs. (4), (5), respectively.
| (4) |
| (5) |
2.4.2. Post-compression evaluation of LSH tablets
Compressed tablets were evaluated for diameter, thickness, hardness, friability, and weight variation (Lachman et al., 1987). Uniformity of moxifloxacin in prepared tablets was also determined.
Prepared tablets were assessed for diameter, thickness, and hardness using a hardness tester (Pharma Test, PTB 311E, Germany). Each test was performed on randomly selected ten tablets from each formulation and mean value was reported along with standard deviation (SD).
To determine weight variation in each formulation, twenty tablets were selected randomly and carefully weigh each tablet on an analytical balance (Shimadzu, Japan). The mean weight and SD were calculated and reported.
Ten tablets from each formulation were randomly selected, accurately weighed, placed in a chamber of friability tester (Pharma Test, PTF 10E, Germany) and allowed to rotate at 25 rpm for 4 min. Tablets were removed from the chamber, gently cleaned from dust particles and accurately weighed. Friability was calculated in terms of percentage weight loss using Eq. (6):
| (6) |
where Wi and Wf are the weight of the tablet before and after friability test, respectively.
To determine the amount of moxifloxacin in the formulations, ten tablets from each formulation were picked randomly and separately crushed in pestle and mortar. Crushed powder (800 mg) of each formulation was mixed vigorously with methanol in a volumetric flask, filtered, diluted (if required) and analyzed on UV spectrophotometer (UV PharmSpec 1700, Shimadzu, Japan) at 295 nm. The amount of moxifloxacin was calculated from the calibration curve. Procedures were repeated three times and the mean value was reported.
2.5. Swelling study
The swelling study was carried out to determine the swelling capacity of the prepared formulation (El-Zahabay et al., 2014, Awasthi and Kulkarni, 2012). Tablets from each formulation were enclosed in Tea bag and placed in separate beakers filled with a buffer of pH 1.2 (900 mL) maintained at 37 ± 0.5 °C. After 12 h, Tea bags containing swollen tablets were removed from the beakers, hung for some time to drain out the excessive media, accurately weighed and calculated the swelling capacity using Eq. (7):
| (7) |
where Ws, We and Wi represented the weight of wet Tea bag having swollen tablet, weight of empty Tea bag and initial weight of the tablet, respectively (Haseeb et al., 2017).
The swelling capacity of all these formulations was also determined at pH 4.5 and 6.8, and in deionized water. To observe the swelling behavior of tablet at different time intervals, swelling capacity of optimum formulation (FF) was determined at pH 1.2, 4.5, 6.8 and in deionized water using Eq. (7).
2.6. Tablet density
The density of tablets of all formulations was calculated using Eq. (8) (Fukuda et al., 2006).
| (8) |
where D is the density of the tablet, w is the weight of the tablet, d is the diameter of the tablet, h is the height of the tablet and π is the circular constant. This experiment was performed thrice and the average values are reported.
2.7. In vitro buoyancy study
In vitro buoyancy study was carried out to determine the buoyancy lag time and the duration of buoyancy. Tablet was placed in a buffer of pH 1.2 and time taken by the tablet to come at the top of the medium was noted which was the buoyancy lag time. The duration of the buoyancy was also determined (Yang et al., 1999).
2.8. In vitro drug release study
In vitro drug release study was performed in USP Dissolution Apparatus Type II (Pharma Test, PT-DT7, Germany). Drug release study was performed in two steps. First, the pH change method was adopted to observe the release of drug mimicking the transit time and pH of the GIT, i.e., 2 h at pH 1.2, 3 h at pH 4.5 and remaining time at pH 6.8 (Wang, 1978, Chavanpatil et al., 2005). Further, it is not possible to find the drug release kinetics and release mechanism through transit time and pH change method, therefore, a drug release study was carried out at pH 1.2 for a maximum period of 24 h (Chavanpatil et al., 2006). Dissolution media of pH 1.2, 4.5 and 6.8 (900 mL) were prepared according to USP 37, maintained at 37 ± 0.1 °C and 100 rpm. After selected time intervals, an aliquot (5 mL) was withdrawn from vessels, filtered and diluted (if required) and determine the concentration of moxifloxacin using UV spectrophotometer at a wavelength of 295 nm. Dissolution media was replenished immediately with freshly prepared buffer.
2.9. Drug release kinetics and mechanism
Moxifloxacin release from tablet formulation was analyzed using different kinetic models, i.e., Zero-order (Eq. (9)), First-order (Eq. (10)), Higuchi (Eq. (11)) and Hixon-Crowell (Eq. (12)). For Zero-order, a graph was plotted between time and cumulative drug release. A graph between log of cumulative drug release and time was plotted for First order. In Hixson-Crowell and Higuchi models, a graph between cube root of percentage remaining and time, and cumulative drug release and the square root of time was plotted, respectively. The coefficient of determination (R2) was calculated from the graph and maximum value (~1) was considered as the best-fitted model.
| (9) |
where K0 is the Zero-order rate constant and Qt is the amount of drug released from the tablet in time t.
| (10) |
where K1 is the First-order rate constant, Q is the remaining amount of drug in the tablet after time t and Q0 is the initial amount of drug in the tablet (Gibaldi and Feldman, 1967, Wagner, 1969).
| (11) |
where KHC is the Hixson-Crowell release constant (Hixson and Crowell, 1931). Q0 and Qt is the initial amount of drug in the tablet and the amount of drug released from the tablet in time t, respectively.
| (12) |
where KH is the Higuchi rate constant and Qt is the amount of drug released in time t (Higuchi, 1963).
Drug release from a polymeric matrix is best explained using Korsmeyer-Peppas model and expressed in Eq. (13) (Korsmeyer et al., 1983, Ritger and Peppas, 1987).
| (13) |
where Mt/M∞ is the fraction of drug release at time t, Kp is the Korsmeyer-Peppas constant and n is the release exponent. The value of n is calculated from the slope of a graph plotted between log Mt/M∞ and log t. The value of n indicates the drug release mechanism from a polymeric matrix system. In case of cylindrical geometry (tablets), the drug release mechanism is considered Fickian, non-Fickian (anomalous transport), case II transport and super case II transport if n ≤ 0.45, 0.45 < n < 0.89, n = 0.89 and n > 0.89, respectively (Korsmeyer et al., 1983, Ritger and Peppas, 1987).
Furthermore, a modified Akaike Information criterion called Model Selection Criterion (MSC) was adopted as expressed in Eq. (14) (Iqbal et al., 2011).
| (14) |
where yi_obs is the ith observed value of y, _obs is the mean of observed data points of y and yi_pre is the ith predicted value of y. wi is the weighting factor and its value is usually equal to 1 for fitting the dissolution data, n is the number of all data points and p is the number of parameters in the model.
All calculations related to drug release kinetics and mechanism were performed using the software, DDSolver (Zhang et al., 2010).
2.10. Scanning electron microscopy
The surface morphology of LSH containing tablet formulations (LSHF and FF) was evaluated through scanning electron microscopy (SEM). For this purpose, a scanning electron microscope (FEI Nova, NanoSEM 450) fortified with low energy Everhart-Thornley detectors (ETD) was used. The microscope was operated at 10 KV to observe the surface texture of the tablets as well as porosity of these tablets before and after swelling in deionized water. Tablets were allowed to swell in deionized water (10 mL) for 60 min followed by sonication for 30 min to remove entrapped air bubbles. These tablets were freeze-dried at −20 °C and cut into longitudinal and transverse sections with the help of a sharp stainless steel blade. These sections were coated with gold using sputter coater (Denton, Desk V HP) operated at 40 mA and observed with SEM after mounted on an aluminium stub. For the preparation of LSHF, LSH (400 mg) was kneaded using a PVP K30 solution and granules were prepared by passing the damp mass through sieve no. 20. After drying, granules were compressed using 11 mm flat surface punches.
2.11. In vivo radiographic evaluation
2.11.1. Preparation of tablets
In vivo X-ray study was performed to observe the effect of physiological conditions and environment of GIT on tablet during floating in the stomach. For better visualization of tablet in an X-ray image, an opaquant barium sulphate (BaSO4) was used in a tablet formulation (FF) by replacing 100 mg (25%) of moxifloxacin. The remaining composition of formulation FF was the same as mentioned in Table 1 (Patil et al., 2014). It was assured that the addition of BaSO4 did not affect any pre- and post-compression parameters along with density, buoyancy time, swelling and drug release behavior. The formulation FF prepared for an X-ray study was assigned as FFX.
2.11.2. In vivo X-ray study
For in vivo X-ray study, four healthy beagle dogs (9–10 Kg each) were selected and kept under fasting overnight before the experiment (Baumgartner et al., 2000). Formulation FFX was administered to stray dogs using a gavage tube followed by ordinary tap water (100 mL). Dogs were only allowed to take water ad libitum during the course of study. X-ray radiograph was taken just before and after the administration of tablet to ensure the absence of any radiopaque material and reach of tablet in the stomach, respectively. Radiographic images of the GIT were taken after regular intervals using X-ray machine (Beam Limiting Device, Model TF-6TL-6, Toshiba Corporation, Japan) until the tablet was totally disappeared in GIT of the dogs. In vivo X-ray study was designed in accordance with the National Institute of Health’s Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 85-23, revised 1985) and approved by Institutional Animal Ethics Committee, The University of Lahore, Lahore vide letter no. IAEC-2016-15A.
3. Results and discussion
3.1. Drug-excipient compatibility study
Results of FTIR analyses of formulations have shown that the characteristic peaks of each ingredient are well defined and neither major shift nor any loss of functional peaks is observed, indicating that they are compatible with each other (Fig. 1). Characteristic peaks of C–C stretching in the aromatic ring and C–H deformation in HPMC K100M can be observed at 1647 cm−1 and 1456 cm−1, respectively. In β-cyclodextrin, peak observed at 3320 cm−1 is due to O–H, absorption band at 2926 cm−1 is due to vibration of C–H bonds in CH2 and CH groups, absorption bands of deformation vibrations of C–H in primary and secondary hydroxyl groups at 1400–1200 cm−1, vibration of C–O–C bonds are present at 1080 cm−1, absorption bands of deformation vibrations of C-H bonds are observed between 950 and 700 cm−1 are due to glycopyranose cycle (Szejtli, 1998, Roik and Belyakova, 2011). FTIR spectra of moxifloxacin have also shown its specific peaks between 1450 and 1620 cm−1 due to C=C stretching and observed C–H bending of substituted benzene at 873 cm−1. Carbonyl stretching in carboxylic acid, C–N stretching and monofluorobenzene stretching were observed at 1705, 1350 and 1183 cm−1, respectively (Pawar et al., 2012). In the FTIR spectra of LSH, the band in the range of 1200–1000 cm−1 recognized as stretching vibrations of (C–O–H) side groups and the (C–O–C) glycosidic linkage of polysaccharide (Kacurakova et al., 2000). The bands of deprotonated carboxylic acid group of uronic acid and CH2 have observed at 1640 and 1420 cm−1, respectively (Boulet et al., 2007). The bands observed at 2900 and 3450 cm−1 are assigned as C–H and O–H stretching, respectively.
Fig. 1.
FTIR spectra of different excipients, physical mixture of LSH and moxifloxacin, and optimized formulation (FF).
3.2. Pre-compression evaluation of formulations
Pre-compression evaluation of the tablet blend is essential to determine the suitability for the compression process. The results of the pre-compression parameters are depicted in Table 2. Values of loose bulk density (1.389–1.742 g/cm3), tapped bulk density (1.742–2.146 g/cm3), compressibility index (11.001–20.792%), Hausner ratio (1.124–1.262) and angle of repose (24.55–29.33) are within the standard range (Boulet et al., 2007, El-Zahabay et al., 2014, Lachman et al., 1987) which indicated the good flow-ability of the prepared granules having suitable mechanical strength.
Table 2.
Pre-compression and post-compression parameters of different moxifloxacin formulations (Mean ± SD).
| Pre-compression parameters (n = 10) |
Post-compression parameters (n = 10) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Formulation code | Angle of repose | Loose bulk density (g/cm3) | Tapped bulk density (g/cm3) | Hausner’s ratio | Compressibility index (%) | Hardness (kg/cm2) | Thickness (mm) | Weight (mg) | Friability (%) | Drug content (%) |
| F0 | 29.33 ± 0.33 | 1.562 ± 0.33 | 1.923 ± 0.18 | 1.231 ± 0.29 | 18.750 ± 0.11 | 8.11 ± 0.05 | 4.48 ± 0.08 | 802.5 ± 2.44 | 0.82 ± 0.11 | 97.11 ± 0.82 |
| FF | 26.41 ± 0.41 | 1.613 ± 0.41 | 1.852 ± 0.21 | 1.148 ± 0.11 | 12.903 ± 0.31 | 8.45 ± 0.07 | 4.46 ± 0.11 | 801.6 ± 3.22 | 0.91 ± 0.56 | 98.88 ± 0.66 |
| F1 | 27.18 ± 0.29 | 1.667 ± 0.51 | 1.873 ± 0.18 | 1.124 ± 0.17 | 11.001 ± 0.29 | 7.87 ± 0.09 | 4.49 ± 0.23 | 798.2 ± 1.67 | 0.90 ± 0.81 | 99.02 ± 0.75 |
| F2 | 29.29 ± 0.19 | 1.515 ± 0.17 | 1.792 ± 0.19 | 1.183 ± 0.09 | 15.454 ± 0.24 | 8.02 ± 0.05 | 4.52 ± 0.11 | 799.8 ± 2.29 | 0.82 ± 0.31 | 98.15 ± 0.59 |
| F3 | 25.46 ± 0.21 | 1.650 ± 0.22 | 2.083 ± 0.38 | 1.262 ± 0.14 | 20.792 ± 0.14 | 7.95 ± 0.01 | 4.53 ± 0.08 | 800.4 ± 1.74 | 0.77 ± 0.23 | 97.47 ± 1.02 |
| F4 | 28.73 ± 0.42 | 1.389 ± 0.18 | 1.742 ± 0.67 | 1.254 ± 0.15 | 20.278 ± 0.18 | 7.88 ± 0.05 | 4.55 ± 0.17 | 802.7 ± 1.59 | 0.83 ± 0.31 | 99.11 ± 0.77 |
| F5 | 24.55 ± 0.41 | 1.742 ± 0.19 | 2.008 ± 0.15 | 1.153 ± 0.11 | 13.240 ± 0.31 | 8.23 ± 0.05 | 4.47 ± 0.14 | 798.2 ± 0.99 | 0.88 ± 0.19 | 98.77 ± 0.79 |
| F6 | 26.97 ± 0.57 | 1.695 ± 0.26 | 1.992 ± 0.26 | 1.175 ± 0.34 | 14.915 ± 0.27 | 8.72 ± 0.03 | 4.43 ± 0.21 | 801.4 ± 2.56 | 0.91 ± 0.19 | 98.34 ± 0.96 |
| F7 | 24.72 ± 0.81 | 1.724 ± 0.39 | 2.146 ± 0.16 | 1.245 ± 0.21 | 19.655 ± 0.41 | 8.45 ± 0.02 | 4.49 ± 0.19 | 800.6 ± 2.89 | 0.75 ± 0.22 | 98.97 ± 0.88 |
| F8 | 28.51 ± 0.92 | 1.661 ± 0.08 | 1.887 ± 0.18 | 1.136 ± 0.19 | 11.960 ± 0.35 | 7.52 ± 0.03 | 4.51 ± 0.13 | 797.3 ± 2.52 | 0.83 ± 0.29 | 97.34 ± 0.83 |
3.3. Post-compression evaluation of formulations
Compressed tablets were evaluated through different post-compression parameters and results are shown in Table 2. Mean values of weight (797.3–802.7 mg), thickness (4.46–4.55 mm), hardness (7.52–8.72 kg/cm2) and friability (0.75–0.91%) are within the normal range. Moxifloxacin contents in all formulations were also in the range between 97.11 and 99.11%.
3.4. Swelling study
The swelling capability of the prepared tablet formulations was evaluated in buffer solutions of pH 1.2, 4.5 and 6.8, and in deionized water and results are shown as a graphical representation in Fig. 2a–d, respectively. Change in the concentration of excipients directly affect the swelling capacity of these formulations as depicted in Fig. 2. It was observed that as the amount of LSH is increased from 30 mg (FF) to 40 mg per tablet (F2), the swelling capacity is decreased from 2.64 to 1.87. Upon decreasing the amount of LSH from 30 mg to 20 mg per tablet (F1), swelling capacity slightly increases from 2.64 to 3.03. This variation in swelling capacity is due to the change in concentration of LSH indicating an inverse relationship between swelling and concentration of LSH which is in accordance with a previous work (Haseeb et al., 2017). LSH has a negligible tendency to swell in acidic pH, therefore, its presence in the tablet has rendered the swelling of tablets (Haseeb et al., 2016, Haseeb et al., 2017). At higher concentration of LSH (F2), the penetration of swelling media (pH 1.2 buffer) in the tablet is more difficult as compared to the formulation with less concentration of LSH (F1). Therefore, less swelling was observed in F2 as compared to F1. However, the swelling ability of formulation F1 and F2 depends upon HPMC (swelling agent) and β-cyclodextrin (channeling agent). In the absence of LSH, formulation F0, a slight increase in the swelling capacity was observed as compared to FF, which may be due to the presence of HPMC K100. In formulation F7 and F8, the effect of HPMC K100 was observed on the swelling index by altering its concentration. Being a slightly swellable polymer, a slight difference in swelling was observed with the variation in concentration of HPMC K100. It was observed that the change in the concentration of β-cyclodextrin and sodium bicarbonate has shown less effect on the swelling of tablets as indicated in formulations F3 and F4, and F5 and F6, respectively. An increase in the concentration of β-cyclodextrin also increases the swelling of the formulation. Being a highly soluble polymer, it diffuses out of the formulation creating microscopic channels. As a result, the penetration of the media in the deep region of the tablet is being possible and relatively more swelling was observed. These results indicated that the main component in these formulations is LSH and its concentration has shown a marked influence on the swelling of tablets which in turn will affect the release of moxifloxacin.
Fig. 2.
Swelling capacity of all formulations after 12 h study at pH 1.2 (a), pH 4.5 (b), pH 6.8 (c) and in deionized water (DW) (d), and optimum formulation (FF) at different time intervals (e).
The swelling capacity of all these formulations were also evaluated at pH 4.5 (Fig. 2b), 6.8 (Fig. 2c) and in deionized water (Fig. 2d). It was observed that as the pH of the solvent increased, the swelling capacity of the tablets was also increased which was mainly due to the high swelling ability of LSH at these pH values and in deionized water. Swelling capacity of optimum formulation (FF) after different time intervals at pH 1.2, 4.5, 6.8 and deionized water is depicted in Fig. 2e. during 12 h study,
3.5. In vitro buoyancy study
Sodium bicarbonate present in the formulation when comes in contact with an acidic media liberates CO2. Tablet absorbs and then retains this media due to the polymeric composition of the formulation. As a result, the generated gas was entrapped within the polymeric matrix which helps the buoyancy of tablet. As long as this gas is entrapped within the tablet, the duration of buoyancy will be prolonged. The buoyancy lag time of all the formulations is expressed in Table 3. All formulations have shown the buoyancy lag time from 419 s (6.98 min) to 587 s (9.78 min) whereas formulation F0 has an exception with 145 s (2.42 min). The reason of less buoyancy lag time in F0 might be due to the absence of LSH. Without LSH, the penetration of the media was easy which initiated the quick generation and released of CO2, hence, the buoyancy lag time was less as compared to other formulations. Furthermore, it was impossible for formulation F0 to keep the CO2 within the tablet for a longer period of time. Therefore, the time duration of buoyancy was not more than 1 h which was very short as compared to other formulations. The duration of buoyancy is directly related to the amount of CO2 generated within the tablet. Therefore, it was noted that with the increase in the concentration of sodium bicarbonate (F6), the duration of buoyancy was also increased and vice versa (F5). Fig. 3 indicating the buoyancy duration of FF after different time intervals.
Table 3.
Different physical parameters of moxifloxacin tablet formulations.
| Formulation code | Tablet density (g/cm3) | Duration of buoyancy (h) | Buoyancy lag time (s) |
|---|---|---|---|
| F0 | 1.014 ± 0.08 | <1 h | 145 ± 3.21 |
| FF | 1.018 ± 0.11 | >12 h | 505 ± 4.33 |
| F1 | 1.006 ± 0.07 | >12 h | 478 ± 2.93 |
| F2 | 1.002 ± 0.18 | >12 h | 545 ± 4.78 |
| F3 | 1.000 ± 0.21 | >12 h | 513 ± 2.45 |
| F4 | 0.999 ± 0.22 | >12 h | 491 ± 2.58 |
| F5 | 1.011 ± 0.15 | >11 h | 587 ± 3.11 |
| F6 | 1.024 ± 0.19 | >14 h | 419 ± 6.72 |
| F7 | 1.009 ± 0.21 | >12 h | 521 ± 2.37 |
| F8 | 1.001 ± 0.11 | >12 h | 488 ± 3.44 |
Fig. 3.
Photographs representing the duration of buoyancy of formulation, FF at pH 1.2.
3.6. Tablet density measurement
Densities of all formulations are found ≈1 indicating that all formulations would float (Table 3). Therefore, all tablet formulations took a little bit more time (minimum 7 min.) to start floating.
3.7. Drug release study
Moxifloxacin release from different formulations is depicted in Fig. 4 and Table S1. Prolonged and sustained release of drug from all formulations was observed expect formulation F0 which is formulated without the addition of LSH. Therefore, it can be concluded that the sustained and prolonged release from these formulations was mainly due to the presence of LSH. However, a slightly different release pattern was observed from all these formulations which was due to the variation in the concentration of excipients. The effect of LSH, HPMC K100M, sodium bicarbonate and β-cyclodextrin on drug release is discussed in the subsequent section.
Fig. 4.
Moxifloxacin release study from different formulations at pH 1.2.
3.7.1. Effect of LSH on drug release
Effect of LSH on the release of moxifloxacin is shown in Fig. 5. At the end of desired period (24 h), 92.19% of drug was released from the optimum formulation (FF). It was also observed that the release of drug was decreased from 99.11 to 82.87% as the concentration of LSH increased from 20 to 40 mg per tablet in formulation F1 and F2, respectively (Fig. 5a). Optimized formulation contained 30 mg of LSH which prolonged the release of drug up to 24 h. Upon decreasing the concentration of LSH to 20 mg, not only the release retarding ability was decreased but also the formulation could not able to release the drug up to the desired period of 24 h. This delay release was due to the less swelling ability of the polymer. The acidic groups present in the polymeric chains of LSH are present in a protonated form in an acidic media and the presence of strong bonding between these groups produce the hindrance for the penetration of dissolution media. Therefore, the dissolution media was unable to penetrate the polymeric matrix and hence retarded the drug release. For comparison, the maximum drug release from F0 was observed after 4 h which was due to the absence of LSH. This comparatively fast release also indicates the release retarding behavior of LSH in the acidic environment.
Fig. 5.
Effect of LSH concentration on moxifloxacin release (a) and in vitro release study of moxifloxacin during transit time and pH of gastrointestinal tract (b).
In case, if the gastroretentive tablet pumps to the intestine as it appears in some cases (Shin et al., 2019), then a study at the physiological pH of different sections of the intestine should also be taken into the account. Therefore, a drug release study was also carried out according to the pH and transit time of the GIT, i.e., at pH 1.2 for 2 h, pH 4.5 for 3 h and pH 6.8 for 7 h (Fig. 5b). It can be seen that the sustained release of moxifloxacin was observed at pH 1.2 and 4.5 which was mainly due to the presence of LSH. In the acidic environment, swelling of LSH is less which directly effect on the release of moxifloxacin. At pH 6.8, due to substantial swelling of LSH (9), more release of drug was observed.
3.7.2. Effect of β-cyclodextrin on drug release
With the passage of time, it is possible that the rate of drug release from a polymeric system is reduced due to the greasy nature of the polymers. Due to this, it is difficult for a dissolution media to diffuse in and out of the polymeric matrix. As a result, the release rate at the later stage is reduced. To overcome this difficulty, a channeling agent is introduced in the formulation design which eliminate the chances of a potential decrease in drug release. β-cyclodextrin, a pH-independent highly soluble channeling agent, was used which not only increases the drug release at the later stage of the study but also improves the compressibility and physical texture of the tablets (Chavanpatil et al., 2006). This effect can be seen in Fig. 6a where the difference between the release profiles from formulation F3 and FF was not significant during the first 6 h and in the later stage, the difference is more prominent as the concentration was increased. This increased drug release is mainly due to the formation of channels that facilitates the approach of dissolution media in the deep area of the tablet. In Fig. 6b, the effect of β-cyclodextrin on the release of moxifloxacin at different pH and transit time of GIT.
Fig. 6.
Effect of β-cyclodextrin concentration on moxifloxacin release (a) and in vitro release study of moxifloxacin during transit time and pH of gastrointestinal tract (b).
3.7.3. Effect of sodium bicarbonate on drug release
In floating drug delivery systems, sodium bicarbonate is used as a gas-generating agent that is liberated in contact with the acidic media of the stomach. The generated gas was entrapped into the viscous and greasy polymeric matrix which results in the floating of the formulation. A decrease in drug release was observed as the concentration of sodium bicarbonate increased from 70 to 130 mg per tablet in formulation F5 and F6, respectively (Fig. 7a). Moxifloxacin is soluble in the acidic environment whereas the presence of sodium bicarbonate builds a basic environment in the tablet which not only decreases the solubility of moxifloxacin therin, therefore, retard its release from the tablet. Therefore, the increase in the concentration of sodium bicarbonate has put a negative impact on the release of moxifloxacin. Fig. 7b expressed the effect of different pH and time duration on the release of moxifloxacin from formulations F5 and F6.
Fig. 7.
Effect of sodium bicarbonate concentration on moxifloxacin release (a) and in vitro release study of moxifloxacin during transit time and pH of gastrointestinal tract (b).
3.7.4. Effect of HPMC K100M on drug release
To achieve the desirable release pattern of moxifloxacin in acidic pH, HPMC K100M, a swellable material, was introduced in the formulation. After 24 h, drug release was decreased to 86% from 99% as the concentration of HPMC K100M was increased from 10 to 30 mg per tablet in formulation F7 and F8, respectively (Fig. 8a). This decrease in the drug release is mainly due to the increase in the swelling of the tablet which ultimately increases the diffusion path length. Drug release was also observed at pH and transit time of GIT (Fig. 8b). It was observed that the drug-releasing behavior was directly proportional to the pH of the media. As LSH has high tendency to swell at pH 6.8, therefore, at this pH, profound drug release was observed. However, at pH 6.8, the release of the drug was also dependent on the concentration of LSH and inverse relation was observed.
Fig. 8.
Effect of HPMC K100M concentration on moxifloxacin release (a) and in vitro release study of moxifloxacin during transit time and pH of gastrointestinal tract (b).
3.8. Drug release kinetics and mechanism
Different drug release kinetics models were applied on drug release data and values are expressed in Table 4 (Awasthi and Kulkarni, 2014). Results indicated that drug release followed first-order kinetics and Korysmeyer-Peppas model as evident from the values of R2 (mostly near to 1) and MSC (having the highest values). Korsmeyer-Peppas model was also applied to find the drug release mechanism and the values indicated that the drug release mechanism form all formulations followed non-Fickian diffusion, i.e., drug release governed by swelling and erosion of polymeric matrix.
Table 4.
Values of drug release kinetics models of different formulations.
| F0 | FF | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zero order | R2 | 0.9932 | 0.7620 | 0.7448 | 0.8374 | 0.7664 | 0.7788 | 0.7743 | 0.8379 | 0.8912 | 0.8480 |
| K0 | 19.673 | 4.860 | 7.096 | 4.263 | 4.539 | 5.708 | 5.628 | 4.654 | 6.559 | 4.380 | |
| MSC | 4.5845 | 1.2816 | 1.1839 | 1.6627 | 1.3001 | 1.3420 | 1.3220 | 1.6657 | 2.0368 | 1.7298 | |
| First order | R2 | 0.8954 | 0.9962 | 0.9948 | 0.9978 | 0.9894 | 0.9943 | 0.9946 | 0.9934 | 0.9835 | 0.9946 |
| K1 | 0.341 | 0.099 | 0.150 | 0.076 | 0.087 | 0.114 | 0.111 | 0.089 | 0.121 | 0.079 | |
| MSC | 1.8573 | 5.4171 | 5.0847 | 5.1448 | 4.3892 | 5.0085 | 5.0472 | 4.8647 | 3.9226 | 5.0679 | |
| Higuchi | R2 | 0.8242 | 0.9452 | 0.9542 | 0.9941 | 0.9281 | 0.9391 | 0.9435 | 0.9294 | 0.9169 | 0.9332 |
| KH | 37.118 | 18.947 | 23.422 | 16.479 | 17.673 | 20.418 | 20.146 | 17.987 | 21.320 | 16.916 | |
| MSC | 1.3381 | 2.7510 | 2.9020 | 2.5364 | 2.4791 | 2.6322 | 2.7066 | 2.4964 | 2.3058 | 2.5525 | |
| Hixson-Crowell | R2 | 0.9573 | 0.9875 | 0.9881 | 0.9833 | 0.9727 | 0.9884 | 0.9831 | 0.9925 | 0.9933 | 0.9877 |
| KHC | 0.086 | 0.028 | 0.041 | 0.022 | 0.025 | 0.032 | 0.031 | 0.025 | 0.034 | 0.022 | |
| MSC | 2.4868 | 4.2314 | 4.2504 | 3.9403 | 3.4485 | 4.2861 | 3.9122 | 4.7413 | 4.8170 | 4.2445 | |
| Korsmeyer-Peppas | R2 | 0.9998 | 0.9848 | 0.9955 | 0.9770 | 0.9780 | 0.9833 | 0.9799 | 0.9837 | 0.9719 | 0.9749 |
| KKP | 19.230 | 9.545 | 12.551 | 8.101 | 7.815 | 10.117 | 10.146 | 7.697 | 10.067 | 8.585 | |
| n | 1.082 | 0.845 | 0.866 | 0.823 | 0.813 | 0.877 | 0.880 | 0.806 | 0.872 | 0.802 | |
| MSC | 7.4141 | 3.6839 | 4.7321 | 3.3298 | 3.3187 | 3.5219 | 3.3345 | 3.6136 | 3.0023 | 3.2409 | |
3.9. Scanning electron microscopy
The morphology of the LSH powder, a tablet containing only LSH (LSHF) and formulation FF was evaluated through SEM and images are depicted in Fig. 9. LSH powder appeared as irregular flakes having a wide range of size distribution. Upon compression of LSH powder into the tablet, these flakes were broken and formed an uneven and rough surface of the tablet. SEM image of a proper formulation (FF) revealed that the surface of the tablet was smooth with micro-cracks as indicated with white arrows in Fig. 9. Swollen then freeze-dried tablets of the formulations LSHF and FF were observed through SEM and images exposed the symmetrical distribution of microscopic pores and channels on the surface of tablets. These channels and pores are responsible for the intake of a large amount of swelling or drug release media which contributed to the swelling of LSH and sustained release of moxifloxacin, respectively.
Fig. 9.
SEM images of LSH powder, tablet surface of the formulation composed of only LSH, surface of formulation FF showing micro-cracks indicated through white arrows, the surface of swollen then freeze-dried tablet of LSHF and swollen then freeze-dried tablet surface of formulation FF.
3.10. In vivo radiographic evaluation
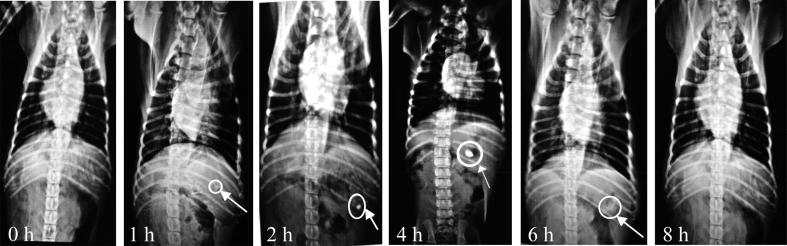
X-rays images captured during the in vivo studies of formulation FFX are depicted in Fig. 10. This study was performed to ascertain the gastric retention of the LSH based tablet formulation and also determined the maximum retention time in the stomach. After 1 h of ingestion, the tablet can be seen in the stomach of the animal. The dull appearance of the tablet indicated the non-swelling or less swelling of the tablet which can be accessed from the size of the tablet as the size of the tablet was not significantly increased. After 2 h, the brightness of the tablet was increased which might be due to the more exposure of radiopaque material on the surface as a result of swelling of the tablet. The size of the tablet was increased after 4 h which was due to more swelling of the tablet, as well as the brightness of the tablet, which was enhanced. Image taken after 6 h indicated the disintegration of the tablet as evident from the fragments of tablet in the stomach. This study also proved the retention of tablet in the stomach for at least 6 h.
Fig. 10.
X-ray images of the gastrointestinal tract (GIT) captured before and after the ingestion of formulation FFX by beagle dog after different time intervals indicated the presence of tablet at upper GIT for approximately 6 h before disintegration.
4. Conclusions
LSH based gastroretentive drug delivery system was successfully developed which sustained the release of moxifloxacin in the stomach and tablets remained floating for 6 h. As well as, sustained-release was noted at all pH of GIT indicates the worth of this newly developed formulation. The results of in vitro drug release at various physiological pH of GIT revealed that our newly designed LSH based gastro-retentive formulations offered therefore double advantage; as in the case, if tablet pumps to the intestine, even then it shows sustained release of moxifloxacin. In vitro drug release study also proved the release of moxifloxacin in acidic environment. In vivo X-ray study also proved the real-time floating of tablets as well as disintegration behavior in the stomach. These findings recommend the fitness of LSH for the development of highly valuable gastroretentive drug delivery system with the additional advantage of sustained release at intestinal pH if the tablets pump to the intestine.
Acknowledgments
Acknowledgements
We are grateful to Don Valley Pharmaceuticals (Pvt.) Ltd. Lahore for providing a generous gift of moxifloxacin. We recognize the cooperation of the radiology section of the Mubarak Medical Complex, Sargodha, Pakistan for the provision of X-Ray analysis and expert opinion. Instruments Laboratories, University of Sargodha (UOS) are also being acknowledged for providing the facilities of spectroscopic and chromatographic characterization. Photography is the courtesy of one of the author M. T. Haseeb. Animal house of The University of Lahore (UOL) and UOS were utilized for animal hosting and caring, therefore, we are thankful to both of them. Authors are thanful to Mr. Zajif Hussain, LUMS, Lahore for the provision of SEM analysis.
Declaration of Competing Interest
No potential conflict of interest was reported by the authors.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2020.03.005.
Contributor Information
Muhammad Ajaz Hussain, Email: majaz172@yahoo.com.
Muhammad Tahir Haseeb, Email: mtahir212@yahoo.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ashraf M.U., Hussain M.A., Bashir S., Haseeb M.T., Hussain Z. Quince seed hydrogel (glucuronoxylan): evaluation of stimuli responsive sustained release oral drug delivery system and biomedical properties. J. Drug Deliv. Sci. Technol. 2018;45:455–465. [Google Scholar]
- Ashraf M.U., Hussain M.A., Muhammad G., Haseeb M.T., Bashir S., Hussain S.Z., Hussain I. A superporous and superabsorbent glucuronoxylan hydrogel from quince (Cydonia oblanga): stimuli responsive swelling, on-off switching and drug release. Int. J. Biol. Macromol. 2017;95:138–144. doi: 10.1016/j.ijbiomac.2016.11.057. [DOI] [PubMed] [Google Scholar]
- Awasthi R., Kulkarni G.T. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: where do we stand? Drug Deliv. 2016;23:378–394. doi: 10.3109/10717544.2014.936535. [DOI] [PubMed] [Google Scholar]
- Awasthi R., Kulkarni G.T. Development of novel gastroretentive floating particulate drug delivery system of gliclazide. Curr. Drug Deliv. 2012;9:437–451. doi: 10.2174/156720112802650716. [DOI] [PubMed] [Google Scholar]
- Awasthi R., Kulkarni G.T. Development of novel gastroretentive drug delivery system of gliclazide: hollow beads. Drug Dev. Ind. Pharm. 2014;40:398–408. doi: 10.3109/03639045.2013.763817. [DOI] [PubMed] [Google Scholar]
- Barbary O.M., Al-Sohaimy S.A., El-Saadani M.A., Zeitoun A.M.A. Extraction, composition and physicochemical properties of flaxseed mucilage. J. Adv. Agric. Res. 2009;14:605–620. [Google Scholar]
- Basu S., Chakraborty S., Bandyopadhyay A.K. Development and evaluation of a mucoadhesive nasal gel of midazolam prepared with Linum usitatissimum L. seed mucilage. Sci. Pharm. 2007;77:899–910. [Google Scholar]
- Baumgartner S., Kristl J., Vrecer F., Vodopivec P., Zorko B. Optimisation of floating matrix tablets and evaluation of their gastric residence time. Int. J. Pharm. 2000;195:125–135. doi: 10.1016/s0378-5173(99)00378-6. [DOI] [PubMed] [Google Scholar]
- Boulet J.C., Williams P., Doco T. Fourier transform infrared spectroscopy study of wine polysaccharides. Carbohydr. Polym. 2007;69:79–85. [Google Scholar]
- Chavanpatil M.D., Jain P., Chaudhari S., Shear R., Vavia P.R. Development of sustained release gastroretentive drug delivery system for ofloxacin: in vitro and in vivo evaluation. Int. J. Pharm. 2005;304:178–184. doi: 10.1016/j.ijpharm.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Chavanpatil M.D., Jain P., Chaudhari S., Shear R., Vavia P.R. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 2006;316:86–92. doi: 10.1016/j.ijpharm.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Cui W., Mazza G., Biliaderis C.G. Chemical structure, molecular size distributions, and rheological properties of flaxseed gum. J. Agric. Food Chem. 1994;42:1891–1895. [Google Scholar]
- El-Zahabay S.A., Kassem A.A., El-Kamel A.H. Formation and in vitro evaluation of size expanding gastro-retentive system of levofloxacin hemihydrate. Int. J. Pharm. 2014;464:10–18. doi: 10.1016/j.ijpharm.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Peppas N.A., McGinity J.W. Floating hot-melt extruded tablets for gstroretentive controlled drug release system. J. Control. Release. 2006;115:121–129. doi: 10.1016/j.jconrel.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Gibaldi M., Feldman S. Establishment of sink conditions in dissolution rate determinations-theoretical considerations and application to non-disintegrating dosage forms. J. Pharm. Sci. 1967;56:1238–1242. doi: 10.1002/jps.2600561005. [DOI] [PubMed] [Google Scholar]
- Haseeb M.T., Bashir S., Hussain M.A., Ashraf M.U., Erum A., Hassan M.N. Acute toxicity study of a polysaccharide based hydrogel from linseed for potential use in drug delivery system. Braz. J. Pharm. Sci. 2018;54:e17459. [Google Scholar]
- Haseeb M.T., Hussain M.A., Abbas K., Youssif B.G.M., Bashir S., Yuk S.H., Bukhari S.N.A. Linseed hydrogel-mediated green synthesis of silver nanoparticles for antimicrobial and wound-dressing applications. Int. J. Nanomed. 2017;12:2845–2855. doi: 10.2147/IJN.S133971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseeb M.T., Hussain M.A., Bashir S., Ashraf M.U., Ahmad N. Evaluation of superabsorbent linseed-polysaccharides as a novel stimuli-responsive oral sustained release drug delivery system. Drug Dev. Ind. Pharm. 2017;43:409–420. doi: 10.1080/03639045.2016.1257017. [DOI] [PubMed] [Google Scholar]
- Haseeb M.T., Hussain M.A., Yuk S.H., Bashir S., Nauman M. Polysaccharides based superabsorbent hydrogel from linseed: dynamic swelling, stimuli responsive on–off switching and drug release. Carbohydr. Polym. 2016;136:750–756. doi: 10.1016/j.carbpol.2015.09.092. [DOI] [PubMed] [Google Scholar]
- Haseeb M.T., Khaliq N.U., Yuk S.H., Hussain M.A., Bashir S. Linseed polysaccharides based nanoparticles for controlled delivery of docetaxel: design, in vitro drug release and cellular uptake. J. Drug Deliv. Sci. Technol. 2019;49:143–151. [Google Scholar]
- Hasnain M.S., Rishishwar P., Rishishwar S., Ali S., Nayak A.K. Isolation and characterization of Linum usitatissimum polysaccharide to prepare mucoadhesive beads of diclofenac sodium. Int. J. Biol. Macromol. 2018;116:162–172. doi: 10.1016/j.ijbiomac.2018.04.151. [DOI] [PubMed] [Google Scholar]
- Higuchi T. Mechanism of rate of sustained action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- Hixson A.W., Crowell J.H. Dependence of reaction velocity upon surface and agitation. Ind. Eng. Chem. 1931;23:1160–1168. [Google Scholar]
- Hoffman A., Stepensky D. Pharmacodynamic aspects of modes of drug administration for optimization of drug therapy. Crit. Rev. Ther. Drug Carrier Syst. 1999;16:571–639. [PubMed] [Google Scholar]
- Hwang S.J., Park H., Park K. Gastric retentive drug-delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 1998;15:243–284. [PubMed] [Google Scholar]
- Imre B., Pukanszky B. From natural resources to functional polymeric biomaterials. Eur. Polym. J. 2015;68:481–487. [Google Scholar]
- Iqbal M.S., Akbar J., Hussain M.A., Saghir S., Sher M. Evaluation of hot-water extracted arabinoxylans from ispaghula seeds as drug carriers. Carbohydr. Polym. 2011;83:1218–1225. [Google Scholar]
- Kacurakova M., Capek P., Sasinkova V., Wellner N., Ebringerova A. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000;43:195–203. [Google Scholar]
- Kaewmanee T., Bagnasco L., Benjakul S., Lanteri S., Morelli C.F., Speranza G., Cosulich M.E. Characteristics of mucilages extracted from seven Italian cultivars of flax. Food Chem. 2014;148:60–69. doi: 10.1016/j.foodchem.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Klausner E.A., Lavy E., Friedman M., Hoffman A. Expandable gastroretentive dosage forms. J. Control. Release. 2003;90:143–162. doi: 10.1016/s0168-3659(03)00203-7. [DOI] [PubMed] [Google Scholar]
- Korsmeyer R.W., Gurny R., Doelker E., Buri P., Peppas N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15:25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- Lachman L., Liberman H.A., Kanig J.L. 3rd ed. Varghese Publishing House; Mumbai, India: 1987. The Theory and Practice of Industrialpharmacy. [Google Scholar]
- Lodhi, B.A., Hussain, M.A., Sher, M., Haseeb, M.T., Ashraf, M.U., Hussain, S.Z., Hussain, I., Bukhari, S.N.A., 2019. Polysaccharide-based superporous, superabsorbent, and stimuli responsive hydrogel from sweet basil: A novel material for sustained drug release. Adv. Polym. Technol. Article ID 9583516, 11 pages.
- Mandal U.K., Chatterjee B., Senjoti F.G. Gastro-retentive drug delivery systems and theirin vivosuccess: a recent update. Asian J. Pharm. Sci. 2016;11:575–584. [Google Scholar]
- Mansuri S., Kesharwani P., Jain K., Tekade R.K., Jain N.K. Mucoadhesion: a promising approach in drug delivery system. React. Funct. Polym. 2016;100:151–172. [Google Scholar]
- Muhammad G., Hussain M.A., Ashraf M.U., Haseeb M.T., Hussain S.Z., Hussain I. Polysaccharide based superabsorbent hydrogel from Mimosa pudica: Swelling–deswelling and drug release. RSC Adv. 2016;6:23310–23317. [Google Scholar]
- Nerkar P.P., Gattani S. In vivo, in vitro evaluation of linseed mucilage based buccal mucoadhesive microspheres of venlafaxine. Drug Deliv. 2011;18:111–121. doi: 10.3109/10717544.2010.520351. [DOI] [PubMed] [Google Scholar]
- Patil S.H., Talele G.S. Natural gum as mucoadhesive controlled release carriers: evaluation of Cefpodoxime Proxetil by D-Optical design technique. Drug Deliv. 2014;21:118–129. doi: 10.3109/10717544.2013.834416. [DOI] [PubMed] [Google Scholar]
- Pawar P.K., Katara R., Majumdar D.K. Design and evaluation of moxifloxacin hydrochloride ocular inserts. Acta Pharm. 2012;62:93–104. doi: 10.2478/v10007-012-0002-5. [DOI] [PubMed] [Google Scholar]
- Qian K.Y., Cui S.W., Nikiforuk J., Goff H.D. Structural elucidation of rhamnogalacturonans from flaxseed hulls. Carbohydr. Res. 2012;362:47–55. doi: 10.1016/j.carres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Qian, K.Y., Cui, S.W., Wu, Y., Goff, H.D., 2012b. Flaxseed gum from flaxseedhulls: extraction, fractionation, and characterization. 28, 275–283.
- Rao G.K., Mandapalli P.K., Manthri R., Reddy V.P. Development and in vivo evaluation of gastroretentive delivery systems for cefuroxime axetil. Saudi Pharm. J. 2013;21:53–59. doi: 10.1016/j.jsps.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritger P.I., Peppas N.A. A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J. Control. Release. 1987;5:37–42. [PubMed] [Google Scholar]
- Roik N.V., Belyakova L.A. IR Spectroscopy, X-ray diffraction and thermal analysis studies of solid “β-cyclodextrin-para-aminobenzoic acid” inclusion complex. Phys. Chem. Solid State. 2011;12:168–173. [Google Scholar]
- Shelke N.B., James R., Laurencin C.T., Kumbar S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014;25:448–460. [Google Scholar]
- Shin S., Kim T.H., Jeong S.W., Chung S.E., Lee D.Y., Kim D.H., Shin B.S. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE. 2019;14:e0216875. doi: 10.1371/journal.pone.0216875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B.N., Kim H.N. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J. Control. Release. 2000;63:235–259. doi: 10.1016/s0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998;98:1743–1753. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- Urguhart, J., Theeuwes, F., 1994. Drug delivery system comprising a reservoir containing a plurality of tiny pellets. US Patent No. US 4434153.
- Wagner J.G. Interpretation of percent dissolved-time plots derived from in-vitro testing of conventional tablets and capsules. J. Pharm. Sci. 1969;58:1253–1257. doi: 10.1002/jps.2600581021. [DOI] [PubMed] [Google Scholar]
- Wang Y. Evaluation of an oral prolonged-release antibiotic formulation. J. Pharm. Sci. 1978;67:1620–1622. doi: 10.1002/jps.2600671130. [DOI] [PubMed] [Google Scholar]
- Waterman K.C. A critical review of gastric retentive controlled drug delivery. Pharm. Dev. Technol. 2007;12:1–10. doi: 10.1080/10837450601168680. [DOI] [PubMed] [Google Scholar]
- Yang L., Eshraghi J., Fassihi R. A new intragastric delivery system for the treatment of Helicobacter pylori associated gastric ulcer: in vitro evaluation. J. Control. Release. 1999;57:215–222. doi: 10.1016/s0168-3659(98)00066-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Huo M., Zhou J., Zou A., Li W., Yao C., Xie S. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12:263–271. doi: 10.1208/s12248-010-9185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.