Abstract
Aims
Methicillin-resistant Staphylococcus aureus (MRSA) can cause wound infections via a ‘Trojan Horse’ mechanism, in which neutrophils engulf intestinal MRSA and travel to the wound, releasing MRSA after apoptosis. The possible role of intestinal MRSA in prosthetic joint infection (PJI) is unknown.
Methods
Rats underwent intestinal colonization with green fluorescent protein (GFP)-tagged MRSA by gavage and an intra-articular wire was then surgically implanted. After ten days, the presence of PJI was determined by bacterial cultures of the distal femur, joint capsule, and implant. We excluded several other possibilities for PJI development. Intraoperative contamination was excluded by culturing the specimen obtained from surgical site. Extracellular bacteraemia-associated PJI was excluded by comparing with the infection rate after intravenous injection of MRSA or MRSA-carrying neutrophils. To further support this theory, we tested the efficacy of prophylactic membrane-permeable and non-membrane-permeable antibiotics in this model.
Results
After undergoing knee surgery eight or 72 hours after colonization, five out of 20 rats (25.0%) and two out of 20 rats (10.0%) developed PJI, respectively. Strikingly, 11 out of 20 rats (55.0%) developed PJI after intravenous injection of MRSA-carrying neutrophils that were isolated from rats with intestinal MRSA colonization. None of the rats receiving intravenous injections of MRSA developed PJI. These results suggest that intestinal MRSA carried by neutrophils could cause PJI in our rat model. Ten out of 20 (50.0%) rats treated with non-membrane-permeable gentamicin developed PJI, whereas only one out of 20 (5.0%) rats treated with membrane-permeable linezolid developed PJI.
Conclusion
Neutrophils as carriers of intestinal MRSA may play an important role in PJI development.
Cite this article: Bone Joint Res. 2020;9(4):152–161.
Keywords: Trojan Horse mechanism, Prosthetic joint infection, Methicillin-resistant Staphylococcus aureus
Article focus
Whether intestinal methicillin-resistant Staphylococcus aureus (MRSA) colonization is able to cause prosthetic joint infection (PJI).
Whether a ‘Trojan Horse’ mechanism plays an important role in this MRSA gut-wound metastasis.
What antibiotics could prevent this gut-wound metastasis effectively?
Key messages
Intestinal MRSA colonization is able to cause PJI in our rat model.
Intestinal MRSA invades knee joints by hiding in neutrophils (Trojan Horse mechanism) and subsequently causes PJI.
Membrane-permeable antibiotics are effective against PJI caused by a Trojan Horse mechanism by killing intracellular pathogens.
Strengths and limitations
Our study, for the first time, provides evidence for the role of a Trojan Horse mechanism in PJI. Clearly, conclusions of the current animal study need to be further validated by future clinical investigations.
Introduction
Prosthetic joint infection (PJI) remains one of the most challenging complications after arthroplasty, as it is difficult to diagnose and treat, and the outcomes are typically poor.1 Indeed, PJIs are associated with decreased quality of life, functional loss, and increased morbidity.2,3 Moreover, the management of PJIs may require surgical debridement, two-stage revision, resection arthroplasty, or even amputation. Although the incidence of PJIs is low after primary (1%) and revision arthroplasty surgery (up to 5%),4–8 the costs for treating PJIs are tremendously high. In the USA, for example, annual treatment costs for PJIs are estimated to reach well over $1.62 billion by 2020.9
The mechanism underlying the pathogenesis of PJIs has become clearer over the last decade. Most cases of PJI derive from intraoperative bacterial contamination and postoperative bacteraemia caused by several typical pathogens such as methicillin-resistant Staphylococcus aureus (MRSA).10–14 One emerging idea for pathogenesis of surgical infection is embodied in the ‘Trojan Horse’ theory. According to this theory, pathogenic Staphylococcus aureus, phagocytosed by neutrophils, enters the general circulation masked and undetected. Without causing septicaemia or sepsis, masked bacteria finally reach distant sites, where they are released when the neutrophils undergo apoptosis and ultimately lysis.15–17 This theory has gained support from experiments showing that injection of neutrophils carrying S. aureus can directly cause infection without any other bacterial exposure.18 In another study, an investigation based on a mouse partial hepatectomy model has shown that intestinal MRSA phagocytosed by neutrophils travels from the gut to the surgical site to cause infection.19 We therefore hypothesized that intestinal carriage of MRSA might be able to cause PJI in an animal model with intra-articular implant via this Trojan Horse mechanism.
Methods
Experimental animals and ethical approval
Eight-week-old female Sprague-Dawley rats were used for this study. All operations were performed under sterile conditions. All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of our hospital.
Bacterial inoculum preparation
In this study, green fluorescent protein (GFP)-tagged MRSA (ATCC BAA-1556; ATCC, Manassas, Virginia, USA) was used as a representative pathogen for PJI in our rat model. We maintained the MRSA culture on tryptic soy agar (BD Tryptic Soy Agar, BA-256665; BD, Franklin Lakes, New Jersey, USA) supplemented with 5% sheep blood (BD) and 10 µg/ml chloramphenicol (Sigma-Aldrich, St. Louis, Missouri, USA). Several colonies (commonly three to five colonies) were harvested, suspended in 5 ml of tryptic soy broth supplemented with 10 µg/ml chloramphenicol, and incubated overnight on a shaker maintained at 37°C. We then washed the suspended bacteria with phosphate-buffered saline (PBS) three times by successively centrifuging, discarding the supernatant, and resuspending. After the final wash, we adjusted the cells to a concentration of 1 × 108 colony-forming units (CFUs) per millilitre, according to a standard curve produced by measuring optical density (OD).
The minimum inhibitory concentration determinations for the GFP-tagged MRSA strain
Minimum inhibitory concentration (MIC) for GFP-tagged MRSA strain was determined by preparing serial dilutions of the antibiotic in tryptic soy broth (32, 16, 8, 4, 2, 1, 0.8, 0.6, 0.4, 0.2 µg/ml) in triplicate. MRSA was added to the serial dilutions at a concentration of 1 × 104 CFUs/ml. The bacteria were then cultured for 24 hours with shaking at 37°C. Bacterial growth was assessed by the OD at 630 nm. The MIC was determined to be the dose of antibiotic that inhibited bacterial growth by > 90%. The MICs of gentamicin and linezolid were 0.8 µg/ml and 1 µg/ml, respectively.
MRSA intestinal colonization
We developed the protocol for MRSA colonization in rats based on a previous report and our prior pilot work (Figure 1a).19 Briefly, the rats were deprived of all food during the MRSA intestinal colonization procedure. We first disrupted the normal intestinal microbiota by treating the rats with 200 mg/kg metronidazole (Catalog No. S1907; Selleck Chemicals, Houston, Texas, USA) by oral gavage and 50 mg/kg ampicillin (Catalog No. S4148; Selleck Chemicals) by intramuscular injection every eight hours for 24 hours (four times total). Oral gavage of 1 ml of MRSA solution (1 × 108 CFUs per ml) was performed two hours after each antibiotic treatment for a total four times. To validate successful colonization, we cultured the shed stool (post-colonization day 1, 3, 5, 7, and 9) on tryptic soy agar (BD Diagnostic Systems Europe, Franklin Lakes, New Jersey, USA) supplemented with 5% sheep blood (BD Diagnostic Systems Europe) and 10 µg/ml chloramphenicol (Sigma-Aldrich). The latter was used to select for MRSA colonies.
Fig. 1.

a) A schematic protocol of methicillin-resistant Staphylococcus aureus (MRSA) intestinal colonization. Rats received oral gavage of 200 mg/kg metronidazole and intramuscular injection of 50 mg/kg ampicillin every eight hours (solid line). Oral gavage of 1 ml of MRSA solution (1 × 108 CFU per ml) was performed two hours after each antibiotic treatment (dashed line). b) The stools collected from 40 rats were cultured at day 1, 3, 5, 7, and 9 after MRSA intestinal colonization. The intestine tissues were harvested and cultured after final sacrifice.
Sterile surgical procedures and postoperative management
A total of 40 rats were divided into two groups, with one group receiving surgery at eight hours and the other at 72 hours after intestinal MRSA colonization. After the rat was anaesthetized, the surgical site was shaved clean of fur and thoroughly scrubbed with povidone-iodine. A fenestrated sterile drape was positioned over the surgical field, and the patella was exposed by making a longitudinal skin incision over knee. The joint was opened through a suprapatellar approach to expose the intercondylar notch of the distal femur. A 22-gauge needle was first inserted into the femoral canal in a retrograde fashion at the notch, followed by bone reaming with 20- and 18-gauge needles. To simulate a prosthetic joint implant, we used a 20 mm-long Kirschner wire (Kirschner wire (K-wire); 0.088 mm in diameter). We then inserted the sterile K-wire through the reamed tract and left a 1 mm protruding segment in the knee joint (Figure 2). The joint and incision were closed with interrupted 4-0 sutures.
Fig. 2.
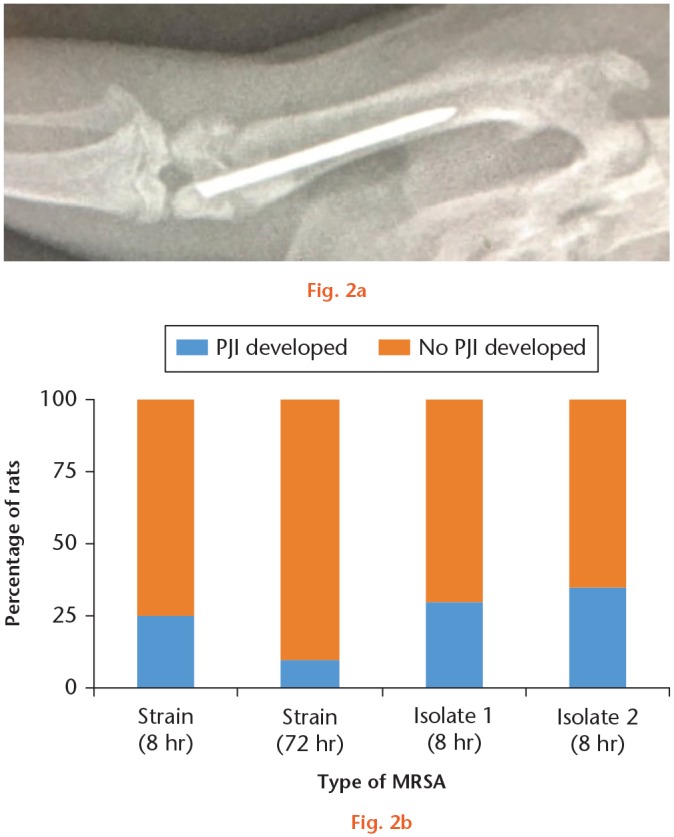
a) Representative anteroposterior radiograph of a rat femur showing a surgically inserted Kirschner wire within 1 mm of the intra-articular prominence. b) With methicillin-resistant Staphylococcus aureus (MRSA) strain, five out of 20 rats (25.0%) in the eight-hour group developed prosthetic joint infection (PJI) and two out of 20 rats (10.0%) in the 72-hour group developed PJI. With two MRSA isolates, PJI was detected in six and seven out of 20 rats (30.0% and 35.0%), respectively.
To exclude the possibility of intraoperative bacterial contamination, we obtained intra-joint specimens for subsequent culturing with a sterile cotton swab before wound closure.
Acquisition of specimens for bacterial culture
Rats were sacrificed with an overdose of anaesthetic (pentobarbital sodium, 200 mg/kg) ten days after implant insertion in order to obtain specimens for bacterial culturing. Sterile instruments, surgical techniques, and tissue preparations were used throughout the entire specimen harvesting process. The surgical site was first draped in a fashion identical to that done for the simulated implant surgery. Specimens of the knee joint capsule, the femur, and the implanted K-wire were harvested. Tissues were placed in sterile vessels for subsequent culturing. For the bone and joint capsule specimens, the tissues were homogenized with a tissue grinder (Boster Biological Technology, Wuhan, China), and K-wires were sonicated to release bacteria from the biofilm on the wire before culturing.
Bacteria culturing and identification
All specimens were cultured on tryptic soy agar (BD Diagnostic Systems Europe) supplemented with 5% sheep blood (BD Diagnostic Systems Europe) and 10 µg/ml chloramphenicol (Sigma-Aldrich) for 48 hours at 37°C. The presence of PJI was defined as any positive bacterial culture from the bone, joint capsule, or implant. Antibiotic-resistant MRSA colonies, if present, were harvested for bacterial identification by 16S ribosomal DNA and GFP gene expression assessment to rule out contamination. Bacterial identification was conducted with 16S ribosomal DNA sequencing by using the MicroSeq 500 microbial identification system (ThermoFisher, Waltham, Massachusetts, USA). The expression of GFP in MRSA was assessed via real-time polymerase chain reaction (PCR) (Applied Biosystems (ABI); ThermoFisher) to confirm that the MRSA in the gut was the same as that isolated from the joint that caused PJI. Notably, the reliability of PJI diagnosis would be suspected if the sum of three colony counts (bone, joint capsule, and K-wire) was below 20. If present, the outcome from this subject would be abandoned. In this study, no such condition was encountered.
GFP-positive neutrophil isolation by flow cytometry
Neutrophils were first isolated from whole blood with a protocol used in previous studies.20,21 Briefly, a 5 ml syringe prepared with heparin (ten units) was prepared for blood collection. Rats were then euthanized with pentobarbital sodium (200 mg/kg) and whole blood was collected by cardiac puncture with a 20-gauge needle. We then centrifuged the blood at 200 g for ten minutes at room temperature. The whitish buffy coat layer at the plasma interface was collected after centrifugation. After washing with PBS, the pellet of white blood cells was resuspended in Hank’s balanced salt solution and layered on top of 1079 and 1119 Histopaque density gradient in a centrifuge tube. The solution was centrifuged at 700 g for 20 minutes at room temperature. The neutrophil-rich layer was collected at the interface between the Histopaque 1079 and 1119. Contaminating red blood cells were then removed by briefly lysing with hypotonic lysis buffer, followed by washing with PBS and additional centrifugation. The purity of isolated neutrophils was assessed using a flow cytometer (BD) with allophycocyanin (APC)-conjugated CD11b and PE-Cy7-conjugated CD 45 antibodies (BD) (Supplementary figure a).
After GFP-tagged MRSA is phagocytosed by neutrophils, the neutrophils can be sorted by flow cytometry on the basis of their GFP label fluorescence. Neutrophils were sorted into GFP-positive and GFP-negative subpopulations. A representative image of GFP-positive neutrophils is shown in Figure 3a. In this study, shown in Figure 3b, we used MRSA-carrying neutrophils isolated from other rats injected intravenously with MRSA (MNiv) and MRSA-carrying neutrophils isolated from other rats that had undergone intestinal MRSA colonization (MNin).
Fig. 3.
a) A representative image of green fluorescent protein (GFP)-positive neutrophils after flow cytometry isolation. GFP-negative cells obtained from isolation were used as control. b) Schematic definitions of methicillin-resistant Staphylococcus aureus (MRSA)-carrying neutrophils isolated from other rats injected intravenously with MRSA (MNiv) and MRSA-carrying neutrophils isolated from other rats that had undergone intestinal MRSA colonization (MNin).
To determine whether extracellular S. aureus was attached to neutrophils, isolated MNiv and MNin were incubated with S. aureus-specific antibody (Abcam, Cambridge, UK), followed by secondary antibodies conjugated with Cy5 (BosterBio). Cells were then harvested after incubation and analyzed using flow cytometry.
Assessment of intra-neutrophil MRSA viability
From the above isolation experiment, 10,000 MRSA-containing neutrophils (MNin and MNiv) and non-MRSA-containing neutrophils were lysed with 100 µl of autoclaved water, and then inoculated on tryptic soy agar (BD) supplemented with 5% sheep blood (BD) and 10 µg/ml chloramphenicol (Sigma-Aldrich). Bacterial colonies were quantified using ImageJ analysis (National Institute of Health and LOCI, Madison, Wisconsin, USA).
Prevention efficacy of membrane- and non-membrane-permeable antibiotics against PJI
We assessed whether MRSA carried in neutrophils can lead to PJI in the presence or absence of two antibiotics: gentamicin (a non-membrane-permeable antibiotic) and linezolid (a membrane-permeable antibiotic). Previously operated rats (see above, Sterile Surgical Procedures) were injected with MNin, and then two hours later were injected intravenously with either gentamicin (8 mg/kg) or linezolid (10 mg/kg). The strain of MRSA we used is normally susceptible to both gentamicin and linezolid. After ten days, the operated knee joints of the rats were cultured for MRSA, as before.
Statistical analysis
Differences in the proportion of MRSA-positive cultures between the two surgical groups were evaluated statistically using Fisher’s exact test. For comparing colony counts between the different groups, we used the non-parametric Mann-Whitney U test. SPSS v24.0 (SPSS, Armonk, New York, USA) was used for all statistical analyses. Statistical significance was established at p < 0.05.
Results
Establishment of intestinal MRSA colonization
Results from culturing of the shed stool were obtained for days 1, 3, 5, 7, and 9 after intestinal colonization of MRSA. MRSA was detected in all (n = 40) stools on days 1, 3, 5, and 7 after receiving antibiotics, being food-deprived, and undergoing gavage of MRSA. For day 9, the stools of 12 out of 40 rats (30.0%) were MRSA-positive (Figure 1b).Once ten days had passed after implant surgery, the rats were euthanized and samples from their intestines were cultured for the presence of MRSA. The intestines of all 40 rats were cultured after final sacrifice and all were positive for MRSA, suggesting that MRSA colonization of the intestines may be persistent, although we at times could not detect the bacteria with stool cultures (Figure 1b).
Intestinal MRSA colonization causes PJI
To determine whether intestinal MRSA carriage can cause PJI, a total of 40 rats were divided into two groups, with one group receiving surgery at eight hours and the other at 72 hours after intestinal MRSA colonization. Rats in both groups were sacrificed ten days after implant insertion. We cultured the operated joints of rats that underwent previous intestinal MRSA colonization for determining the presence of PJI. As a control for inadvertent surgical site MRSA contamination, we cultured tissue samples prior to wound closure. The intraoperative cultures from all 40 rats were negative, indicating the absence of intraoperative contamination. By contrast, cultures of the operated joints (culturing bone, joint capsule, and implant) revealed that five out of 20 rats (25.0%) in the eight-hour group developed PJI and two out of 20 rats (10.0%) in the 72-hour group developed PJI (Figure 2b). We then repeated the eight-hour group experiments with two MRSA isolates from PJI patients. The intraoperative cultures from all 40 rats were negative and PJI was detected in six and seven out of 20 rats (30.0% and 35.0%) respectively for the two isolates (Figure 2b). These results suggest that colonized MRSA originating from the gut can cause infections at distal surgical sites, in our experiment, at the operated knee joint with an intra-articular implant.
Wound-site MRSA exposure is not the cause of PJI after intestinal MRSA colonization
To exclude the possibility that postoperative wound site contamination with MRSA contributed to PJI in our paradigm, we assessed operated joints of 20 rats lacking prior experimental intestinal MRSA colonization. In this control, MRSA solution (1 ml/day of 1 × 108 CFUs/ml MRSA) was applied daily on the rats around the skin incision for ten days. Post-mortem cultures of the operated joints revealed that none of these rats developed PJI after ten days of wound site MRSA exposure.
Extracellular MRSA bacteraemia is not the cause of PJI after intestinal MRSA colonization
We then sought to exclude the possibility that PJI caused by intestine-derived MRSA actually occurs via extracellular bacteraemia. To address this possibility, we injected 20 rats with MRSA (1 × 108 CFUs) intravenously on three consecutive days after knee joint surgery. None of the 20 rats developed PJI, indicating that extracellular bacteraemia does not cause PJI in this experimental paradigm.
MRSA-carrying neutrophils isolated after intestinal MRSA colonization harbours viable MRSA
To determine whether intestinal MRSA reaches knee joints via a Trojan Horse mechanism, we analyzed the neutrophils of rats that had undergone intestinal MRSA colonization. Whole blood samples were collected from rats after 1, 3, 5, and 7 days of GFP-MRSA colonization, and neutrophils were isolated. Flow cytometry revealed that 15.3%, 7.5%, 5.6%, and 5.1% of the circulating neutrophils were GFP-MRSA positive at day 1, 3, 5, and 7, respectively (Figure 4a).
Fig. 4.

a) Bar graph showing the mean percentage of methicillin-resistant Staphylococcus aureus (MRSA)-harbouring neutrophils in rats 1, 3, 5, and 7 days after intestinal colonization with MRSA and eight hours after intravenous injection of MRSA. b) Bar graph showing the mean number of colony-forming units (CFUs) of neutrophil samples 1, 3, 5, and 7 days after intestinal colonization with MRSA and eight hours after intravenous injection of MRSA. Error bars: SD.
Next, we cultured cellular lysates derived from 10,000 of these MNin and non-MRSA-containing neutrophils. We also cultured samples of blood plasma from the rats to exclude the possibility of extracellular bacteraemia following intestinal MRSA colonization. As expected, no MRSA-positive colonies formed on either the cultures of plasma or lysates from non-MRSA-containing neutrophils. By contrast, MRSA-positive colonies formed on the cultures of lysates from MNin. The colony counts per thousand MNin were stable throughout the seven-day study period (Figure 4b).
Intravenous injection of MNin causes PJI
To further confirm that PJI develops from MRSA carried within neutrophils, we intravenously injected naïve rats with MNin (1 × 106 cells). The naïve rats received injections for three consecutive days after knee surgery. Samples from the rats’ joints were cultured ten days after surgery as before, and we observed that 11 out of 20 rats (55.0%) developed PJI. These results suggest that intestinal MRSA carried by neutrophils could cause PJI in our rat model.
MRSA-carrying neutrophils isolated after intravenous injection of MRSA harbour fewer viable MRSA
As mentioned above, direct intravenous injection of MRSA leads to no PJI development. We next explored the potential mechanism. After intravenous injection of MRSA (1 × 108 CFUs), GFP-tagged MRSA was detected by flow cytometry in 55.2% of circulating neutrophils eight hours later (Figure 4a). This result indicated that circulating neutrophils would also harbour MRSA after intravenous injection of MRSA.
We then assessed whether these MNiv could also cause PJI in naïve rats. To test this idea, we repeated the above experiment but this time injected naïve rats with MNiv instead of MNin. None of the rats developed PJI.
To understand the different infectivity of MNin and MNiv, we next assessed the viability of MRSA in these rats by culturing cell lysates derived from MNiv. The number of resulting MRSA-positive colonies was less than 1% of MNin (Figure 4b). The mean colony number was 2.2 (SD 2.5), and the mean colony number in each of the four MNin groups (days 1, 3, 5, and 7) was 250.6 (SD 113.6), 234.7 (SD 98.3), 245.7 (SD 95.4), and 267.3 (SD 123.5), respectively. We recollected MRSA derived from MNin and MNiv. We then established intestinal colonization with either one of these two MRSA isolates (MNin-derived or MNiv-derived). After surgery, PJI was detected in four out of 20 rats for both groups. Direct intravenous injection of either one of these two MRSA isolates would not cause PJI in our rat model. These results together suggested that injection of MNiv did not lead to PJI mainly because MNiv carried fewer viable MRSA.
Prevention efficacy of membrane-permeable and non-membrane-permeable antibiotics
To further confirm that the gut-to-implant metastasis of MRSA takes place via a Trojan Horse mechanism, we assessed the ability of MNin injection to cause PJI in the presence or absence of two antibiotics: gentamicin (a non-membrane-permeable antibiotic) and linezolid (a membrane-permeable antibiotic). Membrane-permeable antibiotics are able to kill intracellular bacteria in contrast to non-membrane-permeable antibiotics. If the Trojan Horse mechanism operates in our paradigm, we would expect a lower incidence of PJI among linezolid-treated rats.
First, rats were injected with MNin, and then two hours later, injected intravenously with either gentamicin (8 mg/kg) or linezolid (10 mg/kg). The strain of MRSA we used is susceptible to both gentamicin and linezolid. Of the rats that received gentamicin, ten out of 20 (50.0%) developed PJI. By contrast, of the rats that received linezolid, only one out of 20 (5.0%) developed PJI. Furthermore, we treated MNin with control, gentamicin (16 μg/ml), or linezolid (20 μg/ml) for four hours before intravenous injection. After intravenous injection of these pretreated MNin, no rat (zero out of 20) developed PJI in the linezolid group in contrast to seven out of 20 in the control group and six out of 20 in the gentamicin group.
These findings show that the membrane-permeable antibiotic linezolid is more effective in preventing PJI in this model than the non-membrane-permeable antibiotic gentamicin. The higher incidence of PJI among gentamicin-treated rats is consistent with the Trojan Horse hypothesis in that MRSA sequestered in neutrophils was shielded from this non-membrane-permeable antibiotic and thus able to eventually reach and infect the knee joints. The incidence of PJI among control and gentamicin- and linezolid-treated rats is summarized in Table I.
Table I.
Percentage of positive cultures by treatment group (n = 20 per group)
| Result of bacterial culture | Numbers of indicated cultures (%) | ||
|---|---|---|---|
| Control | Gentamicin | Linezolid* | |
| MRSA-positive | 11 (55) | 10 (50) | 1 (5) |
| MRSA-negative | 9 (45) | 10 (50) | 19 (95) |
| p-value vs control† | N/A | 0.996 | < 0.001 |
p < 0.05 versus control.
Fisher’s exact test.
MRSA, methicillin-resistant Staphylococcus aureus; N/A, not applicable.
All bacteria grown on culture in this study were identified as the identical strain of inoculated MRSA without colonization of any other bacteria, indicating that there was no unexpected microbial contamination. No extracellular MRSA was detected from MNiv and MNin (Supplementary figure b). Different treatments and their PJI incidence are summarized in Table II. Key findings of this study are summarized in Figure 5.
Table II.
Summary of treatment and prosthetic joint infection incidence (n = 20 per group). No intraoperative contamination was found in any of the groups
| Group | Treatment | PJI incidence, % (positive n/total n) | p-value* |
|---|---|---|---|
| 1 | Preoperative intestinal MRSA colonization and implant insertion after eight hours | 25 (5/20) | 0.007 |
| 2 | Preoperative intestinal MRSA colonization and implant insertion after 72 hours | 10 (2/20) | 0.007 |
| 3 | Implant insertion and postoperative application of MRSA on wound skin | 0 (0/20) | 0.007 |
| 4 | Implant insertion and postoperative intravenous injection of MRSA | 0 (0/20) | 0.007 |
| 5 | Implant insertion and postoperative intravenous injection of MNin | 50 (10/20) | < 0.001 |
| 6 | Implant insertion and postoperative intravenous injection of MNiv | 0 (0/20) | < 0.001 |
| 7 | Treatment of Group 5 and as control for Groups 8 and 9 | 55 (11/20) | 0.001 |
| 8 | Treatment of Group 5 and prevention attempt with gentamicin | 50 (10/20) | 0.001 |
| 9 | Treatment of Group 5 and prevention attempt with linezolid | 5 (1/20) | 0.001 |
| 10 | Implant insertion and postoperative intravenous injection of control MNin | 35 (7/20) | < 0.001 |
| 11 | Implant insertion and postoperative intravenous injection of linezolid-treated MNin | 0 (0/20) | < 0.001 |
| 12 | Implant insertion and postoperative intravenous injection of gentamicin-treated MNin | 30 (6/20) | < 0.001 |
Fisher’s exact test.
MNin, intestinal MRSA colonization; MNiv, MRSA-carrying neutrophils isolated from other rats injected intravenously with MRSA; MRSA, methicillin-resistant Staphylococcus aureus; PJI, prosthetic joint infection.
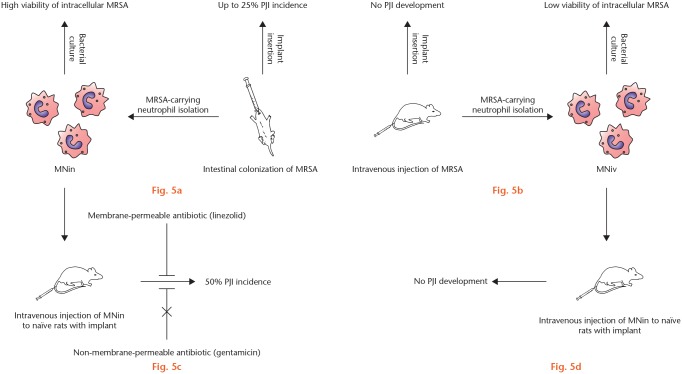
Fig. 5.
Summary of key findings. a) Preoperative intestinal methicillin-resistant Staphylococcus aureus (MRSA) colonization and implant insertion after eight or 72 hours. b) Implant insertion and postoperative intravenous injection of MRSA. c) Implant insertion and postoperative intravenous injection of MRSA-carrying neutrophils isolated from other rats that had undergone intestinal MRSA colonization (MNin). d) Implant insertion and postoperative intravenous injection of MRSA-carrying neutrophils isolated from other rats injected intravenously with MRSA (MNiv). PJI, prosthetic joint infection.
Discussion
In the present study, we sought to determine whether intestine-colonized MRSA was capable of infecting the knee joint after prosthetic implant surgery, and if so, by what mechanism. We found that rats with experimental MRSA intestinal colonization were able to cause PJI. Moreover, we found that neutrophils (and MRSA sequestered within them) may be responsible for covertly delivering MRSA distally to the knee joint to cause infection.
Haematogenous pathogens are an important source in PJIs.22 Nasal-carried MRSA is a preoperative risk factor for PJI.23–25 However, the nasal cavity is not necessarily the direct origin of pathogens that cause PJI because the nasal carriage and carriage in other sites including the intestine usually overlap.26,27 It was found that patients receiving liver transplants with both nasal and intestinal colonization had significantly increased rates of S. aureus infection of 40% (21 out of 52) compared with an infection rate of 18% (8 out of 44) in patients with nasal carriage without intestinal carriage.28 Like many other infections, intestinal MRSA carriage might be an important but previously ill-defined source of MRSA in PJI.
The bacteraemia is previously considered to be responsible for the metastasis of pathogen from carriage to surgical site.29 However, a recent study revealed that in one mouse model of liver resection, intestinal MRSA was shown to cause surgical site infections via a Trojan Horse mechanism instead of extracellular bacteraemia.19 Our present findings are consistent with results from the mouse study. We demonstrated that metastasis of intestinal MRSA could cause distal infections via infected neutrophils in a rat model with intra-articular implant. Together, we just showed a possibility that intestinal colonization of MRSA might underlie the association between nasal carriage of MRSA and PJI. Further clinical evidence is clearly needed to strengthen the hypothesis of an association between PJI and intestinal carriage of MRSA.
We observed in our study that intravenous injection of MRSA (mimicking extracellular bacteraemia) failed to cause PJI. Although more than half of the neutrophils phagocytosed MRSA after the intravenous MRSA injection, the viability of these MRSA was very low. This was consistent with the experiment in which rats received injections of MRSA-carrying neutrophils isolated from other rats intravenously injected with MRSA. These rats did not develop PJI, suggesting that the host immune system could well defend the extracellular bacteraemia. These findings contrast widely with those obtained from rats that had undergone intestinal colonization of MRSA. Although the proportion of MRSA-carrying neutrophils in these rats was relatively low, the viability of intracellular MRSA was much higher. In fact, rats developed PJI after receiving intravenous injection of MRSA-carrying neutrophils that were isolated from rats after intestinal colonization. Taken together, these results suggest that the gut MRSA may differ from other sources of MRSA in their ability to remain hidden within neutrophils for longer periods of time, long enough to be carried to and cause infections in distant locations within the host.
The relationship between an antibiotic and its effect is complicated in vivo, especially for those intracellular bacteria. We demonstrated that the incidence of PJI is higher in rats treated with gentamicin, a non-membrane-permeable antibiotic, than rats treated with linezolid, a membrane-permeable antibiotic. Although gentamicin could kill intracellular bacteria after prolonged culture at a high concentration, the in vivo efficacy of gentamicin is usually poor due to the limitation of host tolerance.17,30 In this study we therefore described the gentamicin as a “non-membrane-permeable antibiotic” like many previous studies.17
Most previous studies are focused on the elimination of nasal rather than intestinal carriage.31 As a result, no reliable method has been reported for eradication of intestinal MRSA carriage. The use of oral rifampin and vancomycin for treatment of intestinal carriage of S. aureus for various patient and healthy populations showed limited success in MRSA elimination and infection prevention.32,33 Development and evaluation of protocols for decolonization of intestinal MRSA are urgently needed.
Although our study provided strong evidence for the Trojan Horse mechanism in PJI, our study had some limitations. First, rats clearly have a different immune response compared to humans, so the applicability of our results to humans is limited. Despite tremendous efforts, we are currently unable to create a model with persistent positive stool culture. The rate of MRSA-positive stool culture decreased dramatically after nine days. In contrast, the mean detection rate of intestinal carriage in healthy individuals is 20% (349/1,766) for S. aureus and 9% (112/1,538) for MRSA according to a previous report.26 Although the Trojan Horse mechanism has been well documented in human cells,34 more clinical evidence is needed to support the reality of this mechanism, especially evidence related to the PJI context. Second, we focused on neutrophils as being the Trojan Horse instead of monocytes. Monocytes are a type of phagocyte that are also capable of engulfing MRSA and may contribute to the gut-to-implant metastasis of MRSA in PJI. We did not assess the role of monocytes in PJI, because isolating a sufficient quantity of monocytes needed for the types of experiments we did is extremely challenging, as monocytes account for less than 5% of white blood cells in rats. In addition, other non-phagocytic immune cell types could also be involved in this Trojan Horse mechanism. Finally, we actually increased the bacterial burden of MRSA in gut to an extremely high level. This is quite different from a clinical setting. The virulence of intestinal MRSA in patients who are not exposed to such a high bacterial burden, and who remained protected by commensal intestinal microbiota, is expected to be significantly lower. This might be true given the high rate of MRSA carriage and much lower PJI incidence in a general population.
In conclusion, neutrophils as covert carriers of intestinal MRSA may play an important role in PJI development.
Acknowledgments
Hongyi Zhu and Hanqiang Jin contributed equally to this study. We thank Dr. Xiaolin Liu for providing the green fluorescent protein (GFP)-carrying methicillin-resistant Staphylococcus aureus (MRSA) strains.
Footnotes
Author contributions: H. Zhu: Analyzed the data, Wrote the manuscript.
H. Jin: Performed the experiments.
C. Zhang: Designed the study.
T. Yuan: Acquired the funding.
ICMJE COI statement: None declared
Ethical review statement: All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of our hospital.
Follow us @BoneJointRes
Supplementary material
Figure a demonstrates the purity of neutrophils after isolation with the Histopaque method. Figure b shows that no Staphylococcus aureus was attached to the cell membrane of isolated neutrophils.
Funding statement
This study was supported by the National Natural Science Foundation of China (81572239).
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Kheir MM, Tan TL, Kheir M, Maltenfort MG, Chen AF. Postoperative Blood Glucose Levels Predict Infection After Total Joint Arthroplasty. J Bone Joint Surg Am. 2018;100(16):1423-1431. [DOI] [PubMed] [Google Scholar]
- 2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J. 2015;97-B(6):793-801. [DOI] [PubMed] [Google Scholar]
- 4. Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87(8):1746-1751. [DOI] [PubMed] [Google Scholar]
- 5. Gwam CU, Mistry JB, Mohamed NS, et al. Current Epidemiology of Revision Total Hip Arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J Arthroplasty. 2017;32(7):2088-2092. [DOI] [PubMed] [Google Scholar]
- 6. Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128-133. [DOI] [PubMed] [Google Scholar]
- 7. Delanois RE, Mistry JB, Gwam CU, Mohamed NS, Choksi US, Mont MA. Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J Arthroplasty. 2017;32(9):2663-2668. [DOI] [PubMed] [Google Scholar]
- 8. Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of Total Hip and Knee Replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 Suppl):61-5.e1. [DOI] [PubMed] [Google Scholar]
- 10. McConoughey SJ, Howlin R, Granger JF, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014;9(8):987-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Block JE, Stubbs HA. Reducing the risk of deep wound infection in primary joint arthroplasty with antibiotic bone cement. Orthopedics. 2005;28(11):1334-1345. [DOI] [PubMed] [Google Scholar]
- 12. Springer BD. The Diagnosis of Periprosthetic Joint Infection. J Arthroplasty. 2015;30(6):908-911. [DOI] [PubMed] [Google Scholar]
- 13. Lora-Tamayo J, Senneville É, Ribera A, et al. Group of Investigators for Streptococcal Prosthetic Joint Infection. The Not-So-Good Prognosis of Streptococcal Periprosthetic Joint Infection Managed by Implant Retention: The Results of a Large Multicenter Study. Clin Infect Dis. 2017;64(12):1742-1752. [DOI] [PubMed] [Google Scholar]
- 14. Bloch BV, Shah A, Snape SE, Boswell TCJ, James PJ. Primary hip and knee arthroplasty in a temporary operating theatre is associated with a significant increase in deep periprosthetic infection. Bone Joint J. 2017;99-B(7):917-920. [DOI] [PubMed] [Google Scholar]
- 15. Richards RL, Haigh RD, Pascoe B, et al. Persistent Staphylococcus aureus isolates from two independent cases of bacteremia display increased bacterial fitness and novel immune evasion phenotypes. Infect Immun. 2015;83(8):3311-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol. 2014;192(10):4709-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thwaites GE, Gant V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol. 2011;9(3):215-222. [DOI] [PubMed] [Google Scholar]
- 18. Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164(7):3713-3722. [DOI] [PubMed] [Google Scholar]
- 19. Krezalek MA, Hyoju S, Zaborin A, et al. Can Methicillin-resistant Staphylococcus aureus Silently Travel From the Gut to the Wound and Cause Postoperative Infection? Modeling the “Trojan Horse Hypothesis”. Ann Surg. 2018;267(4):749-758. [DOI] [PubMed] [Google Scholar]
- 20. Swamydas M, Luo Y, Dorf ME, Lionakis MS. Isolation of Mouse Neutrophils. Curr Protoc Immunol. 2015;110:3.20.1-3.20.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar S, Jyoti A, Keshari RS, Singh M, Barthwal MK, Dikshit M. Functional and molecular characterization of NOS isoforms in rat neutrophil precursor cells. Cytometry A. 2010;77(5):467-477. [DOI] [PubMed] [Google Scholar]
- 22. Parvizi J, Shohat N, Gehrke T. Prevention of periprosthetic joint infection: new guidelines. Bone Joint J. 2017;99-B(4 Supple B):3-10. [DOI] [PubMed] [Google Scholar]
- 23. Ratto N, Arrigoni C, Rosso F, et al. Total knee arthroplasty and infection: how surgeons can reduce the risks. EFORT Open Rev. 2017;1(9):339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sousa RJ, Barreira PM, Leite PT, Santos AC, Ramos MH, Oliveira AF. Preoperative Staphylococcus aureus Screening/Decolonization Protocol Before Total Joint Arthroplasty-Results of a Small Prospective Randomized Trial. J Arthroplasty. 2016;31(1):234-239. [DOI] [PubMed] [Google Scholar]
- 25. Tsang STJ, McHugh MP, Guerendiain D, et al. Underestimation of Staphylococcus aureus (MRSA and MSSA) carriage associated with standard culturing techniques: one third of carriers missed. Bone Joint Res. 2018;7(1):79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Acton DS, Plat-Sinnige MJ, van Wamel W, de Groot N, van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis. 2009;28(2):115-127. [DOI] [PubMed] [Google Scholar]
- 27. Miko BA, Uhlemann AC, Gelman A, et al. High prevalence of colonization with Staphylococcus aureus clone USA300 at multiple body sites among sexually transmitted disease clinic patients: an unrecognized reservoir. Microbes Infect. 2012;14(12):1040-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Squier C, Rihs JD, Risa KJ, et al. Staphylococcus aureus rectal carriage and its association with infections in patients in a surgical intensive care unit and a liver transplant unit. Infect Control Hosp Epidemiol. 2002;23(9):495-501. [DOI] [PubMed] [Google Scholar]
- 29. Marshall C, McBryde E. The role of Staphylococcus aureus carriage in the pathogenesis of bloodstream infection. BMC Res Notes. 2014;7:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang L, Greene MK, Insua JL, et al. Clearance of intracellular Klebsiella pneumoniae infection using gentamicin-loaded nanoparticles. J Control Release. 2018;279:316-325. [DOI] [PubMed] [Google Scholar]
- 31. Vergnano S. Decolonization and decontamination: what’s their role in infection control? Curr Opin Infect Dis. 2015;28(3):207-214. [DOI] [PubMed] [Google Scholar]
- 32. Falagas ME, Fragoulis KN, Bliziotis IA. Oral rifampin for prevention of S. aureus carriage-related infections in patients with renal failure—a meta-analysis of randomized controlled trials. Nephrol Dial Transplant. 2006;21(9):2536-2542. [DOI] [PubMed] [Google Scholar]
- 33. Silvestri L, van Saene HK, Milanese M, et al. Prevention of MRSA pneumonia by oral vancomycin decontamination: a randomised trial. Eur Respir J. 2004;23(6):921-926. [DOI] [PubMed] [Google Scholar]
- 34. Flannagan RS, Heit B, Heinrichs DE. Intracellular replication of Staphylococcus aureus in mature phagolysosomes in macrophages precedes host cell death, and bacterial escape and dissemination. Cell Microbiol. 2016;18(4):514-535. [DOI] [PubMed] [Google Scholar]