Highlights
-
•
A sub-group of bipolar patients have extensive blood-brain barrier leakage
-
•
These patients have a more chronic form of illness, greater depression and anxiety
-
•
All patients with extensive blood-brain barrier leakage are also insulin resistant
Keywords: Blood-brain barrier, Dynamic contrast-enhanced MRI, Bipolar disorder, Depression, Anxiety, Insulin resistance
Abstract
Bipolar disorder affects approximately 2% of the population and is typically characterized by recurrent episodes of mania and depression. While some patients achieve remission using mood-stabilizing treatments, a significant proportion of patients show progressive changes in symptomatology over time. Bipolar progression is diverse in nature and may include a treatment-resistant increase in the frequency and severity of episodes, worse psychiatric and functional outcomes, and a greater risk of suicide. The mechanisms underlying bipolar disorder progression remain poorly understood and there are currently no biomarkers for identifying patients at risk. The objective of this study was to explore the potential of blood-brain barrier (BBB) imaging as such a biomarker, by acquiring the first imaging data of BBB leakage in bipolar patients, and evaluating the potential association between BBB dysfunction and bipolar symptoms. To this end, a cohort of 36 bipolar patients was recruited through the Mood Disorders Clinic (Nova Scotia Health Authority, Canada). All patients, along with 14 control subjects (matched for sex, age and metabolic status), underwent contrast-enhanced dynamic MRI scanning for quantitative assessment of BBB leakage as well as clinical and psychiatric evaluations. Outlier analysis has identified a group of 10 subjects with significantly higher percentages of brain volume with BBB leakage (labeled the “extensive BBB leakage” group). This group consisted exclusively of bipolar patients, while the “normal BBB leakage” group included the entire control cohort and the remaining 26 bipolar subjects. Among the bipolar cohort, patients with extensive BBB leakage were found to have more severe depression and anxiety, and a more chronic course of illness. Furthermore, all bipolar patients within this group were also found to have co-morbid insulin resistance, suggesting that insulin resistance may increase the risk of BBB dysfunction in bipolar patients. Our findings demonstrate a clear link between BBB leakage and greater psychiatric morbidity in bipolar patients and highlight the potential of BBB imaging as a mechanism-based biomarker for bipolar disorder progression.
1. Introduction
Bipolar disorder affects approximately 2% of the population, and is characterized by episodes affecting mood, activity levels, and ability to carry out day-to-day tasks (Kessler et al., 2005). A growing body of evidence suggests that treatment-resistant disease progression is common in bipolar patients, and may include a shift towards more frequent and severe episodes, worse depression, anxiety, socio/occupational dysfunction and increased risk of suicide (Hui et al., 2015). While the pathophysiology of bipolar disorder remains poorly understood, converging evidence points to the presence of neuroinflammation in bipolar patients (Naaldijk et al., 2016; Patel and Frey, 2015). In light of the increasingly recognized role of the brain's microvasculature in neuroinflammation, here we set out to examine the potential link between blood brain barrier (BBB) dysfunction and bipolar disorder progression.
Under normal conditions the BBB restricts the entry of most blood-derived factors into the brain. This tight regulation of the brain's environment is necessary for proper neuronal activity and is performed by the tightly connected membrane of endothelial cells within brain microvessels, and the mural and astrocytic cells engulfing it (Abbott et al., 2010). Hence, disruption of this complex interface allows the extravasation of blood-derived factors into the brain, causing neuroinflammatory responses which can initiate various pathways of neural dysfunction and degeneration (Sweeney et al., 2018).
In the past two decades dynamic contrast-enhanced (DCE-) magnetic resonance imaging (MRI) has been gaining popularity as the method of choice for assessing BBB leakage in living subjects (Heye et al., 2014). In this method, repeated scans of the brain are acquired to capture signal changes due to cross-BBB extravasation of an intravenously injected contrast agent. As the current gold standard for BBB assessment still relies on post-mortem tissue analysis, cross-validation of the two methods in the same individuals is not a trivial task. However, DCE-MRI studies have been used to identify BBB dysfunction in disorders such as multiple sclerosis (Ingrisch et al., 2012; Cramer et al., 2014; Varatharaj, 2019; Haider et al., 2019), stroke (Merali et al., 2017; Kassner et al., 2009; Serlin et al., 2019), brain tumors (Bergamino et al., 2013), epilepsy (Bar-Klein et al., 2017), traumatic brain injury (Weissberg et al., 2014; Tomkins et al., 2008), and dementia (Sweeney et al., 2018; Nation et al., 2018) — all of which were previously linked to BBB impairment in post-mortem studies. Moreover, while a large-scale validation of this approach is yet to be performed, two recent studies have successfully demonstrated the method's reproducibility (Wong et al., 2017) and biological validity (Varatharaj, 2019).
To date DCE-MRI has not been applied to the study of psychiatric disorders and the clinical correlates of BBB dysfunction in these disorders remain unknown. Thus, the goal of the present study was to obtain the first imaging evidence of BBB dysfunction in bipolar patients, and to test the association between BBB pathology and disease severity.
2. Results
2.1. Participants
A cohort of 36 bipolar patients was recruited for the study (23 bipolar type I and 13 bipolar type II). The average duration of illness among the patients was 28 ± 13 years, with an average onset at 22 ± 10 years of age. Bipolar disorder started with a depressive episode in 76% of patients, and about half (55%) have progressed to a chronic course of illness. The average age of the group was 49.1 ± 11.3 years and 70.6% were females. Control subjects were matched for sex, age and metabolic syndrome (Table 1). Compared to controls, bipolar patients scored significantly worse on scales of depression (Montgomery-Ǻsberg Depression Rating Scale), anxiety (Hamilton Anxiety Rating Scale), and capacity of carrying out day-to-day functions (Global Assessment of Functioning, Table 1). No differences in anthropometric or metabolic measures were identified between the groups (Table 1).
Table 1.
Participant characteristics.
| Bipolar Patients | Controls | p value | |
|---|---|---|---|
| Demographics | |||
| Age | 49.1 (1.9) | 47.6 (2.9) | 0.666 |
| Sex (% female) | 70.6 | 71.4 | 1.000 |
| Anthropometric and metabolic measures | |||
| Body mass index (BMI) | 30.1 (1.1) | 28.2 (1.5) | 0.358 |
| Waist-to-hip ratio | 0.9 (0.02) | 0.9 (0.03) | 0.230 |
| Risk of cardiovascular disease (Framingham risk score, Wilson et al., 1998) | 8.6 (1.5) | 4.9 (0.8) | 0.469 |
| Framingham heart age (Wilson et al., 1998) | 53.0 (5.3) | 47.7 (2) | 0.602 |
| Metabolic syndrome (Alberti et al., 2009) (% subjects) | 27.8 | 15.4 | 0.474 |
| Insulin resistance (HOMA-IR score, Esteghamati et al., 2010) | 2.7 (0.3) | 1.7 (0.2) | 0.056 |
| Psychiatric characteristics | |||
| Depression severity (MADRS score, Hawley et al., 2002) | 18.1 (2.4) | 1.9 (0.4) | <0.001 |
| Anxiety severity (HAM-A score, Leentjens et al., 2011) | 11.8 (1.5) | 2.0 (0.4) | <0.001 |
| Global Assessment of Functioning (GAF score, Hall, 1995) | 66.8 (0.5) | 92.1 (0.7) | <0.001 |
| Medication use (% patients) | |||
| Lithium | 72 | .. | .. |
| Antiepileptics | 67 | .. | .. |
| Atypical antipsychotics | 56 | .. | .. |
| Antidepressants | 44 | .. | .. |
| Benzodiazepines | 56 | .. | .. |
| Melatonin | 19 | 0 | 0.169 |
| Blood pressure medication | 14 | 14 | 1.000 |
| Cholesterol medication | 14 | 0 | 0.304 |
Mean (standard error), unless otherwise indicated. Continuous variables were compared using the Wilcoxon rank sum test, and categorical variables were compared using Fisher's Exact Test. MADRS, Montgomery-Ǻsberg Depression Rating Scale; HAM-A, Hamilton Anxiety Rating Scale; HOMA-IR, homeostatic model assessment of insulin resistance.
2.2. A sub-group of bipolar patients have a significantly higher level of BBB dysfunction
All participants underwent DCE-MRI scanning and quantitative maps of leakage rates were calculated for each brain voxel (Veksler et al., 2014; Chassidim et al., 2013) (Fig. 1A). The percent of brain tissue with pathological leakage rates was used as an overall measure of BBB dysfunction, revealing a high variability of values among the bipolar cohort (Fig. 1B). In order to identify subjects with significantly higher levels of BBB dysfunction, an outlier analysis was performed (based on the median + two standard deviations of all 50 subjects). Ten of the 50 subjects were identified as outliers (subjects with over 12.2% of the brain affected by leakage). The outlier group consisted exclusively of bipolar patients, and was labeled the “extensive BBB leakage” group. The group with the lower level of BBB dysfunction included the entire control cohort as well as the remaining 26 bipolar patients. Since there were no differences between the controls and patients within this group (Fig. 1C), it was collectively referred to as the “normal BBB leakage” group. To examine whether the differences between bipolar patients with extensive vs normal leakage were widespread (diffuse) or restricted to specific brain regions (focal), region-specific leakage was quantified in 126 anatomically/functionally significant brain regions and compared between the two groups. The comparison revealed a diffuse rather than focal difference, with 112 of the 126 regions showing significantly higher leakage in the “extensive BBB leakage” group (Fig. 1D, p < 0.05, corrected for multiple comparisons).
Fig. 1.
A sub-group of bipolar patients have extensive BBB leakage. A. The rate of BBB leakage was quantified for every brain voxel, with shades of blue representing tissue with non-permeable BBB and shades of red representing contrast agent accumulation due to BBB leakage. Representative leakage maps of five bipolar patients showcase the different extents of leakage among the bipolar cohort (displayed slices were selected to represent maximal BBB leakage in each subject). B. The overall percent of brain tissue with pathological leakage was calculated for all patients and controls, revealing a high variability of values among the bipolar cohort. C. Outlier analysis of all 50 subjects has identified a group with “extensive BBB leakage”, consisting of ten bipolar patients, and a group with “normal BBB leakage”, consisting of 26 patients and 14 controls (p < 0.0001). D. Compared to bipolar patients with normal BBB leakage, the “extensive BBB leakage” group had significantly higher levels of leakage in 112 of the 126 regions (Wilcoxon rank sum test with a false discovery rate correction for multiple comparisons).
2.3. Extensive BBB leakage in bipolar patients is associated with greater psychiatric morbidity
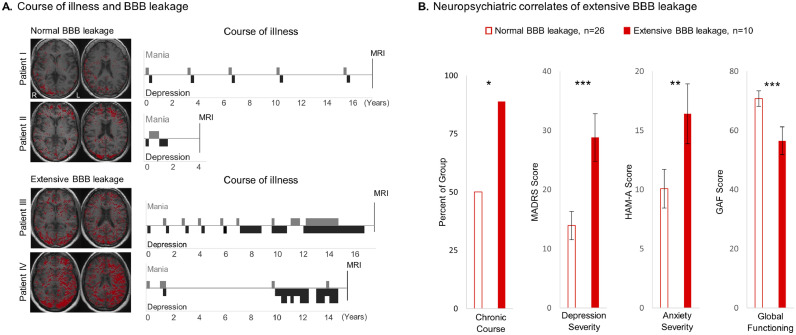
To examine whether a higher level of BBB dysfunction corresponds to a worse bipolar outcome, we next compared the course of illness among the groups (episodic versus chronic), as well as levels of depression, anxiety, and socio/occupational functioning. Bipolar patients with extensive BBB leakage were found to have higher rates of chronic illness with more frequent and/or severe manic/depressive episodes (as exemplified in Fig. 2A and quantified in Fig 2B). Moreover, extensive BBB leakage was found to be associated with greater severity of depression, anxiety, and socio/occupational dysfunction (Fig. 2B). No associations between BBB pathology and age or disease duration were found in our cohort.
Fig. 2.
Extensive BBB leakage in bipolar patients is associated with a worse neuropsychiatric status. A. Representative courses of illness show an episodic course in patients with normal BBB leakage (patients I and II, red pixels representing tissue with leaky BBB), and a progression towards a chronic course in patients with extensive BBB leakage (patients III and IV). B. Quantitative analysis confirmed the higher incidence of a chronic (vs episodic) course of illness among patients with extensive BBB leakage. Extensive BBB leakage was also associated with a greater severity of depression (Montgomery–Åsberg Depression Rating Scale, MADRS), elevated anxiety (Hamilton Anxiety Rating Scale, HAM-A), and worse socio/occupational functioning (Global Assessment of Functioning, GAF). The Wilcoxon rank sum test and the Chi square test were used for comparisons of continuous and categorical variables, respectively. Error bars denote standard error of the mean. Asterisks denote level of significance, with * for p ≤ 0.05, ** for p ≤ 0.01, and *** for p ≤ 0.001.
2.4. Extensive BBB leakage is associated with metabolic dysregulation, yet not with class of mood-stabilizing drugs
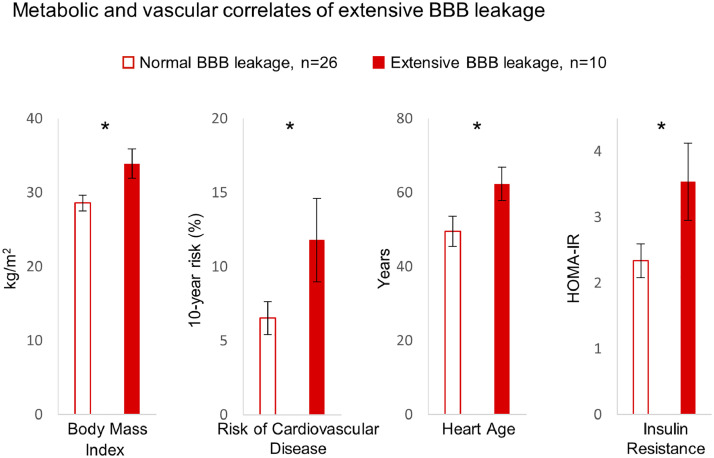
Bipolar patients with extensive BBB leakage were found to have higher body-mass indices, elevated risk of cardiovascular disease, and advanced heart age (Fig. 3). Furthermore, all patients within the “extensive BBB leakage” group were also found to have comorbid insulin resistance (homeostatic model assessment of insulin resistance >1.8, Esteghamati et al., 2010). Notably, while all subjects with extensive BBB leakage had insulin resistance, not all subjects with insulin resistance had extensive BBB leakage (with four insulin resistant controls and 12 insulin resistant bipolar patients having BBB leakage within the normal range). No patients were receiving anti-diabetic or insulin sensitizing drugs. No differences in the class of mood stabilizing treatments were found between the normal and extensive BBB leakage groups.
Fig. 3.
Extensive BBB leakage is associated with metabolic dysregulation. Bipolar patients with extensive BBB leakage were found to have higher body mass indices, increased risk of cardiovascular disease, advanced heart age, and higher levels of insulin resistance. Statistical comparisons were conducted using the Wilcoxon rank sum test. Error bars denote standard error of the mean. Asterisks denote p ≤ 0.05. HOMA-IR, homeostatic model assessment of insulin resistance.
3. Discussion
This study presents the first imaging of BBB leakage in bipolar patients. Using dynamic contrast-enhanced MRI, we show that a higher level of dysfunction (i.e. extensive BBB leakage), affects 28% of the bipolar cohort and none of the controls. The study further shows that patients with extensive BBB leakage experience a more chronic form of illness with greater severity of depression, anxiety and socio/occupational dysfunction. Our findings suggest that BBB imaging holds potential as a mechanism-based biomarker for the psychiatric deterioration experienced by a sub-group of bipolar patients.
We propose that BBB leakage may impact the functionality of the affected brain regions, leading to region-associated symptomatology. This hypothesis is supported by a recent imaging study in subjects with cognitive decline, linking cognitive impairment to BBB dysfunction in the hippocampus (Nation et al., 2018). Larger cohort studies are needed to determine the association between region-specific BBB dysfunction and the diverse symptoms of bipolar progression. Moreover, future large-scale prospective studies are needed for assessing the sensitivity and specificity of DCE-MRI based BBB assessment, before it can become part of routine medical practice.
Our study also links BBB damage in bipolar patients to insulin resistance, in line with recent evidence associating insulin resistance with vascular dysfunction in the brain and increased risk of dementia (Hughes and Craft, 2016). Insulin resistance is known to be more common in bipolar patients compared to the general population (Brietzke et al., 2011; Calkin et al., 2015), yet the mechanisms underlying this phenomenon remain poorly understood (Calkin, 2019). While atypical antipsychotics were suggested to cause insulin resistance in patients with bipolar disorder and schizophrenia (Vancampfort et al., 2016; Burghardt et al., 2018; Correll et al., 2008), we found no association between the use of atypical antipsychotics and insulin resistance or BBB leakage. Future studies are warranted to better understand the high rates of insulin resistance observed in bipolar patients.
While our study is the first to show direct imaging evidence of BBB dysfunction in psychiatric patients, future studies are needed to fully elucidate the mechanisms mediating the psychiatric decline associated with BBB dysfunction and to determine whether vascular-protecting therapies may prove beneficial for bipolar disorder treatment.
4. Conclusions
Our study reveals an association between BBB pathology and worse psychiatric morbidity in bipolar patients. Our findings further suggest that BBB imaging offers promise as a new biomarker for bipolar disorder progression.
5. Methods
5.1. Participants
The study was approved by the Nova Scotia Health Authority Research Ethics Board (1021507). A total of 36 adult patients (over 18 years of age) were recruited to the study through the Mood Disorders Clinic (Nova Scotia Health Authority, Canada). Subjects underwent a detailed psychiatric interview using the schedule for affective disorders and schizophrenia (SADS-L, Endicott and Spitzer, 1978) to diagnose bipolar disorder. Diagnoses required a consensus of at least 3 psychiatrists and were based on the DSM-5 criteria (American Psychiatric Association 2013). As patients with type I versus II bipolar disorder differ primarily in severity of manic episodes, we did not exclude patients based on this criterion. Mood symptoms were rated using the Montgomery-Ǻsberg Depression Rating Scale (MADRS, Hawley et al., 2002), Hamilton Anxiety Rating Scale (HAM-A, Leentjens et al., 2011), and the Global Assessment of Functioning Scale (GAF, reflecting illness effects on social, occupational, and psychological functioning, Hall, 1995). Course of illness was determined using the affective morbidity index (AMI, rating the severity and length of manic/depressive episodes, Berghofer et al., 2008), patient interviews, detailed review of medical records, and analysis of daily mood ratings. Additional data collection included: blood pressure, body mass index (BMI), the homeostatic model assessment of insulin resistance (HOMA-IR, calculated based on fasting levels of blood glucose and insulin, Esteghamati et al., 2010), and Framingham risk scores (heart age and risk of cardiovascular disease, Wilson et al., 1998).
A group of 14 control subjects was also recruited and was matched for sex, age and metabolic status to the bipolar cohort. The same schedule used for diagnosing bipolar disorder (SADS-L), was used to confirm a lack of psychiatric history in this group. The control group also underwent the above-mentioned protocol of interviews and assessments.
Participants with diabetes, pregnancy, or contradiction to MRI or contrast-enhancement (estimated glomerular filtration rate < 60) were excluded from the study. All participants provided informed consent prior to enrollment.
5.2. BBB imaging
5.2.1. DCE-MRI acquisition and preprocessing
Images were acquired using a 3T MRI scanner (Discovery MR750, GE Healthcare, Waukesha, WI), with a 32-channel MR Instruments head coil. The sequences acquired for BBB assessment included: (1) a T1- weighted 3D sagittal anatomical scan (BRAVO, TE/TR = 2/6 ms, TI = 450 ms, FOV 22.4 cm, acquisition matrix 224 × 224 × 168, voxel size 1 × 1 × 1 mm, acceleration 2, averages 2, scan time 5min 42s); (2) a T1-weighted 3D tilted axial sequence with variable flip angles (2-10-30°,DESPOT1, TE/TR = 2/10 ms, flip angle 15°, averages 2, FOV 24 cm, acquisition matrix 192 × 192 × 34, voxel size 1.25 × 1.25 × 6 mm, scan time 6 min 39 s) for the calculation of pre-contrast T1 map (Deoni et al., 2005); and (3) a T1-weighted 3D axial dynamic scan (LAVA, TE/TR = 2/4 ms, FOV 24 cm, acquisition matrix: 192 × 192 × 34, voxel size 1.25 × 1.25 × 6 mm, flip angle 15°, averages 1, Δt = 20 Sec) acquired between minutes 6 and 20 after intravenous injection enhanced of the magnetic contrast Gadobenate Dimeglumine (0.1 mmol/kg, MultiHance, Bracco Imaging Canada, Montreal, QC). All sequences were registered and normalized to MNI coordinates using SPM12 (University College London, www.fil.ion.ucl.ac.uk/spm).
5.2.2. Image analysis
As extravasation of contrast agent due to cross-BBB leakage leads to increased T1 signaling in the affected tissue, it allows quantitative assessment of contrast accumulation in the tissue and hence the contrast leakage rate. To achieve this, T1 intensities are first converted to contrast concentration values (Deoni et al., 2005), and concentration-time curves are constructed for every brain voxel. The concentration-time curves can next be fitted to one of several pharmacokinetic models, allowing the calculation of parameters corresponding to leakage rates. Here we used the linear model (Serlin et al., 2019; Wong et al., 2017; Veksler et al., 2014; Chassidim et al., 2013), which estimates the leakage rate Ki (mMol/min) by calculating the slope of each concentration-time curve between 6-20 min. To compensate for inter-subject variabilities (due to heart rate, blood flow, or rate of contrast injection), each voxel's leakage rate was normalized to that of the superior sagittal sinus (Serlin et al., 2019; Veksler et al., 2014; Chassidim et al., 2013), resulting in a dimensionless measure of leakage rate. Leakage rates were considered pathological when exceeding 0.02, the 95th percentile of all values in a cohort of control subjects (Weissberg et al., 2014). The percent of suprathreshold voxels was used as a measure reflecting overall BBB leakage. To identify subjects with abnormally high overall leakage an outlier analysis was performed (based on the median + two standard deviations of all 50 subjects).
To quantify region-specific BBB leakage, each scan was segmented into 126 anatomically/functionally significant areas in accordance with the MNI brain atlas (https://github.com/neurodebian/spm12/tree/master/tpm). The number of voxels with abnormally high leakage rates was quantified within each region and divided by the total of voxels comprising the region. This ratio was used as the measure of region-specific BBB leakage.
5.3. Statistical analysis
Continuous variables were compared using the Wilcoxon rank sum test, and categorical variables were compared using either Fisher's or Chi-square test. Corrections for multiple comparisons were performed using the false discovery rate method.
Funding
This study was supported by the European Union's Seventh Framework Program(FP7/EPITARGET), the Nova Scotia Health Research Foundation (NSHRF), Brain Canada (Platform Support Grant), MITACS, and the Brain & Behavior Research Foundation (NARSAD). The funders of the study had no role in study design, patient recruitment, data collection, analysis, interpretation or publication.
Declaration of Competing Interest
The authors have no conflicts of interests to disclose.
References
- Kessler R.C. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Hui, S., Sim, K. & Baldessarini, R. J.Pharmacological approaches for treatment-resistant bipolar disorder. 592–604 (2015). [DOI] [PMC free article] [PubMed]
- Naaldijk Y.M., Bittencourt M.C., Sack U., Ulrich H. Kinins and microglial responses in bipolar disorder: a neuroinflammation hypothesis. Biol. Chem. 2016;397:283–296. doi: 10.1515/hsz-2015-0257. [DOI] [PubMed] [Google Scholar]
- Patel J.P., Frey B.N. Disruption in the blood-brain barrier: The missing link between brain and body inflammation in bipolar disorder. Neural. Plast. 2015 doi: 10.1155/2015/708306. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott N.J., Patabendige A.A.K., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heye A.K., Culling R.D., Hernández C.V., Thrippleton M.J., Wardlaw J.M. Neuroimage : clinical assessment of blood – brain barrier disruption using dynamic contrast-enhanced MRI . A systematic review. YNICL. 2014;6:262–274. doi: 10.1016/j.nicl.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingrisch M., Sourbron S., Morhard D., Ertl-Wagner B., Kümpfel T., Hohlfeld R. Quantification of perfusion and permeability in multiple sclerosis: dynamic contrast-enhanced MRI in 3D at 3T. Invest. Radiol. 2012;47:252–258. doi: 10.1097/RLI.0b013e31823bfc97. [DOI] [PubMed] [Google Scholar]
- Cramer S.P., Simonsen H., Frederiksen J.L., Rostrup E., Larsson H.B.W. NeuroImage : clinical abnormal blood – brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI ☆, ☆☆. NeuroImage. Clin. 2014;4:182–189. doi: 10.1016/j.nicl.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider, L., Naismith, R. T. & Rovira, A.Use of gadolinium for MRI diagnostic or surveillance studies in patients with MS. 239–240 (2019). 10.1212/WNL.0000000000007891. [DOI] [PubMed]
- Merali Z., Huang K., Mikulis D., Silver F., Kassner A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One. 2017;12:1–11. doi: 10.1371/journal.pone.0171558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassner, A., Roberts, T. P. L., Moran, B., Silver, F. L. & Mikulis, D. J.Increases blood-brain barrier disruption in acute ischemic stroke : an MR imaging permeability. (2009). 10.3174/ajnr.A1774. [DOI] [PMC free article] [PubMed]
- Serlin, Y. et al. Blood-brain barrier leakage in TIA. 1266–1269 (2019). 10.1161/STROKEAHA.119.025247. [DOI] [PubMed]
- Bergamino M. Measurement of blood-brain barrier permeability with t1-weighted dynamic contrast-enhanced MRI in brain tumors: a comparative study with two different algorithms. ISRN Neurosci. 2013 doi: 10.1155/2013/905279. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Klein G. Imaging blood–brain barrier dysfunction as a biomarker for epileptogenesis. Brain. 2017;140:1692–1705. doi: 10.1093/brain/awx073. [DOI] [PubMed] [Google Scholar]
- Weissberg I. Imaging blood-brain barrier dysfunction in football players. JAMA Neurol. 2014;71 doi: 10.1001/jamaneurol.2014.2682. [DOI] [PubMed] [Google Scholar]
- Tomkins O. Blood-brain barrier disruption in post-traumatic epilepsy. J. Neurol. Neurosurg. Psychiatry. 2008;79:774–777. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- Nation D. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2018 doi: 10.1038/s41591-018-0297-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.M. Measuring subtle leakage of the blood – brain barrier in cerebrovascular disease with DCE-MRI : test – retest reproducibility and its influencing factors. et al. 2017:159–166. doi: 10.1002/jmri.25540. [DOI] [PubMed] [Google Scholar]
- Wilson, P. W. F. et al. Prediction of coronary heart disease using risk factor categories. (1998). [DOI] [PubMed]
- Alberti K.G.M.M. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; World Heart Federation; International. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Esteghamati A. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007) Nutr. Metab. (Lond). 2010;7:26. doi: 10.1186/1743-7075-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley C.J., Gale T.M., Sivakumaran T. Defining remission by cut off score on the MADRS: selecting the optimal value. J. Affect. Disord. 2002;72:177–184. doi: 10.1016/s0165-0327(01)00451-7. [DOI] [PubMed] [Google Scholar]
- Leentjens A.F. Anxiety rating scales in Parkinson's disease: a validation study of the Hamilton anxiety rating scale, the beck anxiety inventory, and the hospital anxiety and depression scale. Mov. Disord. 2011;26:407–415. doi: 10.1002/mds.23184. [DOI] [PubMed] [Google Scholar]
- Hall R.C. Global assessment of functioning. Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Varatharaj A. Blood–brain barrier permeability measured using dynamic contrast-enhanced magnetic resonance imaging: a validation study. J. Physiol. 2019;597:699–709. doi: 10.1113/JP276887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veksler R., Shelef I., Friedman A. Blood-brain barrier imaging in human neuropathologies. Arch. Med. Res. 2014;45:646–652. doi: 10.1016/j.arcmed.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassidim Y. Quantitative imaging assessment of blood-brain barrier permeability in humans. Fluids Barriers CNS. 2013;10:9. doi: 10.1186/2045-8118-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T.M., Craft S. The role of insulin in the vascular contributions to age-related dementia. Biochim. Biophys. Acta - Mol. Basis Dis. 2016;1862:983–991. doi: 10.1016/j.bbadis.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Brietzke E. Insulin dysfunction and allostatic load in bipolar disorder. Expert Rev. Neurother. 2011;11:1017–1028. doi: 10.1586/ern.10.185. [DOI] [PubMed] [Google Scholar]
- Calkin C.V. Insulin resistance and outcome in bipolar disorder. Br. J. Psychiatry. 2015;206:52–57. doi: 10.1192/bjp.bp.114.152850. [DOI] [PubMed] [Google Scholar]
- Calkin C.V. Annals of Medicine Insulin resistance takes center stage : a new paradigm in the progression of bipolar disorder. Ann. Med. 2019;0:1–13. doi: 10.1080/07853890.2019.1659511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15:166–174. doi: 10.1002/wps.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt K.J. Atypical antipsychotics, insulin resistance and weight; a meta-analysis of healthy volunteer studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;83:55–63. doi: 10.1016/j.pnpbp.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll C.U., Frederickson A.M., Kane J.M., Manu P. Equally increased risk for metabolic syndrome in patients with bipolar disorder and schizophrenia treated with second-generation antipsychotics. Bipolar Disord. 2008;10:788–797. doi: 10.1111/j.1399-5618.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- Endicott J., Spitzer R.L. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch. Gen. Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; 2013. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Berghofer A. Long-term effectiveness of lithium in bipolar disorder: a multicenter investigation of patients with typical and atypical features. J. Clin. Psychiatry. 2008;69:1860–1868. doi: 10.4088/jcp.v69n1203. [DOI] [PubMed] [Google Scholar]
- Deoni, S. C. L., Peters, T. M. & Rutt, B. K. High-resolution T 1 and T 2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. 241, 237–241 (2005). [DOI] [PubMed]