Highlights
-
•
fMRI connectivity data improve outcome prediction in youth at risk for psychosis.
-
•
Combining clinical and fMRI measures yields superior predictive performance.
-
•
Default-mode hyperconnectivity is among predictors of poor outcome in at-risk youth.
Keywords: Clinical high risk, Prediction, Cross-validation, Resting-state functional connectivity, Connectome
Abstract
The first episode of psychosis is typically preceded by a prodromal phase with subthreshold symptoms and functional decline. Improved outcome prediction in this stage is needed to allow targeted early intervention. This study assesses a combined clinical and resting-state fMRI prediction model in 137 adolescents and young adults at Clinical High Risk (CHR) for psychosis from the Shanghai At Risk for Psychosis (SHARP) program. Based on outcome at one-year follow-up, participants were separated into three outcome categories including good outcome (symptom remission, N = 71), intermediate outcome (ongoing CHR symptoms, N = 30), and poor outcome (conversion to psychosis or treatment-refractory, N = 36). Validated clinical predictors from the psychosis-risk calculator were combined with measures of resting-state functional connectivity. Using multinomial logistic regression analysis and leave-one-out cross-validation, a clinical-only prediction model did not achieve a significant level of outcome prediction (F1 = 0.32, p = .154). An imaging-only model yielded a significant prediction model (F1 = 0.41, p = .016), but a combined model including both clinical and connectivity measures showed the best performance (F1 = 0.46, p < .001). Influential predictors in this model included functional decline, verbal learning performance, a family history of psychosis, default-mode and frontoparietal within-network connectivity, and between-network connectivity among language, salience, dorsal attention, sensorimotor, and cerebellar networks. These findings suggest that brain changes reflected by alterations in functional connectivity may be useful for outcome prediction in the prodromal stage.
1. Introduction
In the months to years preceding the first psychotic episode, the large majority (i.e., 80–90%) of individuals who are later diagnosed with schizophrenia or a related psychotic disorder experience a prodromal phase characterized by attenuated or transient psychotic symptoms, cognitive and social difficulties, and functional decline (e.g. (Cannon, 2015; Yung and McGorry, 1996)). Research efforts in schizophrenia, and psychosis more broadly, focus increasingly on this early phase of psychotic illness development. These studies aim to understand what happens on the neural, cognitive, and functional level as psychosis first develops and, ultimately, to design methods for early intervention.
Intervening early in the disease course, before recurrent or persistent psychosis and functional impairment have developed, may improve clinical outcomes in those at risk for psychosis. Moreover, early intervention may support social and cognitive development of youth prone to psychosis and thereby mitigate the illness’ impact on their overall functioning. Early identification of those at risk of developing psychosis is crucial to early intervention. Clinical interviews designed to detect individuals at Clinical High Risk (CHR) for psychosis have shown excellent prognostic accuracies comparable to other tests in preventative medicine (Fusar-Poli et al., 2015; Riecher-Rössler and Studerus, 2017). However, their effectiveness is mediated mainly by their ability to rule out psychosis (Fusar-Poli et al., 2015), i.e. to correctly identify those who are not at risk rather than differentiating among at-risk individuals in terms of outcome. Given these drawbacks and the limitations of current psychosis treatments, there is a need for improved outcome prediction in high-risk youth.
With the goal of improving outcome prediction for individual CHR subjects, Cannon and others developed a psychosis-risk calculator that uses clinical and neurocognitive data to compute a more precise probability of conversion to psychosis (Cannon et al., 2016). This risk-calculator was designed with data from the American NAPLS-2 cohort, but has been extensively validated with data from independent cohorts from the US (Carrión et al., 2016), UK (Fusar-poli et al., 2019), and China (Zhang et al., 2018). Moreover, studies suggest that further improvements in outcome prediction in high-risk cohorts may be achieved through the use of neurocognitive, neuroimaging, or neurophysiological data, on their own or in addition to clinical data (Bodatsch et al., 2011; Cannon et al., 2016; de Wit et al., 2017; Kambeitz-Ilankovic et al., 2016; Koutsouleris et al., 2015; Nieman et al., 2014).
The current study aims to assess if fMRI-derived measures of functional connectivity improve outcome prediction in the CHR stage. Long-standing theories posit that psychosis may result from a failure of functional integration in the brain (Collin et al., 2016; Friston et al., 2016; Stephan et al., 2009) and fMRI studies have shown abnormalities in task-related activation and functional connectivity of brain regions in individuals at high-risk for psychosis (Cao et al., 2016; Sabb et al., 2010; Thermenos et al., 2013; Yoon et al., 2015). These alterations may, in part, result from a shared underlying abnormality in the brain's functional organization (Cole et al., 2014), which may be evaluated using resting-state fMRI. Indeed, recent findings suggest that measures of functional connectivity and brain network organization derived from resting-state fMRI may be predictive of conversion to psychosis (Cao et al., 2018; Collin et al., 2018). We hypothesized that adding measures of functional connectivity and network organization to established clinical and neurocognitive predictors of psychosis may improve outcome prediction in the CHR stage above and beyond clinically-based prediction alone. If functional connectivity measures are found to improve outcome prediction, this would suggest that these measures have potential clinical value and may in the future be used to improve outcome prediction for individual patients.
2. Material and methods
2.1. Subjects
This study involved a total of 137 Clinical High Risk (CHR) subjects. These subjects were recruited as part of the Shanghai At-Risk for Psychosis (SHARP) program, an international research effort conducted by the Shanghai Mental Health Center (SMHC) in collaboration with the Beth Israel Deaconess Medical Center (BIDMC) and neuroimaging and other data processing laboratories at Brigham and Women's Hospital, Massachusetts General Hospital, Harvard University and the Massachusetts Institute of Technology. The study was approved by the Institutional Review Boards of BIDMC and the SHMC. All subjects or their legal guardians provided written informed consent, and minor subjects provided assent.
The current study involves a subgroup of a sample reported on previously (Collin et al., 2018). The current subgroup comprises only those individuals for whom good-quality imaging data were available, as well as sufficient follow-up data to determine outcome and complete clinical and neurocognitive data as required for the prediction model (details below).
2.2. Clinical evaluation
2.2.1. Baseline clinical and cognitive assessments
Prodromal symptoms were assessed using a validated Chinese version of the Structured Interview for Prodromal Symptoms (SIPS) (Zheng et al., 2012). Neurocognitive functioning was assessed using the MATRICS Consensus Cognitive Battery (Kern et al., 2008; Nuechterlein et al., 2008), including the Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al., 2004) and Hopkins Verbal Learning Test-Revised (HVLT-R) (Benedict et al., 1998). Overall functioning was assessed using the Global Assessment of Functioning (GAF) (Jones et al., 1995). Table 1 summarizes demographic, clinical, and cognitive variables.
Table 1.
Clinical and cognitive predictor variables. Group-averaged values of clinical and cognitive variables from the psychosis-risk calculator that entered into the prediction model. SIPS P1 and P2 scores are shown separately here to allow easy interpretation, but a combined value was used in the prediction analysis by rescaling each score down to a maximum of 3 (using 0 for values of 0–2) and adding the scores together. Statistical comparison was performed using analysis of variance for continuous and chi-squared tests for categorical variables.
| Good outcome (N = 71) | Intermediate outcome (N = 30) | Poor outcome (N = 36) | Statistics | |
|---|---|---|---|---|
| Age in years, mean (sd) | 18.6 (4.9) | 19.3 (5.3) | 18.6 (4.8) | F(2134) = 0.28 |
| [range] | [13 to 32] | [14 to 32] | [14 to 34] | p = .76 |
| Positive family history, N (%) | 6 (9%) | 3 (10%) | 5 (14%) | χ2 = 0.77 |
| p = .68 | ||||
| GAF change in percentage, mean | −29.7 (11.6) | −28.3 (6.2) | −33.5 (8.5) | F(2134) = 2.71 |
| (sd) [range] | [−73 to −7] | [−43 to −20] | [−47 to −4] | p = .07 |
| SIPS P1 score, mean (sd) | 3.3 (1.8) | 3.2 (1.7) | 3.7 (1.8) | F(2134) = 0.88 |
| [range] | [0 to 6] | [0 to 6] | [0 to 6] | p = .42 |
| SIPS P2 score, mean (sd) | 3.3 (1.7) | 3.6 (1.7) | 3.4 (1.7) | F(2134) = 0.19 |
| [range] | [0 to 6] | [0 to 6] | [0 to 6] | p = .83 |
| HVLT-R score, mean (sd) | 23.4 (5.7) | 23.2 (5.4) | 20.5 (4.7) | F(2134) = 3.6 |
| [range] | [9 to 33] | [11 to 33] | [11 to 29] | p = .03 |
| BACS-SC score, mean (sd) | 59.8 (8.7) | 56.5 (11.1) | 55.3 (10.0) | F(2134) = 2.9 |
| [range] | [42 to 75] | [18 to 75] | [28 to 73] | p = .05 |
2.2.2. Clinical predictor selection
As clinical predictors, we selected variables included in the psychosis-risk calculator developed for the CHR stage (Cannon et al., 2016) and validated in the SHARP study (Zhang et al., 2018). Following Zhang et al. (2018), we used 6 out of the original 8 risk factors in the calculator, including age at baseline, BACS symbol coding raw scores, HVLT-R raw scores, unusual thought content and suspiciousness (quantified as the sum of rescaled SIPS items P1 and P2), change in global functioning (measured as the change in current GAF relative to the preceding year), and having a first-degree family member with psychosis (yes/no). For details on clinical predictors and validation in our sample see Zhang et al. (2018).
2.3. Neuroimaging
2.3.1. Image acquisition
Magnetic Resonance Imaging (MRI) scans were acquired on a 3T Siemens MR B17 (Verio) system, 32-channel head coil at the SMHC and included a resting-state functional MRI (rs-fMRI) scan (149 functional volumes; TR = 2500 ms, TE = 30 ms, FA = 90°, FOV = 224 mm, voxel size 3.5 × 3.5 × 3.5 mm3, 37 contiguous axial slices, duration 6′19″) and an anatomical T1-weighted MRI scan (MP-RAGE; TR = 2300 ms, TE = 2.96 ms, FA = 9°, FOV = 256 mm, voxel size 1 × 1 × 1 mm3, 192 contiguous sagittal slices, duration 9′14″).
2.3.2. Image preprocessing
Image preprocessing was performed using Conn (v17d) software (Whitfield-Gabrieli and Nieto-Castanon, 2012) and included segmentation of gray and white matter tissue, realignment, slice-timing correction, normalization to Montreal Neurological Institute (MNI) space, and smoothing (6 mm FWHM Gaussian filter). Potential spurious correlations in rs-fMRI time-series were assessed using the Artifact Detection Tool (ART; http://www.nitrc.org/projects/artifact_detect). Outliers were defined as volumes showing head displacement in the x, y, or z direction greater than 1 mm relative to the previous frame or a mean global intensity greater than 3 standard deviations from the mean intensity for the entire rs-fMRI scan. These outlier scans were removed from the data through linear regression. Time-series were corrected for motion (captured by 3 rotational, 3 translational, and 1 composite motion parameter), artefactual covariates, and signals within white matter (i.e., 3 principle component analysis (PCA) parameters) and cerebrospinal fluid (3 PCA parameters) masks through linear regression. Resulting time-series were band-pass filtered (0.008 Hz–0.09 Hz).
2.3.3. Imaging predictor selection
From our previous study on functional connectome organization in CHR (Collin et al., 2018), we selected Rand similarity coefficients (SR) for each subject in the current study. Here, SR values (between 0 and 1) reflect how typical or atypical the modular organization of a subject's connectome organization is relative to an average healthy network, with lower values reflecting a more atypical modular organization. For the current study, the SR measure obtained using the Harvard-Oxford atlas included in the default Conn processing pipeline was used (for details see Collin et al., 2018). In addition to this measure of overall connectome organization, we included measures of functional connectivity (i.e., average Fisher-transformed correlation coefficients) within and between 8 established rs-networks, including default-mode (DMN), salience (SAL), language (LAN), dorsal attention (DA), fronto-parietal (FP), sensorimotor (SM), visual (VIS), and cerebellar (CER) networks. These 8 rs-networks are part of Conn's default processing pipeline, as obtained from ICA analysis of 497 subjects from the Human Connectome Project (https://www.nitrc.org/projects/conn/). Figure S1 illustrates all included connectome and connectivity measures per group (supplementary materials).
2.4. Outcome assessment
Subjects were reassessed at one-year follow-up. As the CHR stage has a large heterogeneity in clinical outcome ranging from psychotic conversion to symptom remission (Cannon, 2015), we differentiated multiple outcome levels: (1) good outcome or symptom remission, i.e. no longer meeting CHR criteria at follow-up (N = 71); (2) intermediate outcome, i.e. still meeting CHR criteria at follow-up (N = 30), and (3) poor outcome (N = 36), including CHR converters and ‘treatment-refractory’ CHR individuals, the latter being characterized by worsened symptoms despite antipsychotic treatment but without meeting formal criteria for psychosis.
2.5. Prediction analysis
2.5.1. Logistic regression analysis and leave-one-out cross-validation
The multinomial logistic regression analysis was performed in three steps. First, the logistic regression model was run using only clinical predictors. Second, an imaging-only prediction model that included measures of connectome organization and within/between-network functional connectivity was assessed. Third, the clinical and fMRI measures were combined in one prediction model. Prior to the logistic regression analysis, a PCA analysis was performed to reduce the dimensionality of the predictor data (i.e. up to 43 predictor variables in the combined model). As the resulting PCA components are linearly uncorrelated, this step also averted potential collinearity between variables. A component threshold of 13 was chosen to reach a 10:1 subjects-by-predictor ratio (Harrell et al., 1996; Peduzzi et al., 1996). Next, leave-one-out cross-validation was performed by fitting all participants except one and predicting out-of-sample outcomes. This was done in an iterative fashion for each participant to build cross-validated predictions. The out-of-sample predictions resulting from the cross-validation were used to estimate measures of model performance.
2.5.2. Assessing model performance
Three measures of model performance were assessed. First, positive predictive values (PPV) were computed for each group as the probability that participants with a predicted outcome label indeed had that outcome. A weighted average PPV was computed as a measure of overall model performance, reflecting the average probability across groups that an outcome label predicted by the model was in fact accurate. Note that as we evaluated three outcome categories with varying prevalence, the chance-level average PPV is approximately 37% here. Second, the sensitivity (or true positive rate) was computed as the proportion of each outcome category that was correctly predicted as such. From these, the model F1-measure (harmonic mean of the positive predictive value and sensitivity, separately for each outcome category) was used to characterize overall model performance. This measure was compared to expected chance levels using a permutation test with 1000 sampled permutations in order to evaluate the statistical significance of the model's prediction. In addition, to perform a statistical comparison of the three models in terms of performance, a resampling with replacement analysis was used to compute the expected distribution of accuracy values for each model. These were compared across models yielding p-values reflecting the difference in prediction performance for each pairwise comparison.
2.5.3. Identifying predictor variables driving outcome prediction
A secondary analysis was performed to explore which variables added most predictive information to the combined model. To this end, prediction coefficients were computed for each predictor variable by multiplying the regressor coefficients of the PCA components with the corresponding component loadings. The directionality of these coefficients is such that positive values indicate that increases in the predictor variable predict better outcome (i.e. a relative increase in the likelihood of symptom remission) and negative values imply that increases in the predictor variable predict worse outcome (i.e. an increased likelihood of poor outcome including conversion to psychosis). Associated standard error and p-values were computed to identify predictors with high influence (details in supplementary materials). Note that this is not a proper confirmatory test, but rather a way to compare the relative influence of predictors and highlight those with the greatest influence.
2.5.4. Validation analyses
As the PCA component threshold of 13 components (chosen to limit the subjects-by-predictor ratio to 10:1) is arbitrary, we performed a validation analysis in which the combined prediction model was rerun for a range of PCA component thresholds from 10 to 15 components.
Motion parameters (i.e., composite motion parameter and the number of removed outlier scans) were compared between groups, and included in the combined prediction model to assess the possible influence of head motion on our results.
Moreover, to test for possible systematic group-differences in other factors that may contribute to differential patterns of functional connectivity, outcome groups were compared on their scores on SIPS item D4 (trouble with focus and attention) and G4 (tolerance to normal stress), as measures of attention and anxiety/stress-levels.
3. Results
Fig. 1 illustrates the performance of each of the three models in the logistic regression analysis, i.e. clinical-only, imaging-only, and combined model. In addition, the confusion matrix obtained for each prediction model is provided in the supplementary materials.
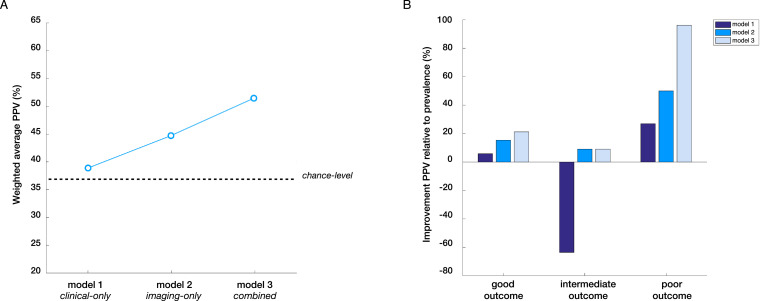
Fig. 1.
Step-wise performance of prediction models. (A) Weighted average positive predictive value (PPV) across groups for each of the three models as a measure of overall model performance, reflecting the average probability that an outcome label predicted by the model was in fact accurate (relative to 37% chance-level). Note that the clinical-only model includes both clinical and cognitive predictors and that the imaging-only model includes both connectome and connectivity measures. (B) changes in outcome prediction relative to the prevalence of each outcome category in the overall sample, illustrating that outcome prediction improved in a step-wise fashion from the clinical-only to the combined model for good and poor outcome groups, with the steepest improvement in the poor outcome group.
3.1. Clinical-only prediction model
Using only clinical and cognitive predictors, the model was unable to predict the three outcome categories to a significant degree (F1 = 0.32, F1-chance = 0.26 ± 0.06, p = .154).
3.2. Imaging-only prediction model
Using only rs-fMRI derived measures of connectome organization and within/between-network functional connectivity yielded a significant prediction model (F1 = 0.41, F1-chance = 0.29 ± 0.06, p = .016). Positive predictive values were 60% for good, 24% for intermediate, and 39% for poor outcome (weighted average PPV of 47%), with sensitivities of 52%, 30%, and 42% respectively. The imaging-only model showed a trend-level improvement in prediction performance relative to the clinical-only model (p = .06).
3.3. Combined prediction model
Combining clinical and imaging measures improved the performance of the prediction model (F1 = 0.46, F1-chance = 0.29 ± 0.06, p < .001). The combined model yielded positive predictive values of 63% for good, 24% for intermediate, and 51% for poor outcome, with sensitivities of 52%, 30%, and 58% respectively. The weighted average PPV was 51%, which is an improvement of 39% relative to chance-level and 32% relative to the clinical-only model. In direct comparison, the combined model significantly outperformed the clinical-only-model (p = .02), but was not statistically better than the imaging-only model (p = .16).
3.4. Identifying variables driving outcome prediction
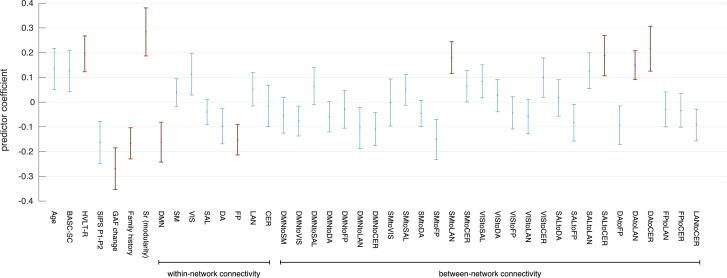
Analyzing prediction coefficients for each variable in the combined prediction model (Fig. 2) showed that GAF functional decline, verbal learning performance (HVLT-R), and a family history of psychosis were the most influential clinical variables. Greater functional decline and a positive family history predicted worse outcome, while higher verbal learning scores predicted better outcome at one-year follow-up. For fMRI-derived measures, influential predictors included modular connectome organization, DMN and FP within-network connectivity, and between-network connectivity among LAN, DA, CER, SM, and SAL networks. Higher within-network connectivity predicted worse outcome, while higher between-network connectivity and more typical modular connectome organization predicted better outcome. Beta- and p-values for each predictor variable in each model are provided in the supplementary materials.
Fig. 2.
Predictor coefficients for combined prediction model. Plot showing the mean prediction coefficient and associated standard error for each individual predictor variable in the combined model. Positive values indicate that increases in the predictor variable are associated with a relative increase in the likelihood of good outcome, while negative values imply that increases in the predictor variable are associated with a relative increase in the likelihood of poor outcome. Predictors with greatest influence (p < .05) are highlighted in red. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Validation analyses
A validation analysis testing the combined model across a range of PCA component thresholds confirmed the main results. Specifically, each threshold yielded a significant level of outcome prediction and the variables identified as the most influential predictors in the model were largely stable across thresholds (supplementary materials, Fig. S2).
There were no significant group-differences in the composite motion parameter (F2,134 = 0.88, p = .416) or the number of removed outlier scans (F2,134 = 1.19, p = .307). Including these measures in the combined prediction model did not change our main results (F1 = 0.46, p = .01 and F1 = 0.43, p = .01 for motion parameter and number of removed scans respectively).
Assessing other factors that could have contributed to differential patterns of functional brain connectivity did not show significant group-differences in focus/attention problems (F2,134 = 0.40, p = .673) or tolerance to stress (F2,134 = 0.18, p = .836).
4. Discussion
The results of this study in a large sample of adolescents and young adults at CHR for psychosis indicate that rs-fMRI data contain predictive information that may help to improve outcome prediction in the prodromal stage beyond validated clinical and cognitive predictors of psychosis alone. An imaging-only and combined (clinical/imaging) prediction model were both found to yielde a significant level of outcome prediction, while a clinical-only model was unable to predict good, intermediate, or poor outcome to a significant degree. Relative to the clinical-only model, the combined model showed a 32% improvement in average PPV, reflecting the probability that a predicted outcome label matched the actual outcome. These findings suggest two main points. First, that brain abnormalities reflected by alterations in functional connectivity precede and possibly drive subsequent changes in clinical functioning. And second, that neuroimaging markers of functional connectivity may be useful for improving early identification and clinical decision-making in prodromal psychosis.
In our current study, baseline clinical and cognitive measures alone were unable to predict outcome to a significant degree. The clinical and cognitive predictors were taken from the NAPLS-2 psychosis risk calculator (Cannon et al., 2016) that was previously validated by our group in a larger cohort that included our current sample (Zhang et al., 2018). We note that the sample size of this previous study was approximately 50% larger because our current sample was limited to subjects for whom we had both complete clinical and imaging data. Another important distinction between the previous investigation and our current study is that the previous study distinguished CHR individuals who developed psychosis from those who did not convert to psychosis, rather than predicting clinical outcome more broadly as in our current study. As the risk-calculator from which the clinical predictors were taken was developed for predicting psychosis specifically, these predictors may not be equally effective in predicting outcome beyond conversion to psychosis.
Our findings show that fMRI-derived measures of functional connectivity may be useful for improving outcome prediction in prodromal psychosis. A number of previous studies support the hypothesis that changes in functional brain connectivity are predictive of outcome in at-risk individuals. In a study in CHR individuals from the NAPLS-2 study, hyperconnectivity in a cerebello-thalamo-cortical network predicted time to conversion to full psychosis (Cao et al., 2018). Moreover, a study comparing high-risk subjects who subsequently converted to psychosis to controls and nonconverters found increased midbrain-prefrontal cortex functional connectivity in converters only (Allen et al., 2012). Another study comparing converters and nonconverters showed that reduced functional connectivity between the bilateral insula was most pronounced in converters (Wang et al., 2016). Finally, a graph theoretical analysis of functional connectivity data showed reduced topological centrality of the anterior cingulate gyrus in high-risk subjects who later converted to psychosis (Lord et al., 2012). There is thus some, albeit limited, data to suggest that abnormalities in functional brain organization predate the onset of psychosis, and may be predictive of transition to psychosis in at-risk individuals.
The notion that abnormal functional brain organization precedes and possibly drives the manifestation of full-blow psychosis is further supported by a graph theoretical study by our own group in a larger sample that includes the current CHR sample (Collin et al., 2018). This study showed that abnormal modular organization of the functional connectome is more common in CHR individuals who subsequently develop psychosis and predictive of shorter time to conversion. Our current findings support these previous results as they show that the predictive effect of the modular connectome measure holds up in cross-validation and extends to outcome more broadly. Combined with validated clinical and cognitive predictors of psychosis, the combined model achieved a weighted average PPV of 51%, which is a 39% improvement relative to chance-level (i.e. with chance-level here being 37% given that we assessed three outcome categories with varying prevalence). Outcome prediction was particularly enhanced for the poor-outcome group, for which the model yielded a positive predictive value of 51%, given a prevalence of poor outcome in the overall sample of just 26%. It is this poor outcome group for whom enhanced outcome prediction is most important, as higher prognostic certainty may allow more targeted early intervention and thereby hopefully improve outcome.
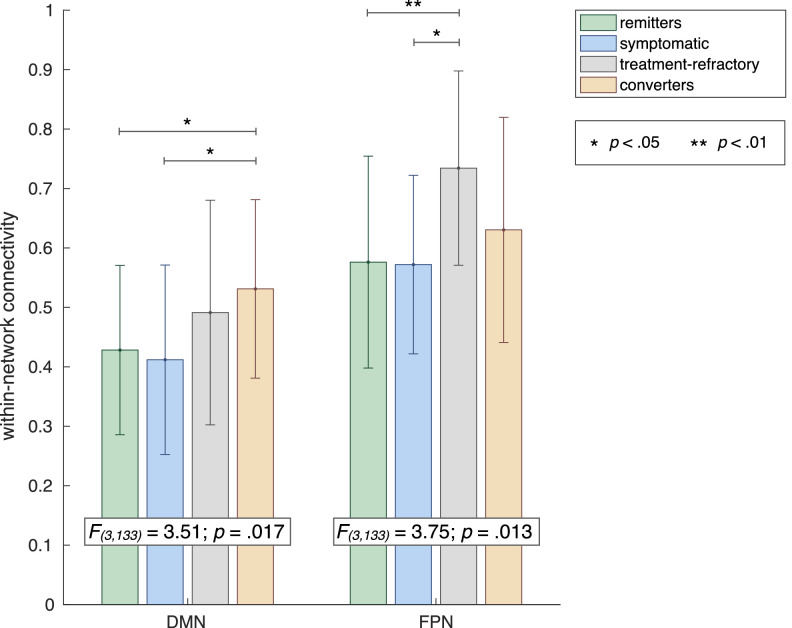
Combined with previously identified clinical and cognitive predictors of outcome in prodromal psychosis, we found several influential measures of within and between-network connectivity in the combined prediction model. First, higher DMN and FP within-network connectivity was associated with a higher likelihood of poor outcome. For the DMN, this finding fits well within the existing literature showing DMN hyperconnectivity in schizophrenia and high-risk individuals (Anticevic et al., 2015; Brandl et al., 2019; Satterthwaite et al., 2015; Shim et al., 2010; Whitfield-Gabrieli et al., 2009). The association between higher connectivity within the FP network (FPN) and poor outcome is reported less frequently. Hyperconnectivity involving the FPN has been shown in schizophrenia and CHR, but mostly between FPN and other regions including (auditory) SM and DMN (Brandl et al., 2019; Cao et al., 2018; Chahine et al., 2017; Sha et al., 2019). Intriguingly, however, a recent study showed that higher cohesiveness of the FPN is predictive of poor response to antipsychotic treatment (Doucet et al., 2018). As our poor-outcome group comprised both CHR converters and treatment-refractory subjects (i.e., showing worsened symptoms despite antipsychotic treatment, without meeting formal criteria for psychosis), we performed a post-hoc analysis to assess group-differences in FPN and DMN connectivity across CHR remitter, symptomatic, treatment-refractory, and converter groups. This analysis showed that while the association between DMN hyperconnectivity and poor outcome is indeed driven by the converter group, the association between FPN hyperconnectivity and poor outcome seems to be driven mainly by the treatment-refractory group, who show significantly higher FPN within-network connectivity than both remitter and symptomatic groups (Fig. 3). Our study thus supports previous findings that increased connectivity in the frontoparietal central-executive network is predictive of poor response to antipsychotic treatment (Doucet et al., 2018).
Fig. 3.
Post-hoc group-comparison of DMN and FPN within-network connectivity. Bar chart showing result of post-hoc analysis comparing within-network connectivity for DMN and FP networks across four outcome groups, confirming the hypothesis that the association between DMN hyperconnectivity and poor-outcome was driven particularly by converters, while FPN hyperconnectivity was mainly associated with treatment-refractory status.
In terms of between-network variables, our secondary analysis of predictor coefficients showed that between-network connectivity between the LAN and the SM and DA network, as well as between DA and SAL networks and the CER network were among the most influential predictors in the combined model. Interestingly, whereas higher within-network connectivity was associated with a higher chance of poor outcome, between-network measures showed the opposite association, with higher connectivity predicting a higher likelihood of good outcome. This finding is of interest in light of hypotheses that schizophrenia symptoms may stem from a failure to establish adequate integration between functional brain systems in development (Collin and Keshavan, 2018). Furthermore, studies have suggested that the transition from adolescence to early adulthood, when psychosis tends to develop, is characterized by a fundamental reorganization of large-scale functional brain networks (Keshavan et al., 2014; Uhlhaas et al., 2009; Uhlhaas and Singer, 2011). Abnormalities in the development of precise temporal coordination between distributed brain networks may thus contribute to the emergence of cognitive deficits and psychotic symptoms in schizophrenia (Uhlhaas and Singer, 2011). In line with these hypotheses, our current findings suggest that higher functional coherence between large-scale brain networks is predictive of better outcome in prodromal psychosis.
There are a number of limitations to our study. First, our clinical-only prediction model failed to reach a significant level of prediction, even though we selected clinical variables aimed specifically at predicting outcome in the prodromal stage. The importance of variable selection in multimodal prediction models in the CHR stage has been stressed in previous literature (Nelson et al., 2019). Specifically, an important concern is that a too limited scope of variables included in the clinical model may overemphasize the additive predictive power of imaging data. As we aimed to predict outcome in a broader sense, including psychotic conversion as well as ongoing subclinical symptoms and symptomatic remission, our clinical-only model may have performed better if it had also included variables associated with resilience to psychosis. However, there is a paucity of research into factors conferring resilience to psychosis. Future studies including resilience factors in addition to risk factors may help improve outcome predictions in at-risk groups. The clinical-only model may have also performed better if we had included a healthy control group, in line with the notion that the effectiveness of clinical predictors of psychosis is mediated primarily by their ability to ‘rule out’ psychosis (Fusar-Poli et al., 2015). However, the purpose of our current study was specifically to differentiate among high-risk individuals in terms of psychosis, rather than identifying those not at risk. Second, rs-fMRI-derived measures of functional connectivity are known to be sensitive to physiological and head motion artifacts (Power et al., 2012; Satterthwaite et al., 2012). We dealt with these issues to the best of our ability by using the anatomical CompCor (aCompCor) method to reduce physiological noise (Behzadi et al., 2007) and the Artifact Detection Tool (art) to identify motion and artifactual timepoints and mititgate their effects (Whitfield-Gabrieli and Nieto-Castanon, 2012), but we cannot rule out the possibility that these confounders influenced our results. Similarly, other factors such as levels of stress, anxiety, and attention/awareness, which are known to influence functional brain patterns (Bilevicius et al., 2018; Pearlson, 2017; Simpson et al., 2001; Soares et al., 2013) may have influenced our results, although we found no significant group-differences in SIPS items measuring tolerance to stress and attention problems. Third, we reiterate that our post-hoc analysis identifying variables that contributed most predictive information to the overall model is not a proper confirmatory test. Our analysis is intended to allow a meaningful comparison of the relative influence of predictor variables and highlight those with high influence, but we note that predictor coefficients of several other clinical and imaging variables were similar in magnitude. Validation in an independent sample is the only proper way to ascertain the relative importance of the predictor variables in our current study to outcome prediction in CHR more generally. Collaborations among CHR consortia are warranted to allow external validation of neuroimaging findings in the high-risk stage. Such future studies would benefit from improved resolution of rs-fMRI acquisition.
In conclusion, the current findings suggest that rs-fMRI-derived measures of connectome organization and functional connectivity, on their own or in addition to clinical predictors of psychosis, improve outcome prediction in the prodromal stage. Outcome prediction was found to be particularly enhanced for poor outcome CHR individuals, which is a promising result as improved prediction of those at the highest risk for poor outcome may contribute to targeted early intervention aimed at improving clinical and functional outcome. Moreover, our findings support the notion that changes in functional connectivity and brain network organization precede and possibly drive subsequent progression of illness in prodromal psychosis.
CRediT authorship contribution statement
Guusje Collin: Conceptualization, Formal analysis, Visualization, Writing - original draft. Alfonso Nieto-Castanon: Software, Formal analysis, Validation, Visualization, Writing - original draft. Martha E. Shenton: Supervision, Writing - review & editing. Ofer Pasternak: Writing - review & editing. Sinead Kelly: Writing - review & editing. Matcheri S. Keshavan: Writing - review & editing. Larry J. Seidman: Conceptualization, Funding acquisition. Robert W. McCarley: Funding acquisition. Margaret A Niznikiewicz: Funding acquisition. Huijun Li: Funding acquisition. Tianhong Zhang: Investigation. Yingying Tang: Investigation. William S. Stone: Funding acquisition, Writing - review & editing. Jijun Wang: Funding acquisition, Investigation, Resources. Susan Whitfield-Gabrieli: Conceptualization, Resources, Supervision, Writing - review & editing.
Acknowledgments
This work was supported by the United States National Institute of Mental Health (R21 MH 093294, R01 MH 101052, R01 MH 111448, R01 MH 108574, and R01 MH 64023), the Ministry of Science and Technology of China (2016 YFC 1306803), the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 749201 (to GC), and VA Merit Awards from the US Department of Veterans Affairs (to MES and RWM).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102108.
Contributor Information
Guusje Collin, Email: gcollin@mit.edu.
Jijun Wang, Email: jijunwang27@163.com.
Appendix. Supplementary materials
References
- Allen P., Luigjes J., Howes O.D., Egerton A., Hirao K., Valli I., Kambeitz J., Fusar-Poli P., Broome M., McGuire P. Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophr. Bull. 2012;38:1268–1276. doi: 10.1093/schbul/sbr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Hu X., Xiao Y., Hu J., Li F., Bi F., Cole M.W., Savic A., Yang G.J., Repovs G., Murray J.D., Wang X.-J., Huang X., Lui S., Krystal J.H., Gong Q. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35:267–286. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R.H.B., Schretlen D., Groninger L., Brandt J. Hopkins verbal learning test – revised : normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 1998;12:43–55. [Google Scholar]
- Bilevicius E., Smith S.D., Kornelsen J. Resting-state network functional connectivity patterns. Brain Connect. 2018;8:40–48. doi: 10.1089/brain.2017.0520. [DOI] [PubMed] [Google Scholar]
- Bodatsch M., Ruhrmann S., Wagner M., Müller R., Schultze-lutter F., Frommann I., Brinkmeyer J., Gaebel W., Maier W., Klosterkötter J., Brockhaus-dumke A. Prediction of psychosis by mismatch negativity. Biol. Psychiatry. 2011;69:959–966. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- Brandl F., Avram M., Weise B., Shang J., Simões B., Bertram T., Hoffmann Ayala D., Penzel N., Gürsel D.A., Bäuml J., Wohlschläger A.M., Vukadinovic Z., Koutsouleris N., Leucht S., Sorg C. Specific substantial dysconnectivity in schizophrenia: a transdiagnostic multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. Biol. Psychiatry. 2019;85:573–583. doi: 10.1016/j.biopsych.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Cannon T.D. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn. Sci. 2015;19:744–756. doi: 10.1016/j.tics.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon T.D., Yu C., Addington J., Bearden C.E., Cadenhead K.S., Cornblatt B.A., Heinssen R., Jeffries C.D., Mathalon D.H., McGlashan T.H., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Woods S.W., Kattan M. An individualized risk calculator for research in prodromal psychosis. Am. J. Psychiatry. 2016;173:980–988. doi: 10.1176/appi.ajp.2016.15070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Bertolino A., Walter H., Schneider M., Schafer A., Taurisano P., Blasi G., Haddad L., Grimm O., Otto K., Dixson L., Erk S., Mohnke S., Heinz A., Romanczuk-Seiferth N., Mühleisen T.W., Mattheisen M., Witt S.H., Cichon S., Noethen M., Rietschel M., Tost H., Meyer-Lindenberg A. Altered functional subnetwork during emotional face processing a potential intermediate phenotype for schizophrenia. JAMA Psychiatry. 2016;73:598–605. doi: 10.1001/jamapsychiatry.2016.0161. [DOI] [PubMed] [Google Scholar]
- Cao H., Chén O.Y., Chung Y., Forsyth J.K., Mcewen S.C., Gee D.G., Bearden C.E., Addington J., Goodyear B., Cadenhead K.S., Mirzakhanian H., Cornblatt B.A., Carrión R.E., Mathalon D.H., Mcglashan T.H., Perkins D.O., Belger A., Seidman L.J., Thermenos H., Tsuang M.T., Erp T.G.M.Van, Walker E.F., Hamann S., Anticevic A., Woods S.W., Cannon T.D. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat. Commun. 2018;9:3836. doi: 10.1038/s41467-018-06350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión R.E., Ph D., Cornblatt B.A., Ph D., Burton C.Z., Ph D., Tso I.F., Ph D. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP project. Am. J. Psychiatry. 2016;173:989–996. doi: 10.1176/appi.ajp.2016.15121565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine G., Richter A., Wolter S., Goya-maldonado R., Gruber O. Disruptions in the left frontoparietal network underlie resting state endophenotypic markers in schizophrenia. Hum. Brain Mapp. 2017;38:1741–1750. doi: 10.1002/hbm.23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.W., Bassett D.S., Power J.D., Braver T.S., Petersen S.E. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G., Keshavan M.S. Connectome development and a novel extension to the neurodevelopmental model of schizophrenia. Dialogues Clin. Neurosci. 2018;20:101–110. doi: 10.31887/DCNS.2018.20.2/gcollin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G., Seidman L.J., Keshavan M.S., Stone W.S., Qi Z., Zhang T., Tang Y., Li H., Anteraper S.A., Niznikiewicz M.A., McCarley R.W., Shenton M.E., Wang J., Whitfield-Gabrieli S. Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Mol. Psychiatry. 2018 doi: 10.1038/s41380-018-0288-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G., Turk E., van den Heuvel M.P. Connectomics in schizophrenia: from early pioneers to recent brain network findings. Biol. Psychiatry CNNI. 2016;1:199–208. doi: 10.1016/j.bpsc.2016.01.002. [DOI] [PubMed] [Google Scholar]
- de Wit S., Ziermans T.B., Nieuwenhuis M., Kahn S., Schothorst P.F., van Engeland H., Durston S., Schnack H.G. Individual prediction of long-term outcome in adolescents at ultra-high risk for psychosis: applying machine learning techniques to brain imaging data. Hum. Brain Mapp. 2017;38:704–714. doi: 10.1002/hbm.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G.E., Moser D.A., Luber M.J., Leibu E., Frangou S. Baseline brain structural and functional predictors of clinical outcome in the early course of schizophrenia. Mol. Psychiatry. 2018 doi: 10.1038/s41380-018-0269-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K., Brown H.R., Siemerkus J., Stephan K.E. The dysconnection hypothesis (2016) Schizophr Res. 2016;176:83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Cappucciati M., Rutigliano G., Schultze-Lutter F., Bonoldi I., Borgwardt S., Riecher-Rössler A., Addington J., Perkins D., Woods S.W., McGlashan T.H., Lee J., Klosterkötter J., Yung A.R., McGuire P. At risk or not at risk? A meta-analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry. 2015;14:322–332. doi: 10.1002/wps.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-poli P., Werbeloff N., Rutigliano G., Oliver D., Davies C., Stahl D., Mcguire P., Osborn D. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent national health service trust. Schizophr. Bull. 2019;45:562–570. doi: 10.1093/schbul/sby070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell F.E.J., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Jones S.H., Thornicroft G., Coffey M., Dunn G. A brief mental health outcome scale: reliability and validity of the global assessment of functioning (GAF) Br. J. Psychiatry. 1995;166:654–659. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- Kambeitz-Ilankovic L., Meisenzahl E.M., Cabral C., von Saldern S., Kambeitz J., Falkai P., Möller H., Reiser M., Koutsouleris N. Prediction of outcome in the psychosis prodrome using neuroanatomical pattern classification. Schizophr. Res. 2016;173:159–165. doi: 10.1016/j.schres.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Keefe R.S.E., Goldberg T.E., Harvey P.D., Gold J.M., Poe M.P., Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kern R.S., Nuechterlein K.H., Green M.F., Baade L.E., Fenton W.S., Gold J.M., Keefe R.S.E., Mesholam-Gately R., Mintz J., Seidman L.J., Stover E., Marder S.R. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am. J. Psychiatry. 2008;165:214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- Keshavan M.S., Giedd J., Lau J.Y.F., Lewis D.A., Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N., Riecher-Rössler A., Meisenzahl E.M., Smieskova R., Studerus E., Kambeitz-ilankovic L., von Saldern S., Cabral C., Reiser M., Falkai P., Borgwardt S. Detecting the psychosis prodrome across high-risk populations using neuroanatomical biomarkers. Schizophr. Bull. 2015;41:471–482. doi: 10.1093/schbul/sbu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord L., Allen P., Expert P., Howes O., Broome M., Lambiotte R., Fusar-poli P., Valli I., Mcguire P., Turkheimer F.E. Functional brain networks before the onset of psychosis: a prospective fMRI study with graph theoretical analysis. NeuroImage Clin. 2012;1:91–98. doi: 10.1016/j.nicl.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B., Yung A.R., McGorry P.D. Importance of variable selection in multimodal prediction models in patients at clinical high risk for psychosis and recent-onset depression. JAMA Psychiatry. 2019;76:339. doi: 10.1001/jamapsychiatry.2018.4234. [DOI] [PubMed] [Google Scholar]
- Nieman D.H., Ruhrmann S., Dragt S., Soen F., Tricht M.J.van, Koelman J.H.T.M., Bour L.J., Velthorst E., Becker H.E., Weiser M., Linszen D.H., Haan L.De. Psychosis prediction: stratification of risk estimation with information-processing and premorbid functioning variables. Schizophr. Bull. 2014;40:1482–1490. doi: 10.1093/schbul/sbt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein K.H., Green M.F., Kern R.S., Baade L.E., Barch D.M., Cohen J.D., Essock S., Fenton W.S., Frese F.J.I., Gold J.M., Goldberg T., Heaton R.K., Keefe R.S.E., Kraemer H., Mesholam-gately R., Stover E., Weinberger D.R., Young A.S., Zalcman S., Marder S.R. The MATRICS consensus cognitive battery, Part 1: Test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Pearlson G.D. Applications of resting state functional MR imaging to neuropsychiatric diseases. Neuroimaging Clin. NA. 2017;27:709–723. doi: 10.1016/j.nic.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecher-Rössler A., Studerus E. Prediction of conversion to psychosis in individuals with an at-risk mental state : a brief update on recent developments. Curr. Opin. Psychiatry. 2017;30:209–219. doi: 10.1097/YCO.0000000000000320. [DOI] [PubMed] [Google Scholar]
- Sabb F.W., van Erp T.G.M., Hardt M.E., Dapretto M., Caplan R., Cannon T.D., Bearden C.E. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophr. Res. 2010;116:173–183. doi: 10.1016/j.schres.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T., Vandekar S., Wolf D., Bassett D., Ruparel K., Shehzad Z., Craddock R.C., Shinohara R., Moore T., Gennatas E., Jackson C., Roalf D., Milham M., Calkins M., Hakonarson H., Gur RC, Gur RE. Connectome-wide network analysis of youth with Psychosis-Spectrum symptoms. Mol. Psychiatry. 2015:1–8. doi: 10.1038/mp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H., Gur R.C., Gur R.E. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z., Wager T.D., Mechelli A., He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol. Psychiatry. 2019;85:379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Shim G., Oh J.S., Jung W.H., Jang J.H., Choi C.H., Kim E., Park H.Y., Choi J.S., Jung M.H., Kwon J.S. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav. Brain Funct. 2010;6:58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J.R., Drevets W.C., Snyder A.Z., Gusnard D.A., Raichle M.E. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. PNAS. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M., Sampaio A., Ferreira M., Santos N.C., Marques P., Marques F., Palha J.A., Cerqueira J.J., Sousa N. Stress impact on resting state brain networks. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0066500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos H.W., Whitfield-Gabrielie S., Seidman L.J., Kuperberg G., Juelich R.J., Divatia S., Riley C., Jabbar G.A., Shenton M.E., Kubicki M., Manschreck T., Keshavan M.S., DeLisi L.E. Altered language network activity in young people at familial high-risk for schizophrenia. Schizophr. Res. 2013;151:229–237. doi: 10.1016/j.schres.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Singer W., Haenschel C., Sireteanu R., Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. PNAS. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr. Bull. 2011;37:514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Ji F., Hong Z., Poh J.S., Krishnan R., Lee J., Rekhi G., Keefe R.S.E., Adcock R.A., Wood S.J., Fornito A., Pasternak O., Chee M.W.L., Zhou J. Disrupted salience network functional connectivity and white-matter microstructure in persons at risk for psychosis: findings from the LYRIKS study. Psychol. Med. 2016;46:2771–2783. doi: 10.1017/S0033291716001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., Tsuang M.T., Faraone S.V., McCarley R.W., Shenton M.E., Green A.I., Nieto-Castanon A., LaViolette P., Wojcik J., Gabrieli J.D.E., Seidman L.J. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. PNAS. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y.B., Yun J.Y., Jung W.H., Cho K.I.K., Kim S.N., Lee T.Y., Park H.Y., Kwon J.S. Altered fronto-temporal functional connectivity in individuals at ultra-high-risk of developing psychosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung A.R., McGorry P.D. The Prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr. Bull. 1996;22:353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- Zhang T., Li H., Tang Y., Niznikiewicz M.A., Shenton M.E., Keshavan M.S., Stone W.S., McCarley R.W., Seidman L.J., Wang J. Validating the predictive accuracy of the NAPLS-2 psychosis risk calculator in a clinical high-risk sample from the SHARP (Shanghai At Risk for Psychosis) program. Am. J. Psychiatry. 2018;175:906–908. doi: 10.1176/appi.ajp.2018.18010036. [DOI] [PubMed] [Google Scholar]
- Zheng L., Wang J., Zhang T., Li H., Li C., Jiang K. The Chinese version of the SIPS/SOPS: a pilot study of reliability and validity. Chin. Ment. Heal. J. 2012;26:571–576. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.