ABSTRACT
Aim
This study aimed to evaluate and compare the antimicrobial efficacy of root canal sealers of different bases when mixed with herbal extracts.
Materials and methods
An evaluation of three sealers (Endomethasone, AH plus, Apexit plus) mixed with three herbal extracts [Emblica officinalis (Amla), Myristica fragrans (Nutmeg) and Salvadora persica (Miswak)] was done against nine strains of bacteria at various time intervals using the agar diffusion test. Moreover, measurement of the mean zones of inhibition was done.
Results
The largest zones of bacterial growth inhibition were observed with Endomethasone mixed with Myristica fragrans (Nutmeg) followed in the descending order by AH plus mixed with Salvadora persica (Miswak) and Apexit plus mixed with Salvadora persica (Miswak) respectively. The differences between zones of inhibition among different groups were found to be statistically significant.
Conclusion
Zinc-oxide-eugenol-based sealer (Endomethasone) mixed with herbal extracts produced the largest inhibitory zones followed in the descending order by resin-based sealer (AH plus) and calcium-hydroxide-based sealer (Apexit plus), respectively.
Clinical significance
Herbal plants such as [Emblica officinalis (Amla), Myristica fragrans (Nutmeg) and Salvadora persica (Miswak)] are rich sources of bioactive compounds that possess antimicrobial properties. Mixing their extracts with endodontic sealers can produce additive antimicrobial effect against microbes found in inflamed pulp.
How to cite this article
Devi MT, Saha S, Tripathi AM, et al. Evaluation of the Antimicrobial Efficacy of Herbal Extracts Added to Root Canal Sealers of Different Bases: An In Vitro Study. Int J Clin Pediatr Dent 2019;12(5):398–404.
Keywords: Amla, Antimicrobial efficacy, Miswak, Nutmeg, Root canal sealers, Root canal treatment
INTRODUCTION
The goal of root canal treatment is to eliminate bacterial infection. As the microorganism remain in the canal and dentinal tubules after instrumentation, only chemomechanical preparation of canal cannot accomplish the aim of root canal treatment.1
Therefore, it is desirous to use sealers with a good sealing ability and antimicrobial activity to eliminate residual microorganisms.2 However, controversies around antimicrobial effects of sealers on common isolated bacteria in infected teeth as well as their variable degree of cytotoxicity complicates clinicians’ decision in choosing a suitable sealer.3,4
Additionally, the constant increase in antibiotic resistant strains and side effects caused by synthetic drugs has prompted researchers to look for herbal alternatives.5 Though there are various uses of herbs in medicinal field, less studies have been done in the field of dentistry.6 Hence, the aim of the study was to evaluate and to compare the antimicrobial efficacy of root canal sealers mixed with herbal extracts against microbes found in the inflamed pulp or pulp necrosis.
MATERIALS AND METHODS
The present study was conducted in the Department of Pedodontics and Preventive Dentistry at Sardar Patel Postgraduate Institute of Dental and Medical Sciences, in collaboration with Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), and CSIR–Central Institute of Medicinal and Aromatic Plants, Lucknow, Uttar Pradesh.
In the present study, evaluation of antimicrobial efficacy of three commercially available root canal sealers of different bases (zinc oxide eugenol based sealer, epoxy resin based sealer and calcium hydroxide based sealer) when mixed with three herbal extracts [Emblica officinalis (Amla), Salvadora persica (Miswak) and Myristica fragrans (Nutmeg)] was done against nine strains of bacteria (aerobes, facultative and obligate anaerobes) known to be common isolates in necrotic pulps and endodontic lesions, at various time intervals using the agar diffusion test.
To overview the proper study design and to take care of the possible constraints during the main study, a pilot study was carried out in the same departments previously.
Tested Sealers (Fig. 1)
Figs 1A to C.
Three commercially available root canal sealers: (A) Endomethasone; (B) AH Plus; (C) Apexit Plus
Three commercially available root canal sealers of different bases used in the present study were (Table 1).
Table 1.
Sources of three tested sealers
| Sealer | Manufacturer | Batch no. |
|---|---|---|
| Endomethasone | Septodont, Saint-Maur-des-Fosses Cedex, France | 612000679 |
| AH plus | Dentsply De Tray GniBH, Konstanz, Germany | 60620112 |
| Apexit plus | Ivoclar Vivadent, Schaan, Liechtenstein | # 593991 |
Endomethasone (zinc-oxide-eugenol-based sealer)
AH plus (epoxy-resin-based sealer)
Apexit plus (calcium-hydroxide-based sealer)
Preparation of the Sealers
The preparation of sealers was done in strict compliance with the manufacturer's recommendations.
Tested Herbal Extracts
Methanolic extracts of Amla, Miswak, and Nutmeg were used in the study.
Collection of herbal plant samples and preparation of methanolic extract of the tested herbal plants was carried out at (CSIR) Central Institute of Medicinal and Aromatic Plants, Lucknow, Uttar Pradesh (Table 2).
Table 2.
Ethnobotanical data of plants and their extract yield percentage
| Botanical name | Family | Common name | Plant part used | Extract yield (%) |
|---|---|---|---|---|
| Emblica officinalis | Phyllanthaceae | Amla | Fruit | 12 |
| Myristica fragrans | Myristicaceae | Nutmeg | Seed kernel | 10 |
| Salvadora persica | Salvadoraceae | Miswak | Twig | 7 |
Preparation of Herbal Extracts
The dried plant materials of each plant species were grounded into a coarse powder. An estimated 200 g of the powder was soaked in 500 mL of methanol with stirring for 24 hours at room temperature and filtered through double layers of a muslin cloth and the process of cold maceration had been repeated three times with fresh solvent using same powdered drug. The filtrates were evaporated and dried at 40°C under reduced pressure using rotatory vacuum evaporator. The extract yields were weighted and then stored in small bottles in a fridge at 5°C, and their yield percentages were calculated using the following formula:
where R = weight of extracted plants residues and S = weight of plant raw sample.
Tested Microorganisms
Antibacterial activities of the sealers mixed with herbal extracts were evaluated against the bacterial isolates (Table 3).
Table 3.
Test microorganisms
| Microorganisms | Source | Morphotype |
|---|---|---|
| Staphylococcus aureus | ATCC 25923 | Gram-positive cocci |
| Streptococcus ß haemolyticus | ATCC 10556 | Gram-positive cocci |
| Enterococcus faecalis | ATCC 29212 | Gram-positive cocci |
| Escherichia coli | ATCC 25922 | Gram-negative bacilli |
| Pseudomonas aeruginosa | ATCC 27853 | Gram-negative bacilli |
| Peptostreptococcus sp. | NCTC 9821 | Gram-positive cocci |
| Bacteroides fragilis | ATCC 35406 | Gram-negative bacilli |
| Lactobacillus casei | ATCC 393 | Gram-positive bacilli |
| Veillonella parvula | ATCC 10790 0211122101011810MATERIAL | Gram-negative cocci |
ATCC, American type culture collection; NCTC, national culture type collection
Procedure
Cultures of each strain of bacteria were obtained from the laboratory stock, Department of Microbiology, (SGPGIMS), Lucknow.
Growth Conditions and Bacterial Culture
The bacterial strains namely Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Pseudomonas aeruginosa were grown aerobically to logarithmic phase from their respective stock cultures in Brain Heart Infusion Broth (BHIB, Difco Laboratories, Detroit, Michigan) at 37°C. Trypticase soy broth was used for growing Streptococcus β haemolyticus under the same conditions. Obligate anaerobes (Peptostreptococcus sp., Bacteroides fragilis, Lactobacillus casei and Veillonella parvula) were grown overnight at 37°C anaerobically in a medium consisting of Brain Heart Infusion Broth, beef extract (0.5% Difco), hemin, and menadione.
Preparation of the Inoculums
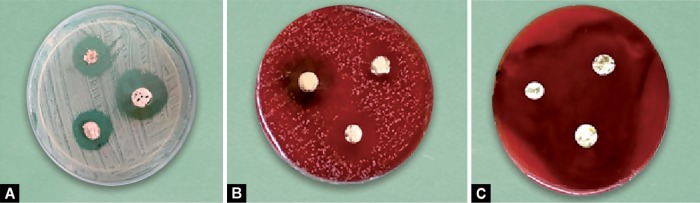
Inoculums for each bacterial strain were prepared by picking up four to five colonies with the help of a circular, previously sterilized loop of 4 mm internal diameter and dissolving them into respective test tubes containing 5 mL of 0.85% saline solution, producing a turbidity of 0.5 on McFarland scale corresponding to a concentration of 108 colony-forming units per milliliter. Except Streptococcus β haemolyticus, for all the aerobes and facultative anaerobes, Petri dishes of 90 mm diameter containing 4-mm-thick Mueller-Hinton agar were used for inoculation (Fig. 2A). Blood agar plates were used for Streptococcus β haemolyticus (Fig. 2B). Agar plates containing Wilkins-Chalgren medium were used for all the obligates anaerobes (Fig. 2C)
Figs 2A to C.
Culture plates showing zone of inhibition on the following: (A) Mueller–Hinton agar plates; (B) Blood agar plates; (C) Wilkins – Chalgren agar plates
The respective bacterial dilutions were then swabbed evenly onto freshly prepared respective agar plates using the “lawn technique” to ensure even distribution of the inoculums. Three equal sections were divided in each plate (for every individual bacterial strain). Wells of 6 mm diameter and depth 5 mm were created with the help of previously fabricated and sterilized copper wells in each section of the plate. The three tested sealers added to three different herbal extracts were then filled into wells in each section until the material was flushed with the surface of the agar medium in the Petri dishes.
Incubation
To allow the diffusion of the sealers mixed with herbal extracts through the agar, the inoculated plates were kept for two hours at room temperature. The incubation of MH agar plates was done at 37°C. CO2 incubator (Jouan, Saint Herblain, France) in an atmosphere of 10% CO2 was used for the blood agar plates inoculated with Streptococcus β haemolyticus strain and plates with strict anaerobes were immediately placed into GasPak anaerobic jars [nitrogen (90%) and CO2 (10%)].
The plates for aerobes and facultative anaerobes were read at 24 hours, 48 hours, 72 hours, and lastly 7 days for size of the zone of inhibition, while readings for obligate anaerobes were carried out after 48 hours, 7 days, and 15 days. The whole experiment was repeated six times for each isolate and the mean zone of inhibition was then calculated.
Measuring the Size of Zone of Inhibition
The growth of inhibitory zones around each sealer mixed with herbal extract for all the plates of each bacterial strain (aerobes, facultative anaerobes, and obligate anaerobes) were evidenced by a lack of bacterial colonization (clearing of agar) adjacent to each sealer mixed with herbal extract. The most uniform diameter segment of the zone of inhibition was measured with an endodontic millimeter ruler and the 6 mm diameter of the well was extracted from the measurement as the cut-off value.
All measurements above this value were considered indicative of significant bacterial growth inhibition. Wider zones of inhibition were interpreted to indicate greater antimicrobial activity of the involved sealers mixed with herbal extracts.
Statistical Analysis
All the statistical analysis were performed using SPSS (version 21, IBM, USA). All the data were presented in tabular and bar diagram form. Kruskal–Wallis test along with Mann–Whitney U test was used for intergroup comparison. While the intragroup comparison was done using Friedman test and Wilcoxon signed rank test. The level of statistical significance was set at 0.05.
RESULT
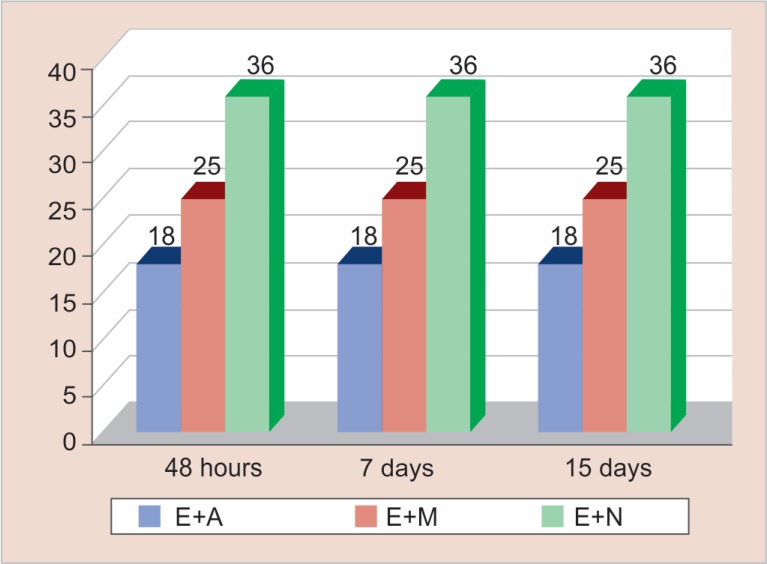
Figure 3 reveals the antimicrobial efficacy of zinc-oxide-eugenol-based sealer (Endomethasone) mixed with the three herbal extracts. It was seen that Endomethasone combined with Myristica fragrans (mean zone of inhibition was 36 mm) showed the highest antimicrobial efficacy followed in the descending order by its combination with Salvadora persica (mean zone of inhibition was 25 mm) and Emblica officinalis (mean zone of inhibition was 18 mm), respectively. The highest zone of inhibition of Endomethasone mixed with Myristica fragrans was shown by Bacteroides fragilis (36 mm), Peptostreptococcus species (35 mm), Veillonella parvula (34 mm) Staphylococcus aureus (33 mm), Lactobacillus casei (30 mm), Escherichia coli (29 mm), Streptococcus β haemolyticus (20 mm), Pseudomonas aeruginosa (16.03 mm), and Enterococcus faecalis (15 mm) respectively in descending order. The zone of inhibitions were measured after 48 hours for the obligate anaerobes and for aerobes and facultative anaerobes, zone of inhibitions were measured after 24 hours.
Fig. 3.
Antimicrobial efficacy of zinc oxide eugenol-based sealer mixed with herbal extracts
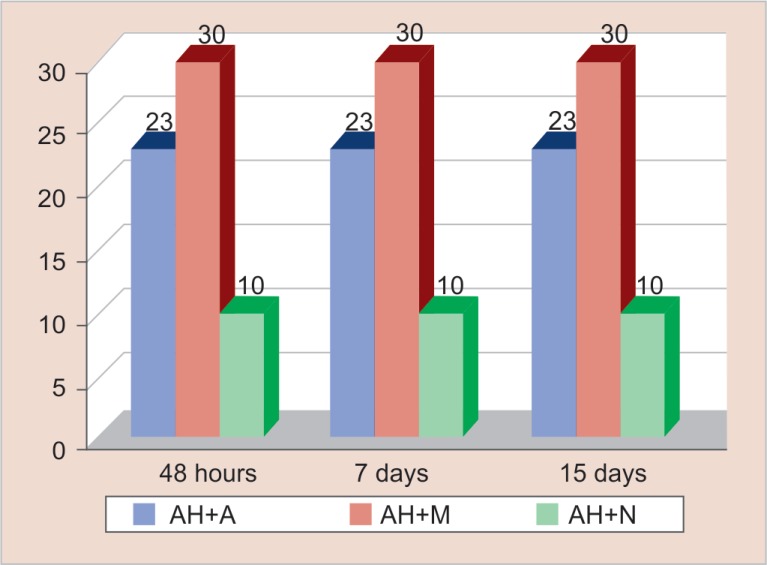
Figure 4 reveals the antimicrobial efficacy of resin-based sealer (AH plus) mixed with the three herbal extracts. It was observed that AH plus mixed with Salvadora persica (Miswak) (mean zone of inhibition was 30 mm) showed maximum antimicrobial efficacy followed in descending order by its combination with Emblica officinalis (Amla) (mean zone of inhibition was 23 mm) and Myristica fragrans (Nutmeg) (mean zone of inhibition was 10.03 mm), respectively. The highest zone of inhibition of AH plus mixed with Salvadora persica was shown by Lactobacillus casei (30 mm), Streptococcus β haemolyticus (23 mm), Pseudomonas aeruginosa (17 mm), Staphylococcus aureus (16 mm), Peptostreptococcus species (15 mm), Bacteroides fragilis (12 mm), and Escherichia coli (11 mm) respectively in descending order. AH plus mixed with Salvadora persica (Miswak) did not show antimicrobial efficacy against Enterococcus faecalis and Veillonella parvula.
Fig. 4.
Antimicrobial efficacy of resin-based sealer mixed with herbal extracts
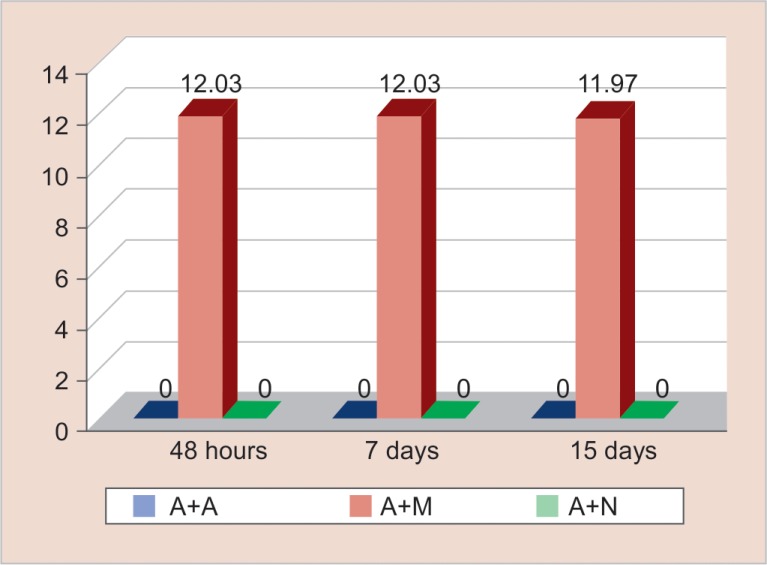
Figure 5 reveals the antimicrobial efficacy of calcium-hydroxide-based sealer (Apexit plus) mixed with herbal extracts. Antimicrobial efficacy was seen against Peptostreptococcus strains when Apexit plus was mixed with Salvadora persica (Miswak) and the mean zone of inhibition was 12.01 mm. It was seen that Apexit plus mixed with three different herbal extracts Emblica officinalis (Amla), Salvadora persica (Miswak), and Myristica fragrans (Nutmeg) demonstrated no antimicrobial efficacy against the remaining eight microorganisms tested.
Fig. 5.
Antimicrobial efficacy of calcium hydroxide-based sealer mixed with herbal extracts
DISCUSSION
Elimination of bacteria by cleaning, shaping, and obturation of the root canal system is the objective of root canal treatment. Therefore root canal sealers with good sealing ability and antimicrobial activity with low toxic effects on surrounding periapical tissue are desired to kill residual microorganisms inside the canal.2 Hypersensitivity, immune suppression, and allergic reactions are some of the adverse effects associated with antimicrobial agents, and the continuous evolution of bacterial resistance has necessitated the search for medicinal plant alternatives as antimicrobial agents.7
Medicinal plants are rich sources of bioactive compounds such as alkaloids, flavonoids, and phenolic compounds.8 Owing to their safety and ability to combat new developing drug resistant pathogens, herbal preparations are always welcome in present-day science.9
There are insufficient scientific reports that indicate the antimicrobial activities of herbal extracts mixed with endodontic root canal sealers. Hence, the aim of the study was to evaluate and compare the antimicrobial efficacy of endodontic sealers of different bases mixed with herbal extracts.
The most commonly used root canal sealers in endodontics are mainly of three types, namely zinc-oxide-eugenol-based, epoxy-resin-based and calcium-hydroxide-based root canal sealers.10–12
Great care had been taken regarding the reading of zone of inhibition; hence, the whole experiment was repeated six times to standardize it.
Methanolic extracts were shown to have a greater activity compared to ethanolic or aqueous extracts because more phytoconstituents are leached out from it when compared to ethanolic extracts. Hence, methanolic extracts of three herbal plants were prepared as test medicaments.13
Streptococcus β haemolyticus or facultative anaerobes as a group have been reported to be one of the most prevalent groups associated with persistent infections, pulp necrosis, and postendodontic treatment disease.14,15 Hence, it had been included in the present study, as it represents a standard against which the antibacterial action of a sealer mixed with herbal extracts should be studied.
Enterococcus faecalis (a gram-positive coccus) was chosen as the test organism because it is a facultative anaerobe that is the most resistant species in the oral cavity and the possible cause of failure of root canal treatment.14
Pseudomonas aeruginosa is a motile, nonfermenting, gram-negative organism belonging to the family Pseudomonadaceae.16 P. aeruginosa has been recovered from primary and persistent endodontic infections. Leonardo et al. reported that growth of P. aeruginosa was not inhibited by several of the commonly used root canal sealers and pastes. Hence, it was chosen as a test microorganism.17
Other recovered microorganisms were mainly gram-negative rods such as Escherichia coli.14 Gomes et al. considered Enterococcus faecalis and Staphylococcus aureus to be the most resistant species in the oral cavity and the causative organisms for the failure of root canal treatment.14 The root canal flora consisting of strict (obligate) anaerobes dominates by the time pulpal necrosis and a radiolucent periapical lesion develops.18 Ercan et al. stated that Peptostreptococcus spp. was the most predominantly isolated microbial genera from necrotic pulp tissues and from failed endodontic treatments in infected root canals, which is followed by Lactobacillus spp. and Veillonella spp., respectively.19 Balto stated that Bacteroides species play a significant role in clinical signs and symptoms of pulpal and periradicular diseases.20
The results of the present study revealed that zinc-oxide-eugenol-based root canal sealer, Endomethasone when mixed with Myristica fragrans (Nutmeg) showed the largest inhibitory zones of inhibition followed in the descending order by its combination with Salvodora persica (Miswak) and Emblica officinalis (Amla) respectively against all microorganisms studied at all-time intervals. The highest zone of inhibition was shown by Bacteroides fragilis, Peptostreptococcus species, Veillonella parvula, Staphylococcus aureus, Lactobacillus casei, Escherichia coli, Streptococcus β haemolyticus, Pseudomonas aeruginosa, and Enterococcus faecalis, respectively. The highest zone of inhibition shown with Endomethasone is in accordance to studies conducted by Bodrumlu et al.,21 Saha et al.,1 Poggio et al.,22 Ahamed and Geetha23 and Hasheminia et al.24 who found zinc-oxide-eugenol based sealer (Endomethasone) to have a greater antibacterial activity than resin-based sealers (AH plus). Hasheminia et al. stated that the antibacterial activity of Endomethasone is due to the existence of components such as paraformaldehyde, thymol iodide, and zinc oxide in the composition of this sealer.24 The antimicrobial effect of Endomethasone is additionally attributed to free eugenol liberated from these sealers even after their setting and this eugenol however inhibits the growth, or even induces death of bacterial cells in relatively high concentrations.25 But Badole et al. stated that use of Endomethasone could attribute to cytotoxicity.26 To overcome its cytotoxicity, endodontic sealers should be mixed with some biocompatible substances for which herbal extracts were used in the present study. When Endomethasone was mixed with Myristica fragrans (Nutmeg), the mixture showed the largest inhibitory zones of microbial growth followed by its combination with Salvodora persica (Miswak) and Emblica officinalis (Amla), respectively. The high antimicrobial activity of Myristica fragrans is attributable to the bioactive compounds present in it such as tannins, saponins, essential oils, phenolic compounds, and flavonoids.27,28 The presence of bioactive compounds and their ability to inactivate microbial adhesion, enzymes, and cell envelope protein account for its high antimicrobial activity. Reports by Narashimhan and Dhake stated that Trimyristin (an active compound obtained from Myristica fragrans) is responsible for its good antibacterial properties against both gram-positive and gram-negative bacteria.29 Omoruyi and Emefo also stated that essential oils containing eugenol (as in the case of Myristica fragrans) possess a significant antimicrobial activity due to hydrophobicity and partitioning effect on the microbial plasma membrane.30 Because both Endomethasone and Myristica fragrans possess eugenol as their constituents, the antibacterial efficacy of this combination of sealer produced the maximum zone of inhibition.
The results of the present study also revealed that zinc oxide eugenol based sealer, Endomethasone when mixed with Salvodora persica (Miswak) showed better antibacterial efficacy when compared to Endomethasone when mixed with Emblica officinalis (Amla) but less than Endomethasone mixed with Myristica fragrans. This may be attributed to the fact that Salvodora persica (Miswak) contains many phytochemical constituents and active compounds such as flavonoids, sterols, saponins, tannins, basic alkaloids, coumarins, terpenoids, essential oils, resin, lectins, and polypeptides.31 They also have a volatile compound called benzyl isothiocyanate (BITC), which has the ability to penetrate through the outer bacterial membrane and possibly interfere with the bacterial redox systems and thus hamper the ability of the bacterium to maintain its membrane potential.32 On the other hand, Emblica officinalis (Amla) when compared to Salvodora persica (Miswak) contains a fewer number of phytochemical constituents such as tannins, flavonoids, phenols, and saponins, which are responsible for its less antimicrobial efficacy.33
The results of the present study showed that resin-based sealer (AH plus) when mixed with Salvodora persica (Miswak) showed the maximum antibacterial efficacy followed in the descending order by its combination with Emblica officinalis (Amla) and Myristica fragrans (Nutmeg), respectively. This result was lesser in comparison when Endomethasone was mixed with the three herbal extracts. The antimicrobial activity revealed by AH plus seems to be correlated to its compositional components (i.e. bisphenol A diglycidyl ether, epoxy resin and amines that are present in it).34 The material also releases a small quantity of formaldehyde epoxy and amine ingredients during the polymerization process, which may also add to its antimicrobial effect.1 The highest antimicrobial efficacy exerted by resin-based sealer (AH plus) mixed with Salvodora persica (Miswak) when compared to its combination with Emblica officinalis (Amla) and Myristica fragrans (Nutmeg) may be due to the fact that resin, which is one of the active ingredients of the sealer in the composition, is also present in Salvodora persica (Miswak). This additive effect of the presence of resin in both the sealer and Salvodora persica (Miswak) may be responsible for the highest antimicrobial efficacy of resin-based sealer in combination with Salvodora persica (Miswak) in comparision to its combination with other two herbal extracts [Emblica officinalis (Amla) and Myristica fragrans (Nutmeg)].
The results of the present study showed that calcium-hydroxide-based sealer (Apexit plus) when mixed with three different herbal extracts [Emblica officinalis (Amla), Salvadora persica (Miswak) and Myristica fragrans (Nutmeg)] demonstrated no antimicrobial activity against eight of the microorganisms tested. Only slight antimicrobial activity was seen against Peptostreptococcus strains. This slight antimicrobial activity against Peptostreptococcus strains was shown only when Apexit plus was mixed with Salvadora persica (Miswak) and not evident with Apexit plus mixed with the other two herbal extracts [Emblica officinalis (Amla) and Myristica fragrans (Nutmeg)]. Very slight antimicrobial effect with calcium-hydroxide-based sealers might be explained by the too slow release of hydroxyl ions during the duration of contact and also lack of sufficient release of hydroxyl ions from calcium hydroxide to inhibit the growth pH of the above microorganisms.35 Additionally, those artificial media that contain blood also exert a buffering capacity that further produces a reduction in the high pH of calcium hydroxide. Clinically this same action can be produced by the buffering action of tissue fluids and blood.1 Owing to these drawbacks evidenced by calcium-hydroxide-based sealer, researchers have recommended methods such as direct contact test (DCT) for evaluation of antibacterial efficacy of calcium-hydroxide-based sealer.3
The limitation of the present study could be attributed to the short duration of the study. To overcome this, more studies for a longer duration should be taken up to achieve the main aim. Further, to make it clear about the active proposition regarding the antimicrobial activity of the present herbal extracts, both qualitative analysis and quantitative screening of the herbal extracts is needed. The possible interplay between different properties (pharmacological and physical) of these herbal extracts when mixed with root canal sealers are still not clear. Hence coming to a conclusion on its usage when mixed with root canal, sealers should be made after further research have been performed on animal/human models.
CONCLUSION
On the basis of the results, observations, and statistical analysis, the following conclusions could be drawn:
Zinc-oxide-eugenol-based root canal sealer (Endomethasone) when mixed with the three tested herbal extracts showed maximum zone of inhibition followed in the descending order by AH plus and Apexit plus when mixed with three herbal extracts, respectively.
Among the combination of zinc-oxide-eugenol-based sealer (Endomethasone) with the three herbal extracts, its mixture with Myristica fragrans (Nutmeg) showed the largest inhibitory zones against all the microorganisms studied at all-time intervals followed in descending order by its mixture with Salvadora persica (Miswak) and least by its mixture with Emblica officinalis (Amla).
Epoxy-resin-based sealer (AH plus) when mixed with Salvadora persica (Miswak) showed the largest inhibitory zones against all microorganisms studied at all-time intervals followed in the descending order by epoxy-resin-based sealer (AH plus) mixed with Emblica officinalis (Amla) and least when mixed with Myristica fragrans (Nutmeg).
When calcium-hydroxide-based sealer (Apexit plus) was mixed with extract of Salvadora persica (Miswak), only the zone of inhibition was shown against Peptostreptococcus sp. No zones of inhibition were found when calcium-hydroxide-based sealer (Apexit plus) was mixed with Emblica officinalis (Amla) or when mixed with Myristica fragrans (Nutmeg).
CLINICAL SIGNIFICANCE
Bioactive compounds present in the herbal plant extracts used in this study are potent antimicrobial agents. Their antimicrobial properties can be beneficial in the field of dentistry in combating dental diseases. Extracts of [Emblica officinalis (Amla), Myristica fragrans (Nutmeg), and Salvadora persica (Miswak)] can be mixed with root canal sealers of different bases (zinc oxide eugenol based sealer, epoxy resin based sealer, calcium hydroxide based sealer), enabling the enhancement of the antimicrobial properties and decreasing the cytotoxicity of these sealers by additive effect of the combination.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.Saha S, Samadi F, et al. Antimicrobial activity of different endodontic sealers: An in vitro evaluation. J Indian Soc of Pedod Prev Dent. 2010 Oct;28(4):251–257. doi: 10.4103/0970-4388.76151. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Kayaoglu G, Erten H, et al. Short – term antibacterial activity of root canal sealers towards Enterococcus faecalis. Int Endod J. 2005 Jul;38(7):483–488. doi: 10.1111/j.1365-2591.2005.00981.x. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Shantiaee Y, Dianat O, et al. In Vitro Evaluation of the Antibacterial Activity of Three Root Canal Sealers. Iran Endod J. 2010;5(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 4.da Silva PT, Pappen FG, et al. Cytotoxicity Evaluation of Four Endodontic Sealers. Braz Dent J. 2008;19(3):228–231. doi: 10.1590/S0103-64402008000300010. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Vinothkumar TS, Rubin MI, et al. In vitro evaluation of five different herbal extracts as an antimicrobial endodontic irrigant using real time quantitative polymerase chain reaction. J Conserv Dent. 2013 Mar-Apr;16(2):167–170. doi: 10.4103/0972-0707.108208. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbaszadegan A, Dadolahi S, et al. Antimicrobial and Cytotoxic Activity of Cinnamomum zeylanicum, Calcium Hydroxide, and Triple Antibiotic Paste as Root Canal Dressing Materials. J Contem Dent Pract. 2016 Feb;17(2):105–113. doi: 10.5005/jp-journals-10024-1811. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Upadhyay K, Kencheppa M, et al. Comparison of Antibacterial Efficacy of Combination of Turmeric and Calcium Hydroxide with Three Intracanal Medicaments against Various Endodontic Bacteria: An in vitro Study. J Orofac Res. 2015 Oct-Dec;5(4):113–117. doi: 10.5005/jp-journals-10026-1193. DOI: [DOI] [Google Scholar]
- 8.Yamani HA, Pang EC, et al. Antimicrobial Activity of Tulsi (Ocimum tenuiflorum) Essential Oil and Their Major Constituents against Three Species of Bacteria. Front Microbiol. 2016 May;7:1–10. doi: 10.3389/fmicb.2016.00681. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dausage P, Dhirawani RB, et al. A Comparative Study of Ion Diffusion from Calcium Hydroxide with Various Herbal Pastes through Dentin. Int J Clin Pediatr Dent. 2017 Jan;10(1):41–44. doi: 10.5005/jp-journals-10005-1405. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahangari Z, Ashraf H, et al. Antibacterial activity of three endodontic sealers with various bases. Beheshti Univ Dent J. 2005;22:1–6. (Special Issue): [Google Scholar]
- 11.al-Khatib ZZ, Baum RH, et al. The antimicrobial effect of various endodontic sealers. Oral Surg Oral Med Oral Pathol. 1990 Dec;70(6):784–790. doi: 10.1016/0030-4220(90)90022-K. DOI: [DOI] [PubMed] [Google Scholar]
- 12.De Almeida WA, Leonardo MR, et al. Evaluation of apical sealing of three endodontic sealers. Int Endod J. 2000 Jan;33(1):25–27. doi: 10.1046/j.1365-2591.2000.00247.x. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Mistry KS, Sanghvi Z, et al. The antimicrobial activity of Azadirachta indica, Mimusops elengi, Tinospora cardifolia, Ocimum sanctum and 2% chlorhexidine gluconate on common endodontic pathogens: An in vitro study. Eur J Dent. 2014 Apr-Jun;8(2):172–177. doi: 10.4103/1305-7456.130591. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Almeida Gomes BPF, Pedroso JA, et al. In Vitro Evaluation of the Antimicrobial Activity of Five Root Canal Sealers. Braz Dent J. 2004;15(1):30–35. doi: 10.1590/S0103-64402004000100006. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Rôças IN, Siqueira JF. Characterization of Microbiota of Root Canal-Treated Teeth with Post treatment Disease. J Clin Microbiol. 2012 Mar;50(5):1721–1724. doi: 10.1128/JCM.00531-12. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alhazmi A. Pseudomonas aeruginosa – Pathogenesis and Pathogenic Mechanisms. Int J Biol. 2015;7(2):44–67. doi: 10.5539/ijb.v7n2p44. DOI: [DOI] [Google Scholar]
- 17.Singh G, Elshamy FMM, et al. An in vitro Comparison of Antimicrobial Activity of Three Endodontic Sealers with Different Composition. J Cont Dent Pract. 2016 Jul;17(7):553–556. doi: 10.5005/jp-journals-10024-1888. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Stevens RH, Grossman LI. Antimicrobial effect of root canal cements on an obligate anaerobic organism. J Endod. 1981 Jun;7(6):266–267. doi: 10.1016/S0099-2399(81)80004-0. DOI: [DOI] [PubMed] [Google Scholar]
- 19.Ercan E, Dalli M, et al. Investigation of Microorganisms in Infected Dental Root Canals. Biotechnol & Biotechnol Equip. 2006;20(2):166–172. [Google Scholar]
- 20.Balto H. Ecology of pulpal and periapical flora. Afr J Microbiol Res. 2013 Oct;7(40):4754–4761. doi: 10.5897/AJMR2013.5399. DOI: [DOI] [Google Scholar]
- 21.Bodrumlu E, Semiz M. Antibacterial Activity of a New Endodontic Sealer against Enterococcus faecalis. J Can Dent Assoc. 2006 Sep;72(7):637a–637c. [PubMed] [Google Scholar]
- 22.Poggio C, Lombardini M, et al. Antibacterial effects of six endodontic sealers. Int J Artif Organs. 2011;34(9):908–913. doi: 10.5301/ijao.5000055. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Ahamed ST, Geetha RV. Comparative Effect of Commercially Available Endodontic Sealers against Enterococcus faecalis. Int J Pharm Sci Rev Res. 2017 May;44(2):186–187. [Google Scholar]
- 24.Hasheminia M, Razavian H. In vitro evaluation of the antibacterial activity of five sealers used in root canal therapy. Dent Res J. 2017 Jan-Feb;14(1):62–67. doi: 10.4103/1735-3327.201141. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawińska M, Szczurko G, et al. In vitro evaluation of the antibacterial effect of various root canal sealers on selected anaerobic bacteria. J Stomat. 2016;69(5):521–530. doi: 10.5604/00114553.1230106. DOI: [DOI] [Google Scholar]
- 26.Badole GP, Warhadpande MM, et al. A comparative evaluation of cytotoxicity of root canal sealers: an in vitro study. Restor Dent & Endod. 2013;38(4):204–209. doi: 10.5395/rde.2013.38.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osho MB, Ojo EE, et al. Antimicrobial Activities of Jatropha curcas and Myristica fragrans Seeds Extracts against Pathogenic Isolates from Barber Clippers in Shomolu Local Council Development Area, Lagos State. Int J Microbiol Biotechnol. 2016;1(1):25–32. [Google Scholar]
- 28.Kapilan R. Determination of Antibacterial Activity of Some Important Spices. Int J Res–Granthaalayah. 2015 Oct;3(10):57–60. [Google Scholar]
- 29.Narasimhan B, Dhake AS. Antibacterial Principles from Myristica fragrans seeds. J Med Food. 2006;9(3):395–399. doi: 10.1089/jmf.2006.9.395. DOI: [DOI] [PubMed] [Google Scholar]
- 30.Omoruyi IM, Emefo OT. In Vitro evaluation of the antibiogramic activities of the seeds of Myristica fragrans on food borne pathogen. Malays J Microbiol. 2012;8(4):253–258. doi: 10.21161/mjm.42312. DOI: [DOI] [Google Scholar]
- 31.Mohammed SG. Comparative Study of In vitro Antibacterial Activity of Miswak Extracts and Different Toothpastes. Am J Agric Biol Sci. 2013;8(1):82–88. doi: 10.3844/ajabssp.2013.82.88. DOI: [DOI] [Google Scholar]
- 32.Sofrata A, Santagelo EM, et al. Benzyl Isothiocynate, a Major Component from the Roots of Salvadora Persica Is Highly Active against Gram Negative Bacteria. Plos ONE Aug. 2011;6(8):1–10. doi: 10.1371/journal.pone.0023045. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patil SG, Deshmukh AA, et al. In vitro antibacterial activity of Emblica officinalis fruit extract by tube Dilution Method. Int J Toxicol Appl Pharmacol. 2012;2(4):49–51. [Google Scholar]
- 34.Saini P, Kumar M, et al. Evaluation of Antimicrobial Efficacy of Root Canal Sealers against Endodontic Pathogens: An in vitro Study. Int J Oral Care Res. 2016 Oct-Dec;4(4):267–272. doi: 10.5005/jp-journals-10051-0060. DOI: [DOI] [Google Scholar]
- 35.Kaplan AE, Picca M, et al. Antimicrobial effects of six endodontic sealers: an in vitro evaluation. Endod Dental Traumatol. 1999;15(1):42–45. doi: 10.1111/j.1600-9657.1999.tb00748.x. DOI: [DOI] [PubMed] [Google Scholar]