Abstract
Autologous chondrocytes in vitro expanded, are used as tools of regenerative therapies for cartilage injuries. However, inability to maintain the hyaline phenotype both in vitro and post in vivo transplantation, remains one of the major hurdles for long term efficacy under clinical settings. We have reported earlier, hyaline phenotype maintenance of both human and rabbit chondrocytes for a long duration both in vitro when cultured conditions using a Thermo-reversible Gelation Polymer (TGP) scaffold-based methodology and in vivo post-transplantation animal model of cartilage damage. Having intrigued by such encouraging outcome, we in this study, analysed the similar TGP culture environment whether would be able to allow in vitro expansion of severe osteoarthritis affected cartilage tissue from elderly patients and evaluated the cells using lectin microarray characterization for pluripotency. Cartilage tissue were obtained from patients (n = 7; age: 60–85 years) undergoing total knee arthroplasty for severe osteoarthritis. Chondrocytes were isolated and cultured in two groups: i. conventional culture without scaffold (2D) and ii. using a TGP scaffold-based culture (3D) up to 18 weeks. In addition to earlier reported findings such as maintenance of hyaline phenotype having been confirmed in this study as well, surface glycoprotein analysis by lectin microarray demonstrated that the α1-2 Fuc recognition lectin (UEA-1) (marker reported in literature for pluripotent stem cells) was found to be more highly expressed in 3D culture compared to 2D culture and even increased over time in 3D culture. We have developed an environment where osteoarthritis affected chondrocytes from the elderly could be cultured up to 18 weeks in vitro using TGP scaffold which express pluripotent cell associated surface glycoproteins compared to the conventional methodology.
Keywords: Cartilage, Chondrocytes, Osteoarthritis, Thermo-reversible gelation polymer (TGP) scaffold, Hyaline phenotype, Pluripotency, Lectin microarray
Abbreviations: ACI, Autologous chondrocyte implantation; MACI, matrix-associated chondrocyte implantation; TGP, Thermo-reversible gelation polymer; iPSC, Induced pluripotent stem cells; ESC, Embryonic stem cells; 2D, Two-dimensional; 3D, Three-dimensional; PBS, Phosphate-buffered saline; CO2, Carbon dioxide; hPSCs, Human pluripotent stem cells
Highlights
-
•
Good quality chondrocytes were grown from cartilage tissue of elderly with severe osteoarthritis for 18 weeks in vitro.
-
•
Inflamed donor chondrocytes could be revived to form normal tissue in a 3D in vitro TGP scaffold environment.
-
•
Pluripotent stem cell marker UEA-1 in Lectin microarray was positive in TGP- Polymer scaffold grown cartilage.
1. Introduction
Autologous chondrocyte implantation (ACI) and matrix-associated chondrocyte implantation (MACI) have long been used as regenerative therapies for cartilage damage, and several clinical trials have been conducted to establish the safety of these methods. However, the clinical outcome of these methods has yet to be elucidated; the transplanted articular chondrocytes have been reported to revert back to a fibroblast phenotype in vitro and in vivo, leading to complications [1,2]. Our earlier work examining chondrocytes in vitro culture using Thermo-reversible Gelation Polymer (TGP) and their subsequent transplantation resulted in the following outcomes (which we consider vital in overcoming the aforementioned hurdles).
-
i.
Bovine chondrocytes can be expanded for up to 16 weeks without growth factors while glycosaminoglycan and hydroxyproline contents increase as a function of time [3].
-
ii.
Human chondrocytes from non-weight bearing cartilage of young donors can be expanded for 16 weeks while maintaining the hyaline phenotype throughout the culture [4].
-
iii.
Rabbit chondrocytes can be grown in vitro while maintaining the hyaline phenotype for 10 weeks [5].
-
iv.
In vitro cultured rabbit chondrocytes 6 months post transplantation maintained the hyaline phenotype in biopsy [5].
Due to the abovementioned findings, we examine cells cultured in a TGP-based environment and transplanted, and we aimed to determine whether the same environment could also be nurturing multipotent or pluripotent cells among the chondrocytes cultured. To evaluate the pluripotency of the cells, we chose lectin microarray as a tool because lectins or cell surface glycans are rightly named as “cell signatures”; any process a cell undergoes, such as activation, differentiation, malignant transformation or inflammation, is reflected in these glycans. A study conducted by Tateno et al. [6] on 114 cell types of induced pluripotent stem cells (iPSC) and nine cell types of embryonic stem cells (ESCs) revealed that iPSCs that initially carried the glycome signature of the somatic cell from which they were derived expressed increased levels of α2–6-sialylation, α1–2-fucosylation, and type 1 LacNAc and decreased levels of α2–3-sialylation and tetra-antennary N-glycans upon the induction of pluripotency. This phenomenon was similar to the glycan signature of ESCs.
In this study, we determined the glycan signature of chondrocytes isolated from the diseased articular cartilage of elderly donors (age > 60 years) grown without a scaffold (termed two-dimensional (2D) culture) and TGP-based culture (termed three-dimensional (3D) culture) using lectin microarray.
2. Materials and methods
This research has been approved by the IRB of the authors' affiliated institutions. The cartilage specimens were collected from the articular defect of seven (n = 7) patients aged between 60 and 85 years old. Based on the guidance and recommendations of the IRB, informed consent was obtained from all the patients. Human cartilage biopsies were obtained from these patients who underwent total knee arthroplasty for severe osteoarthritis. The tissue samples were collected in sterile phosphate-buffered saline (PBS) with antibiotics and transported in cool conditions to the laboratory for processing. The harvested cartilage tissue in each patient was weighed and then washed with 10 ml of PBS and subjected to digestion with 0.25% Trypsin for 30 min in an orbital shaker at 150 rpm at 37 °C. The tissues were then subjected to 2 mg/ml collagenase digestion for 12–18 h in a 5% carbon dioxide (CO2) incubator at 37 °C on top of an orbital shaker. After digestion, D-MEM containing autologous plasma processed from blood that was collected from the patients at time of cartilage biopsy collection was added to arrest enzyme activity. The digested suspension was filtered with a 70-μm nylon mesh; undigested tissues were discarded, and the filtrate was centrifuged at 1000 rpm for 10 min. The cell pellets were washed with PBS, and the cell population was counted by the trypan blue dye exclusion method. For 6 out of 7 samples, the cells were first seeded without a scaffold (2D culture) in media containing low glucose D-MEM, 10% autologous plasma, 1% Penicillin Streptomycin, 50 μg/ml Gentamicin, and 0.25 μg/ml amphotericin B l-ascorbic acid (50 mg/ml) for 2 weeks at 37 °C with 5% CO2. After 2 weeks, the cells were cultured with a 3D-TGP scaffold [[3], [4], [5]] using the same media composition. For the seventh sample, the cells were seeded in both 2D and 3D cultures simultaneously after digestion. At the end of the culture period (up to 31 days for 2D cultures and up to 18 weeks for 3D cultures), the cells were counted, and a portion of the cells were stored for characterization using H & E staining, immunohistochemistry and glycan analysis by lectin microarray. Routine protocols were employed for H & E staining and immunohistochemistry. Immunohistochemistry staining was against a CD 44 antibody. Differential profiling of cell glycoproteins based on lectin microarray was conducted using the protocol of Kuno et al. [7]. Briefly, the cells from both 2D and 3D cultures were labeled with the fluorescent dye Cy3, subjected to gel filtration, applied to a LecChip™ to initiate the reaction between lectin and glycan, and scanned with a GlycoStation ™ Reader 1200. The results were analyzed using Glycostation™ ToolsPro Suite 1.5.
3. Results
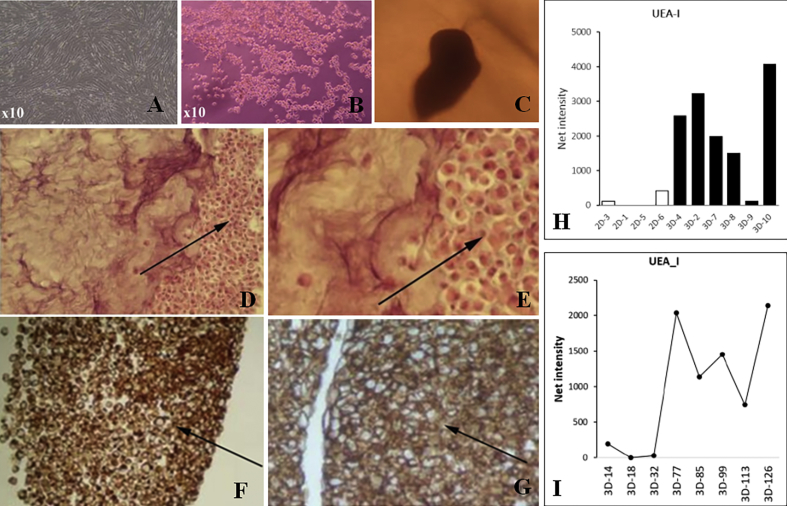
The cells in 2D culture grew as individual cells and cells showed fibroblast-like reversal of morphology by day 31 (Fig. 1A), while the cells in the periphery migrated out of the tissue-like aggregate in the 3D -TGP scaffold and were found to have rounded morphology, indicating an undifferentiated state even at 10 weeks of culture (Fig. 1B). Tissue-like aggregation of cells in 3D culture can be observed from Fig. 1C. In H & E (Fig. 1D and E) & CD 44 immunohistochemistry staining (Fig. 1F, G), a hyaline phenotype and tissue-like morphology was apparent in the 3D culture in comparison to loosely arranged cells that are observed in the 2D culture. Surface glycoprotein analysis by lectin microarray demonstrated that the α1-2 Fuc recognition lectin (UEA-1) was found to be more highly expressed in 3D culture compared to 2D culture (Fig. 1H) and even increased over time in 3D culture (Fig. 1I). The UEA-1 lectin has already been established as a biomarker for human pluripotent stem cells (hPSCs) [8].
Fig. 1.
A- Cells de-differentiated to fibroblast morphology in 2D culture, B- Cells migrated out of TGP scaffold showing rounded morphology even after 10 weeks; C- Tissue like aggregation in 3D; D, E− H & E staining showing hyaline phenotype, Arrows show individual cell morphology in 2D culture (D) and tissue like aggregation in 3D culture (E); F, G-Immunohistochemistry staining showing positivity for CD 44, Arrows show individual cell morphology in 2D culture (F) and tissue like aggregation in 3D culture (G); H - Comparison of UEA-I expression in 2D Vs 3D cultures in Lectin microarray; I- Time course of expression of UEA-1 in 3D cultures. 2D or 3D in the x-axis of Figure H & I refers to the group cultured and the number after 2D or 3D indicates the number of days in culture.
4. Discussion
In the present study, the cartilage tissue was not obtained from the non-weight bearing relatively healthy portion of the knee but rather the osteoarthritic, diseased portion from the subjects. This is in contrast with conventional ACI procedures [9]. In this study, we were able to confirm our earlier finding of the hyaline phenotype being maintained due to the utilization of TGP, even at the longer expansion time point of 18 weeks, while chondrocytes reverted to the fibroblast phenotype in 2D culture. There was higher UEA-1 lectin expression in the 3D culture group, a known characteristic of hPSCs [8]. The fucose-specific lectin UEA-I has been reported to be a potential tool for isolating viable hPSCs with sustained pluripotency from heterogeneous populations [8]. In the present study, UEA-1 not only is expressed more in 3D cultures but also increases over time in the 3D cultures (Fig. 1H and I). This important finding further suggests that our culture methodology of employing TGP results in maintaining an undifferentiated phenotype for a longer period of time in vitro [3]. Further research should examine whether multipotent or pluripotent stem cells are present in the native cartilage tissue itself [10], which gets expanded under in vitro 3D conditions, or that the 3D culture environment provides cues for reprogramming adult chondrocytes to a more potent stem cell state.
5. Conclusion
TGP scaffold provides an environment where osteoarthritis-affected cartilage tissue-derived chondrocytes can be cultured up to 18 weeks in vitro while maintaining functionality, as shown by the expression of pluripotent cell-associated surface glycoproteins. This outcome suggests that researcher should examine future applications for TGP being transplanted along with in vitro cultured cells, thereby making the post-transplant environment favorable for pluripotent cell expansion in vivo to enhance the repair of damaged cartilage.
Declaration of Competing Interest
1. Dr. Katoh is an employee of Edogawa Hospital, Japan and is an applicant/inventor to several patents on biomaterials and cell culture methodologies, some of them described in this manuscript.
2. Dr. Fujimaru is an employee of Edogawa Hospital, Japan.
3. Dr. Senthilkumar & Dr. Preethy are employees of Nichi-In Centre for Regenerative Medicine (NCRM).
4. Dr. Abraham is a share holder in GN Corporation Co. Ltd., Japan and is an applicant/inventor to several patents on biomaterials and cell culture methodologies, some of them described in this manuscript.
Acknowledgements
The authors wish to acknowledge Dr. Jun Makino, Department of Orthopaedic Surgery, Edogawa Hospital, Tokyo, Japan; Dr. Fumihiro Ijima of Hasumi International Research Foundation, Asagaya, Tokyo, Japan for his assistance with the cell culture work described in the manuscript; Mr. Mathaiyan Rajmohan and Mr. Ramalingam Karthick from the Fujio-Eiji Academic Terrain (FEAT), Nichi-In Centre for Regenerative Medicine (NCRM), Chennai, Tamil Nadu, India, for their assistance with data collection of the study described in the manuscript.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Bartlett W., Skinner J.A., Gooding C.R., Carrington R.W., Flanagan A.M., Briggs T.W. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 2.Horas U., Pelinkovic D., Herr G., Aigner T., Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003 Feb;85:185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda A., Kojima K., Tinsley K.W., Yoshioka H., Mori Y., Vacanti C.A. In vitro culture of chondrocytes in a novel thermoreversible gelation polymer scaffold containing growth factors. Tissue Eng. 2006 May;12:1237–1245. doi: 10.1089/ten.2006.12.1237. [DOI] [PubMed] [Google Scholar]
- 4.Arumugam S., Manjunath S., Senthilkumar R., Srinivasan V., Rajendiran S., Yoshioka H. In vitro expansion and characterization of human chondrocytes using a novel Thermoreversible Gelation Polymer (TGP) J Orthopaedics. 2011;8:e5. [Google Scholar]
- 5.Arumugam S., Bhupesh Karthik B., Chinnuswami R., Mori Y., Yoshioka H., Senthilkumar R. Transplantation of autologous chondrocytes ex-vivo expanded using Thermoreversible Gelation Polymer in a rabbit model of articular cartilage defect. J Orthop. 2017;14:223–225. doi: 10.1016/j.jor.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tateno H., Toyota M., Saito S., Onuma Y., Ito Y., Hiemori K. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J Biol Chem. 2011;286:20345–20353. doi: 10.1074/jbc.M111.231274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuno A., Matsuda A., Ikehara Y., Narimatsu H., Hirabayashi J. Differential glycan profiling by lectin microarray targeting tissue specimens. Methods Enzymol. 2010;478:165–179. doi: 10.1016/S0076-6879(10)78007-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y.C., Nakagawa M., Garitaonandia I., Slavin I., Altun G., Lacharite R.M. Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis. Cell Res. 2011;21:1551–1563. doi: 10.1038/cr.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niemeyer P., Pestka J.M., Kreuz P.C., Salzmann G.M., Köstler W., Südkamp N.P. Standardized cartilage biopsies from the intercondylar notch for autologous chondrocyte implantation (ACI) Knee Surg Sports Traumatol Arthrosc. 2010;18:1122–1127. doi: 10.1007/s00167-009-1033-4. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y., Tuan R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11:206–212. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]