Abstract
Introduction
Regenerative therapy using chondrocyte sheets is effective for osteoarthritis. The clinical application of chondrocyte sheet therapy is expected to be further advanced by the use of a feasible cryopreservation technique. Previously, we developed a chondrocyte sheet vitrification method; however, it was too complex to be used for routine clinical application. Here, we aimed to develop a prototype method for vitrifying chondrocyte sheets for clinical practice.
Methods
We developed a “circulating vitrification bag” as a container to process cell sheets for vitrification in an efficient and sanitary fashion. Moreover, we invented the “vitrification storage box”, which is useful for the vitrification of cell sheets, long-term preservation, and transportation. These devices were used to vitrify rabbit chondrocyte sheets, which were then assessed for their structural characteristics and the viability of the component cells after rewarming.
Results
In all cell sheet samples (n = 7) vitrified by the circulating vitrification bag method, the integrity of the sheet structure was maintained, and the cell survival rate was similar to that of non-vitrified samples (91.0 ± 2.9% vs. 90.0 ± 3.0%). Proteoglycan and type II collagen, which are major components of cartilage, were densely and evenly distributed throughout the chondrocyte sheet subjected to vitrification similarly to that observed in the non-vitrified sheet. After long-term storage using the vitrification storage box, the cell sheets maintained normal structure and cell viability (survival rate: 81.2 ± 1.0% vs. 84.3 ± 1.8%) compared to the non-vitrified sheet.
Conclusion
Our results indicate that the circulating vitrification bag method is an effective approach for realizing the clinical application of vitrified chondrocyte sheets. The vitrification storage box is also useful for the long-term preservation of vitrified cell sheets, further enhancing the feasibility of the clinical application of cryopreserved chondrocyte sheets.
Keywords: Chondrocyte, Cell sheet, Vitrification, Cryopreservation, Osteoarthritis
Abbreviations: DMSO, dimethyl sulfoxide; EG, ethylene glycol; FBS, fetal bovine serum; LN, liquid nitrogen; PBS, phosphate buffered saline
Highlights
-
•
We developed a new device and method for the cryopreservation of cell sheets.
-
•
The method and device efficacy were evaluated using vitrified rabbit chondrocyte sheets.
-
•
Our approach allows efficient clinical application of vitrified cell sheets.
1. Introduction
Regenerative medicine using autologous chondrocyte sheets has been reported to be effective for the treatment of osteoarthritis [1]. Allogeneic cell sheets created from polydactyly derived chondrocytes obtained from polydactyly surgery were reported to have similar treatment effects as autologous chondrocyte sheets [2,3].
Cryopreservation of cell sheets would help expand the clinical application of cell sheet therapy and promote its industrialization. We previously developed a vitrification method that allows for the cryopreservation of chondrocyte sheets [4]. Through this method, cryopreserved chondrocyte sheets were found to maintain their cartilage repair ability [5]. However, this previous method involves complex work processes [4], and it would be desirable to improve it to develop a simpler and more practical method for clinical application. In addition, for the expansion of the use of chondrocyte sheet therapy, it is important to establish technologies for the long-term preservation and transportation of cryopreserved cell sheets. To meet these requirements, we developed the “circulating vitrification bag” as a prototype device for allowing the practical use of the chondrocyte sheet vitrification method. Moreover, as a device that can maintain the stable vitrified state of cell sheets, the “vitrification storage box” was developed. The results of the performance of these devices based on the vitrification of rabbit chondrocyte sheets are reported.
2. Materials and methods
2.1. Preparation of rabbit chondrocyte sheets
Rabbit chondrocyte sheets were prepared according to our previous report [4]. Briefly, commercially available primary cultured cells derived from the knee cartilage of a Japanese white rabbit (CHC04C; Cosmo Bio Co., Ltd., Hokkaido, Japan) were plated onto temperature-responsive culture dishes (UpCell®, diameter: 35 mm; CellSeed, Tokyo, Japan) at a density of 5.9 × 104 to 7.5 × 104 cells/dish and cultured in DMEM/F12 medium (11320; Thermo Fisher Scientific K.K., Tokyo, Japan) supplemented with 20% fetal bovine serum (FBS; SH30070.03, GE Healthcare, Tokyo, Japan) at 37.5 °C in a humidified atmosphere of 5% CO2 in air. Upon reaching confluence, the medium in each dish was replaced with RPMI1640 medium (11875; Thermo Fisher Scientific K.K.) supplemented with 10% FBS and 100 μM l-ascorbic acid (3140401A1039; FUJIFILM Pharma Co., Ltd., Tokyo, Japan). The cells formed a single thin layer 2 weeks after plating, at which time the UpCell® dishes were placed at 25 °C for 30 min to promote detachment of the cell sheet from the bottom surface of the dish. The cell sheet was then removed from the dish using a cell shifter (CSD001; CellSeed). Two cell sheets were layered together to form a double-layered sheet, which was further cultured for 1 week.
2.2. Vitrification and rewarming solutions
Vitrification and rewarming solutions were prepared according to our previous report [4] (Table 1). HEPES (20 mM)-buffered Tissue Culture Medium-199 (05909; Nissui Pharmaceutical, Tokyo, Japan) supplemented with 20% calf serum (12133C; Sigma-Aldrich, St. Louis, MO, USA) was used as the basal solution. Dimethyl sulfoxide (DMSO; 13407-45, NACALAI TESQUE, Kyoto, Japan) and ethylene glycol (EG; 15209-85, NACALAI TESQUE) were used as permeable cryoprotectants. Sucrose (30404-45; NACALAI TESQUE) and carboxylated poly-l-lysine (Bio Verde, Kyoto, Japan) were used as nonpermeable cryoprotectants.
Table 1.
Composition of each solution and the time and temperature of each step.
| DMSO (v/v%) | EG (v/v%) | COOH-PLL (w/v%) | Sucrose (mol) | Time (min) | Temperature | |
|---|---|---|---|---|---|---|
| Solutions for pre-vitrification treatment | ||||||
| Equilibration solution-1 | 10 | 10 | – | – | 5 | RT |
| Equilibration solution-2 | 10 | 10 | – | – | 12.5 | RT |
| Vitrification solution-1 | 20 | 20 | 10 | 0.5 | 5 | IT |
| Vitrification solution-2 | 20 | 20 | 10 | 0.5 | 7.5 | IT |
| Solutions for post-vitrification treatment | ||||||
| Rewarming solution | – | – | – | 1 | 1 | RT |
| Dilution solution | – | – | – | 0.5 | 3 | RT |
| Washing solution-1 | – | – | – | – | 5 | RT |
| Washing solution-2 | – | – | – | – | 5 | RT |
COOH-PLL: Carboxylated poly-l-lysine; DMSO: Dimethyl sulfoxide; EG: Ethylene glycol; IT: Ice-cold temperature; RT: Room temperature.
An equilibration solution consisting of 10% (v/v) DMSO and 10% (v/v) EG in basal solution and a vitrification solution containing 20% (v/v) DMSO, 20% (v/v) EG, 10% (w/v) carboxylated poly-l-lysine, and 0.5 M sucrose were prepared. A rewarming solution and a dilution solution containing 1 M and 0.5 M sucrose, respectively, were prepared, and the basal solution was used as the washing solution. The vitrification solution was used at an ice-cold temperature (on crushed ice). All other solutions were used at room temperature (24–27 °C).
2.3. Vitrification and rewarming procedures
2.3.1. Circulating vitrification bag method
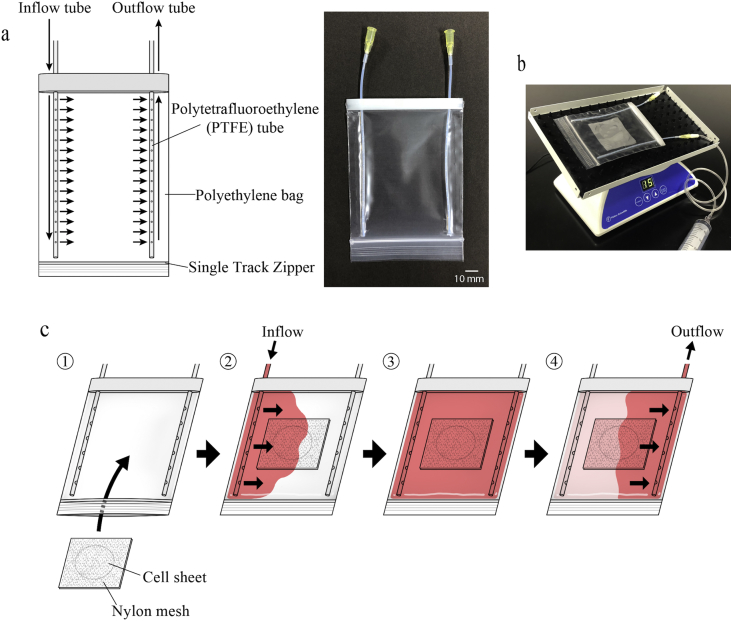
As a container for the vitrification of cell sheets, we developed the circulating vitrification bag, which allows sequential flushing of solutions during the pre- and post-vitrification processes. As shown in Fig. 1a, the circulating vitrification bag is a sealable polyethylene bag to which inflow and outflow tubes for injecting or discharging various solutions, respectively, are attached. The cell sheet was sandwiched between two nylon meshes (45 × 45 mm, 95 μm thread diameter, 140 μm opening diameter), and the four corners were fixed with a stapler. After inserting the cell sheet into the circulating vitrification bag, the zipper was closed while removing air in the bag (Fig. 1c1). The circulating vitrification bag was attached to the stage of an orbital shaker (05-450-213; SANSYO Co., Ltd., Tokyo, Japan), and the solutions shown in Table 1 were injected sequentially using a 20-ml syringe (Fig. 1b) while shaking the bag with the following conditions; rotation number: 15 rpm, rocking angle: 20°, turning angle: 360°, under room temperature (24–27 °C). First, 10 ml equilibration solution-1 was injected via the inflow tube into the circulating vitrification bag (pre-equilibration; Fig. 1c2). The cell sheet was kept in equilibration solution-1 for 5 min while shaking the bag (Fig. 1c3). Then, the solution was discharged from the outflow tube using a 20-ml syringe (Fig. 1c4). By using this same procedure, the cell sheet was exposed to equilibration solution-2 for 12.5 min. After discharging the solution, vitrification solution-1 was injected while shaking the bag. After confirming the permeation of the solution into every part of the bag, the bag was removed from the shaker and placed on ice. After exposure of the cell sheet to vitrification solution-1 for 5 min (pretreatment with vitrification solution), the bag was attached to the shaker once again, and the solution was discharged. Based on the same procedure described above, vitrification solution-2 was injected into the bag, and the cell sheet was exposed to the solution on ice for 7.5 min. After the discharge of the solution, the circulating vitrification bag was held 1 cm above the surface of liquid nitrogen (LN) in a 1-L Styrofoam container and vitrified by exposure to the vapor (approximately −150 °C). After exposure to the LN vapor for at least 1 min, the cell sheet was rewarmed. The circulating vitrification bag was placed on a heating plate at 45 °C. At the same time, the bag was covered by a gel pack that had been preheated to 38 °C for rapid rewarming. After 1 min, when the cell sheet had completely rewarmed, the circulating vitrification bag was attached again to the shaker. While shaking the circulating vitrification bag, the solutions for rewarming, dilution/removal of cryoprotectants, and washing (shown in Table 1) were successively injected and discharged. The cell sheet was exposed to rewarming solution for 1 min, dilution solution for 3 min, washing solution-1 for 5 min (prewash), and finally washing solution-2 for 5 min. Following the washing process, the cell sheet sandwiched between nylon meshes was removed from the bag using forceps and placed into a 100-mm dish. The cell sheet was then retrieved from the nylon meshes using forceps and immersed in 5 ml of Ca++- and Mg++- free phosphate buffered saline (PBS(−)) in a 60-mm dish. After checking for visible damage (cracks), the sheet was enzyme-treated for the cellular viability assessment.
Fig. 1.
The structure of the circulating vitrification bag and operation procedure. a: (Left) The structure of the circulating vitrification bag. An inflow tube and an outflow tube (polytetrafluoroethylene tube, inner diameter: 1.2 mm) are attached to the polyethylene bag (110 × 85 mm; film thickness: 0.063–0.064 mm). The inflow tube has 15 holes (hole diameter: 0.6 mm) to allow quick inflow of the solutions (indicated by arrows). The outflow tube has 30 holes (hole diameter: 0.6 mm), allowing for smooth solution discharge (indicated by arrows). (Right) A photograph of the circulating vitrification bag. b: A circulating vitrification bag attached on an orbital shaker that was operated with the following conditions; rotation number: 15 rpm, rocking angle: 20°, turning angle: 360°, under room temperature (24–27 °C). c: Operation procedure of the circulating vitrification bag. (1) Insert the cell sheet sandwiched between two nylon meshes into the circulating vitrification bag and close the opening. Then, attach the bag to the shaker. (2) While shaking the circulating vitrification bag, inject the equilibration solution-1 via the inflow tube (arrows). (3) Keep the cell sheet in solution for a given amount of time (Table 1). (4) Discharge the solution in the circulating vitrification bag from the outflow tube (arrows). Repeat steps (2) to (4) to successively inject and discharge the pre- and post-vitrification solutions shown in Table 1.
2.3.2. Envelope method
For comparison with the circulating vitrification bag method, the envelope method that we reported previously [4] was used to vitrify and rewarm chondrocyte sheets. Briefly, a cell sheet was successively immersed in 5 ml of the solutions used for pretreatment prior to vitrification (Table 1) in a 60-mm dish. The dishes with vitrification solution-1 and -2 were placed on ice. When picking up the cell sheet using forceps, we treated the cell sheet with extra care to avoid breaking it. After treating the cell sheet with vitrification solution-2, it was placed on a 90 × 50 mm rectangular piece of aluminum foil (thickness: 12 μm; Mitsubishi Aluminum Co., Ltd., Tokyo, Japan) using forceps. The aluminum foil was folded to enclose the cell sheet [4] and was held 1 cm above the LN surface in a 1-L Styrofoam container, and the cell sheet was vitrified by exposure to vapor (approximately −150 °C). After exposure to LN vapor for more than 1 min, the cell sheet was rewarmed.
For rapid rewarming, the cell sheet wrapped with aluminum foil was placed on a heating plate at 38 °C, and it was covered by a gel pack that had been preheated to 38 °C. One minute later, the aluminum foil was carefully unfolded, and the cell sheet was retrieved using forceps without breaking it and successively immersed in 5 ml of the solutions used for rewarming, dilution/removal of cryoprotectants, and washing (Table 1) in a 60-mm dish. After washing, the cell sheet was immersed in 5 ml of PBS(−) in a 60-mm dish, and after checking it for visible damage (cracks), it was enzyme-treated for the cellular viability assessment.
2.4. Evaluation of the cells composing the cell sheet
The cell sheet viability assessment was conducted according to the method used in our previous study [4]. Briefly, the cell sheet was cut into 1–2 mm2 pieces with ophthalmic scissors and incubated in DMEM/F12 medium containing 2 mg/ml collagenase II (17101; Thermo Fisher Scientific K.K.) at 37 °C for 60 min to isolate the component cells. The suspension of the isolated cells was centrifuged at 1000 rpm for 5 min, and the precipitated cells were resuspended in DMEM/F12 medium; the cell viability was determined after trypan blue staining [viability (%) = live cells/live and dead cells × 100].
2.5. Histological examination and immunohistochemical staining
Histological examination and immunohistochemical staining were performed according to our previous report [4]. Double-layered cell sheets were harvested after culture or cryopreservation and fixed in 4% paraformaldehyde solution for 1 week. The specimens were embedded in paraffin, sectioned, and placed on glass slides. After deparaffinization and rehydration, the sections were stained for proteoglycan with 0.1% Safranin-O or immunostained with type II collagen antibody. For immunohistochemistry, the slides were incubated with a diluted primary anti-human type II collagen antibody (F-57; Daiichi Fine Chemical, Toyama, Japan) for 16 h at 4 °C, followed by incubation with the EnVision + Mouse/HRP secondary antibody (K4000; DAKO, Glostrup, Denmark) for 1 h at room temperature. Finally, the sections were stained with diaminobenzidine (K3466; DAKO) and counterstained with hematoxylin. Coverslips were mounted onto slides and sealed with nail polish. The slides were then examined under a microscope, and images were captured (Biozero BZ-X710; KEYENCE, Osaka, Japan).
2.6. Experimental design
2.6.1. Verification of the circulating vitrification bag method
To verify the performance of the newly developed circulating vitrification bag method, a group of rabbit chondrocyte sheets were vitrified using this new method and the previously reported envelope method. Fresh cell sheets were used as non-vitrified controls. The sheet structure, viability of cells composing the sheet, and distributions of proteoglycan and type II collagen, which are major components of cartilage, were examined in vitrified and non-vitrified control sheets.
2.6.2. Long-term preservation of cell sheets using the vitrification storage box
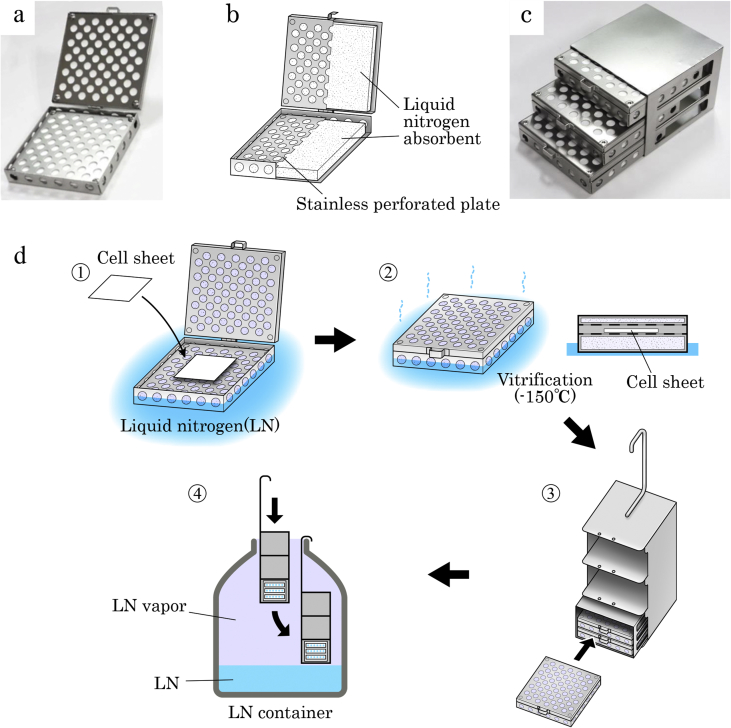
For the long-term storage and handling of vitrified cell sheets, which are vulnerable to physical damage due to devitrification, we developed a vitrification storage box (Fig. 2a–c). As shown in Fig. 2b, the box is a thin stainless steel container with built-in LN absorbents in the main body and the lid. In this experiment, we aimed to demonstrate that the vitrification storage box allows for long-term storage of the rabbit chondrocyte sheet. The cell sheets wrapped with aluminum foil following pretreatment with cryoprotectants were vitrified in accordance with the envelope method [4] using the vitrification storage box. The vitrification storage box containing the vitrified sheet was transferred to and stored in LN vapor (−150 °C) in an LN container (Fig. 2d) for a storage period of one or six months. The cell sheets after the long-term storage periods were compared with the cell sheets rewarmed immediately after vitrification (vitrified controls) and fresh cell sheets (non-vitrified controls) in terms of the cell sheet structure and cellular viability.
Fig. 2.
The structure and operation procedure of the vitrification storage box. The vitrification storage box (a) and its internal structure (b). b: The main body of the vitrification storage box (75 × 75 × 12 mm) and its lid (75 × 75 × 5 mm) are both made of stainless steel perforated plates (1 mm in thickness), and they are connected by a hinge. Both the main body and the lid have a built-in liquid nitrogen (LN) absorbent. c: Three vitrification storage boxes can be accommodated in a stainless storage rack (76 × 78 × 57 mm). The storage rack can be accommodated in an LN container canister (d-3). d: The operation procedure of the vitrification storage box. (1) Place the cell sheet wrapped in the vitrification package on the perforated plate of the main body of the box that was immersed and cooled in LN. (2) Vitrify the cell sheet in the vitrification storage box by exposing it to LN vapor (−150 °C) discharged from the LN absorbents. (3, 4) Place up to three vitrification storage boxes in a canister using the storage rack (3) and store them in LN vapor in the LN container (4). The vitrification storage box was prepared on a special order by Umihira Co. Ltd., Kyoto, Japan.
3. Results
3.1. Characteristics of post rewarming rabbit chondrocyte sheets vitrified with the circulating vitrification bag method
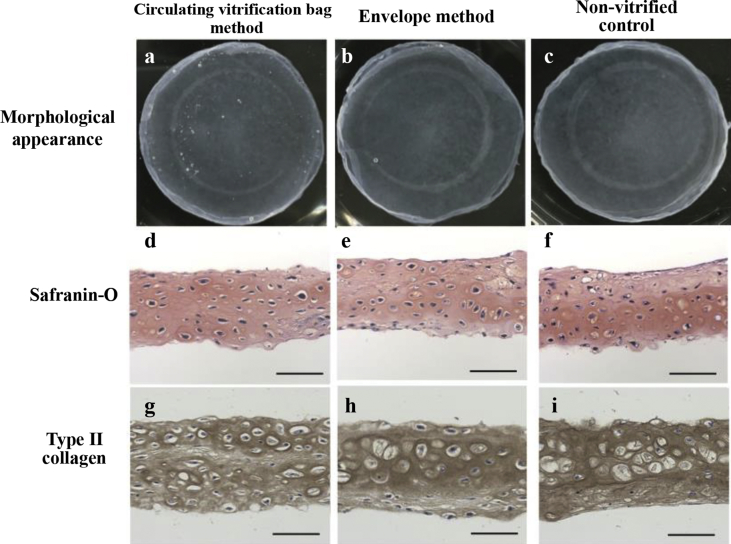
The circulating vitrification bag method was used to vitrify rabbit chondrocyte sheets, and the integrity of the sheet structure as well as the viability of the component cells were examined after rewarming. All of the vitrified sheets (n = 7) showed no visible damage and had the same appearance as non-vitrified cell sheets (Fig. 3a–c), and the average cell viability was 91.0 ± 2.9% (Table 2). This cell survival rate was equal to that of the cell sheets vitrified by the envelope method (n = 7; 89.5 ± 1.4%) and the non-vitrified control (n = 7; 90.0 ± 3.0%).
Fig. 3.
Comparative analysis of the characteristics of rabbit chondrocyte sheets before and after vitrification. Comparison of the morphological appearance (a–c) of rabbit chondrocyte sheets and distribution of proteoglycan (d–f) and type II collagen (g–i) in cross-sections of the cell sheets. a: There was no visible crack in the cell sheet vitrified by the circulating vitrification bag method. The extracellular matrix of the vitrified cell sheet contained abundant proteoglycan (d) and type II collagen (g) with a dense and even distribution. These characteristics of the vitrified cell sheets were similar to those of the cell sheets vitrified by the envelope method (b, e, h) and non-vitrified controls (c, f, i). (Scale bar = 50 μm).
Table 2.
Structural maintenance and cell viability after rewarming of rabbit chondrocyte sheets vitrified by the circulating vitrification bag method.
| Vitrification method | No. of cell sheets recovered without fracture/No. of cell sheets examined (%) | Cell viabilitya |
|---|---|---|
| Circulating vitrification bag method | 7/7 (100) | 91.0 ± 2.9% (n = 7) |
| Envelope method | 7/7 (100) | 89.5 ± 1.4% (n = 7) |
| Non-vitrified control | 7/7 (100) | 90.0 ± 3.0% (n = 7) |
Mean ± S. D.
In addition, portions of the cell sheets recovered after vitrification and rewarming were isolated to investigate the distributions of proteoglycan and type II collagen using histochemical staining and immunostaining. Similar to the cell sheet vitrified with the envelope method and the non-vitrified cell sheet, the cell sheets vitrified with the circulating vitrification bag method showed strong safranin-o staining throughout the tissue section (Fig. 3d–f). These results indicated that proteoglycan was densely and evenly distributed throughout the chondrocyte sheet. Moreover, the cell sheet also showed an even and abundant distribution of type II collagen (Fig. 3g–i). These data showed that the extracellular matrix of the cell sheets had been maintained after cryopreservation with the circulating vitrification bag method.
3.2. Post rewarming structure and cellular viability of the cell sheet stored for long periods using the vitrification storage box
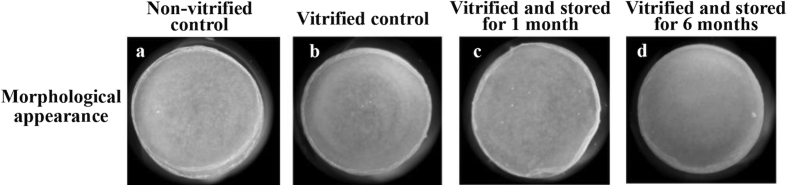
The vitrification storage box was used to vitrify and store rabbit chondrocyte sheets for long periods, and the sheet structure and cellular viability of post rewarming cell sheets were assessed (Table 3). There was no visible damage in the vitrified cell sheets stored for both 1 and 6 months in LN vapor in the LN container (Fig. 4). Furthermore, the rates of cell survival after storage for one and six months (79.2 ± 2.6% and 81.2 ± 1.0%, respectively) were equal to those of the cell sheets rewarmed immediately after vitrification (80.1 ± 2.5%) and the non-vitrified controls (84.3 ± 1.8%).
Table 3.
Structural maintenance and cell viability of rabbit chondrocyte sheets preserved for long periods in the vitrification storage box.
| Storage period | No. of cell sheets recovered without fracture/No. of cell sheets examined (%) | Cell viabilityb | |
|---|---|---|---|
| Non-vitrified control | – | 6/6 (100) | 84.3 ± 1.8% (n = 6) |
| Vitrified controla | – | 5/5 (100) | 80.1 ± 2.5% (n = 5) |
| Vitrified and stored | 1 month | 3/3 (100) | 79.2 ± 2.6% (n = 3) |
| 6 months | 3/3 (100) | 81.2 ± 1.0% (n = 3) |
Vitrified control represents the cell sheet that was rewarmed immediately after vitrification.
Mean ± S. D.
Fig. 4.
Morphological appearance of rabbit chondrocyte sheets after long-term vitrification preservation. The morphological appearance of rabbit chondrocyte sheets that were stored for long periods in the vitrification storage box was observed. a: Non-vitrified control cell sheet. b: The cell sheet vitrified with the envelope method and immediately rewarmed (vitrified control). c, d: Intact morphology of the cell sheets vitrified in the vitrification storage box and stored in the LN container for one month (c) and six months (d).
4. Discussion
Vitrification techniques permit cryopreservation of cells and tissues with a high concentration of cryoprotectants. The benefit of vitrification techniques is that the solution turns into a glassy (amorphous) state at ultralow temperatures without the formation of ice crystals that cause physical damage to the cells [6]. In the vitrification method, samples are exposed to high concentrations of cryoprotectants. During the pretreatment process of cell sheet vitrification, samples are exposed to various solutions to allow cryoprotectants to permeate into the cells in a stepwise fashion at increasing concentrations between the isotonic solution and the vitrification solution, which has an osmolarity of 11000 mOsmol. Next, in the post-vitrification process, the cell sheet in ultrahypertonic vitrification solution is then exposed in a stepwise fashion to solutions with decreasing concentrations, during which cryoprotectants are diluted and eliminated. To complete these processes, the previously reported vitrification method [4] required a cumbersome, manual process to transfer the cell sheet successively from one solution to another; this method is unrealistic to implement in actual clinical settings.
In contrast, the circulating vitrification bag method developed in the current study allows the cell sheet to be exposed successively to various solutions with different components and osmolarity by keeping the cell sheet in a sealed bag while successively replacing the solution in the bag. The circulating vitrification bag method minimizes the number of occasions the operator comes in contact with the cell sheet, which is helpful in reducing labor and enhancing sterility when applying the cell sheet vitrification technique in clinical settings.
The main concern in the application of this new method was that the residual solution in the preceding step within the bag may not be completely replaced by the solution in the following step. However, in our preliminary experiment, the solution in the bag was confirmed to be almost completely replaced by the new solution after just one round of preflushing (Suppl. Fig. 1). It can be interpreted that, in the circulating vitrification bag method, the cell sheet was properly exposed to each solution in the vitrifying-rewarming process, which allowed it to achieve similar performances as a sheet treated by the conventional method that requires manual handling of cell sheets.
In the cell sheet vitrification procedure, cells are exposed to large changes in the osmolarity of the solutions used. Rapid changes in osmolarity can be a stress factor for cells [7]. However, the use of the circulating vitrification bag is thought to be beneficial for reducing the osmotic stress to cells during cell sheet treatment. The reason for this is that in the circulating vitrification bag method, the cell treatment solution used in the preceding step is gradually replaced by the solution in the subsequent step (Suppl. Fig. 1). Therefore, the change in osmolarity during this process is estimated to be milder. In other words, a well-designed solution replacement method can minimize the stress due to osmotic changes while improving the survival rates of the component cells of vitrified cell sheets. As the bag used in this study was a prototype, the optimization of both the inflow and outflow rates will be required in the future.
This study found that the circulating vitrification bag method was able to maintain the morphological integrity of the vitrified and rewarmed cell sheet. The survival rates of component cells and components of the extracellular matrix were also maintained after vitrification and were on par with those of the non-vitrified sheets. The recovery of cartilage damage using the chondrocyte sheet was shown to involve bioactive substances secreted from the cell sheet, including transforming growth factor beta 1, melanoma inhibitory activity, and prostaglandin E2 [5,8,9]. We have already confirmed in our previous study that these factors could be generated from vitrified and rewarmed rabbit chondrocyte sheets [5]. In the future, we need to check whether the cell sheet vitrified using the circulating vitrification bag method has the same cartilage damage recovery function.
In the present study, we did not test the applicability of the circulating vitrification bag method to other cell sheet types than rabbit chondrocytes. The vitrification protocol used in the circulating vitrification bag method has been proven to be effective for cryopreserving human myoblast cell sheet [10]. It is, therefore, likely that the circulating vitrification bag method is applicable to various cell sheets.
In the development of vitrification methods for animal embryos, including those of humans, the minimum volume cooling (MVC) concept [11] has been widely used. By considering that the utility of the MVC concept has been proven in embryo vitrification, we developed the cell sheet vitrification method based on this concept [4]. In methods based on the MVC concept, the sample is cryopreserved along with a minimum volume of vitrification solution. By minimizing the amount of vitrification solution used, the speed of the temperature decrease or increase of the sample is maximized. During the temperature fall and rise, the solution quickly passes through the temperature range at which ice crystals are more likely to form. In this way, the risk of ice crystal formation in the solution is reduced [12], and a high sample survival rate can be maintained.
On the other hand, one drawback of methods based on the MVC concept is that they are more likely to result in devitrification. A cell sheet vitrified with a small amount of vitrification solution devitrifies even with a slight rise in temperature. Devitrification under non-optimal conditions may result in cell damage associated with ice crystal formation in solution. Such devitrification tends to occur when pulling the vitrified cell sheet out of the LN container or transferring it within the laboratory or between facilities. Here, we developed a vitrification storage box that is effective in the prevention of devitrification in the abovementioned situations. Moreover, the vitrification storage box can be used in the entire process, from vitrification of the cell sheet to storage and transportation. The characteristics of the cell sheet vitrification method that we previously developed [4] allow it to induce vitrification by exposing the sample to LN vapor (approximately −150 °C) rather than by immersing it in LN. The internal temperature of the vitrification storage box filled with LN absorbents is maintained stably at approximately −150 °C, which produces the desired vitrification conditions and preservation conditions of the vitrified cell sheet.
5. Conclusions
In conclusion, the results of this study indicated that the circulating vitrification bag method was potentially capable of realizing the clinical application of vitrified chondrocyte sheets. The vitrification storage box was also shown to be useful for the long-term preservation of vitrified cell sheets, making the clinical application of cryopreserved chondrocyte sheets even more feasible.
Declaration of Competing Interest
Asuka Hayashi, Miki Maehara, Ayuko Uchikura and Hitomi Matsunari declare that they have no conflict of interest. Kazuaki Matsumura and Suong-Hyu Hyon are cofounders of Bioverde Inc. Masato Sato receives research funding from CellSeed Inc. Hiroshi Nagashima is a founder and shareholder of PorMedTec Co., Ltd.
Acknowledgments
This work was supported by the Meiji University International Institute for Bio-Resource Research (MUIIBR), and the Research Project for Practical Applications of Regenerative Medicine (No. JP19bk0104063 to MS) from the Japan Agency for Medical Research and Development. The authors thank Mr. Y. Tokuyama for his technical assistance.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2020.04.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sato M., Yamato M., Mitani G., Takagaki T., Hamahashi K., Nakamura Y. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. npj Regen Med. 2019;4:4. doi: 10.1038/s41536-019-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toyoda E., Sato M., Takahashi T., Maehara M., Okada E., Wasai S. Transcriptomic and proteomic analyses reveal the potential mode of action of chondrocyte sheets in hyaline cartilage regeneration. Int J Mol Sci. 2019;21(1) doi: 10.3390/ijms21010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maehara M., Sato M., Toyoda E., Takahashi T., Okada E., Kotoku T. Characterization of polydactyly-derived chondrocyte sheets versus adult chondrocyte sheets for articular cartilage repair. Inflamm Regen. 2017;37:22. doi: 10.1186/s41232-017-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maehara M., Sato M., Watanabe M., Matsunari H., Kokubo M., Kanai T. Development of a novel vitrification method for chondrocyte sheets. BMC Biotechnol. 2013;13:58. doi: 10.1186/1472-6750-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tani Y., Sato M., Maehara M., Nagashima H., Yokoyama M., Yokoyama M. The effects of using vitrified chondrocyte sheets on pain alleviation and articular cartilage repair. J Tissue Eng Regen Med. 2017;11:3437–3444. doi: 10.1002/term.2257. [DOI] [PubMed] [Google Scholar]
- 6.Rall W.F., Fahy G.M. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985;313:573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura K., Hyon S.H. Polyampholytes as low toxic efficient cryoprotective agents with antifreeze protein properties. Biomaterials. 2009;30:4842–4849. doi: 10.1016/j.biomaterials.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Kaneshiro N., Sato M., Ishihara M., Mitani G., Sakai H., Mochida J. Bioengineered chondrocyte sheets may be potentially useful for the treatment of partial thickness defects of articular cartilage. Biochem Biophys Res Commun. 2006;349:723–731. doi: 10.1016/j.bbrc.2006.08.096. [DOI] [PubMed] [Google Scholar]
- 9.Hamahashi K., Sato M., Yamato M., Kokubo M., Mitani G., Ito S. Studies of the humoral factors produced by layered chondrocyte sheets. J Tissue Eng Regen Med. 2015;9:24–30. doi: 10.1002/term.1610. [DOI] [PubMed] [Google Scholar]
- 10.Ohkawara H., Miyagawa S., Fukushima S., Yajima S., Saito A., Nagashima H. Development of a vitrification method for preserving human myoblast cell sheets for myocardial regeneration therapy. BMC Biotechnol. 2018;18(1):56. doi: 10.1186/s12896-018-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamawaki A., Kuwayama M., Hamano S. Minimum volume cooling method for bovine blastocyst vitrification. Theriogenology. 1999;51(1):165. [Google Scholar]
- 12.Wowk B. Thermodynamic aspects of vitrification. Cryobiology. 2010;60:11–22. doi: 10.1016/j.cryobiol.2009.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.