Abstract
Soft tissue calcifications associated with various connective tissue diseases such as dermatomyositis and scleroderma have been well documented Plaque-like sheets of subcutaneous calcifications presenting as an indurated soft tissue mass in a patient with primary Sjogren syndrome have been rarely documented in the literature. We present the magnetic resonance and conventional radiographic findings of calcinosis cutis and calcinosis circumscripta of a 47-year-old woman with biopsy proven Sjogren syndrome. We also delineate various types of soft tissue calcification, histopathology of calcinosis cutis, and current treatment options. Recognizing the magnetic resonance characteristics of this phenomenon may prove useful to radiologists, especially in the absence of clinical history and conventional radiographs.
Keywords: Calcinosis cutis, Calcinosis circumscripta, Soft tissue calcifications, Connective tissue disorder, Sjogren syndrome
Introduction
Soft tissue calcification associated with autoimmune disease has often been described with entities such as systemic sclerosis, dermatomyositis, and mixed connective tissue disorders [1].
The moniker calcinosis cutis has been used to describe various insoluble calcific salt deposits of soft tissue that were originally described by Virchow in 1855 [2], [26]. The calcifications have been categorized into 5 types—metastatic, iatrogenic, idiopathic, dystrophic, and calciphylaxis [3].
The etiology of the calcium deposition varies depending on the specific subtype, for example, metastatic calcinosis cutis is characterized by abnormal calcium and phosphate levels in the serum. Iatrogenic calcinosis cutis is associated with a therapeutic or diagnostic procedure such as subcutaneous injection of calcium-containing heparins, extravasation of calcium gluconate, and use of calcium-containing electrode compounds for electromyographic or electroencephalographic examination [2]. Idiopathic occurs without any underlying tissue damage or metabolic disorder [3]. Dystrophic calcinosis cutis is the most common type and occurs as the result of local tissue damage with normal calcium and phosphate levels in serum. Calciphylaxis, also called calcific uremic arteriolopathy, is a net-like or mesh-like network of small vessel calcification affecting the dermis or subcutaneous fat [4].
The term “tumoral calcinosis” describes another type of calcification that is typically periarticular and has primary and secondary etiologies [5,6]. This term has generated consternation in the literature as has been “liberally and imprecisely used to describe any massive collection of periarticular calcification, although this term actually refers to a hereditary condition associated with massive periarticular calcification. The inconsistent use of this term has created confusion throughout the literature [7].” Tumoral calcinosis can usually be distinguished from calcinosis cutis as the former is periarticular, often with a “sediment sign,” secondary to fluid–calcium levels. Calcinosis cutis, as its name implies, primarily affects the subcutaneous fat and dermis.
Calcinosis cutis of connective tissue disease is categorized as the dystrophic type [6]. Dystrophic calcification of connective tissue disease is distinguished from ossification due to the lack of zonal ossification unlike processes such as myositis ossificans [8]. While the exact mechanism of this pathology is unknown, the soft tissues of connective tissue disease are predisposed to calcification in the setting of a normal serum calcium and phosphorous [3]. It has been postulated that the pathophysiology of dystrophic calcinosis may be the result of structural defects, hypoxemia, or chronic tissue inflammation [3]. The denatured proteins of necrotic cells may bind to calcium and phosphorous, acting as a substrate for dystrophic calcinosis in tissues affected by trauma or chronic inflammation [9].
Case presentation
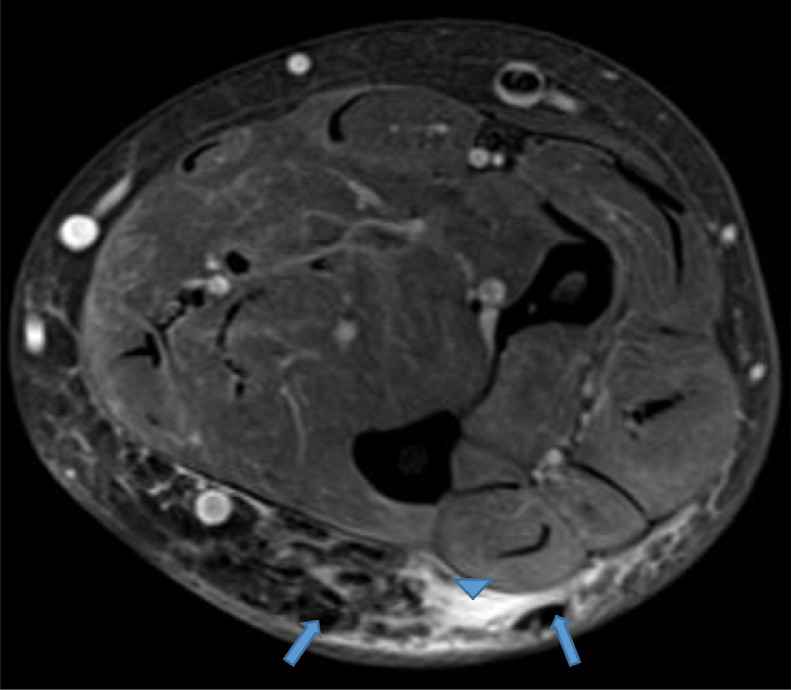
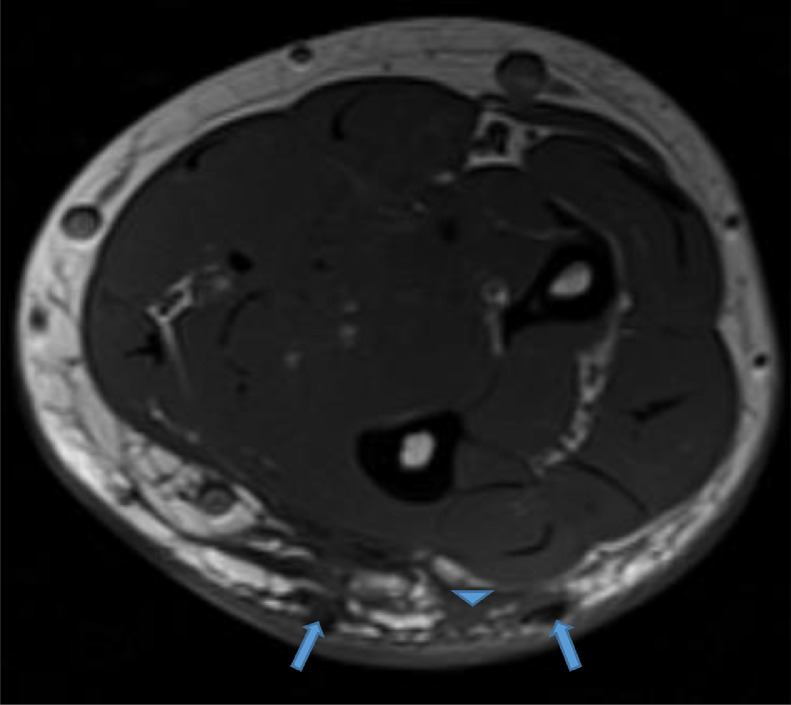
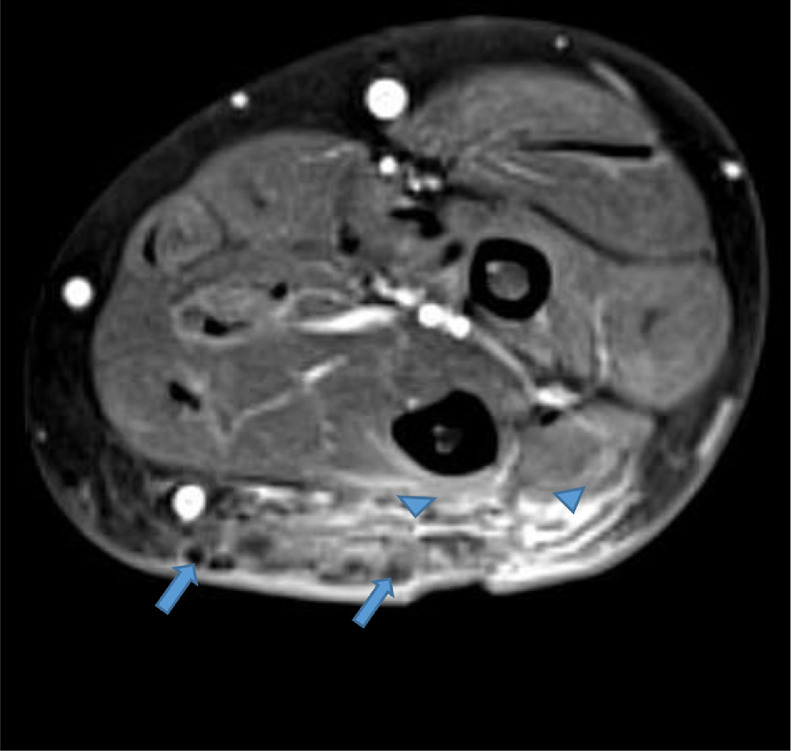
Our case is of a 47-year-old woman with a history of polyarticular joint pain and swelling who presented with symptoms of dry mouth and eyes. In addition, she described an indurated and erythematous mass of her left posterior forearm that had increased in size over the past year and caused her increasing discomfort (Fig. 1). Her social and travel history were noncontributory with the exception of being a current smoker with 20 pack per year smoking history. The patient's laboratory values were as follows rheumatoid factor negative, anti-nuclear antibody (ANA) positive titer of 1:160 with a homogenous cytoplasmic pattern, and a positive Sjogren Anti-SS-A of >8.0. The Sjogren Anti-SS-B was normal at <0.2. C-reactive protein was elevated to 13.2. Serum calcium and phosphorus were normal. All other laboratory values were normal, including her renal function. Physical exam revealed mild swelling of the joints of the hands. No rashes were evident, specifically her facial complexion was normal. A magnetic resonance (MR) of the left forearm was obtained to evaluate an enlarging mass and to rule out sarcoma. The MR revealed a mass within the extensor surface of the forearm, contained within the subcutaneous fat. The mass exhibited numerous rounded foci, the largest measuring 0.4 cm, that were low signal on all sequences. On T2-weighted fat-saturated images, there was high T2 fluid-like signal that infiltrated the subcutaneous fat surrounding the small round low-signal foci (Fig. 2, Fig. 3, Fig. 4). There was subtle thickening of the skin overlying the mass but there was no penetration of the underlying deep fascia by the mass. On T1 sequences, the small, rounded low signal foci within the subcutaneous fat were surrounded by intermediate signal (Fig. 5, Fig. 6). After the administration of IV gadolinium contrast, there was significant enhancement evident around the periphery of the mass, with additional enhancement around the small, rounded, low-signal foci (Fig. 7, Fig. 8). The low-signal structures were suspicious for calcification; however, given the degree of enhancement, phleboliths of a vascular malformation were not excluded and conventional radiographs were subsequently recommended.
Fig. 1.
Indurated erythematous mass of left forearm (elbow adjacent to lower border of photograph for positional reference).
Fig. 2.
Axial proton density (PD) fat saturation image reveals low signal intensity foci (blue arrows) surrounded by high signal intensity fluid infiltrating the subcutaneous fat (blue arrowhead). Color version available online
Fig. 3.
Sag short-TI inversion recovery (STIR) sequence reveals multiple low signal intensity foci surrounded by high signal intensity infiltration of the subcutaneous fat (white oval). The area of designated palpable concern is bracketed with cutaneous skin markers. Elbow at top of image for positional reference
Fig. 4.
Coronal PD fat saturation image reveals multiple low signal intensity foci in the area of designated concern, bracketed by skin markers. The surrounding subcutaneous fat is infiltrated by high signal. Elbow at top of image for reference
Fig. 5.
Axial T1 image reveals low signal intensity foci surrounded by intermediate signal infiltration of the subcutaneous fat (blue arrowhead). Color version available online
Fig. 6.
Sagittal T1 image reveals multiple hypointense foci within the subcutaneous fat of the extensor surface of the forearm (white oval). Note skin thickening and low signal intensity infiltration of the subcutaneous fat in the affected area. The area of designated palpable concern is bracketed with cutaneous skin markers. Elbow at top of image for positional reference
Fig. 7.
Axial T1 fat saturation image with IV contrast reveals intense enhancement in the subcutaneous fat (arrowheads) surrounding the low signal intensity foci in the affected area (arrows). Note the skin thickening overlying the affected area. IV, intravenous
Fig. 8.
Sag T1 fat saturation image with IV contrast reveals enhancement of subcutaneous fat surrounding the low signal intensity foci (white oval). Elbow at bottom of image for orientation. IV, intravenous
Radiographs of the left forearm revealed a plaque-like sheet of calcification along the extensor surface that abutted the skin surface and followed the contours of the underlying deep fascia (Fig. 9, Fig. 10, Fig. 11). The dystrophic, nonossified appearance of the mass ruled out the possibility of phleboliths and thus a vascular mass such as a soft tissue hemangioma was excluded. A diagnosis of calcinosis cutis was posited, and additional rheumatologic tests were ordered, including a biopsy of the lower lip and additional conventional radiographs of the hands and feet. The lip biopsy results revealed multifocal plasmacytic and lymphocytic sialadenitis consistent with Sjogren syndrome. Radiographs of the hands revealed periarticular dystrophic calcifications without erosions, most consistent with digital calcinosis circumscripta (Fig. 12, Fig. 13, Fig. 14) [10]. Given the constellation of findings, the patient was diagnosed with primary Sjogren syndrome.
Fig. 9.
Oblique radiograph of the left forearm revealing plaque-like calcifications within the subcutaneous fat, depicted as low signal intensity foci on the aforementioned MR scan. MR, magnetic resonance
Fig. 10.
AP radiograph of the left forearm revealing plaque-like calcifications within the subcutaneous fat, depicted as low signal intensity foci on the aforementioned MR scan. MR, magnetic resonance
Fig. 11.
Lateral radiograph of the left forearm revealing plaque-like calcifications within the subcutaneous fat, depicted as low signal intensity foci on the aforementioned MR scan. MR, magnetic resonance
Fig. 12.
AP radiograph of the right hand reveals multiple periarticular calcifications most conspicuously at the proximal interphalangeal (PIP) joints and adjacent to the distal ulna consistent with calcinosis circumscripta. Scattered calcifications are also noted adjacent to the distal interphalangeal (DIP) joints. DIP, distal interphalangeal
Fig. 13.
Oblique radiograph of the right hand reveals multiple periarticular calcifications most conspicuously at the PIP joints and adjacent to the distal ulna consistent with calcinosis circumscripta. PIP, proximal interphalangeal
Fig. 14.
Lateral radiograph of the right hand reveals periarticular calcifications dorsal to the wrist and periarticular to the PIP joints consistent with calcinosis circumscripta. PIP, proximal interphalangeal
Given the symptomatic mass and above findings a wide local excision of the mass was performed; the specimen measured 15 × 8 × 2 cm (Fig. 15). Except for a small area in the center, the wound was approximated primarily. Three months after the excision, using wet-to-dry dressing changes twice per day, the wound was completely healed. Gross pathology examination of the resected specimen revealed calcific granular deposits within the subcutaneous fat (Figs. 16 A and B). Hematoxylin and Eosin staining revealed dark blue calcium deposits with perigranular basophilic inflammatory infiltrate and fibrosis (Figs. 17 A-C).
Fig. 15.
Photograph of the forearm status post wide local excision of subcutaneous mass. A small length of the incision could not be closed primarily due to tissue tension and reveals exposed fascia (white tissue). The incision was completely healed after 3 months, with wet-to-dry dressing exchanges twice daily. Elbow at top of image for reference
Fig. 16.
(A) Mass-like firm tissue excised from forearm. Fresh sections have chalky white fluid inside the soft tissue. The calcific areas have a firm consistency. (B) Serially sectioned skin with subcutis showing dense calcification after formalin fixation
Fig. 17.
(A-C) Hematoxylin & Eosin staining at standard nonmagnified (A), 25× (B), and 200× (C), revealed dark blue calcium deposits with perigranular basophilic inflammatory infiltrate and fibrosis. Color version available online
Discussion
“Sicca syndrome” is a term coined by Henrik Sjogren, to describe a series of 19 patients that he treated for dry eyes and mouth during his training as an ophthalmologist in Sweden in 1933. While “sicca syndrome” (ie, xerostomia and xerophthalmia) and “Sjogren syndrome” has become nearly synonymous, it is known that most sicca syndrome patients do not have Sjogren syndrome. Specifically, to be designated as Sjogren syndrome, the etiology of the dry eyes and mouth must be due to autoimmune-induced inflammation of the lacrimal and salivary glands that causes measurable impairment of tear and saliva production [11], [27]. This is in contradistinction to nonautoimmune sicca in which therapies directed to the immune system may be ineffective or even deleterious.
“Sjogren's syndrome is an autoimmune disease characterized by a lymphoplasmacytic infiltration of the exocrine glands, which ultimately leads to their atrophy and destruction [6].” This pathology manifests with a daily unremitting sensation of dry eyes and dry mouth that interferes with activities of daily living. A minority of patients, 20%, experience extraglandular manifestations of the disease that can lead to end-organ damage. Renal impairment secondary to interstitial nephritis, glomerulonephritis, and renal tubular acidosis is known to be associated with Sjogren syndrome. Graves’ disease and Hashimoto's thyroiditis have also been reported more frequently in patients with Sjogren's syndrome. Similarly, autoimmune hepatitis and primary biliary cirrhosis affect Sjogren syndrome patients more frequently than control populations. A low-grade, usually indolent, lymphoma is also a known but rare complication [12]. The development of peripheral neuropathy is also of concern with patients affected with Sjogren's syndrome [13].
The discovery of subcutaneous dystrophic calcifications often elicits a differential diagnosis of connective tissue disease such as progressive systemic sclerosis, mixed connective tissue disease, dermatomyositis, polymyositis, and systemic lupus erythematosus [7]. Dystrophic calcifications associated with Sjogren syndrome are reported far less frequently. Tsuchida reported massive calcinosis cutis associated with primary Sjogren's syndrome [14]. Llamas-Velasco et al and Fueki reported the other 2 cases as Fueki et al describing a mechanism of calcium deposition involving the interplay between osteonectin and matrix Gla protein [6,15]. Our case involves a patient with biopsy-proven primary Sjogren's syndrome and a description of her imaging findings, including MR imaging of calcinosis cutis. Calcinosis cutis universalis is a descriptive term used to describe extensive plaque or sheet-like calcification that can be seen on radiographs in patients with various connective tissue diseases; however, the degree and extent of calcification that must be present to invoke this term remains ill defined. Juvenile dermatomyositis is known to be associated with extensive superficial soft tissue calcifications that resemble an exoskeleton and one could suggest that the term “universalis” would aptly apply [3]. Calcinosis cutis circumscripta is a localized form of calcinosis cutis universalis that often affects the hands and feet [7,10].
The deposition of macroscopic calcium deposits in close proximity with the skin poses an inherent risk of skin ulceration and infection. The etiology of this process is uncertain but is likely due to combination of focally increased pressure from a noncompliant, abnormally ridged mineralized structure within the subcutaneous fat and a foreign-body type reaction [16,17]. Increased vascularity derived from an inflammatory foreign body type reaction could explain the increased enhancement we observed after the administration of intravenous gadolinium contrast on the MR scan. Ultimately, these calcific deposits can erode through the dermis resulting in the exudation of chalky deposits through patient's skin, increasing the risk for infection [6,18].
“While no pharmacological treatment has been generally accepted as standard therapy, although various treatments have been reported to be beneficial, including warfarin, bisphosphonates, minocycline, ceftriaxone, aluminum hydroxide, probenecid, intralesional corticosteroids, intravenous immunoglobulin, carbon dioxide laser, and extracorporeal shock wave lithotripsy [19].” In addition, calcium channel blockers such as diltiazem have been tried with varying effects. It is thought that calcium antagonists have an “immunomodulatory or dysregulatory effect on lymphocytes and can suppress superoxide generation and phagocytic action of neutrophils. Moreover mast cell degranulation and platelet aggregation may also be impaired [20].” Tajalli and Qureshi found that a topical solution of 20% sodium thiosulfate in a petrolatum base 3 times per day was effective in healing a calcinosis cutis of a fingertip ulcer in a patient with limited scleroderma (CREST syndrome) [21]. Song et al reported that intravenous sodium thiosulfate had no effect on 3 patients with extensive connective tissue disease associated dystrophic calcinosis cutis [22]. Given the variable results of pharmacologic therapy, surgical excision has been described as the treatment of choice [23,24].
While typically not a diagnostic dilemma on conventional radiographs, our case illustrates how calcinosis cutis can have an unusual appearance on MR that many radiologists may be unfamiliar with. In the era of increased access to MR imaging and the widespread trend of radiologists interpreting imaging studies from areas remote to their locale, radiologists may not have the luxury of having all or even the correct sequence of imaging modalities at their disposal. For this reason, it is important for the radiologists to be able to use the imaging evidence at their disposal to formulate a concise differential diagnosis and confirm, if necessary, their differential with additional imaging modalities. In this case, conventional radiography was used in a retrograde manner to cinch the findings suspected on the MR imaging. MR was useful to confirm the extent of disease, whether a soft tissue component was present, and whether the underlying fascia had been violated, all of which proved useful for presurgical planning. In the future, presumably with the widespread use of ACR Select as a clinical decision support tool for advanced imaging, the process of ordering imaging studies will be less cumbersome for the clinician and more streamlined for the patient [25].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
All work was performed at: UF Health Jacksonville, 655 West 8th Street, Jacksonville, FL 32209.
References
- 1.Aggarwal N., Shrestha S. Images in clinical medicine. Dystrophic calcinosis cutis. N Engl J Med. 2013;368(21):e28. doi: 10.1056/NEJMicm1211227. [DOI] [PubMed] [Google Scholar]
- 2.Carrascosa M.F., Pascual Velasco, Corrales Martinez A, Fernandez-Ayala Novo M, Casuso Saenz E, Salcines Caviedes J.R. Calcinosis cutis. BMJ Case Rep. 2011;2011:1–2. doi: 10.1136/bcr.01.2011.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiter N., El-Shabrawi L, Leinweber B, Berghold A, Aberer E. Calcinosis cutis: part I. Diagnostic pathway. J Am Acad Dermatol. 2011;65(1):1–12. doi: 10.1016/j.jaad.2010.08.038. quiz 13-4. [DOI] [PubMed] [Google Scholar]
- 4.Shmidt E., Murthy N.S., Knudsen J.M., Weenig R.H., Jacobs M.A., Starnes A.M. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67(6):1296–1301. doi: 10.1016/j.jaad.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Girard C.J., 2nd, Wasserman P.L., Lenchik L. Secondary Tumoral Calcinosis with Intraosseous Penetration. Radiol Case Rep. 2009;4(1):213. doi: 10.2484/rcr.v4i1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llamas-Velasco M., Eguren C, Santiago D, Garcia-Garcia C, Fraga J, Garcia-Diez A. Calcinosis cutis and Sjogren’s syndrome. Lupus. 2010;19(6):762–764. doi: 10.1177/0961203309355298. [DOI] [PubMed] [Google Scholar]
- 7.Olsen K.M., Chew F.S. Tumoral calcinosis: pearls, polemics, and alternative possibilities. Radiographics. 2006;26(3):871–885. doi: 10.1148/rg.263055099. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal S., Atwal S.S., Mondal D, Garga U.C. Radiographic patterns of soft tissue calcinosis in juvenile dermatomyositis and its clinical implications. J Clin Diagn Res. 2014;8(12):RD08–RD11. doi: 10.7860/JCDR/2014/10787.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulman N., Slobodin G., Rozenbaum M., Rosner I. Calcinosis in rheumatic diseases. Semin Arthritis Rheum. 2005;34(6):805–812. doi: 10.1016/j.semarthrit.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Ardolino A.M., Milne B.W., Patel P.A., Fairhurst J., Clarke N.M. Digital calcinosis circumscripta: case series and review of the literature. J Pediatr Orthop B. 2012;21(5):443–447. doi: 10.1097/BPB.0b013e3283484c15. [DOI] [PubMed] [Google Scholar]
- 11.Baer A.N., Walitt B. Update on Sjogren syndrome and other causes of sicca in older adults. Rheum Dis Clin North Am. 2018;44(3):419–436. doi: 10.1016/j.rdc.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malladi A.S., Sack K.E., Shiboski S.C., Shiboski C.H., Baer A.N., Banushree R. Primary Sjogren’s syndrome as a systemic disease: a study of participants enrolled in an international Sjogren’s syndrome registry. Arthritis Care Res (Hoboken) 2012;64(6):911–918. doi: 10.1002/acr.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCoy S.S., Baer A.N. Neurological complications of Sjogren's syndrome: diagnosis and management. Curr Treatm Opt Rheumatol. 2017;3(4):275–288. doi: 10.1007/s40674-017-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuchida Y., Sumitomo S., Fujio K., Yamamoto K. Massive calcinosis cutis associated with primary Sjogren’s syndrome. BMJ Case Rep. 2016;2016:1–2. doi: 10.1136/bcr-2015-214006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fueki H., Hino R., Yoshioka M., Nakamura M., Tokura Y. Calcinosis cutis associated with primary Sjogren’s syndrome: strong expression of osteonectin and matrix Gla protein. Rheumatology (Oxford) 2011;50(12):2318–2320. doi: 10.1093/rheumatology/ker304. [DOI] [PubMed] [Google Scholar]
- 16.Kim S.Y., Choi H.Y., Myung K.B., Choi Y.W. The expression of molecular mediators in the idiopathic cutaneous calcification and ossification. J Cutan Pathol. 2008;35(9):826–831. doi: 10.1111/j.1600-0560.2007.00904.x. [DOI] [PubMed] [Google Scholar]
- 17.Touart D.M., Sau P. Cutaneous deposition diseases. Part II. J Am Acad Dermatol. 1998;39(4):527–546. doi: 10.1016/s0190-9622(98)70001-5. [DOI] [PubMed] [Google Scholar]
- 18.Rabens S.F., Bauer M. Minimal scleroderma with extensive calcinosis cutis. Calif Med. 1973;118(5):69–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter N., El-Shabrawi L., Leinweber B., Berghold A., Aberer E. Calcinosis cutis: part II. Treatment options. J Am Acad Dermatol. 2011;65(1):15–22. doi: 10.1016/j.jaad.2010.08.039. quiz 23-4. [DOI] [PubMed] [Google Scholar]
- 20.Palamaras I., Kyriakis K. Calcium antagonists in dermatology: a review of the evidence and research-based studies. Dermatol Online J. 2005;11(2):8. [PubMed] [Google Scholar]
- 21.Tajalli M., Qureshi A.A. Successful treatment of calcinosis cutis of fingertip in the setting of CREST syndrome with topical 20% sodium thiosulfate. JAAD Case Rep. 2019;5(11):988–990. doi: 10.1016/j.jdcr.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song P., Fett N.M., Lin J., Merola J.F., Costner M., Vleugels R.A. Lack of response to intravenous sodium thiosulfate in three cases of extensive connective tissue disease-associated calcinosis cutis. Br J Dermatol. 2018;178(6):1412–1415. doi: 10.1111/bjd.15783. [DOI] [PubMed] [Google Scholar]
- 23.Valdatta L., Buoro M., Thione A., Mortarino C., Tuinder S., Fidanza C. Idiopathic circumscripta calcinosis cutis of the knee. Dermatol Surg. 2003;29(12):1222–1224. doi: 10.1111/j.1524-4725.2003.29391.x. [DOI] [PubMed] [Google Scholar]
- 24.Guermazi A., Grigoryan M., Cordoliani F., Kerob D. Unusually diffuse idiopathic calcinosis cutis. Clin Rheumatol. 2007;26(2):268–270. doi: 10.1007/s10067-005-0135-8. [DOI] [PubMed] [Google Scholar]
- 25.Radiology, A.C.o. Clinical Decision Support Tool Includes ACR Select. 2018; Available from:https://www.acr.org/Practice-Management-Quality-Informatics/Quality-Care-News/Newsletter/Quality-and-Safety-eNews-June-2018/Clinical-Decision-Support-Tool-Includes-ACR-Select.
- 26.Virchow R. Kalk-metastasen. Arch Path Anat. 1855;8:103–113. [Google Scholar]
- 27.Sjogren H. Zur Kenntnis der Keratoconjunctivitis sicca (Keratitis filiformis bei Hypofunktion der Träonendrüsen. Ophthalmol (Copenh). 1933;1(11):1–151. [Google Scholar]