Abstract
Introduction
Adipose-derived stem cells (ASCs) are potential cell sources for cartilage tissue engineering. Chitosan has been shown to enhance the stemness and differentiation capability of ASCs, and the native extracellular matrix (ECM) derived from articular cartilage has been also reported to induce chondrogenic differentiation of ASCs. Here we tested the hypothesis that a porous three-dimensional (3D) hybrid scaffold composed of chitosan and cartilage ECM can provide a better environment to induce ASC chondrogenesis.
Methods
Mixed solution composed of chitosan and cartilage ECM was frozen and lyophilized to form a composite construct. The porous 3D scaffolds were further crosslinked by genipin and used for ASC culture.
Results
Cultivation of ASCs in the chitosan/cartilage ECM composite 3D scaffolds induced the formation of cell spheroids with profound glycosaminoglycan production after 14 and 28 days culture. Chondrogenesis of ASCs seeded in the 3D scaffolds was also evident by mRNA expressions of cartilage-specific gene COL2A1 and ACAN on day 14. Histology and immunohistochemistry on day 28 also showed abundant cartilage-specific macromolecules, namely collagen type II and proteoglycan, deposited in a surface layer of the composite scaffold with tangential layer, transitional layer, and lacunae-like structures. Otherwise, hypertrophic markers collagen type I and X were concentrated in the area beneath the surface.
Conclusion
Our findings demonstrated spatial chondrogenic differentiation of ASCs in the chitosan-cartilage ECM composite scaffolds. This 3D hybrid scaffold exhibits great potentials for ASC-based cartilage tissue engineering.
Keywords: Adipose-derived stem cell, Chitosan, Chondrogenesis, Extracellular matrix, Tissue-engineered cartilage
Highlights
-
•
Cultivation of ASCs in the chitosan and cartilage ECM hybrid scaffold induced chondrogenesis.
-
•
ASCs in composite scaffold expressed cartilage-specific genes COL2A1 and ACAN.
-
•
Histologic inspections showed abundant cartilage-specific collagen type II and proteoglycan productions.
-
•
Chitosan-cartilage ECM hybrid scaffold exhibits great potentials for ASC-based cartilage tissue engineering.
1. Introduction
Cartilage regeneration is a challenging clinical problem in plastic and orthopedic surgery following tissue damage due to trauma, developmental anomalies, or age-related degeneration, such as osteoarthritis. Since current treatment modalities exhibit limitations, tissue engineering has emerged as a promising treatment for cartilage regeneration. Various types of stem cells have been investigated for application in tissue engineered cartilage. Among them, adipose-derived stem cells (ASCs) are multipotent stem cells that can be easily accessed from subcutaneous adipose tissue via liposuction [1,2]. During in vitro expansion, ASCs can be expanded and induced toward a chondrogenic phenotype by exogenous delivery or genetic overexpression of growth factors [3].
The selection of biomaterial is another issue for cartilage tissue engineering. The multifunctional nature of the native extracellular matrix (ECM) has caught increasing attention in the design and fabrication of tissue engineering scaffolds [4]. It has been shown that cartilage-specific ECM components such as collagen type II and glycosaminoglycans (GAGs) are crucial in regulating chondrogenic phenotype and supporting chondrogenesis [5]. Chitosan has the characters of positively-charged natural polysaccharide, good biocompatibility, and similar molecular structure to GAGs, which make it widely used as a scaffold material for cartilage tissue engineering [6]. In addition, the newly-secreted chondroitin sulfates, which produced by differentiated chondrocytes, can be captured in the chitosan scaffold through the formation of ionic complexes of positively-charged chitosan and the negatively-charged GAGs, which may provide a protective effect to tissue engineered cartilage against GAG hydrolysis [7]. Recent studies further showed that culturing ASCs on chitosan films enhance stemness and chondrogenic differentiation capability [[8], [9], [10]].
It was found that the cell type and deacetylation degree of chitosan could affect the cell adhesion and proliferation, therefore, the mixture of chitosan and other molecules with adhesion motifs is commonly used to increase the cellular adhesiveness of chitosan [11]. For example, primary chondrocytes cultured on chondroitin 4-sulfate-augmented chitosan maintained the synthesis of cartilage-specific collagens [7]. In addition to the major macromolecules in cartilage, namely collagen type II and GAGs, other endogenous content of ECM molecules may also play important roles in supporting chondrogenic differentiation [12,13]. The cartilage-derived ECM is previously shown to induce in vitro chondrogenesis of stem cells to chondrocytes without exogenous delivery of growth factors [[14], [15], [16]]. Herein we proposed to integrate cartilage-derived ECM components with chitosan to fabricate a three-dimensional (3D) composite scaffold for cartilage tissue engineering. We hypothesized that this cartilage-derived component could serve as a scaffold material for attachment and chondrogenic differentiation of ASCs to further promote cartilage formation.
2. Materials and methods
2.1. Preparation of the chitosan-cartilage ECM films
Full thickness porcine cartilage was harvested from femoral condyles of freshly sacrificed porcine knee joints, and cartilage-derived matrix was prepared as previously described [14]. The matrix was further pulverized by a freezer mill to form fine powder. One gram of cartilage ECM powder and 100 mg of pepsin (Sigma–Aldrich, St. Louis, MO) were mixed in 100 mL of 0.01 M HCl and kept at a constant stir for 72 h at room temperature. The resultant viscous solution of digested cartilage ECM had a pH of approximately 3.0–4.0. Modified from a previous protocol, the ECM solution was neutralized by mixing 0.1 N NaOH (1/10 of the volume of ECM solution) and 10x phosphate buffered saline (PBS, 1/9 of the volume of ECM solution) to pH 7.4 [17]. The activity of pepsin was irreversibly inactivated when the pH was raised to 7.4, and the resultant cartilage ECM gel was adjusted to a concentration of 0.4% by adding PBS.
Chitosan solution (2%; C-3646, Sigma–Aldrich) was prepared by dissolving chitosan in 1% acetic acid. The 2% chitosan solution was mixed with 0.4% cartilage ECM gel or PBS 1:1 in volume. The resulting chitosan and chitosan-cartilage ECM mixture solutions were added into 24-well culture plates (Corning, Corning, NY) as 0.5 mL per well. The solution was then allowed to dry at 50 °C for 2 days to form a thin membrane. Each well was then neutralized by 0.1 N NaOH aqueous solution for 15 min and washed thoroughly with distilled water. The resultant composite films were composed of 5 parts of chitosan and 1 part of cartilage ECM, with pure chitosan films served as a control. Before cell culture, the coated wells were sterilized in 70% ethanol overnight and rinsed extensively with PBS, followed by treatment under ultraviolet light overnight.
2.2. Preparation of 3D composite scaffolds
Mixed solution composed of 1% chitosan and 0.2% cartilage ECM was frozen overnight and lyophilized to create a porous composite construct. This 3D scaffolds were stabilized in 70% ethanol overnight and neutralized in 0.5 N NaOH/ethanol (4:1 in volume) solution for 2 h [8]. The composite constructs were further crosslinked in 0.05% genipin solution (Wako, Richmond, VA) at 37 °C for 3 days [18]. All constructs were thoroughly washed with distilled water and lyophilized again. The resulting sponge-like scaffolds were cut using a biopsy punch and scalpel to form constructs 6 mm in diameter and 1.5 mm in height. These specimens were sterilized using ethylene oxide and outgassed for one week before cell culture.
Crosslinked chitosan-cartilage ECM constructs were examined by scanning electron microscope (SEM). The pore sizes of imaged samples were estimated by tracing the periphery of the pores using the Image J software. We selected scaffold pores with a long-to-short axis ratio of not more than 1.5 for measurements, and the cross-section area of at least 30 pores were estimated for each sample.
2.3. Cell culture
Human ASCs (Zen-Bio, Durham, NC) were plated at an initial density of 8000 cells/cm2 in expansion medium consisting of Dulbecco's modified Eagle's medium (DMEM)-F-12 (Gibco, Grand Island, NY), 10% fetal bovine serum (FBS; Atlas Biologicals, Ft. Collins, CO), 1% penicillin-streptomycin (Gibco), and 1 ng/mL basic fibroblast growth factor (Roche Diagnostics). The cells were cultured at 37 °C in 5% CO2, and the media were changed every 2–3 days. Passage 4 ASCs were used for chondrogenic differentiation experiments.
2.4. ASC culture on 2D chitosan-cartilage ECM films
For 2D cell culture, 10,000 ASCs per 0.5 mL control medium were plated in each well of the 24-well tissue culture plate coated with chitosan or chitosan-cartilage ECM. The control medium consisted of DMEM-high glucose (Gibco), 10% FBS, and 1% penicillin-streptomycin. At post-seeding day 1, 2 and 4, cell proliferation assay was performed according to the manufacturer's protocol (CellTiter-Blue, Promega, Madison, WI). Briefly, 0.1 mL of CellTiter-Blue reagent was added into each well containing 0.5 mL of control medium. After incubating at 37 °C for 3 h, 0.1 mL of medium was aspirated from each well for fluorescence measurement.
2.5. ASC culture in 3D chitosan-cartilage ECM composite scaffolds
For 3D culture, 500,000 ASCs were re-suspended in 30 μL chondrogenic medium consisting of DMEM-high glucose, 10% FBS, 1% penicillin-streptomycin, 37.5 μg/mL l-ascorbic acid 2-phosphate (Sigma–Aldrich), 1% ITS + Premix (Becton Dickinson, Bedford, MA), 100 nM dexamethasone (Sigma–Aldrich) and 10 ng/mL TGF-β1 (R&D Systems). Cells were seeded by directly pipetting on a crosslinked chitosan-cartilage ECM scaffold that was placed in 24-well low-attachment plates (Corning). The constructs were incubated at 37 °C for 1 h to allow cell attachment, and then 1 mL of chondrogenic medium was added in each well. Medium was changed every 2–3 days. Cultures were terminated at defined time points during the study for evaluation.
2.6. Live cell/matrix stain for 3D culture
After cultured in chondrogenic medium for 2 and 5 days, in situ discrimination of ASCs on the chitosan-cartilage ECM scaffolds was performed. The live cells were stained with the vital stain Calcein AM (Invitrogen, Carlsbad, CA) and the green fluoresce was visualized by fluorescence microscopy. The scaffold material was counter-stained simultaneously by Texas Red C2-dichlorotriazine (Invitrogen), which appeared yellow by fluorescence microscopy.
2.7. Biochemical analysis for 3D culture
Three cells/scaffold constructs per time points (1, 7, 14, and 28 days) were digested by 1 mL of papain solution consisting of 125 μg/mL papain (Sigma–Aldrich), 100 mM phosphate buffer, 10 mM cysteine, and 10 mM EDTA at pH 6.3 for 24 h at 65 °C. Total DNA content was measured using the PicoGreen fluorescent double-stranded DNA (dsDNA) assay (Molecular Probes, Eugene, OR) according to the manufacturer's protocol. GAG content in the samples was measured by the dimethylmethylene blue assay using bovine chondroitin sulfate as a standard. Total collagen content was determined by assaying for hydroxyproline in scaffolds after acid hydrolysis and reaction with p-dimethylaminobenzaldehyde and chloramine-T, using 0.134 as the ratio of hydroxyproline to collagen.
2.8. RNA isolation and real-time quantitative PCR
Three ASC-seeded 3D composite scaffolds per time point (day 1 and 14) were snap frozen, pulverized and lysed in the supplied Trizol reagent (Invitrogen). Total RNA was isolated using RNeazy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. Total RNA concentration was determined by optical density at 260 nm (OD260) using a spectrophotometer (Nanodrop ND-1000, Wilmington, DE). Once RNA was isolated, complementary DNA was synthesized from RNA using iScript reverse transcriptase (Bio-Rad, Hercules, CA). Real-time PCR (iCycler, Bio-Rad) with commercial primer probes (Applied Biosystems, Foster City, CA) were used to compare transcript levels for 5 different genes: 18s ribosomal RNA (endogenous control; assay ID Hs99999901_s1), aggrecan (ACAN; assay ID Hs00153936_m1), collagen type I (COL1A1; assay ID Hs00164004_m1), collagen type II (COL2A1; custom assay: FWD Primer 5-GAGACAGCATGACGCCGAG-3; REV primer 5-GCGGATGCTCTCAATCTGGT-3; Probe 5-FAM-TGGATGCCACACTCAAGTCCCTCAAC-TAMRA-3) [19], and collagen type X (COL10A1; assay ID Hs00166657_m1). PCR was performed in triplicate for each sample for each gene. Using 18s as a house-keeping gene, data were analyzed by normalizing to the day 0 ASC samples.
2.9. Histological and immunohistochemical analysis
Cells/scaffold constructs were fixed overnight at 4 °C in a solution containing 4% paraformaldehyde, dehydrated in graded ethanol solutions, embedded in paraffin, cut into 5 μm-thick sections, and mounted on SuperFrost microscope slides (Microm International AG, Volketswil, Switzerland). To stain for sulfated GAGs, sections were treated with hematoxylin for 3 min, 0.02% fast green for 3 min, 0.1% aqueous Safranin O solution for 5 min, rinsed with distilled water, and dehydrated with xylene.
Immunohistochemical analysis was also performed using monoclonal antibodies against collagen type I (Abcam, Cambridge, MA), collagen type II (Developmental Studies Hybridoma Bank, University of Iowa), collagen type X (Sigma–Aldrich), and chondroitin 4-sulfate (Bio-Rad). Digest-All (Zymed, South San Francisco, CA) was used for pepsin digestion on sections for collagen type I, II, and X. Sections labeled chondroitin 4-sulfate were treated with trypsin, then with soybean trypsin inhibitor, and subsequent the chondroitinase (all from Sigma–Aldrich). The Histostain-Plus ES Kit (Zymed) was used on all sections for serum blocking before secondary antibody labeling (anti-mouse IgG antibody, Sigma–Aldrich), and subsequent linking to horseradish peroxidase. Aminoethyl carbazole (Zymed) was used as the enzyme substrate/chromogen. Negative controls without utilizing primary antibodies were also prepared to rule out nonspecific labeling.
2.10. Statistical analysis
All measurements are presented as mean ± standard deviation (SD). Data involving two groups or time points were evaluated by Student's t test, while data involving more than three groups or time points were evaluated by ANOVA with Scheffé's post-hoc test using STATA software (Stata Inc, College Station, TX) to determine significance. The cut-off level of significance was set at p = 0.05.
3. Results
3.1. ASC culture on 2D films with control medium
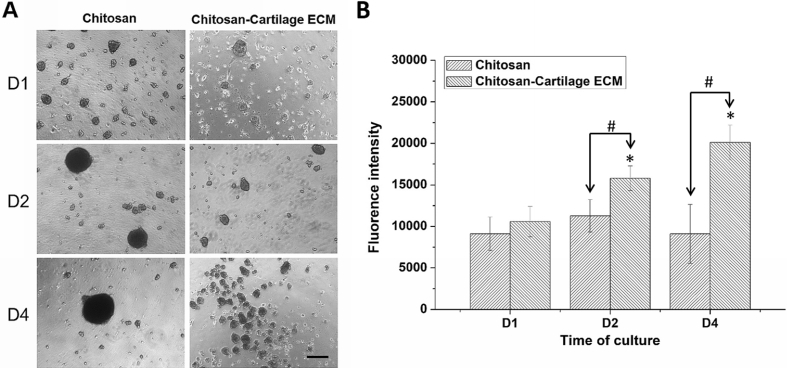
ASCs aggregated to form a few big spheroids on chitosan films after cell seeding, while multiple smaller spheroids were found on chitosan-cartilage ECM films (Fig. 1A). The size of ASC spheroids on chitosan films increased as time, while the size of ASC spheroids on chitosan-cartilage ECM films did not grew from day 2. The diameter of ASC spheroids on chitosan films was significantly larger than those on chitosan-cartilage ECM films at day 4 (134.4 ± 22.7 vs. 51.6 ± 17.3 μm, p < 0.001). Cell proliferation assay was performed for ASCs cultured on pure chitosan and hybrid chitosan-cartilage ECM films. On day 2 and 4, respectively, ASCs on chitosan-cartilage ECM films showed significantly higher cell proliferation than those on pure chitosan film as well as day 1 samples (p < 0.05, Fig. 1B).
Fig. 1.
Human ASC culture on chitosan-cartilage ECM films and chitosan films. (A) ASCs aggregated to form a few big spheroids on chitosan films, while ASCs on chitosan-cartilage ECM film formed multiple smaller spheroids. Scale bar = 100 μm (B) ASC proliferation on the films was measured by CellTiter-Blue assay. Data presented as mean ± SD. ∗p < 0.05 relative to day 1 samples; #p < 0.05 between the indicated groups.
3.2. ASC culture in 3D composite scaffold
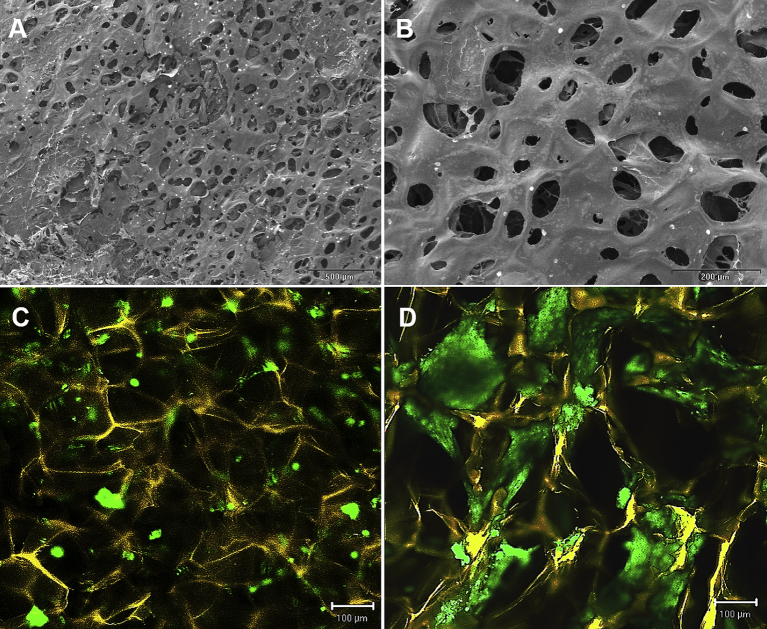
An interconnected porous structure was found in the chitosan-cartilage ECM composite scaffold under SEM inspection, and the average pore size of the scaffold was measured to be 75.8 ± 18.0 μm (Fig. 2A and B). Two days after seeding ASCs on the composite scaffold, confocal microscopy of live/matrix stain showed multiple small ASC spheroids formation (42.3 ± 18.3 μm) at the surface of the composite scaffold (Fig. 2C), which was similar to the phenomenon observed on the 2D chitosan-cartilage ECM films. At day 5, ASC spheroids appeared to enlarge and connect to larger cellular aggregates (123.1 ± 48.6 μm) within the interstices of the composite scaffolds (Fig. 2D).
Fig. 2.
Representative scanning electron microscopic images showed a porous morphology of the chitosan-cartilage ECM composite scaffold with an average pore size of 75.8 ± 18.0 μm. (A) low-power view, bar = 500 μm. (B) high-power view, bar = 200 μm. Confocal microscopic images of chitosan-cartilage composite scaffolds after cell seeding. The scaffolds were stained yellow, while the viable ASCs were stained green. (C) Day 2 image showed multiple small ASC spheroids formation. (D) Day 5 image demonstrated further aggregation of the ASC spheroids.
3.3. Biochemical analysis of the 3D scaffolds
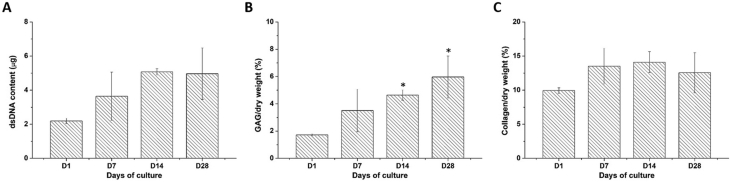
For ASCs cultured in the chitosan-cartilage ECM composite scaffold, no significant differences were noticed in the dsDNA content harvested at different time points. However, a trend toward higher dsDNA content on day 14 and 28 as compared to day 1 samples was observed (2.2 ± 0.2 μg at day 1 vs. 5.1 ± 0.2 μg at day 14, p = 0.058, and 5.0 ± 1.5 μg at day 28, p = 0.068; Fig. 3A). After normalization to dry weight, the scaffolds exhibited a significantly higher GAG content on day 14 and 28 compared to day 1 samples (1.7 ± 0.1% at day 1 vs. 4.6 ± 0.4% at day 14 and 6.0 ± 1.5% at day 28, p < 0.05 respectively; Fig. 3B). Moreover, the normalized collagen content in the ASC-seeded chitosan-cartilage ECM composite scaffolds as determined by the hydroxyproline assay did not exhibit significant changes over the culture period. With an initial collagen fraction of 10.0 ± 0.4% at day 1, the proportion fluctuated from 14.1 ± 1.5% at day 14 to 12.6 ± 2.9% at day 28 (Fig. 3C).
Fig. 3.
Biochemical assays of human ASCs cultured on chitosan-cartilage ECM composite scaffolds. (A) dsDNA content, (B) GAG content normalized by dry weight, and (C) collagen content normalized by dry weight. Data are presented as mean ± SD. ∗p < 0.05 relative to the day 0 sample.
3.4. Gene expression
At day 1, the mRNA levels of COL2A1 was significantly upregulated (1.7 ± 0.2 fold, p < 0.05) and COL1A1 was significantly downregulated (0.6 ± 0.1 fold, p < 0.05) relative to day 0 samples, while transcript levels of COL10A1 and ACAN exhibited no significant change from day 0. On day 14, all 4 genes were significantly upregulated, with 3.8 ± 0.4 fold increase for COL1A1 (p < 0.05), 5.0 ± 1.3 fold increase for COL2A1 (p < 0.01), 37.9 ± 2.2 fold increase for COL10A1 (p < 0.01), and 65.9 ± 11.5 fold increase for ACAN (Fig. 4, p < 0.001).
Fig. 4.
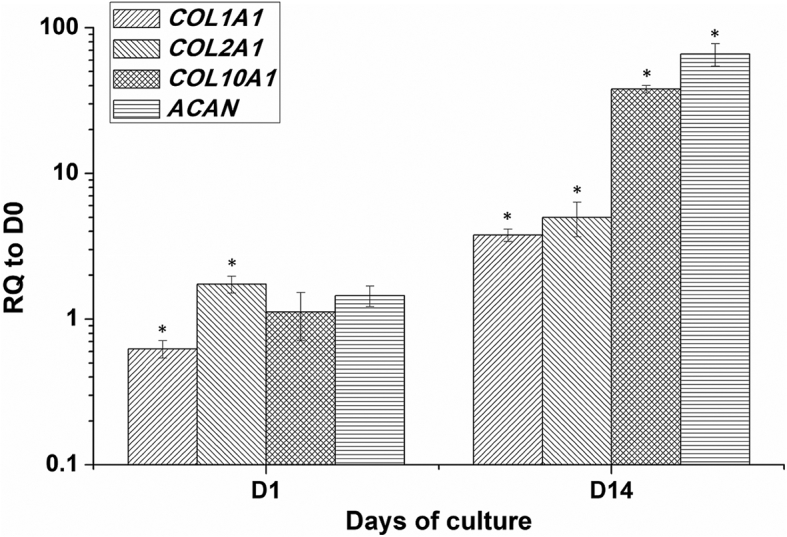
Gene expression of COL1A1, COL2A1, COL10A1, and ACAN in human ASCs cultured in the chitosan-cartilage ECM composite scaffolds. Data presented as the relative quantification (RQ) to day 0 transcript values (mean ± SD). ∗p < 0.05 relative to the day 0 sample for each gene.
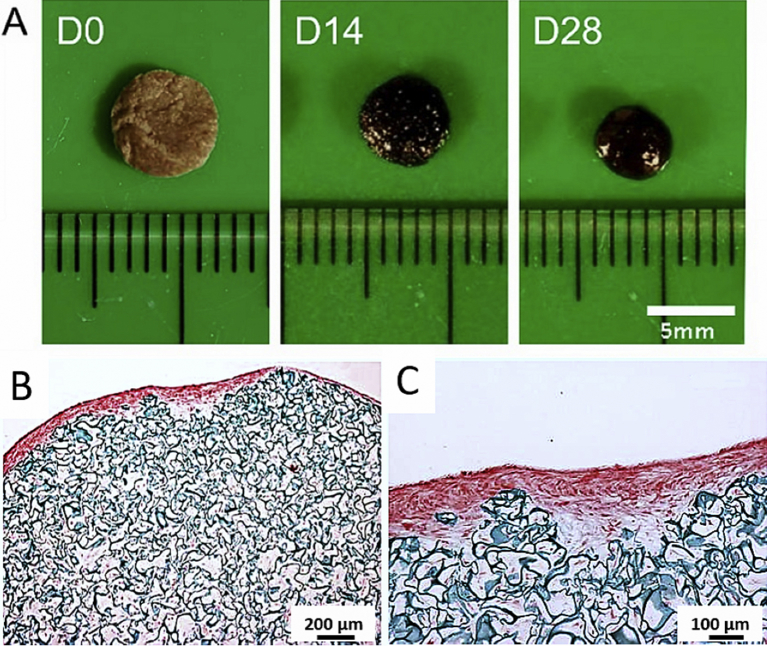
3.5. Gross morphology, immunohistochemistry, and histology
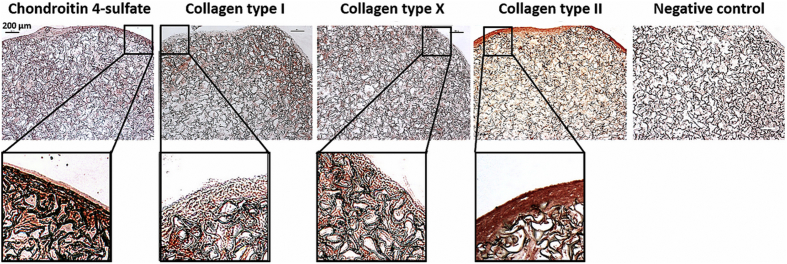
The color of the chitosan-cartilage ECM composite construct was light brown at day 0 and getting darker over the culture period, and the surface of the scaffold also turned smoother at day 28 (Fig. 5A). After in vitro ASC culture for 28 days, histological examination using Safranin O/fast green staining revealed that the neo-matrix filled the interstices of the porous scaffold sparsely (Fig. 5B). Especially, a GAG-rich layer on the surface of the composite scaffold was found (Fig. 5C). Immunohistochemical examinations further showed that the surface layer of the composite scaffold exhibited strong staining for cartilage-specific molecules such as collagen type II and chondroitin 4-sulfate (Fig. 6). Otherwise, collagen type I and collagen type X, the markers of hypertrophic cartilage, were stained positively mainly in the area immediately beneath the GAG-rich surface layer of the chitosan-cartilage ECM composite scaffolds. In the high-power views, the spatial distribution of GAG and collagen type II-positive area overlying collagen type I and X-positive area was clearly delineated.
Fig. 5.
(A) Gross pictures of the genipin-crosslinked chitosan-cartilage ECM composite scaffolds. Over the culture period, the surface of the ASC-seeded composite scaffolds became smoother. (B) Low-power view and (C) high-power view of day 28 safranin O/fast green stain of human ASCs seeded chitosan-cartilage ECM scaffolds. Surface area of the composite construct revealed an intensively red GAG-rich region concentrated on the periphery of the composite scaffold. (B scale bar = 200 μm; C scale bar = 100 μm).
Fig. 6.
Day 28 immunohistochemistry of human ASCs cultured in the composite chitosan-cartilage ECM scaffolds. Immunohistostaining of the composite scaffolds revealed intense staining of chondroitin 4-sulfate and collagen type II in the surface layer of the constructs, while the area underneath was stained positive for type I and collagen type X (scale bar = 200 μm).
4. Discussion
A notable finding of this study is that the porous biomaterial composed of chitosan and native cartilage ECM can induce spatial chondrogenic differentiation of ASCs without special culture conditions. Likewise, the scaffold geometry is also reported to influence ASC chondrogenesis [20]. By measuring the gene expression, protein synthesis, histology, and immunohistochemistry, it was found that ASCs produced significant amounts of cartilaginous tissue in vitro culture, particularly at the surface layer of the composite scaffolds. Although cartilage-specific macromolecules, like collagen type II and GAG, were also distributed sparsely within the ASC-seeded composite scaffold on day 28 of culture, they were primarily located in the cartilaginous surface layer. Moreover, the cartilage hypertrophic phenotypic markers collagen type I and collagen type X were negative in this GAG-rich surface layer, but was positive within the scaffold, particularly in the area right beneath the surface area [21]. In addition, the histological appearances of the ASC-seeded chitosan-cartilage ECM scaffold resembled the tangential layer, transitional layer, and lacunae structures of articular cartilage [22]. Likewise, the SEM images of the composite scaffolds revealed a highly porous surface topography. The large pore size facilitated the migration of ASCs into the composite scaffold, which was evidenced by the neo-matrix deposition in the core of the scaffold after in vitro culture.
In this study, we found that mixing native cartilage ECM with chitosan significantly enhanced ASC proliferation in comparison to pure chitosan films. Moreover, ASCs on chitosan-cartilage ECM films formed multiple small spheroids compared to a few big spheroids formed on chitosan-alone films. The smaller spheroids generated on the composite film may avoid the development of core necrosis, thus maintaining the survival and proliferative activity of ASCs provide benefits for further tissue engineered applications. It has also been reported that ASCs within spheroids exhibited higher differentiation potentials, including differentiation toward osteogenic and chondrogenic lineages [9,10]. Cell shape has been identified as an important determinant for stem cell differentiation, and chondrogenesis of ASCs result from their round cell shape in 3D spheroid culture [5,23]. Therefore, ASC spheroid formation within the interstices of the chitosan-cartilage ECM scaffold may contribute to the prominent chondrogenic differentiation of seeded ASCs.
Tissue engineered osteochondral grafts hold greater potential to repair full-thickness defects of articular cartilage through enhanced biochemical and mechanical interactions with underlying subchondral bone as compared to simple engineered cartilage [24]. Previous studies attempting to engineer osteochondral implants in vitro were used to seed different cells, such as articular chondrocytes and osteoblasts, in the scaffold with layered or graded structures. It has been reported to use a single cell source for both the osteogenic and chondrogenic layer of the engineered construct, however, it requires dual-chamber stirred bioreactors containing chondrogenic and osteogenic media in separate compartments for cell culture [25,26]. This study proposed that it is possible to induce spatial osteochondral differentiation of ASCs by appropriate scaffold design, and the mechanism of this intriguing phenomenon shall be further explored. Otherwise, a similar result was also found in the research by Ye et al. which indicating the infrapatellar fat pad derived-ASCs seeding in 3D printed chitosan scaffold could form a cartilage-like cap under chondrogenic induction [27].
Cellular aggregation is an important step for chondrogenesis of stem cells [28,29]. In the present study, the composite construct showed notable contraction after 28 days of in vitro culture. Confocal microscopy also revealed ASC attachment and spheroid formation on the surface of the 3D chitosan-cartilage ECM composite scaffold. The downregulation of COL1A1 and upregulation of COL2A1 of the seeded ASCs on day 1 further revealed an initial pro-chondrogenic property of the composite scaffold. Along with cellular proliferation and aggregation, cells deposited a dense layer of ECMs in the surface of the composite construct. This highly cellular layer may cause the depletion of oxygen, nutrition and chondrogenic factors in the medium before ASCs access them, which resulting in a different ASC phenotype in the core of the composite scaffold.
For the clinical application of tissue engineered cartilage, mass production for the cartilage constructs is one of the obstacles. In addition to enhancing the exchanges of nutrition/wastes and the uniformly of cell distribution, the bioreactor can provide physical stimuli and modulate mechanotransduction to chondrocytes, which can further improve the ECM component syntheses and realize the mass productions [30]. However, the type of bioreactors and the parameters of setting are still needed to be optimized [31]. Therefore, in combination with using a bioreactor, this chitosan-cartilage ECM scaffold may create a better engineered tissue for the repair of cartilage defects.
5. Conclusion
In summary, we fabricated a chitosan-cartilage ECM scaffold with favorable biological properties for chondrogenic differentiation of ASCs. The deposition of cartilage-specific macromolecules on the surface of the 3D composite scaffolds was an intriguing finding in this study. It was found that scattered neo-matrix deposited within the interstices of the scaffold, and positively stained of hypertrophic cartilage markers was also observed under the GAG and collagen type II-rich surface layer. As showed in the histological result, tangential layer, transitional layer, and lacunae-like structures were found in the ASC-seeded chitosan-cartilage ECM scaffold. The spatial chondrogenic differentiation of ASCs may be mediated by the ASC spheroid formation and oxygen tension throughout the scaffold. Otherwise, it is needed to further refinement of the scaffold composition and architecture that can make an ASC-based osteochondral construct without special culture equipment.
Declaration of Competing Interest
There are no conflicts of interest.
Acknowledgments
This study was supported by grants from Taipei Medical University (Grant no.: TMU104-AE1-B30), Taiwan.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Gimble J.M., Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol. 2003;58:137–160. doi: 10.1016/s0070-2153(03)58005-x. [DOI] [PubMed] [Google Scholar]
- 2.Aust L., Devlin B., Foster S.J., Halvorsen Y.D., Hicok K., du Laney T. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 3.Guilak F., Lott K.E., Awad H.A., Cao Q., Hicok K.C., Fermor B. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206(1):229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 4.Badylak S.F., Gilbert T.W. Immune response to biologic scaffold materials. Semin Immunol. 2008;20(2):109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Z., Doulabi B.Z., Huang C., Bank R.A., Helder M.N. Collagen type II enhances chondrogenesis in adipose tissue-derived stem cells by affecting cell shape. Tissue Eng Part A. 2010;16(1):81–90. doi: 10.1089/ten.TEA.2009.0222. [DOI] [PubMed] [Google Scholar]
- 6.Shi C., Zhu Y., Ran X., Wang M., Su Y., Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185–192. doi: 10.1016/j.jss.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Sechriest V.F., Miao Y.J., Niyibizi C., Westerhausen-Larson A., Matthew H.W., Evans C.H. GAG-augmented polysaccharide hydrogel: a novel biocompatible and biodegradable material to support chondrogenesis. J Biomed Mater Res. 2000;49(4):534–541. doi: 10.1002/(sici)1097-4636(20000315)49:4<534::aid-jbm12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Cheng N.C., Chang H.H., Tu Y.K., Young T.H. Efficient transfer of human adipose-derived stem cells by chitosan/gelatin blend films. J Biomed Mater Res B Appl Biomater. 2012;100(5):1369–1377. doi: 10.1002/jbm.b.32706. [DOI] [PubMed] [Google Scholar]
- 9.Cheng N.C., Wang S., Young T.H. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33(6):1748–1758. doi: 10.1016/j.biomaterials.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 10.Huang G.S., Dai L.G., Yen B.L., Hsu S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials. 2011;32(29):6929–6945. doi: 10.1016/j.biomaterials.2011.05.092. [DOI] [PubMed] [Google Scholar]
- 11.Hsu S.H., Whu S.W., Hsieh S.C., Tsai C.L., Chen D.C., Tan T.S. Evaluation of chitosan-alginate-hyaluronate complexes modified by an RGD-containing protein as tissue-engineering scaffolds for cartilage regeneration. Artif Organs. 2004;28(8):693–703. doi: 10.1111/j.1525-1594.2004.00046.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed N., Dreier R., Gopferich A., Grifka J., Grassel S. Soluble signalling factors derived from differentiated cartilage tissue affect chondrogenic differentiation of rat adult marrow stromal cells. Cell Physiol Biochem. 2007;20(5):665–678. doi: 10.1159/000107728. [DOI] [PubMed] [Google Scholar]
- 13.Vickers S.M., Squitieri L.S., Spector M. Effects of cross-linking type II collagen-GAG scaffolds on chondrogenesis in vitro: dynamic pore reduction promotes cartilage formation. Tissue Eng. 2006;12(5):1345–1355. doi: 10.1089/ten.2006.12.1345. [DOI] [PubMed] [Google Scholar]
- 14.Cheng N.C., Estes B.T., Awad H.A., Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15(2):231–241. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng N.C., Estes B.T., Young T.H., Guilak F. Engineered cartilage using primary chondrocytes cultured in a porous cartilage-derived matrix. Regen Med. 2011;6(1):81–93. doi: 10.2217/rme.10.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diekman B.O., Rowland C.R., Lennon D.P., Caplan A.I., Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010;16(2):523–533. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freytes D.O., Martin J., Velankar S.S., Lee A.S., Badylak S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29(11):1630–1637. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Cheng N.C., Estes B.T., Young T.H., Guilak F. Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis. Tissue Eng Part A. 2013;19(3–4):484–496. doi: 10.1089/ten.tea.2012.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehlhorn A.T., Niemeyer P., Kaiser S., Finkenzeller G., Stark G.B., Südkamp N.P. Differential expression pattern of extracellular matrix molecules during chondrogenesis of mesenchymal stem cells from bone marrow and adipose tissue. Tissue Eng. 2006;12(10):2853–2862. doi: 10.1089/ten.2006.12.2853. [DOI] [PubMed] [Google Scholar]
- 20.Yang K.C., Chen I.H., Yang Y.T., Hsiao J.K., Wang C.C. Effects of scaffold geometry on chondrogenic differentiation of adipose-derived stem cells. Mater Sci Eng C Mater Biol Appl. 2020;110:110733. doi: 10.1016/j.msec.2020.110733. [DOI] [PubMed] [Google Scholar]
- 21.Caron M.M., Emans P.J., Coolsen M.M., Voss L., Surtel D.A., Cremers A. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20(10):1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Wang C.C., Yang K.C., Lin K.H., Liu Y.L., Liu H.C., Lin F.H. Cartilage regeneration in SCID mice using a highly organized three-dimensional alginate scaffold. Biomaterials. 2012;33(1):120–127. doi: 10.1016/j.biomaterials.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Zanetti N.C., Solursh M. Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J Cell Biol. 1984;99(1 Pt 1):115–123. doi: 10.1083/jcb.99.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam J., Perera P., Rath B., Agarwal S. Dynamic regulation of bone morphogenetic proteins in engineered osteochondral constructs by biomechanical stimulation. Tissue Eng Part A. 2013;19(5–6):783–792. doi: 10.1089/ten.tea.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmoudifar N., Doran P.M. Osteogenic differentiation and osteochondral tissue engineering using human adipose-derived stem cells. Biotechnol Prog. 2013;29(1):176–185. doi: 10.1002/btpr.1663. [DOI] [PubMed] [Google Scholar]
- 26.Scioli M.G., Bielli A., Gentile P., Cervelli V., Orlandi A. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J Tissue Eng Regen Med. 2017;11(8):2398–2410. doi: 10.1002/term.2139. [DOI] [PubMed] [Google Scholar]
- 27.Ye K., Felimban R., Traianedes K., Moulton S.E., Wallace G.G., Chung J. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3d printed chitosan scaffold. PloS One. 2014;9(6) doi: 10.1371/journal.pone.0099410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estes B.T., Diekman B.O., Gimble J.M., Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5(7):1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guilak F., Awad H.A., Fermor B., Leddy H.A., Gimble J.M. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41(3–4):389–399. [PubMed] [Google Scholar]
- 30.Salinas E.Y., Hu J.C., Athanasiou K. A guide for using mechanical stimulation to enhance tissue-engineered articular cartilage properties. Tissue Eng Part B Rev. 2018;24(5):345–358. doi: 10.1089/ten.teb.2018.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li K., Zhang C., Qiu L., Gao L., Zhang X. Advances in application of mechanical stimuli in bioreactors for cartilage tissue engineering. Tissue Eng Part B Rev. 2017;23(4):399–411. doi: 10.1089/ten.TEB.2016.0427. [DOI] [PubMed] [Google Scholar]