Abstract
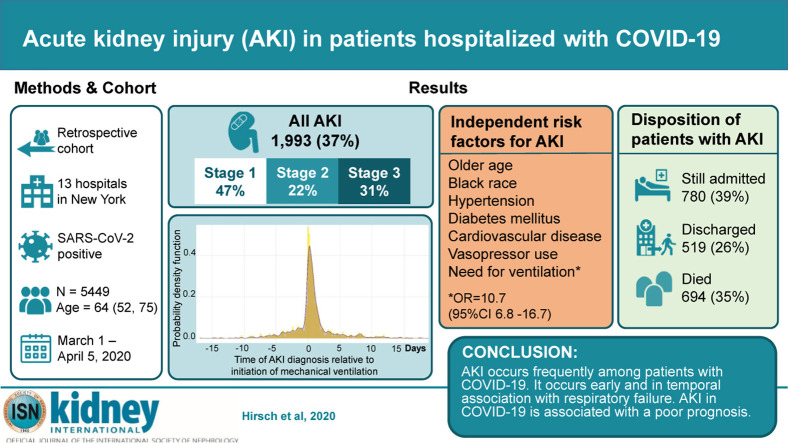
The rate of acute kidney injury (AKI) associated with patients hospitalized with Covid-19, and associated outcomes are not well understood. This study describes the presentation, risk factors and outcomes of AKI in patients hospitalized with Covid-19. We reviewed the health records for all patients hospitalized with Covid-19 between March 1, and April 5, 2020, at 13 academic and community hospitals in metropolitan New York. Patients younger than 18 years of age, with end stage kidney disease or with a kidney transplant were excluded. AKI was defined according to KDIGO criteria. Of 5,449 patients admitted with Covid-19, AKI developed in 1,993 (36.6%). The peak stages of AKI were stage 1 in 46.5%, stage 2 in 22.4% and stage 3 in 31.1%. Of these, 14.3% required renal replacement therapy (RRT). AKI was primarily seen in Covid-19 patients with respiratory failure, with 89.7% of patients on mechanical ventilation developing AKI compared to 21.7% of non-ventilated patients. 276/285 (96.8%) of patients requiring RRT were on ventilators. Of patients who required ventilation and developed AKI, 52.2% had the onset of AKI within 24 hours of intubation. Risk factors for AKI included older age, diabetes mellitus, cardiovascular disease, black race, hypertension and need for ventilation and vasopressor medications. Among patients with AKI, 694 died (35%), 519 (26%) were discharged and 780 (39%) were still hospitalized. AKI occurs frequently among patients with Covid-19 disease. It occurs early and in temporal association with respiratory failure and is associated with a poor prognosis.
Keywords: AKI, continuous RRT, COVID-19, dialysis, renal failure
Graphical abstract
Since late 2019, when the severe acute respiratory coronavirus 2, and the resulting illness, acute respiratory syndrome with coronavirus 2 (coronavirus disease 2019 [COVID-19]), developed in Wuhan, China,1 COVID-19 has become a worldwide pandemic, with 2.3 million cases reported as of April 19, 2020.2 In late January 2020, the United States had its first COVID-19 case in Washington state,3 followed by massive growth of infections in New York, the current epicenter.2 , 4 As had been observed in China and Italy, the disease resulted in a large number of hospitalizations, respiratory failure, and intensive care unit (ICU) admissions.5 , 6 The New York metropolitan area began to see a rapid increase in COVID-19 cases in February 2020. Our health system, with 23 hospitals in counties in and around New York City, rapidly experienced a surge in COVID-19 hospitalizations, with over 9000 at the time of this writing. As we cared for patients with COVID-19, we noticed an alarming number of patients who developed acute kidney injury (AKI), at rates higher than had been reported from China.
Early reports from China and Italy found the rate of AKI to range widely from 0.5% to 29%, with most estimates on the lower end.7, 8, 9, 10, 11, 12, 13, 14, 15 U.S. data has been limited to critically ill patients in the ICU in a Seattle hospital showing a 19% rate of AKI.16 Differences may have resulted from definitions of AKI and the populations studied. Little to date has been published on AKI in COVID-19 beyond rate; for example, wider descriptions of the timing, urine studies, relationship to respiratory failure, detailed analysis of renal replacement therapy (RRT) requirements, risk factors, and outcomes post-AKI are lacking.
Northwell Health is uniquely situated to study COVID-19 based on its location in metropolitan New York and because it has 23 hospitals, with a combination of urban and suburban areas and academic tertiary and community hospitals. In this study, we sought to define the rate of AKI among patients hospitalized with COVID-19 and to describe various aspects of the phenomenology of AKI in this patient population.
Results
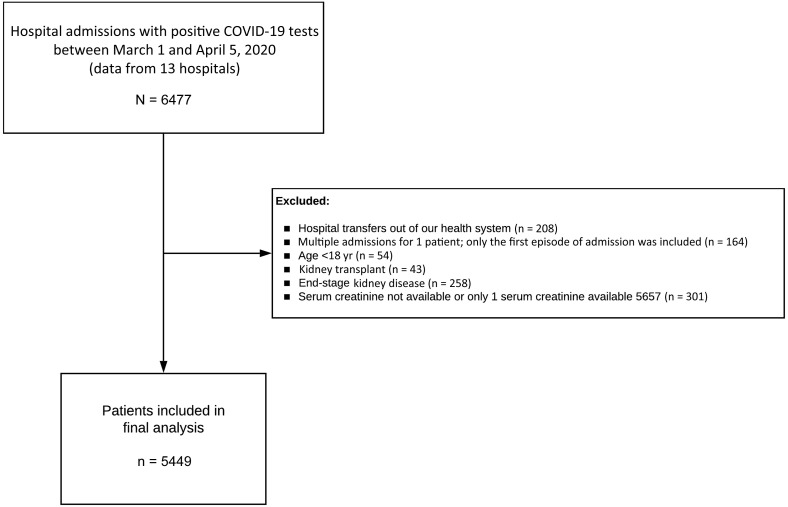
From March 1, 2020, to April 5, 2020, 6477 patients were admitted to 13 Northwell Health hospitals with a diagnosis of COVID-19 present on admission or made during the hospitalization. Of these, 5449 were used as the analysis cohort (Figure 1 ). The baseline characteristics of patients at hospital admission are provided in Table 1 . A total of 1190 patients (21.8%) were treated with mechanical ventilation at some point during the hospitalization. Among the 5449 patients, 888 (16.3%) died, 3280 (60.2%) were discharged to home or to a rehabilitation facility, and 1281 (23.5%) were still in treatment.
Figure 1.
Flowchart of the study. COVID-19, coronavirus disease 2019.
Table 1.
Clinical characteristics of the study cohort
| Variables | Overall (n = 5449) |
|---|---|
| Age (yr) | 64.0 (52.0, 75.0) |
| Male | 3317 (60.9) |
| Race | |
| Asian | 466 (8.6) |
| Black | 1123 (20.6) |
| White | 2112 (38.8) |
| Declined | 32 (0.6) |
| Other/multiracial | 1494 (27.4) |
| Other/unknown | 222 (4.1) |
| Ethnicity | |
| Hispanic/Latino | 1145 (21.0) |
| Not Hispanic/Latino | 3953 (72.5) |
| Other/unknown | 351 (6.4) |
| Language | |
| English | 4433 (81.4) |
| Spanish | 640 (11.7) |
| Other | 376 (6.9) |
| Insurance status | |
| Commercial | 1856 (34.1) |
| Medicaid | 1141 (20.9) |
| Medicare | 2292 (42.1) |
| Self-pay | 114 (2.1) |
| Other | 46 (0.8) |
| Hospital type | |
| Tertiary | 3765 (69.1) |
| Community | 1684 (30.9) |
| Comorbid conditionsa | |
| HTN | 3037 (55.7) |
| CAD | 600 (11.0) |
| HF | 349 (6.4) |
| PVD | 98 (1.8) |
| Diabetes | 1797 (33.0) |
| HIV | 35 (0.6) |
| Chronic liver disease | 114 (2.1) |
| COPD | 296 (5.4) |
| Asthma | 460 (8.4) |
| OSA | 164 (3.0) |
| Cancer | 327 (6.0) |
| Obesity | 1475 (27.1) |
| Morbid obesity | 456 (8.4) |
| BMI (kg/m2) | 28.6 (25.4, 33.1) |
| Admission SCr (mg/dl) | 1.01 (0.80, 1.34) |
| Medications | |
| No. of medications | 4 (1, 8) |
| ACE-I | 654 (13.3) |
| ARB | 902 (18.3) |
| ICU | 1395 (25.6) |
| Mechanical ventilator | 1190 (21.8) |
| ECMO | 10 (0.2) |
| Inotropesb | 54 (1.0) |
| Vasopressorc | 1168 (21.4) |
| CCI | 4 (2, 6) |
| Length of stay (d) | |
| Discharged/expired | 5.7 (3.3, 8.7) |
| Currently hospitalized | 12.0 (9.3, 16.0) |
| Disposition | |
| Discharged | 3280 (60.2) |
| Expired | 888 (16.3) |
| Currently admitted | 1281 (23.5) |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CAD, coronary artery disease; CCI, Charleson Comorbidity Index; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; HF, heart failure; HTN, hypertension; ICU, intensive care unit; OSA, obstructive sleep apnea; PVD, peripheral vascular disease; SCr, serum creatinine.
Values are median (interquartile range) or n (%).
Chronic kidney disease was not included as a comorbid condition because prehospitalization estimated glomerular filtration rate was available only in 15% of patients.
Inotropes include dobutamine and milrinone.
Vasopressors include norepinephrine, epinephrine, vasopressin, dopamine, and angiotensin-2 infusion.
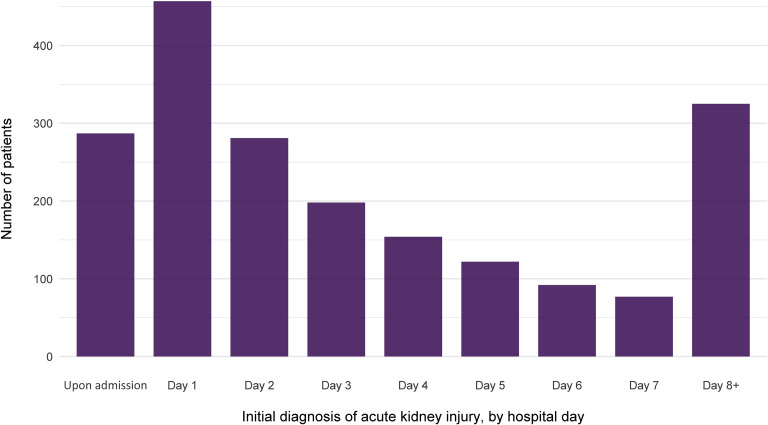
Overall, 1993 of 5449 patients (36.6%) developed AKI during their hospitalization. The peak stages of AKI were stage 1 in 46.5%, stage 2 in 22.4%, and stage 3 in 31.1% (Table 2 ). The baseline laboratory data of patients are shown in Supplementary Table S1. The timing of initial development of AKI with respect to hospital admission is displayed in Figure 2 . Most cases developed early in the course, with 37.3% either arriving with AKI or developing it within 24 hours of admission. The number of patients requiring dialytic support at some point was 285 (overall 5.2% of all patients, representing 14.3% of those with AKI). The modality utilized was intermittent hemodialysis only in 154 patients (54% of all patients requiring RRT), continuous RRT only in 70 (24.6%) and requirement of both treatments at some point in 61 (21.4%). The median time of initiation of dialytic support from hospital admission was 2.0 (interquartile range [IQR]: −1.63, 141) hours. The median number of hours from AKI diagnosis to initiation of dialysis was 0.0 (IQR: 0.0, 79.6).
Table 2.
Baseline characteristics of study cohort, by AKI status
| Variables | No AKI (n = 3456) | AKI (n = 1993) | Stages of AKI |
P valuea (no AKI vs. all AKI | P valueb (trend) | ||
|---|---|---|---|---|---|---|---|
| 1 (n = 927) | 2 (n = 447) | 3 (n = 619) | |||||
| Age (yr) | 61.0 (50.0, 72.0) | 69.0 (58.0, 78.0) | 69.0 (58.0, 79.0) | 71.0 (58.5, 79.0) | 67.0 (58.0, 76.0) | <0.001 | <0.001 |
| Male | 2047 (59.2) | 1270 (63.7) | 556 (60.0) | 266 (59.5) | 448 (72.4) | 0.001 | <0.001 |
| Race | 0.04 | 0.04 | |||||
| Asian | 304 (8.8) | 162 (8.1) | 72 (7.8) | 35 (7.8) | 55 (8.9) | ||
| Black | 708 (20.5) | 415 (20.8) | 190 (20.5) | 106 (23.7) | 119 (19.2) | ||
| White | 1294 (37.4) | 818 (41.0) | 393 (42.4) | 191 (42.7) | 234 (37.8) | ||
| Other/multiracial | 988 (28.6) | 506 (25.4) | 232 (25.0) | 92 (20.6) | 182 (29.4) | ||
| Other/unknown | 138 (4.0) | 84 (4.2) | 38 (4.1) | 21 (4.7) | 25 (4.0) | ||
| Declined | 24 (0.7) | 8 (0.4) | 2 (0.2) | 2 (0.4) | 4 (0.6) | ||
| Ethnicity | 0.03 | 0.03 | |||||
| Hispanic/Latino | 765 (22.1) | 380 (19.1) | 159 (17.2) | 85 (19.0) | 136 (22.0) | ||
| Not Hispanic/Latino | 2474 (71.6) | 1479 (74.2) | 711 (76.7) | 330 (73.8) | 438 (70.8) | ||
| Other/unknown | 217 (6.3) | 134 (6.7) | 57 (6.1) | 32 (7.2) | 45 (7.3) | ||
| Language | 0.52 | 0.12 | |||||
| English | 2825 (81.7) | 1608 (80.7) | 764 (82.4) | 353 (79.0) | 491 (79.3) | ||
| Spanish | 402 (11.6) | 238 (11.9) | 96 (10.4) | 53 (11.9) | 89 (14.4) | ||
| Other | 229 (6.6) | 147 (7.4) | 67 (7.2) | 41 (9.2) | 39 (6.3) | ||
| Insurance status | <0.001 | <0.001 | |||||
| Commercial | 1329 (38.5) | 527 (26.4) | 247 (26.6) | 98 (21.9) | 182 (29.4) | ||
| Medicaid | 792 (22.9) | 349 (17.5) | 159 (17.2) | 78 (17.4) | 112 (18.1) | ||
| Medicare | 1238 (35.8) | 1054 (52.9) | 495 (53.4) | 260 (58.2) | 299 (48.3) | ||
| Self-pay | 67 (1.9) | 47 (2.4) | 19 (2.0) | 8 (1.8) | 20 (3.2) | ||
| Other | 30 (0.9) | 16 (0.8) | 7 (0.8) | 3 (0.7) | 6 (1.0) | ||
| Hospital type | 0.06 | 0.04 | |||||
| Tertiary | 2420 (70.0) | 1345 (67.5) | 635 (68.5) | 312 (69.8) | 398 (64.3) | ||
| Community | 1036 (30.0) | 648 (32.5) | 292 (31.5) | 135 (30.2) | 221 (35.7) | ||
| Comorbid conditionsc | |||||||
| HTN | 1745 (50.5) | 1292 (64.8) | 624 (67.3) | 287 (64.2) | 381 (61.6) | <0.001 | <0.001 |
| CAD | 311 (9.0) | 289 (14.5) | 136 (14.7) | 72 (16.1) | 81 (13.1) | <0.001 | <0.001 |
| HF | 141 (4.1) | 208 (10.4) | 112 (12.1) | 47 (10.5) | 49 (7.9) | <0.001 | <0.001 |
| PVD | 37 (1.1) | 61 (3.1) | 22 (2.4) | 18 (4.0) | 21 (3.4) | <0.001 | <0.001 |
| Diabetes | 967 (28.0) | 830 (41.6) | 368 (39.7) | 193 (43.2) | 269 (43.5) | <0.001 | <0.001 |
| HIV | 25 (0.7) | 10 (0.5) | 5 (0.5) | 4 (0.9) | 1 (0.2) | 0.42 | 0.36 |
| Chronic liver disease | 72 (2.1) | 42 (2.1) | 27 (2.9) | 8 (1.8) | 7 (1.1) | 1.00 | 0.11 |
| COPD | 149 (4.3) | 147 (7.4) | 74 (8.0) | 36 (8.1) | 37 (6.0) | <0.001 | <0.001 |
| Asthma | 317 (9.2) | 143 (7.2) | 74 (8.0) | 35 (7.8) | 34 (5.5) | 0.012 | 0.02 |
| OSA | 99 (2.9) | 65 (3.3) | 27 (2.9) | 20 (4.5) | 18 (2.9) | 0.46 | 0.31 |
| Cancer | 194 (5.6) | 133 (6.7) | 65 (7.0) | 25 (5.6) | 43 (6.9) | 0.13 | 0.30 |
| Obesity | 916 (26.5) | 559 (28.0) | 244 (26.3) | 132 (29.5) | 183 (29.6) | 0.23 | 0.25 |
| Morbid obesity | 276 (8.0) | 180 (9.0) | 81 (8.7) | 42 (9.4) | 57 (9.2) | 0.20 | 0.57 |
| BMI (kg/m2) | 28.4 (25.3, 32.8) | 29.0 (25.4, 33.5) | 28.7 (25.5, 33.2) | 28.7 (24.7, 33.6) | 29.5 (25.9, 34.0) | 0.09 | 0.04 |
| Kidney function | |||||||
| Admission SCr (mg/dl) | 0.95 (0.77, 1.16) | 1.24 (0.91, 1.82) | 1.26 (0.91, 1.83) | 1.30 (0.95, 1.86) | 1.19 (0.90, 1.79) | <0.001 | <0.001 |
| Discharge SCr (mg/dl) | 0.80 (0.66, 0.98) | 1.42 (0.84, 3.16) | 1.02 (0.72, 1.57) | 1.38 (0.80, 2.26) | 4.00 (2.21, 6.12) | <0.001 | <0.001 |
| Peak SCr (mg/dl) | 0.98 (0.80, 1.20) | 2.23 (1.40, 4.12) | 1.50 (1.10, 2.16) | 2.13 (1.59, 2.91) | 5.20 (3.40, 7.24) | <0.001 | <0.001 |
| Median SCr (mg/dl) | 0.86 (0.70, 1.03) | 1.20 (0.82, 2.00) | 1.04 (0.75, 1.53) | 1.10 (0.80, 1.53) | 2.05 (1.11, 3.55) | <0.001 | <0.001 |
| Admission eGFR (ml/min/1.73 m2) | 82.5 (62.0, 98.0) | 56.0 (34.0, 80.0) | 54.0 (33.0, 81.0) | 53.0 (32.0, 79.0) | 62.0 (37.0, 81.0) | <0.001 | <0.001 |
| Discharge eGFR (ml/min/1.73 m2) | 94.0 (77.0, 107.0) | 45.0 (17.0, 86.0) | 69.0 (39.0, 97.0) | 50.0 (27.0, 89.0) | 14.0 (8.0, 27.0) | <0.001 | <0.001 |
| Medications | |||||||
| No. of medications | 3 (1, 7) | 6 (2, 10) | 6 (2, 10) | 6 (2, 10) | 5 (1, 9) | <0.001 | <0.001 |
| ACE-I | 385 (11.9) | 269 (15.9) | 119 (14.4) | 51 (13.4) | 99 (20.2) | <0.001 | <0.001 |
| ARB | 516 (16.0) | 386 (22.8) | 184 (22.3) | 84 (22.1) | 118 (24.0) | <0.001 | <0.001 |
| ICU | 335 (9.7) | 1060 (53.2) | 320 (34.5) | 244 (54.6) | 496 (80.1) | <0.001 | <0.001 |
| Mechanical ventilator | 122 (3.5) | 1068 (53.6) | 288 (31.1) | 262 (58.6) | 518 (83.7) | <0.001 | <0.001 |
| ECMO | 0 (0.0) | 10 (0.5) | 3 (0.3) | 5 (1.1) | 2 (0.3) | <0.001 | <0.001 |
| Inotropesd | 3 (0.1) | 51 (2.6) | 10 (1.1) | 12 (2.7) | 29 (4.7) | <0.001 | <0.001 |
| Vasopressore | 119 (3.4) | 1049 (52.6) | 278 (30.0) | 252 (56.4) | 519 (83.8) | <0.001 | <0.001 |
| CCI | 3.0 (1.0, 5.0) | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.5) | 4.0 (3.0, 6.0) | <0.001 | <0.001 |
| Length of stay (d) | |||||||
| Discharged/ expired | 4.9 (3.0, 7.6) | 7.8 (5.0, 11.6) | 7.4 (4.8, 10.9) | 7.2 (4.2, 12.1) | 8.6 (5.8, 12.3) | <0.001 | <0.001 |
| Currently hospitalized | 10.3 (8.5, 13.0) | 13.2 (10.2, 17.3) | 12.2 (9.4, 16.2) | 14.0 (11.0, 18.1) | 14.0 (11.0, 18.23) | <0.001 | <0.001 |
| Disposition | <0.001 | <0.001 | |||||
| Discharged | 2761 (79.9) | 519 (26.0) | 397 (42.8) | 87 (19.5) | 35 (5.7) | ||
| Expired | 194 (5.6) | 694 (34.8) | 208 (22.4) | 152 (34.0) | 334 (54.0) | ||
| Currently hospitalized | 501 (14.5) | 780 (39.1) | 322 (34.7) | 208 (46.5) | 250 (40.4) | ||
ACE-I, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin II receptor blocker; BMI, body mass index; CAD, coronary artery disease; CCI, Charleson Comorbidity Index; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; HF, heart failure; HTN, hypertension; ICU, intensive care unit; OSA, obstructive sleep apnea; PVD, peripheral vascular disease, SCr, serum creatinine.
Values are median (interquartile range) or n (%).
Comparisons are made between no AKI and AKI using Fisher exact test for categorical variables and or nonparametric Kruskal-Wallis test for continuous variables.
Comparisons are made across the stages of AKI using Kruskal-Wallis rank sum test.
Chronic kidney disease was not included as a comorbid condition because prehospitalization estimated glomerular filtration rate was only available in 15% of patients.
Inotropes include dobutamine and milrinone.
Vasopressors include norepinephrine, epinephrine, vasopressin, dopamine, angiotensin-2 infusion.
Figure 2.
The number of patients with initial diagnosis of acute kidney injury, by hospital day of admission.
The relationship between respiratory failure and development of AKI was substantial and is displayed in Table 3 . Among patients who required mechanical ventilation, 1068 of 1190 (89.7%) developed AKI compared with 925 of 4259 (21.7%) in nonventilated patients. The majority of severe (stage 3) AKI (518 of 619 [83.6%]) and most patients requiring dialytic support (276 of 285 [96.8%]) occurred in patients on mechanical ventilation. RRT was required in 9 of 4259 nonventilated patients (0.2%) compared with 276 of 1190 patients on ventilators (23.2%).
Table 3.
The proportion of patients with AKI, by requirement for invasive mechanical ventilation
| No use of invasive mechanical ventilation (n = 4259) | Required invasive mechanical ventilation (n = 1190) | P valuea | |
|---|---|---|---|
| No AKI | 3334 (78.3) | 122 (10.3) | <0.001 |
| AKI | |||
| Any stage | 925 (21.7) | 1068 (89.7) | <0.001 |
| Stage 1 | 639 (15.0) | 288 (24.2) | <0.001 |
| Stage 2 | 185 (4.3) | 262 (22.0) | <0.001 |
| Stage 3 | 101 (2.4) | 518 (43.5) | <0.001 |
| Required renal replacement therapyb | 9 (0.2) | 276 (23.2) | <0.001 |
AKI, acute kidney injury.
Values are n (%).
Data were compared using the Fisher exact test.
Renal replacement therapy includes intermittent hemodialysis and continuous renal replacement therapy.
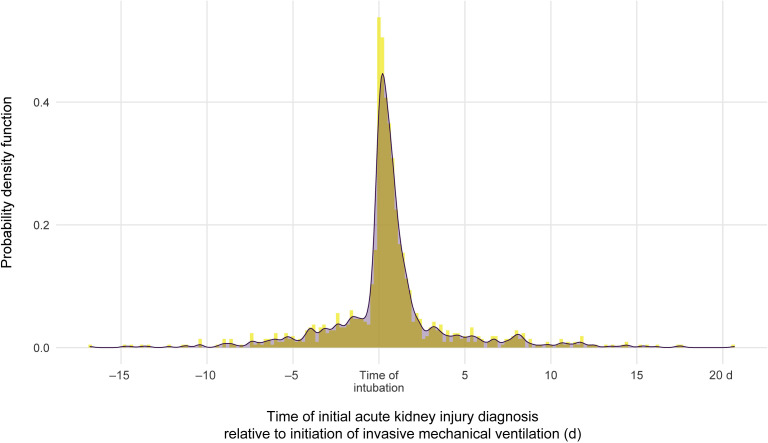
There was a substantial clustering of AKI events at the time of intubation and initiation of mechanical ventilation (Figure 3 ). Of patients who required ventilation and developed AKI, 52.2% had the onset of AKI within 24 hours of intubation. The median time from mechanical ventilation to initiation of dialytic therapy was 0.3 (IQR: −41.1, 92.3) hours.
Figure 3.
The probability of acute kidney injury diagnosis relative to time of mechanical ventilation.
Urine studies were available within 24 hours before or 48 hours after the development of AKI in up to 646 of 1993 patients with AKI (Table 4 ). The median urine-specific gravity was 1.020 (IQR: 1.010, 1.020). By urine dipstick, 36.2% had no heme and 46.1% had 2+ to 3+ positivity. Urine protein was negative in 26.0%, but 2+ to 3+ positive in 42.1%. Automated microscopy detected significant leukocyturia or hematuria in 36.5% and 40.9%, respectively. The urine sodium concentration was <35 mEq/l in 65.6% of patients.
Table 4.
Urine tests results obtained 24 hours before up to 48 hours after the development of acute kidney injury
| Urine studies | n (%) |
|---|---|
| Urine sodium (mEq/l) | |
| <35a | 187 (65.6) |
| 35–50 | 47 (16.5) |
| >50 | 51 (17.9) |
| Specific gravity | 1.020 (1.010, 1.020) |
| Blood | |
| 0, negative or trace | 196 (36.2) |
| 1+, small | 96 (17.7) |
| 2+, moderate | 148 (27.3) |
| 3+, large | 102 (18.8) |
| Protein | |
| 0, negative, trace, or <30 | 168 (26.0) |
| 1+, 30–99 | 206 (31.9) |
| 2+, 100–299 | 194 (30.0) |
| 3+, 300–999 | 78 (12.1) |
| Urine microscopy, automated | |
| Positive with white blood cells, >5 | 221 (36.5) |
| Positive with red blood cells, >5 | 249 (40.9) |
Values are n (%) or median (interquartile range).
Urine sodium was reported as <20 mEq/l in a few of the enterprise hospitals.
We studied various baseline characteristics as potential predictors of AKI in COVID-19 (Table 5 ). By univariate analysis, increased age, male sex, diabetes mellitus, hypertension, history of cardiovascular disease, increased body mass index (BMI), mechanical ventilation, vasopressor medications, and a history of treatment with renal-angiotensin-aldosterone–inhibiting medications were predictors of AKI. Treatment in a tertiary care hospital was associated with marginally reduced risk. Independent predictors, by multivariate analysis included older age, black race, diabetes, hypertension, cardiovascular disease, mechanical ventilation, and use of vasopressor medications. In this analysis, renal-angiotensin-aldosterone–inhibiting drugs were no longer an AKI predictor. As BMI data were missing in 1138 of 4211 patients (27%), we compared 2 multivariate logistic regression models, one including BMI and the other excluding BMI (Supplementary Table S2), with no appreciable change in the risk factors for AKI observed.
Table 5.
Univariate and multivariate logistic regression analyses of risk factors associated with the development of AKI
| Variable | Unadjusted OR | 95% CI | P value | Adjusted ORa | 95% CI | P value |
|---|---|---|---|---|---|---|
| Age (yr) | 1.03 | 1.02–1.03 | <0.001 | 1.03 | 1.03–1.04 | <0.001b |
| Male | 1.21 | 1.08–1.35 | 0.001 | 1.14 | 0.97–1.33 | 0.10 |
| White race | Reference | Reference | Reference | Reference | Reference | Reference |
| Asian | 0.84 | 0.68–1.04 | 0.11 | 0.83 | 0.61–1.12 | 0.23 |
| Black | 0.93 | 0.80–1.08 | 0.32 | 1.23 | 1.01–1.50 | 0.04b |
| Other/mixed | 0.81 | 0.71–0.93 | 0.003 | 0.84 | 0.69–1.03 | 0.09 |
| Unknown | 0.9 | 0.69–1.18 | 0.44 | 0.74 | 0.50–1.11 | 0.15 |
| Tertiary hospital | 0.89 | 0.79–1.00 | 0.05 | 0.90 | 0.77–1.06 | 0.20 |
| Diabetes | 1.84 | 1.64–2.06 | <0.001 | 1.76 | 1.49–2.07 | <0.001b |
| Hypertension | 1.81 | 1.61–2.02 | <0.001 | 1.25 | 1.04–1.50 | 0.02b |
| Cardiovascular diseasec | 2.05 | 1.77–2.37 | <0.001 | 1.48 | 1.22–1.80 | <0.001b |
| Respiratory diseased | 1.09 | 0.93–1.26 | 0.29 | — | — | — |
| Obesity, BMI ≥30 kg/m2 | 1.12 | 1.00–1.26 | 0.05 | 1.11 | 0.94–1.31 | 0.22 |
| HIV | 0.69 | 0.33–1.44 | 0.33 | — | — | — |
| Cancer | 1.2 | 0.96–1.51 | 0.11 | 1.09 | 0.82–1.45 | 0.54 |
| Chronic liver disease | 1.01 | 0.69–1.49 | 0.95 | — | — | — |
| Mechanical ventilation | 31.60 | 25.80–38.60 | <0.001 | 10.7 | 6.81–16.70 | <0.001b |
| Vasoactive medicatione | 31.40 | 25.60–38.40 | <0.001 | 4.53 | 2.88–7.13 | <0.001b |
| ACE-I or ARB use | 1.61 | 1.42–1.82 | <0.001 | 0.87 | 0.73–1.04 | 0.12 |
ACE-I, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin II receptor blocker; BMI, body mass index; CI, confidence interval; OR, odds ratio.
Variables were entered into the model when the α level of risk factor was less than 0.15. Age, sex, and race were added into the model regardless of α level.
Independent risk factors include increased age, black race, diabetes, hypertension, cardiovascular disease, mechanical ventilation, and vasoactive medication.
Cardiovascular diseases include coronary artery disease, heart failure, and peripheral vascular disease.
Respiratory diseases include asthma and chronic obstructive pulmonary disease.
Vasoactive medications include inotropes and vasopressors.
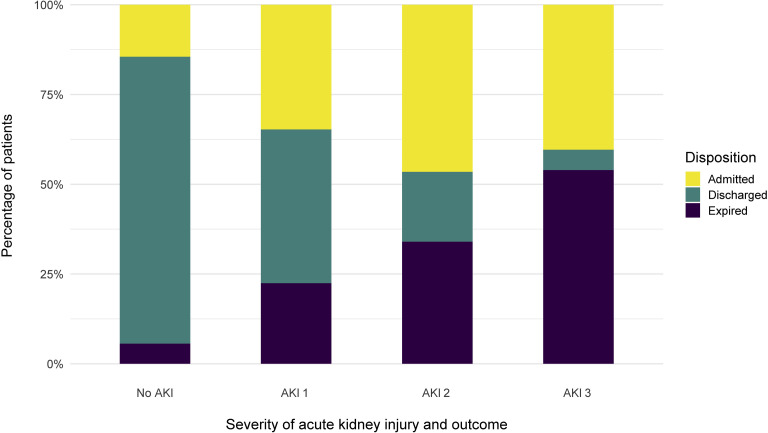
Among 1993 patients who developed AKI during the hospitalization, a total of 780 (39%) were still hospitalized, 519 patients (26%) were discharged and 694 (35%) died. Of those who died, 34% had stage 1, 64% stage 2, and 91% stage 3 (Figure 4 ). Among the 285 patients who required RRT, 157 died and 9 were discharged from the hospital. Another 119 were still undergoing treatment in the hospital, with 108 still on RRT (90.8%). For patients who developed AKI and survived to hospital discharge, the median peak serum creatinine was 2.34 (IQR: 1.42, 4.25) mg/dl with median at hospital discharge of 1.70 (IQR: 0.96, 3.58).
Figure 4.
The proportion of disposition type, by stages (1–3) of acute kidney injury (AKI).
Discussion
Infection with COVID-19 is primarily a respiratory illness, but other organs including the kidneys are often affected.9 We found that in a large cohort of hospitalized patients at both tertiary care and community hospitals the rates of AKI were higher than were reported previously in the literature.
The important relationship between AKI and respiratory failure is indicated by the following findings. First, severe AKI occurred most commonly in close temporal proximity to the time of intubation and mechanical ventilation. Second, the rate of AKI was 89.7% among patients on ventilators compared with 21.7% among other patients. Third, severe (stages 2 and 3) AKI occurred in 65.5% of patients on ventilators compared with 6.7% of nonventilated patients. Finally, almost all of the patients requiring RRT were on ventilator support (276 of 285 [96.8%]). Taken together, these data strongly suggest that AKI, particularly when severe, is a condition that occurs among patients with COVID-19 who also have respiratory failure.
AKI is a common complication among patients hospitalized for a wide range of diagnoses. Among patients hospitalized with COVID-19, we found that 36.6% developed AKI during their hospitalization. This is a higher rate than has been reported previously from China and other areas, from smaller studies, and including various stages of disease. For example, the rate of AKI reported to date has ranged from 0.5% to 29%.7, 8, 9, 10, 11, 12, 13, 14, 15 In particular, Cheng et al. 13 reported from Wuhan, China, a rate of AKI of only 5.1% of 701 patients. While we cannot completely explain this difference, it must be noted that significantly lower rates of comorbidities such as diabetes and hypertension were reported in their patients. In addition, the respiratory disease severity appeared to be less, as only 13.4% of their patients required mechanical ventilation compared with 21.8% of our population. The greater rate found in our study seems consistent with anecdotal reports emerging from other U.S. hospitals.17
In our analysis, the clearest risk factors for the development of AKI were indicators of severe COVID-19, specifically the need for ventilator support or vasopressor drug treatment. Reported risk factors for generally poor outcomes of COVID-19, such as increased age, male sex, and higher BMI12 , 18, 19, 20, 21 were varied in their relationship to AKI risk. Older individuals and men were at greater risk, but there was less of an association to BMI. Individuals from minority communities, particularly African Americans and Hispanics have been disproportionately affected by and have had worse outcomes with COVID-19,22 , 23 and we found black race to be associated with increased risk for AKI. There has been interest in the role of blockers of the renin-angiotensin and aldosterone system and COVID-19.24, 25, 26, 27 We did not find that use of these drugs at hospital admission was related to greater AKI risk.
The etiology of AKI in COVID-19 cases has not been fully elucidated. The close temporal relationship between AKI and respiratory failure occurrence is somewhat suggestive of ischemic acute tubular necrosis, which often happens concordant with systemic collapse. However, other etiologies must continue to be considered. The prothrombotic state that has been observed among patients with COVID-19 suggests other renal pathogenic factors. Our urinary results are provocative. We found a high median urine-specific gravity and a majority of patients with urinary sodium <35 mEq/l at the time of development of AKI. Traditionally these findings are most indicative of prerenal states, but they may also be found with glomerulonephritis and even certain forms of acute tubular necrosis. Because a cytokine storm often occurs in close temporal proximity to respiratory failure, it is possible that circulating substances or other related factors could contribute to AKI. It was beyond the scope of the current work to evaluate for these possibilities. In addition, our findings of fairly high rates of proteinuria and hematuria are noteworthy, but inferences are limited as we were unable to ascertain indwelling urethral catheter status at the time of urine collection. It should be noted that, Su et al.,28 in a study from Wuhan, China, determined autopsy findings among 26 patients, with tubular injury as the primary renal finding.
Although not a primary purpose of this study, we found that the development of AKI among our patients portended a particularly dire prognosis. Of 1993 who developed AKI, 35% of them died. For patients requiring dialytic support, the prognosis appears bleaker. Of 285 patients, 157 died and only 9 were discharged from the hospital at the time of analysis. Another 119 were still hospitalized, but 108 of them were still receiving RRT. The early censoring precludes definitive inferences, but it can be seen that the prognosis of patients on RRT who have COVID-19 is quite guarded.
Our study has certain strengths. To date, this is by far the largest cohort of hospitalized patients with COVID-19 with a focus on AKI. The multicenter design and mixture of community and tertiary hospitals allows examination of a breadth of presentations. To increase the validity of the data, we had prespecified operational definitions for variables and outcomes. Our definition and identification of AKI is consistent with national and international guidelines, is well-validated, and has been automatically calculated in real time for almost 1 year.
The present study has limitations. First, because this is an observational study, we cannot make inferences regarding causal relationships between exposures and AKI. Second, although we have adjusted for potential confounders, there may be potential unmeasured confounders. Third, we relied exclusively on electronic health information systems as the source of data, including identification of AKI. However, the amount of data validation, cleaning, auditing, and quality assurance was substantial. Fourth, we cannot generalize our findings in the outpatient AKI settings because nonhospitalized patients were not part of the present study. In addition, the lack of baseline chronic kidney disease status does not allow us to specifically look at that factor as a risk factor. Finally, the data we presented may not represent the true rate of AKI as the denominator may be different if we had included other health system hospitals in the area.
In conclusion, we found that AKI was a relatively common finding among patients hospitalized with COVID-19. It was strongly linked to the occurrence of respiratory failure and was rarely a severe disease among patients who did not require ventilation. The development of AKI in patients hospitalized for COVID-19 conferred a poor prognosis. Further study will be needed to better understand the causes of AKI and patient outcomes.
Methods
Study design and cohort
This was a retrospective observational cohort study of a large New York health system. Data for this study was obtained from the 13 hospitals using the enterprise inpatient electronic health record Sunrise Clinical Manager (Allscripts, Chicago, IL). All adult patients (age ≥18 years) who tested positive by polymerase chain reaction testing of a nasopharyngeal sample for COVID-19 and were hospitalized from March 1, 2020, to April 5, 2020, were eligible. For patients who had multiple qualifying hospital admissions, we included only the first hospitalization. Patients who were transferred between hospitals within the health system were treated as 1 hospital encounter. Patients were excluded if they were transferred to hospitals out of the health system for which we were unable to obtain data, had end-stage kidney disease (ESKD), had prior kidney transplant, or had <2 serum creatinine levels during the admission.
The Institutional Review Board of Northwell Health approved the study protocol before the commencement of the study. In a previous publication,29 we reported an initial case series on the broad spectrum of clinical characteristics and outcomes among patients hospitalized with COVID-19. In that article, we reported preliminary information on AKI and RRT for the first 2634 patients who died or were discharged from our hospitals.
Data cleaning and preparation process
The electronic health record data were screened for duplicate records and missing data; range checks were done to assess for outliers and erroneous data. Outliers were excluded and duplicate records were removed. To ensure accurate exclusion of ESKD and kidney transplant, additional verification was performed. ESKD diagnosis was defined using International Classification of Diseases, Tenth Revision code N18.6. One study investigator (JSH) adjudicated the ESKD diagnosis through manual chart review of hospital admission notes for the following key search terms: “ESRD,” “ESKD,” “end stage renal,” and “end stage kidney.” Kidney transplant was defined using International Classification of Diseases, Tenth Revision codes T86.1, T86.10, T86.11, T86.12, T86.13, T86.19, and Z94.0. The study investigator (JSH) adjudicated kidney transplant diagnosis using the following key search terms: “kidney transplant,” “renal transplant,” “kidney txp,” “renal txp,” “DDRT,” “LRRT,” and “LURT.”
Definitions and measurements
AKI was defined according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria as follows: stage 1—increase in serum creatinine by 0.3 mg/dl within 48 hours or a 1.5 to 1.9 times increase in serum creatinine from baseline within 7 days; stage 2—2.9 times increase in serum creatinine within 7 days; stage 3—3 times or more increase in serum creatinine within 7 days or initiation of RRT.30 Patients were stratified according to the highest AKI stage attained during their hospital stay.
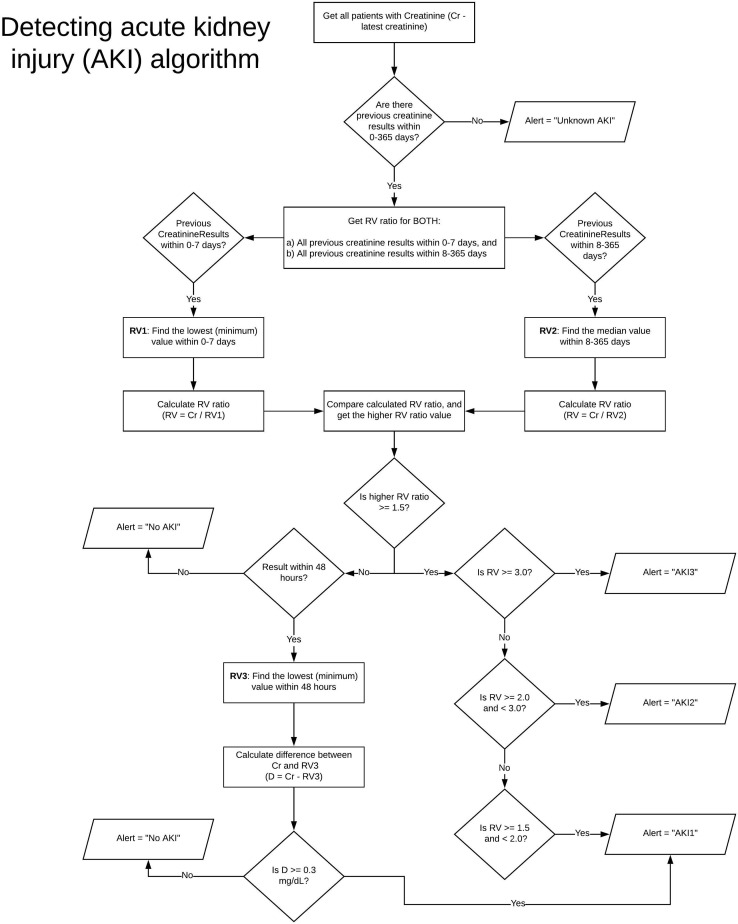
Baseline serum creatinine and adjudication of AKI was automatically calculated from a prebuilt operational algorithm that was available internally since June 2019 (Supplementary Figure S1). This algorithm was based on KDIGO AKI criteria and the U.K. National Health Service AKI algorithm.31 It attempts to calculate baseline kidney function from prehospitalization serum creatinine values and simultaneously recalculates baseline to determine AKI based on the aforementioned KDIGO criteria. The baseline creatinine was defined as the median serum creatinine within 8 to 365 days prior to hospital admission. When there were no prior serum creatinine records, we used the serum creatinine on the day of admission as the baseline serum creatinine, with subsequent baseline recalculated with each new serum creatinine according to a predefined algorithm (Supplementary Figure S1). As only 15% of patients had prehospital baseline serum creatinine measurements available, the baseline was retrospectively adjusted to the median serum creatinine value from the entirety of the hospitalization (this value was chosen, rather than the nadir or final creatinine to provide a more conservative estimate to adjudicate AKI). A ratio of >1.5 from peak to median was classified as AKI. Any patient requiring RRT, regardless of automated AKI stage reporting, was defined as stage 3 AKI, as per KDIGO criteria. We did not use the urine output criteria to define AKI as the documentation of urine output in the electronic health record was unreliable. The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.32
We additionally collected urine test results including urine electrolytes and urinalysis with automated microscopy that were obtained within 24 hours before or 48 hours after the initial development of AKI. Ascertainment of indwelling urethral catheter information was not available for this study.
Outcomes
The primary outcome was the development of AKI. Secondary outcomes included need for RRT and hospital disposition (i.e., discharge or death). The RRT modalities offered to patients with AKI in our health system were intermittent hemodialysis or continuous CRRT. All patients were followed up through April 12, 2020.
Covariates
We collected data on patient demographics, baseline history of comorbid conditions, and home medications. Comorbid conditions and home medications were determined from provider-entered past medical history and admission medication reconciliation. Chronic kidney disease was not included as a comorbid condition because the diagnosis of chronic kidney disease using electronic health record has low sensitivity and low positive predictive value.33 Moreover, prehospitalization estimated glomerular filtration rate was available only in 15% of the patients and thus precludes ascertainment of the diagnosis of chronic kidney disease based on estimated glomerular filtration rate. In addition to baseline clinical data, we collected details of hospital admission such as ICU stay, mechanical ventilation, extracorporeal membrane oxygenation, vasopressor support, and baseline laboratory test results within 48 hours of hospital admission. Due to the COVID-19 pandemic, many additional ICUs were created in nontraditional hospital areas and units. Hence, ICU stay was defined as one of the following: need for invasive mechanical ventilation, need for vasopressor or inotrope support, care from an ICU service, or care in a known ICU location.
Statistical analysis
We performed descriptive statistics including means and SDs for normally distributed continuous measures, medians and IQRs for skewed continuous measures, and proportions for categorical measures. We compared baseline patient characteristics between patients with or without AKI using Fisher exact tests for categorical variables and nonparametric Kruskal-Wallis test for continuous variables. To compare the clinical characteristics of patients across different stages of AKI, we performed Kruskal-Wallis test. To identify risk factors associated with the development of AKI, we performed a logistic regression model, with adjustment for risk factors that differed between those who developed AKI and those who did not. The models were built using stepwise procedure, and variables were entered into the models when the α level of risk factor was <0.15. All statistical tests were 2-sided, and a P value <0.05 was considered statistically significant. We performed sensitivity analysis for missing data. The statistical analyses were performed using R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria).
To ensure a clear and complete report of the study’s design, conduct, and findings, this study followed the Enhancing the Quality and Transparency of Health Research (EQUATOR) reporting guidelines, Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD).34 , 35
Disclosure
KDJ is a consultant for Astex Pharmaceuticals and Natera. All the other authors declared no competing interests.
Acknowledgments
Author Contributions
JSH, JHN, SF, and KDJ conceived of and designed the study, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. JSH and JHN acquired, analyzed, or interpreted the data. JSH, JHN, SF, PS, HHS, ADH, RLB, DWR, and KDJ drafted the manuscript. The Division of Kidney Diseases and Hypertension at Northwell Health Faculty members and authors listed in the Northwell Nephrology COVID-19 Research Consortium Appendix provided critical revision of the manuscript for important intellectual content. JSH and JHN performed statistical analysis. JHN created the graphical abstract.
Footnotes
Northwell Nephrology COVID-19 Consortium authors and affiliations.
Figure S1. Algorithm for detecting AKI using electronic health records.
Table S1. Baseline laboratory blood results within 48 hours of admission.
Table S2. Comparison analysis of risk factors associated with the development of acute kidney injury when including BMI in the multivariate logistic regression model versus excluding BMI in the model.
Contributor Information
Kenar D. Jhaveri, Email: kjhaveri@northwell.edu.
Northwell Nephrology COVID-19 Research Consortium:
Mersema Abate, Hugo Paz Andrade, Richard L. Barnett, Alessandro Bellucci, Madhu C. Bhaskaran, Antonio G. Corona, Bessy Flores Chang, Mark Finger, Steven Fishbane, Michael Gitman, Candice Halinski, Shamir Hasan, Azzour D. Hazzan, Jamie S. Hirsch, Susana Hong, Kenar D. Jhaveri, Yuriy Khanin, Aireen Kuan, Varun Madireddy, Deepa Malieckal, Abdulrahman Muzib, Gayatri Nair, Vinay V. Nair, Jia H. Ng, Rushang Parikh, Daniel W. Ross, Vipulbhai Sakhiya, Mala Sachdeva, Richard Schwarz, Hitesh H. Shah, Purva Sharma, Pravin C. Singhal, Nupur N. Uppal, Rimda Wanchoo, Bessy Suyin Flores Chang, and Jia Hwei. Ng
Appendix
List of Northwell Nephrology COVID-19 Research Consortium
Mersema Abate, Hugo Paz Andrade, Richard L. Barnett, Alessandro Bellucci, Madhu C. Bhaskaran, Antonio G. Corona, Bessy Suyin Flores Chang, Mark Finger, Steven Fishbane, Michael Gitman, Candice Halinski, Shamir Hasan, Azzour D. Hazzan, Jamie S. Hirsch, Susana Hong, Kenar D. Jhaveri, Yuriy Khanin, Aireen Kuan, Varun Madireddy, Deepa Malieckal, Abdulrahman Muzib, Gayatri Nair, Vinay V. Nair, Jia Hwei Ng, Rushang Parikh, Daniel W. Ross, Vipulbhai Sakhiya, Mala Sachdeva, Richard Schwarz, Hitesh H. Shah, Purva Sharma, Pravin C. Singhal, Nupur N. Uppal, and Rimda Wanchoo.
Supplementary Material
Supplementary Figure S1.
References
- 1.World Health Organization Novel coronavirus—China. January 2020. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ Available at:
- 2.COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available at: [DOI] [PMC free article] [PubMed]
- 3.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC COVID-19 Response Team Geographic differences in COVID-19 cases, deaths, and incidence—United States, February 12–April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:465–471. doi: 10.15585/mmwr.mm6915e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [preprint]. medRxiv. https://doi.org/10.1101/2020.03.04.20031120. Accessed April 19, 2020.
- 12.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abelson R, Fink S, Kulish N, Thomas K. An overlooked, possibly fatal coronavirus crisis: a dire need for kidney dialysis. New York Times. April 18, 2020. Available at: https://www.nytimes.com/2020/04/18/health/kidney-dialysis-coronavirus.html. Accessed May 2, 2020.
- 18.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation [e-pub ahead of print]. Obesity (Silver Spring). https://doi.org/10.1002/oby.22831. Accessed April 19, 2020. [DOI] [PMC free article] [PubMed]
- 19.Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China [e-pub ahead of print]. Chest. https://doi.org/10.1016/j.chest.2020.04.010. Accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 20.Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality of COVID-19 patients in Wuhan, China [e-pub ahead of print]. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2020.04.012. Accessed April 19, 2020. [DOI] [PMC free article] [PubMed]
- 22.Johns Hopkins Coronavirus Resource Center Racial data transparency: states that have released breakdowns of Covid-19 data by race. https://coronavirus.jhu.edu/data/racial-data-transparency Available at:
- 23.New York State Department of Health. Fatalities. https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Fatalities?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n Available at:
- 24.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esler M., Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38:781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 27.Diaz J.H. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med. 2020;27:taaa041. doi: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;97:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Workgroup KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 31.NHS England Acute kidney injury (AKI) algorithm. https://www.england.nhs.uk/akiprogramme/aki-algorithm Available at:
- 32.Levey A.S., Stevens L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jalal K., Anand E.J., Venuto R., Eberle J., Arora P. Can billing codes accurately identify rapidly progressing stage 3 and stage 4 chronic kidney disease patients: a diagnostic test study. BMC Nephrol. 2019;20:260. doi: 10.1186/s12882-019-1429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benchimol E.I., Smeeth L., Guttmann A. The Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.EQUATOR Network The Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement. https://www.equator-network.org/reporting-guidelines/record Available at: [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.