Abstract
A patient presented to the emergency department with altered mental status and lower extremity weakness in the setting of nitrous oxide inhalant abuse and Coronavirus Disease-2019 (COVID-19) infection. He subsequently developed hypotension and severe hypoxia, found to have a saddle pulmonary embolus (PE) with right heart strain requiring alteplase (tPA).
1. Case report
A 23-year-old male with a history of nitrous oxide (N2O) abuse presented to the emergency department (ED) after being found face down. Paramedics stated that approximately 150 cartridges of nitrous oxide (N2O) were found in the patient's room. The patient complained of feeling unwell, with weakness in his legs leading to difficulty with walking. There were no known Coronavirus Disease-2019 (COVID-19) risk factors.
Upon arrival to the ED, the patient was afebrile, with a heart rate of 112 beats per minute and a blood pressure of 115/69 mmHg. His pulse oximetry (SpO2) was 97% on room air. On examination, the patient had a normal respiratory effort and clear breath sounds, but was oriented only to the year, and with diffuse weakness in his bilateral lower extremities. Shortly thereafter, the patient developed worsened tachycardia of 139 beats per minute and blood pressure 73/57 mmHg, requiring vasopressor support. His oxygen saturation fell to 92%, with progressively cool and clammy extremities.
Laboratory investigations revealed elevated troponin T-hs of 70 ng/L, creatine kinase of 2414 U/L, d-dimer of 7386 ng/mL, lactate dehydrogenase of 424 U/L, procalcitonin of 0.10 ng/mL, lactate of 3.9 mmol/L, ferritin of 587 μg/L, homocysteine of 104.5 μmol/L, and low vitamin B12 of <150 pg/mL. Initial venous blood gas revealed a PCO2 of 37 mmHg and a venous pH of 7.4. The patient was tested for COVID-19 using polymerase chain reaction (PCR). Although initial testing was negative, a second test was positive. Subsequent hematology studies revealed the presence of lupus anticoagulant (LAC). Electrocardiogram revealed sinus tachycardia with incomplete right bundle branch block. Lower-limb compression ultrasonography was positive for a nonocclusive deep venous thromboses (DVT) in the bilateral popliteal veins. The left internal jugular vein, which had been cannulated for central access, was also noted to subsequently develop thrombosis (Video 1).
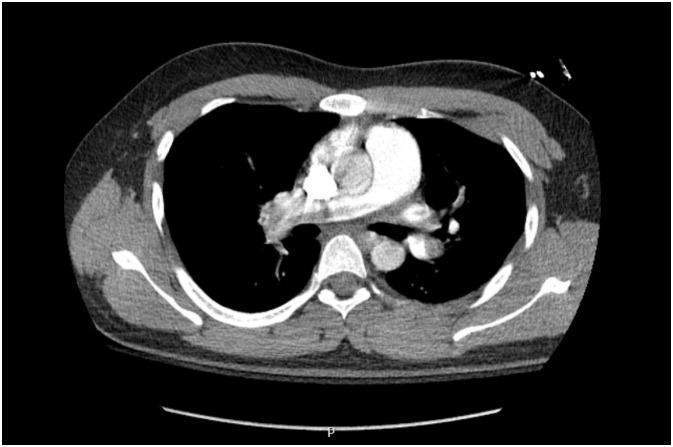
Bedside transthoracic echocardiography (TTE) demonstrated right ventricular dilatation suggestive of right heart strain (Video 2). Given the high concern for pulmonary embolism with the above findings, computed tomography (CT) was performed and revealed saddle pulmonary embolism with dependent ground-glass opacity in the left lower lobe (Fig. 1).
Fig. 1.
Chest computed tomography (CT) demonstrating saddle pulmonary embolism.
Despite intravenous fluids, broad-spectrum antibiotics (vancomycin and cefepime), and vitamin B12, the patient became hemodynamically unstable. He received tPA, with improvement in blood pressure and tachycardia. His course was complicated by a left-sided neck hematoma, which formed at the site of the recently placed central venous catheter, after the administration of tPA. Although the patient had positive LAC, hematology recommended against initiation of chronic anticoagulation, given the diagnosis of antiphospholipid antibody syndrome (APLAS) requires two positive tests separated by at least 12 weeks. They considered his thromboembolic event provoked in the setting of N2O inhalant abuse and COVID-19 positivity.
Emerging reports show a higher prevalence of coagulopathy and thrombosis in cases with COVID-19 [[1], [2], [3]]. The predominant clinical picture appears to be disseminated intravascular coagulation (DIC) with high rates of venous thromboembolism (VTE), elevated d-dimer levels and high fibrinogen levels, in concert with low anti-thrombin levels and pulmonary congestion, with microvascular thrombosis. Recent studies by Zhou et al. and Tang et al. reported a positive correlation between elevated d-dimer levels on hospital admission and in-hospital mortality [2,3]. Tan et al. performed a retrospective analysis of 183 confirmed COVID-19 patients demonstrating an 11.5% death rate. Of the patients who died, 86% had elevated d-dimers of ≥3 μg/mL, and 71% of them developed disseminated intravascular coagulation [3]. In a separate study by Zhou et al., they also found that a d-dimer >1 μg/mL was a predictor of mortality, with an 81% rate of mortality in those having an elevated d-dimer [2]. In a retrospective study of 449 patients with severe COVID-19 pneumonia, 99 patients received heparin. The study showed a decrease in 28-day mortality among the subgroup of patients with a sepsis-induced coagulopathy (SIC) score ≥4 or a d-dimer result that was 6 times the upper limit of normal [4]. Recent guidelines have also suggested that elevated d-dimer levels are associated with higher risk of requiring mechanical ventilation, ICU admission, or death [5].
This patient's coagulopathy may have been secondary to a combination of COVID-19 infection and N2O inhalant abuse leading to hyperhomocysteinemia. It is worth noting that while initial COVID-19 PCR testing was negative, repeat testing was positive, which raises concerns regarding the sensitivity of the COVID-19 PCR. In highly suspicious cases, two tests may be necessary to ensure adequate sensitivity. The role of the previously undetected lupus anticoagulant in the patient's coagulopathy is unclear and may have contributed as well.
Both COVID-19 and nitrous oxide (N2O) could theoretically contribute to a hypercoagulable state. There are case reports illustrating a higher risk of VTE associated with chronic N2O inhalant abuse [6,7]. Nitrous oxide leads to decreased Vitamin B12 levels with chronic abuse, which leads to increased homocysteinemia by inhibition of methionine synthase [7].
The following are the supplementary data related to this article.
Ultrasonographic demonstration of clotted left internal jugular vein (longitudinal and short views) with central venous catheter in place.
Bedside transthoracic echocardiography (TTE) demonstrating right ventricular dilatation suggestive of right heart strain (parasternal long, parasternal short and apical four chamber views); right popliteal vein with DVT also visualized.
Financial support
None.
Declaration of competing interest
None.
Contributor Information
Melanie F. Molina, Email: Melanie.Molina@MGH.HARVARD.EDU.
Ahad A. Al Saud, Email: AALSAUD@mgh.harvard.edu.
Abdullah A. Al Mulhim, Email: AALMULHIM@mgh.harvard.edu.
Andrew S. Liteplo, Email: ALITEPLO@PARTNERS.ORG.
Hamid Shokoohi, Email: hshokoohi@mgh.harvard.edu.
References
- 1.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. [Epub Ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun W., Liao J.P., Hu Y. Pulmonary embolism and deep vein thrombosis caused by nitrous oxide abuse: a case report. World J Clin Cases. 2019;7(23):4057–4062. doi: 10.12998/wjcc.v7.i23.4057. Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Uil S.H. Aortic arch thrombus caused by nitrous oxide abuse. J Vasc Surg Cases Innov Tech. 2018;4(2):80–82. doi: 10.1016/j.jvscit.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ultrasonographic demonstration of clotted left internal jugular vein (longitudinal and short views) with central venous catheter in place.
Bedside transthoracic echocardiography (TTE) demonstrating right ventricular dilatation suggestive of right heart strain (parasternal long, parasternal short and apical four chamber views); right popliteal vein with DVT also visualized.