Background
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that causes coronavirus disease 2019 (COVID-19), has resulted in a global pandemic. Patients with cardiovascular risk factors or established cardiovascular disease are more likely to experience severe or critical COVID-19 illness and myocardial injury is a key extra-pulmonary manifestation. These patients frequently present with ST-elevation on an electrocardiogram (ECG) due to multiple etiologies including obstructive, non-obstructive, and/or angiographically normal coronary arteries. The incidence of ST-elevation myocardial infarction (STEMI) mimics in COVID-19–positive hospitalized patients, and the association with morbidity and mortality is unknown. Understanding the natural history and appropriate management of COVID-19 patients presenting with ST elevation is essential to inform patient management decisions and protect healthcare workers.
Methods
The Society for Cardiovascular Angiography and Interventions (SCAI) and The Canadian Association of Interventional Cardiology (CAIC) in conjunction with the American College of Cardiology Interventional Council have collaborated to create a multi-center observational registry, NACMI. This registry will enroll confirmed COVID-19 patients and persons under investigation (PUI) with new ST-segment elevation or new onset left bundle branch block (LBBB) on the ECG with clinical suspicion of myocardial ischemia. We will compare demographics, clinical findings, outcomes and management of these patients with a historical control group of over 15,000 consecutive STEMI activation patients from the Midwest STEMI Consortium using propensity matching. The primary clinical outcome will be in- hospital major adverse cardiovascular events (MACE) defined as composite of all-cause mortality, stroke, recurrent MI, and repeat unplanned revascularization in COVID-19 confirmed or PUI. Secondary outcomes will include the following: reporting of etiologies of ST Elevation; cardiovascular mortality due to myocardial infarction, cardiac arrest and /or shock; individual components of the primary outcome; composite primary outcome at 1 year; as well as ECG and angiographic characteristics.
Conclusion
The multicenter NACMI registry will collect data regarding ST elevation on ECG in COVID-19 patients to determine the etiology and associated clinical outcomes. The collaboration and speed with which this registry has been created, refined, and promoted serves as a template for future research endeavors.
Background
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), is highly contagious in the community and has resulted in a global pandemic,1 with the largest number of reported cases and deaths being in the United States.2
Patients with cardiovascular risk factors or established cardiovascular disease are more likely to experience severe or critical COVID-19 illness requiring intensive care unit (ICU) care for advanced therapies including mechanical ventilation, vasopressors for hemodynamic support, and mechanical circulatory support including extracorporeal membrane oxygenation (ECMO).3., 4., 5., 6. Myocardial injury, due to underlying ischemia, acute thrombotic occlusion, or myocarditis, is reported in 7–28% of hospitalized COVID-19–positive patients and is associated with higher mortality.7., 8., 9., 10.
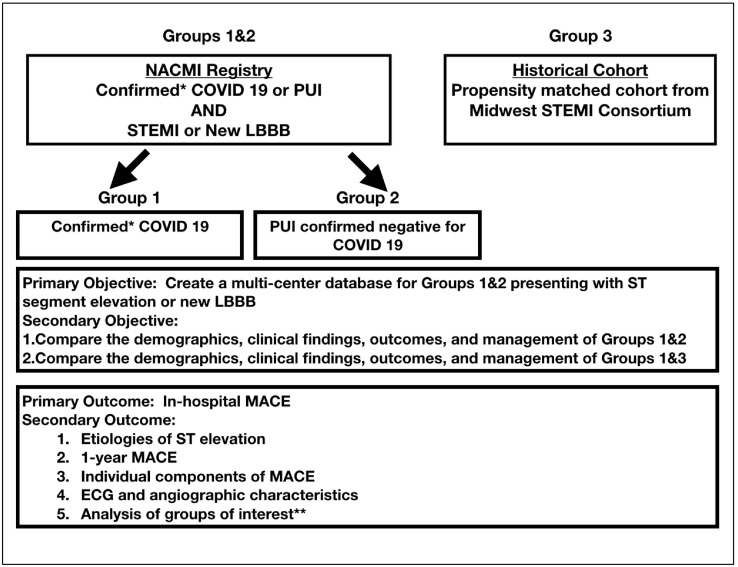
Early reports indicate that in COVID-19 patients with ST-elevation on electrocardiography (ECG), emergent angiography has revealed a variety of findings including classic obstructive coronary artery disease (CAD), non-obstructive CAD, angiographically normal epicardial coronary arteries, and/or left ventricular dysfunction due to myocarditis or stress-induced cardiomyopathy.11 , 12 In an attempt to protect health care providers, some institutions and opinion leaders have advocated for thrombolytic therapy as initial mode of reperfusion.13 , 14 However, as the incidence of these STEMI mimics in COVID-19–positive hospitalized patients and their association with mortality is unknown, risks of fibrinolytic therapy may outweigh potential benefit. As this is a rapidly evolving pandemic with a paucity of data to drive clinical decisions and protect healthcare workers, The Society for Cardiovascular Angiography and Interventions (SCAI) and the Canadian Association of Interventional Cardiology (CAIC) in conjunction with the American College of Cardiology Interventional Council have collaborated to create a multi-center observational registry, NACMI. The primary objective of this registry is to create a multi-center database of patients who present with ST-segment elevation or new left bundle branch block (LBBB) on ECG with a clinical suspicion of myocardial ischemia and are COVID-19–positive or persons under investigation (PUI) (Figure 1 ). The secondary objectives of this registry are to compare the demographics, clinical findings, outcomes and management of confirmed COVID 19 patients with PUI subsequently confirmed negative and with a propensity matched historical control of over 15,000 consecutive STEMI activation patients from the Midwest STEMI Consortium (Figure 1). Ultimately, we plan to develop data-driven treatment plans to inform guidelines. While it is expected some PUI included in the registry will subsequently test negative for COVID-19, they represent an important subset of patients with implications for resource and personal protective equipment (PPE) utilization during this unprecedented pandemic.
Figure 1.
Study design.
LBBB, Left Bundle Branch Block; MACE, major adverse cardiovascular events defined as composite of all-cause mortality, stroke, recurrent MI, and repeat unplanned revascularization; NACMI, North American COVID-19 ST-Segment Elevation Myocardial Infarction; PUI, Persons Under Investigation; STEMI, ST-segment-elevation myocardial infarction.
*Confirmed includes those known to be positive for COVID 19 and PUI subsequently confirmed for COVID 19;
** Groups of interest will include patients developing in-hospital STEMI, those not taken to the cardiac catheterization laboratory, those without a clear culprit artery and those receiving fibrinolytic therapy.
The NACMI registry is a rapid collaboration of multinational societies and medical institutions with a pragmatic study design to encourage widespread participation that will enable development of data-driven guidelines and therapies. Herein, we describe the unique features in the development and implementation of the NACMI registry.
Methods
Overview
The registry will utilize observational methods to assess and compare the characteristics, treatment, and follow-up of confirmed COVID-19 patients presenting with ST elevation on ECG. The registry will enroll patients with confirmed or suspected COVID-19 infection with concomitant ST-segment elevation or new LBBB on ECG with clinical suspicion of myocardial ischemia at the time of presentation to the hospital or that developed while in the hospital. We anticipate approximately 100–150 hospitals from Canada and the United States will participate in the registry. This study will include patients who presented from January 1, 2020 to December 31, 2021 or the end of the pandemic allowing centers to identify eligible patients retrospectively and/or prospectively as long as determination of COVID 10 status has been made. Given the critical nature of the information to be gathered, we believe this warrants waiver of consent and centralized Institutional Review Board (IRB) but all sites are encouraged to work with their local IRB to identify the most appropriate and least burdensome route to local approval of the registry as well as patient recruitment. The Minneapolis Heart Institute Foundation will be the sponsor site for the Central IRB and responsible for coordination of sites, database, and analysis.
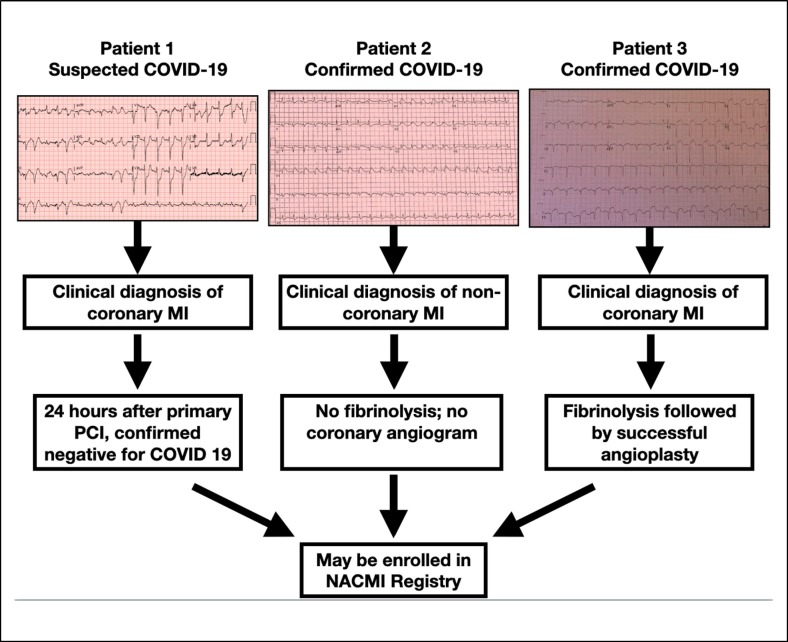
Inclusion criteria for participation are: (1) COVID-19–positive or PUI, (2) ST-segment elevation or new LBBB on 12-lead ECG with clinical suspicion of myocardial ischemia at any time during hospitalization for confirmed or suspected COVID-19, and (3) are ≥18 years of age. Study inclusion will be restricted to those with an accompanying clinical correlate of myocardial ischemia (eg, chest or abdominal discomfort, dyspnea, cardiac arrest, shock, mechanical ventilation). Patients presenting with STEMI or new LBBB who are not COVID-19–positive or PUI are excluded in this registry. PUI is currently defined as presence of fever or respiratory symptoms (cough, shortness of breath, sore throat), myalgias, flu-like illness, pulmonary infiltrates on chest x-ray, loss of smell, or mental status changes, or exposure to a confirmed case or cluster of suspected COVID-19 cases. As Figure 2 illustrates, there are multiple pathways that patients can be enrolled, including: (1) as a PUI even if they ultimately test negative for COVID-19 (Figure 2, Patient 1), (2) the clinical diagnosis reflects non-coronary myocardial injury due to diffuse ST-segment elevation (Figure 2, Patient 2), and (3) regardless of mode of reperfusion strategy (Figure 2, Patient 3). The comparator group will consist of patients in the multicenter Midwest STEMI Consortium, a comprehensive database from four large regional STEMI centers (The Christ Hospital, Cincinnati, OH, Iowa Heart Center, Des Moines, IA, Minneapolis Heart Institute Foundation, Minneapolis, MN, and Prairie Heart Institute Foundation, Springfield, IL) with 5 PCI sites and over 100 non-PCI sites. 15 Currently, over 15,000 consecutive STEMI activations are in the database which includes comprehensive baseline, angiographic, clinical characteristics, and long-term follow up.
Figure 2.
Examples of eligible patients presenting with ST-segment elevation.
Patient 1 presented with fever, a cough and typical ischemic chest pain with inferoposterior STEMI and, while full precautions were taken with PPE, had primary percutaneous coronary intervention (PCI) and subsequently tested negative for COVID-19. Patient 2 was a confirmed COVID-19 who developed diffuse ST-segment elevation in the critical care unit thought to be secondary to myocarditis and was treated with medical therapy. Patient 3 was a confirmed COVID-19 patient presenting with anterior myocardial infarction and had pharmacoinvasive therapy.
All 3 patients are eligible for the North American COVID-19 ST-Segment Elevation Myocardial Infarction (NACMI) registry.
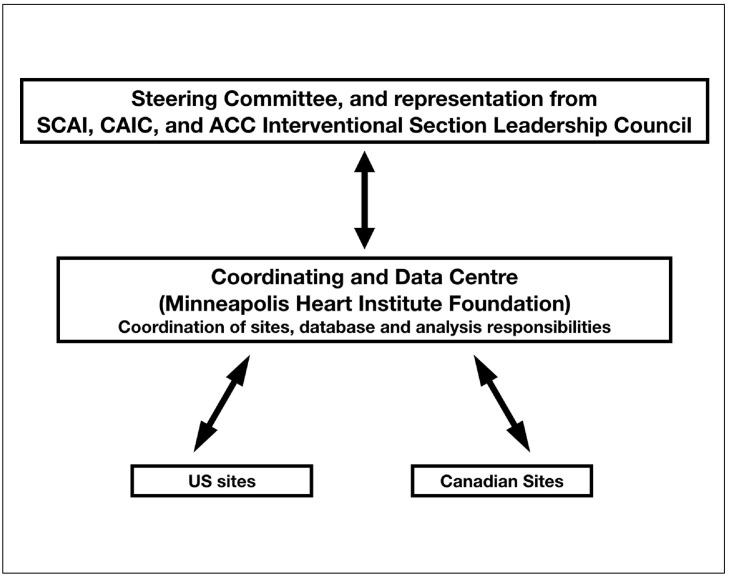
The NACMI registry oversight will be managed by the executive leadership of SCAI, CAIC, and American College of Cardiology (ACC) Interventional Council. The Minneapolis Heart Institute Foundation will serve as the centralized coordinating and data center with the responsibility for registry data analysis. The Lindner Research Centre at The Christ Hospital will coordinate data transfer amongst registry participating sites (Figure 3 ). A blinded ECG core laboratory will be utilized. In subjects undergoing subsequent coronary angiography, core angiographic laboratory analyses will be performed. No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Figure 3.
Overall trial organization.
ACC, American College of Cardiology; CAIC, Canadian Association of Interventional Cardiology; SCAI, Society for Cardiovascular Angiography and Interventions.
Study conception and site recruitment
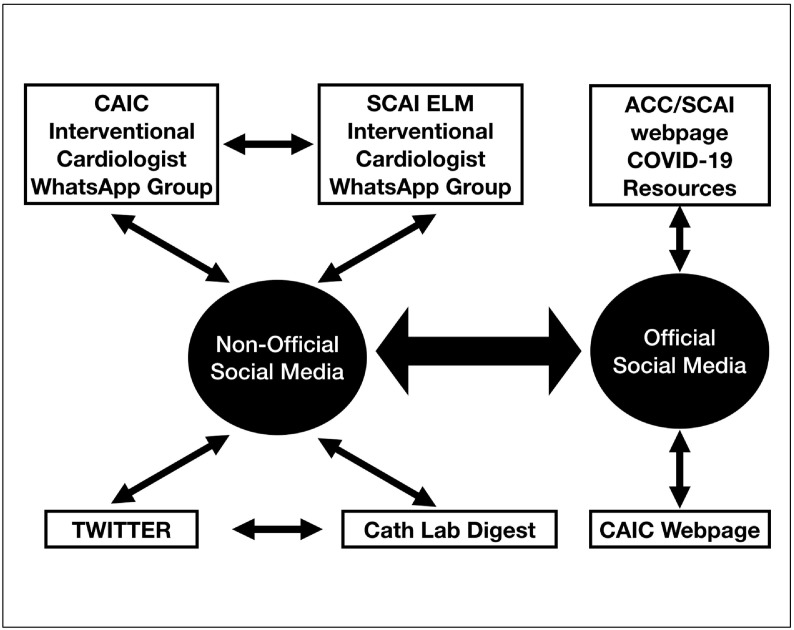
This registry represents a collaborative effort between the interventional cardiology societies of Canada and the United States (CAIC, SCAI, and ACC Interventional Council) to create a multi-center registry of COVID-19–positive or PUI patients who present with STE or new LBBB with clinical suspicion of myocardial ischemia. The rapidity with which this collaboration has occurred, both in the design of the protocol and data form, central IRB approval, and in site recruitment is attributable to both official and non-official social media resources (Figure 4 ). In the non-official social media platform, discussions within and eventually between a 93-member CAIC cardiologist WhatsApp group from Canada, and 49-member SCAI Emerging Leader Mentorship current and former Fellow group representing most of the United States was instrumental in linking like-minded individuals resulting in refining the registry protocol as well as publishing ongoing guidance in approaching COVID-19–positive or PUI patients in the cardiac catheterization laboratory.16 , 17The registry protocol was supported by executive leadership representing CAIC and SCAI who launched official webpages to announce the registry that was then widely reported.18 , 19 Concomitantly, a number of reports on Twitter (#CardioTwitter) of presumed STEMI COVID-19–positive patients whose subsequent angiograms showed non-obstructive CAD were tagged to individuals involved in the NACMI registry. These events led to a unified steering committee, strong multilateral society support from CAIC, SCAI, and ACC, engaged frontline interventional cardiologists, and over 100 enrolled sites, at the time of preparation of this manuscript – all of which occurred within the span of 14 days. The existing network will serve as a platform for rapid knowledge translation with a concerted effort to answer important questions in the midst of an epidemic.
Figure 4.
Social medial collaboration for design and site recruitment.
Both non-official and official platforms in social media were instrumental in synergizing efforts to refine the protocol and recruit sites in both Canada and United States. ACC, American College of Cardiology; CAIC, Canadian Association of Interventional Cardiology; ELM, Emerging Leader Mentorship; SCAI, Society for Cardiovascular Angiography and Interventions.
Data collection
Each cardiac catheterization laboratory enrolled will have a designated person assigned to report cases of patients who meet the inclusion criteria. REDCap (Research Electronic Data Capture), a secure web-based application for building and managing online databases, will be used for data management.20 The following data variables will be collected as co-variates of interest: baseline demographic and clinical characteristics, laboratory data, and pattern of ST-segment elevation (diffuse, focal, with or without ST-segment depression and/or PR-segment depression) transthoracic echocardiography or point-of-care ultrasound findings, commonly used time to treatment metrics in STEMI care (door-to-balloon or door-to-needle times), angiographic characteristics, and adjunctive pharmacological and device therapies including mechanical circulatory support (MCS) (See Appendix A). The REDCap database will be maintained by the Minneapolis Heart Institute Foundation.
Planned analysis
We will compare in- hospital major adverse cardiovascular events (MACE) defined as composite of all-cause mortality, stroke, recurrent MI, and repeat unplanned revascularization in COVID-19 confirmed patients (known positive and PUI subsequently confirmed) to a propensity matched cohort from the Midwest STEMI Consortium (Figure 1). In the PUI COVID-19 patients, we will compare outcomes in those confirmed positive with those confirmed negative. Secondary outcomes will include the following: reporting of etiologies of ST Elevation; cardiovascular mortality due to myocardial infarction, cardiac arrest and /or shock; individual components of the primary outcome; composite primary outcome at 1 year; as well as ECG and angiographic characteristics (Figure 1). Finally, specific groups of interest will be patients developing in-hospital STEMI, those not taken to the cardiac catheterization laboratory, those without a clear culprit artery, those receiving thrombolytic therapy, those in the crisis phase and containment phase of the pandemic. We plan to publish our results after the first 300 cases are entered with an anticipated 100 cases confirmed and 200 cases of PUI confirmed negative.
Statistics
Descriptive characterization will be performed of the experimental group with clinical and demographic variables reported using means ± standard deviation or as median, interquartile range (IQR) when appropriate. Categorical variables will be reported as frequencies and percentages. Continuous and categorical variables will be assessed using appropriate tests and/or models. P < .05 will be considered statistically significant and P values are 2-sided whenever possible. Propensity matched analyses (either inverse probability of treatment weighted analyses or propensity score models) will be utilized to compare clinical characteristics and outcomes of the experimental group to a control comparator group of patients from the Midwest STEMI Consortium during a comparable time frame in the year 2019. All analyses will be performed with Stata (version 15, StataCorp, College Station, TX) or R (R Core Team 2018, Vienna, Austria).
Discussion
Implications for COVID-19–positive patients
Observational registries have gained traction in cardiovascular medicine as they provide important information, including benefits and potential complications of different treatments or procedures.21 For example, consecutive enrollment of patients with well-defined entry criteria in the Chest pain-MI Registry has been a reliable source for outcome-based, quality improvement on high-risk STEMI/NSTEMI patients.22 Immediate access to actionable data is particularly relevant to the current expanding COVID-19 pandemic which is disproportionally affecting cardiovascular patients and healthcare workers.23 At present, there is no data-driven consensus on management of patients with confirmed or suspected COVID-19 infection who present with ST-segment elevation on ECG.17 , 24 , 25 The NACMI registry will be beneficial in identifying etiology, patterns of myocardial injury, developing a risk model for cardiac complications, understanding short and long-term major adverse cardiac events, and designing clinical trials testing different treatment modalities. As such, the NACMI registry has the potential to deliver actionable, real-time data from representative North-American sites.
Implications for STEMI systems of care
As an individual patients' COVID-19 status is unknown at the time of cardiac catheterization laboratory activation, there are significant implications of the findings of this registry for resource utilization, exposure of staff, and clinical decision-making. These implications are amplified by the current supply shortage of PPE. Furthermore, depending on the type of specimen collected, false-negative rates of up to 30% has been reported in detecting COVID 19.26 Therefore, the registry will include both COVID-19–positive patients and PUI with ST elevation. Comparison of PUI subsequently confirmed positive to those subsequently confirmed negative will provide a unique insight into the pathophysiologic mechanism of STEMI from COVID-19, including myocarditis and prothrombotic states. Comparison of patients enrolled in NACMI to propensity matched non-COVID STEMI controls for 1 year prior will provide critical information regarding etiology, treatment strategies and clinical outcomes in the COVID-19 pandemic. As testing for COVID-19 becomes more rapid, this decision-making process is expected to evolve. Additionally, it has been reported that overall STEMI volumes have decreased in the COVID-19 era, without a clear understanding of the reasons.27 , 28 It may be that patients are delaying their presentation to the hospital, potentially leading to need for mechanical circulatory support in the acute setting, mechanical complications of STEMI, sudden death or an increased heart failure population. Additionally, if patients are presenting later in their clinical course, NACMI registry data will allow practitioners to improve care models to improve time to treatment in this unique era.
Limitations
Registry participation in a strained healthcare system environment is challenging at a time in which research services in some institutions are deemed non-essential and closed. This brings into question the role of selection bias and whether our multi-center database will reflect real-world database. However, given that over 100 sites have responded with interest and they vary in geography, prevalence of COVID 19, and systems of health care delivery in United States and Canada, we believe we will get a meaningful look at the presentation and management of ST-segment elevation in this patient population. We recognize that management of COVID 19 confirmed or suspected patients presenting with ST-segment elevation is not static and will change over time due to many factors including availability of PPE, intensive care unit beds, community access to testing, better understanding of disease process, and different phases of enforcing and/or easing of social restrictions. We plan to better appreciate these temporal trends by subsequent analyses of crisis and containment phase. Finally, one may assume that a more accurate comparator to our proposed historical cohort would be a contemporaneous cohort of all patients presenting with STEMI during this pandemic (including those not suspected of having COVID 19). However, it was considered not feasible for many sites to capture all patients with STEMI. Furthermore, the current approach to patients presenting with STEMI is highly variable with some centers treating all patients presenting with STEMI as PUI irrespective of screening results while other centers have instituted no change to their STEMI protocols if patients screen negative for COVID 19.
Conclusion
The multicenter NACMI registry will collect contemporaneous data regarding ST elevation in COVID-19 patients in the midst of a global pandemic. This will allow clinicians to understand the etiologies of this finding and associated clinical outcomes. Information obtained from NACMI can then be used to determine optimal treatment plans for these often critically ill COVID-19 patients and address any barriers to care. The collaboration and speed with which this registry has been created can serve as a template for future research efforts.
The following is the supplementary data related to this article.
NACMI Baseline CRF.
Footnotes
Funding: None.
References
- 1.(Accessed April 26, 2020, at. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-s….)
- 2.(Accessed April 26, 2020, at https://www.worldometers.info/coronavirus/#.)
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried J.A., Ramasubbu K., Bhatt R. The variety of cardiovascular presentations of COVID-19. Circulation. 2020 Apr;3 doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerkin K.J., Fried J.A., Raikhelkar J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020 Mar;21 doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 6.Akhmerov A., Marban E. COVID-19 and the heart. Circ Res. 2020 Apr;7 doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar;27 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [published online ahead of print, 2020 Mar 25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D., Hu B., Hu C. China; JAMA: 2020 Feb 7. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020 Apr;8 doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with COVID-19—a case series. N Engl J Med. 2020 Apr;17 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar;27 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing Z.C., Zhu H.D., Yan X.W. Recommendations from the Peking Union Medical College Hospital for the management of acute myocardial infarction during the COVID-19 outbreak. Eur Heart J. 2020 Mar;31 doi: 10.1093/eurheartj/ehaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels M.J., Cohen M.G., Bavry A.A. 2020 Apr 13. Reperfusion of STEMI in the COVID-19 Era - Business as Usual? Circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry T.D., Sharkey S.W., Burke M.N. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation. 2007 Aug 14;116(7):721–728. doi: 10.1161/CIRCULATIONAHA.107.694141. [DOI] [PubMed] [Google Scholar]
- 16.Wood D.A., Sathananthan J., Gin K. Precautions and procedures for coronary and structural cardiac interventions during the COVID-19 pandemic: guidance from Canadian Association of Interventional Cardiology. Can J Cardiol. 2020 Mar;24 doi: 10.1016/j.cjca.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szerlip M., Anwaruddin S., Aronow H.D. Considerations for cardiac catheterization laboratory procedures during the COVID-19 pandemic perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM) Members and Graduates. Catheter Cardiovasc Interv. 2020 Mar;25 doi: 10.1002/ccd.28887. [DOI] [PubMed] [Google Scholar]
- 18.(Accessed April 26, 2020, at https://mailchi.mp/e50b8caab3b4/scai-caic-acci-webinar-tavr-and-covid-19-experiences-from-the-us-and-canada-and-latest-society-guidance-1461325?e=c01419e814.)
- 19.(Accessed April 26, 2020, at http://www.scai.org/covid-19-resources.)
- 20.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James S., Frobert O., Lagerqvist B. Cardiovascular registries: a novel platform for randomised clinical trials. Heart. 2012 Sep;98(18):1329–1331. doi: 10.1136/heartjnl-2012-301727. [DOI] [PubMed] [Google Scholar]
- 22.(Accessed April 26, 2020, at https://cvquality.acc.org/NCDR-Home/registries/hospital-registries/chest-pain-mi-registry.)
- 23.The Lancet COVID-19: protecting health-care workers. Lancet. 2020 Mar 21;395(10228):922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welt F.G.P., Shah P.B., Aronow H.D. Catheterization laboratory considerations during the Coronavirus (COVID-19) pandemic: from ACC's Interventional Council and SCAI. J Am Coll Cardiol. 2020 Mar;16 doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmud E., Dauerman H.L., Welt F.G. Management of acute myocardial infarction during the COVID-19 pandemic. J Am Coll Cardiol. 2020 Apr;21 doi: 10.1016/j.jacc.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 Mar;11 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia S., Albaghdadi M.S., Meraj P.M. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020 Apr;9 doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzler B., Siostrzonek P., Binder R.K. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020 Apr;16 doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NACMI Baseline CRF.