Highlights
-
•
The optimal antithrombotic regimen to be used in patients with AF and PCI or ACS is still debated.
-
•
Each of the six randomised controlled trials comparing double to triple therapy has limitations.
-
•
None was powered to assess differences between treatment arms in ischaemic event rates.
-
•
The contrasting results regarding ischaemic events within published meta-analyses can be explained by heterogeneity, incompleteness and varying definitions of stent thrombosis.
-
•
The overall reduced bleeding rates, but increased early definite and probable stent thrombosis rates with double versus triple antithrombotic therapy encourage consideration of triple therapy during the first weeks from PCI followed by double therapy.
Keywords: Acute coronary syndrome, Anticoagulant, Antiplatelet, Antithrombotic, Atrial fibrillation, Double, Percutaneous coronary intervention, Triple
1. Introduction
Six randomised controlled trials (RCTs) testing double versus triple antithrombotic therapy (ATT) [1], [2], [3], [4], [5], [6] in patients with atrial fibrillation (AF) and with concomitant acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI) have addressed the crucial question of whether double is superior to triple therapy in terms of ischaemic and bleeding events. International consensuses propose differing recommendations, with North Americans suggesting that oral anticoagulation (OAC) plus a P2Y12 inhibitor ‘should be considered for most patients’ at discharge [7], and Europeans stating that ‘initial triple therapy should be used in most AF patients undergoing PCI’ [8]. Additionally, meta-analyses and reviews [9], [10], [11], [12], [13], [14], [15], [16], [17], [18] report inconsistent efficacy results for double ATT (DAT) versus triple ATT (TAT). To understand these apparent discrepancies and to assist clinical decision-making, we critically review the original evidence and subsequent meta-analyses.
2. General features of trial designs
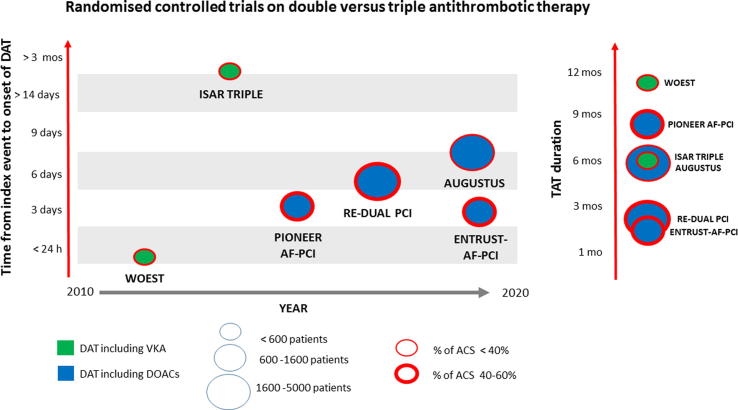
To date, a total of 11,421 patients have been randomised to DAT versus TAT within six RCTs [1], [2], [3], [4], [5], [6]. In 5 of these trials, PCI was performed in all enrolled patients [6], [1], [2], [3], [4]. In 4 of these trials, AF was the reason for anticoagulation in all enrolled patients [1], [4], [5], [6]. The proportion of ACS ranged from 27% to 52%. Five of six trials were open-label [6], [1], [2], [3], [4]. The first two (WOEST and ISAR-TRIPLE) used vitamin K antagonist (VKA) in both the double and triple ATT arm. The last four (RE-DUAL PCI, PIONEER AF-PCI, AUGUSTUS and ENTRUST-AF-PCI), instead, used a direct oral anticoagulant (DOAC) in the double ATT arm. RE-DUAL PCI and ENTRUST-AF-PCI used full dose DOACs in the DAT arm, PIONEER AF-PCI used submaximal DOAC in the DAT arm and low dose DOAC in the TAT arm, whereas AUGUSTUS randomised patients twice: to full dose DOAC or VKA, and to TAT with aspirin or DAT plus placebo. Of the four DOAC trials, RE-DUAL PCI and ENTRUST-AF-PCI compared two strategies, PIONEER AF-PCI compared three strategies, and AUGUSTUS compared 4 strategies. DAT was initiated 4 h to 6 weeks after the index event. TAT lasted from one to 12 months. Follow-up ranged from 6 to 14 months (Fig. 1 and Table 1). Clopidogrel was the P2Y12 inhibitor used in the TAT arms, in agreement with guideline recommendations. Clopidogrel was the most used antiplatelet agent within the DAT arms (88 to 95%), with ticagrelor and prasugrel in a minority.
Fig. 1.
Randomised controlled trials ranked by time from index event to onset of double antithrombotic therapy (DAT) on the left y axis, year of publication on the x axis, and number of patients, anticoagulant used, and clinical presentation indicated by the balloon size, colour and thickness of contour. Duration of triple antithrombotic therapy (TAT) in the right panel. ACS: acute coronary syndrome; DOAC: direct oral anticoagulant; mo: month; VKA: vitamin K antagonist.
Table 1.
Main characteristics and results of randomised controlled trials comparing double versus standard triple antithrombotic therapy in atrial fibrillation (AF) patients with acute coronary syndrome (ACS) or undergoing a percutaneous coronary intervention (PCI). Bid: twice daily; CI: confidence interval; DAT: double antithrombotic therapy; HR: hazard ratio for DAT vs TAT; TAT: triple antithrombotic therapy; VKA: Vitamin K antagonists; vs: versus.
| Trial | Year | Design | Sample (N) | Follow-up | Type of patients | Time from index event to DAT onset | TAT duration | DAT treatment | TAT (control) treatment | TIMI major bleeding (HR, 95% CI) | Myocardial infarction (HR, 95% CI) | Stent thrombosis (HR, 95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WOEST | 2013 | Randomised open-label | 573 | 12 months | PCI: 100% ACS: 27% AF: 69% |
≤4 h | ~12 months | VKA + clopidogrel | VKA + clopidogrel + aspirin | 0.56 (0.25–1.27) |
0.69 (0.29–1.60) |
0.44 (0.14–1.44) |
| ISAR-TRIPLE | 2015 | Randomised open-label (landmark-analysis from 6 weeks to 9 months) | 614 | 9 months | PCI: 100% ACS: 32% AF: 84% |
6 weeks | ~6 months (9 month follow-up) | VKA + aspirin | VKA + clopidogrel + aspirin | 1.01 (0.35–2.88) |
Singlee event in DAT group | No events |
| PIONEER AF-PCI | 2016 | Randomised open-label | 1389 (out of 2124) | 12 months | PCI: 100% ACS: 40% AF: 100% |
≤3 days | ~8 months | Rivaroxaban 15 mg daily + clopidogrel (95%) | VKA + clopidogrel + aspirin | 0.66 (0.33–1.31) |
0.86 (0.46–1.59) |
1.20 (0.32–4.45) |
| RE-DUAL PCI | 2017 | Randomised open-label | 2725 | 14 months | PCI: 100% ACS: 50% AF: 100% |
≤5 days | ~2.7 months | Dabigatran 110/150 mg bid + clopidogrel (88%) | VKA + clopidogrel + aspirin | 0.45 (0.27–0.73) |
1.36 (0.88–2.11) |
1.51 (0.67–3.41) |
| AUGUSTUS | 2019 | Randomised open-label (aspirin vs placebo double-blind) | 4614 | 6 months | PCI: 76% ACS: 38% AF: 100% |
6 days | 6 months | VKA/apixaban 5 mg bid + clopidogrel (93%) | VKA/apixaban 5 mg bid + clopidogrel + aspirin | 0.52 (0.33–0.82) |
1.24 (0.89–1.72) |
1.91 (0.92–3.98) |
| ENTRUST-AF-PCI | 2019 | Randomised open-label | 1506 | 12 months | PCI: 100% ACS: 52% AF: 100% |
~2 days (≤5 days) |
~2 months | Edoxaban 60 mg daily + clopidogrel (92%) | VKA + clopidogrel + aspirin | 0.62 (0.32–1.18) |
1.26 (0.72–2.16) |
1.32 (0.46–3.79) |
3. Specific features of the six randomised trials
3.1. WOEST
Enrollment and design: 573 patients undergoing PCI and requiring OAC were randomised open-label to TAT (VKA + clopidogrel + aspirin) or DAT (VKA + clopidogrel) and followed for 12 months [2]. Time from PCI to randomisation was ≤4 h. TAT was maintained throughout the trial (12 months). PCI was performed mostly by femoral access (74%). Drug eluting stents (DES) were used in 65% of cases. AF was the reason for OAC in 69%. Less than one third (27%) had an ACS (Fig. 1 and Table 1).
Results: At 1 year, a significant reduction in the primary outcome of any bleeding (hazard ratio, HR, 0.36, 95% confidence interval CI 0.26–0.50), but not in Thrombolysis in Myocardial Infarction (TIMI) major or Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO) severe bleeding, was found in the DAT versus TAT group. DAT was associated with a significantly lower risk of death (HR 0.39, 95% CI 0.16–0.93) and a nonsignificant reduction of myocardial infarction (MI) and stent thrombosis (ST) risk [2] (Table 1).
Appraisal: The duration of TAT was longer and the ACS population smaller than in other RCTs. The results can be interpreted as supporting immediate DAT (VKA + clopidogrel) versus long-term TAT on the basis of overall superior safety, although neither TIMI major nor GUSTO severe bleeding were significantly reduced by DAT; the efficacy results in favour of DAT versus TAT, especially in terms of all-cause death reduction, are limited by low statistical power and have not been confirmed by subsequent trials.
3.2. ISAR-TRIPLE
Enrollment and design: 614 patients undergoing PCI (98% femoral access, 99% DES, 32% ACS) and requiring OAC (84% for AF) were randomised open-label to 6 months TAT (VKA + aspirin + clopidogrel) or to 6 weeks TAT followed by clopidogrel discontinuation (i.e., delayed DAT with VKA + aspirin); time from PCI to randomisation was up to 14 days; follow-up was 9 months (Fig. 1 and Table 1). Previous ST or DES in the left main stem were exclusion criteria [3].
Results: Landmark analyses from 6 weeks to 9 months showed similar rates of the primary endpoint (death, MI, ST, stroke or TIMI major bleeding; HR 0.70, 95% CI 0.35–1.42), a significantly lower risk of any Bleeding Academic Research Consortium (BARC) bleeding, but not of TIMI major bleeding alone, for DAT versus TAT. Only one MI occurred in the DAT group, with no ST in either group (Table 1).
Appraisal: This is the only RCT to include both safety and efficacy outcomes in the primary endpoint, and to delay DAT onset at 6 weeks after randomisation; it is the first to use VKA + aspirin instead of VKA + clopidogrel in the DAT arm. The results can be interpreted as supporting either long-term TAT (without major safety concerns) or delayed DAT (without apparent efficacy concerns, accepting the small sample size). Importantly, given the known enhanced rate of ST in the first month/weeks after PCI, DAT was started 6 weeks after PCI; thus, the efficacy outcomes of this trial do not reflect an early DAT strategy.
3.3. PIONEER AF-PCI
Enrollment and design: 2,124 AF patients undergoing PCI (66% DES, 40% ACS) were randomised open-label to either: (a) rivaroxaban 15 mg daily + clopidogrel or (b) 1, 6 or 12 months of dual antiplatelet therapy (DAPT) plus rivaroxaban 2.5 mg twice daily (bid) followed by rivaroxaban 15 mg daily + aspirin or (c) 1, 6 or 12 months of DAPT plus VKA followed by VKA + aspirin (full TAT arm). The 1, 6 or 12 months TAT duration was decided and prespecified by treating clinicians. DAPT consisted in aspirin + clopidogrel (93–96%) and was continued up to 1 year in 49% of patients in the full TAT arm. Time from PCI to study drug was ≤72 h (Fig. 1 and Table 1).
Results: At 1 year, patients on rivaroxaban 15 mg + clopidogrel versus those on full TAT (n = 1,389) showed a significant reduction in the primary safety outcome (composite of TIMI major + minor bleeding or bleeding requiring medical attention), but not in TIMI major bleeding alone. There were no statistically significant differences in the occurrence of stroke, MI or ST [4] (Table 1).
Appraisal: This is the first trial replacing VKA with a DOAC in the DAT group. Two of the three treatment arms (n = 1,418) received either low dose (15 mg daily) or very low dose (2.5 mg bid) rivaroxaban; neither of these regimens correspond to the approved dose for stroke prevention in AF patients (i.e., 20 mg daily for creatinine clearance ≥50 ml/min). The results can be interpreted as supporting early DAT over TAT on the basis of overall superior safety, although TIMI major bleeding was not significantly reduced. The efficacy results are limited by low statistical power (only 11.4% to detect a >15% risk reduction at a two-sided significance level of 0.05 for adverse cardiovascular events) and by non-approved DOAC regimens for stroke prevention in AF.
3.4. RE-DUAL PCI
Enrollment and design: 2,725 AF patients undergoing PCI (50% ACS) were randomised open-label to either TAT (VKA + aspirin + clopidogrel or ticagrelor) for 1 or 3 months (according to bare metal stenting or DES), dropping aspirin thereafter, or to DAT with a DOAC at two different doses (dabigatran 110 mg bid or 150 mg bid) + clopidogrel or ticagrelor. Time from PCI to study drug was ≤120 h (preferably ≤72 h). DES were used in 85% of patients; thus, in the TAT group, aspirin was discontinued after 1 month in 15% and after 3 months in 85% of patients. In the DAT group, the antiplatelet agent was clopidogrel in 88% and ticagrelor in 12% (Fig. 1 and Table 1).
Results: At 1 year, the primary endpoint of the International Society on Thrombosis and Haemostasis (ISTH) major + clinically relevant non-major (CRNM) bleeding (HR 0.61, 95% CI 0.50–0.74) and the secondary endpoints of either TIMI major or ISTH major bleeding were significantly lower with DAT versus TAT, regardless of the 150 or 110 mg dabigatran dosing. When DAT with dabigatran 110 mg bid was compared to TAT, there was a trend towards increased risk of MI (HR 1.51, 95% CI 0.94–2.41) and definite ST (HR 1.86, 95% CI 0.79–4.40) [1]. DAT with dabigatran 150 mg bid showed similar results in terms of MI and ST compared to TAT. The trial was initially powered to assess both safety and efficacy, but the latter was not achieved, given subsequent amendments, namely: (a) sample size reduction from 8,520 to 2,502; (b) addition of unplanned revascularisation (UR) by PCI or surgery to the efficacy endpoint; (c) efficacy switch from primary to secondary endpoint; (d) pooling of dabigatran arms for efficacy analyses.
Appraisal: This is the first trial comparing DAT with systematic short-lasting TAT (≤3 months). Inclusion of UR to the efficacy endpoint contributed significantly to the overall number of events, to the extent that noninferiority for efficacy was lost when UR was excluded (p value from 0.005 to 0.11) [1]. Unlike DAT with dabigatran 150 mg bid, DAT with dabigatran 110 mg bid showed a trend towards increased rates of MI and ST compared to TAT [1]. The overall results can be interpreted as supporting DAT over TAT on the basis of superior safety, whereas the efficacy results are limited by low statistical power, inclusion of UR (not necessarily thrombosis-driven), and a trend towards increased MI and ST rates with the lower dabigatran regimen.
3.5. AUGUSTUS
Enrollment and design: 4,614 AF patients with PCI-treated chronic coronary artery disease (62%) or medically- or PCI-treated ACS (38%) were randomised in a two-by-two factorial design to open-label apixaban 5 mg bid or VKA and to double-blind aspirin or placebo, on top of a P2Y12 inhibitor administered to all patients (93% clopidogrel, 5.4% ticagrelor, 1.2% prasugrel). Follow-up was 6 months. Of interest, 24% (1,094) were medically treated ACS/AF patients. The trial design aimed to assess the contribution of (a) DOAC versus VKA and (b) aspirin vs none on safety and efficacy outcomes. Median time from index event to randomisation was 6 days. TAT duration was 6 months (Fig. 1 and Table 1).
Results: At 6 months, DAT versus TAT, with either apixaban or VKA, was associated with significant reductions of the primary outcome of ISTH major + CRNM bleeding (HR 0.51, 95% CI 0.43–0.61) and of TIMI major, ISTH major and GUSTO severe bleeding. Although the trial was not powered to assess efficacy, there was a trend toward a two-fold increased risk of probable or definite ST and toward an increased rate of MI in the DAT versus TAT arm [5] (Table 1). This trend was not evident in medically treated ACS patients (HR 0.65, 95% CI 0.33–1.30 for MI) [19]. Apixaban compared to VKA, both as DAT or TAT, resulted in a significant reduction of the primary outcome of ISTH major + CRNM bleeding (HR 0.69, 95% CI 0.58–0.81) and ISTH major bleeding, with a nonsignificant reduction of GUSTO severe and TIMI major bleeds. The risk of stroke was halved by apixaban (HR 0.50, 95% CI 0.26–0.97), with a trend towards reduced rates of MI and ST compared to VKA. In AUGUSTUS, 80% of definite or probable ST events occurred within 30 days of PCI [19], [20].
Appraisal: This is the first and only trial testing TAT with a full-dose DOAC [21]. Treatment and follow-up duration was limited to 6 months. Direct comparisons of TAT with apixaban versus TAT with VKA, and of DAT with apixaban versus DAT with VKA have not been fully reported (e.g., for the outcome of ST). The overall results can be interpreted as supporting: (a) DOAC over VKA, both in DAT and in TAT regimens, given the superior safety (although TIMI major and GUSTO severe bleeding did not differ significantly) and a trend towards superior efficacy; (b) DAT over TAT on the basis of superior safety, despite a trend towards increased MI and ST with DAT; (c) DAT over TAT in ACS patients not receiving PCI.
3.6. ENTRUST-AF-PCI
Enrollment and design: 1,506 AF patients undergoing PCI (52% ACS) were randomised open-label to DAT (edoxaban 60 mg daily + P2Y12 inhibitor) or TAT (VKA + P2Y12 inhibitor + aspirin) and followed for 12 months. Time from index event to randomisation was ≤5 days. The antiplatelet agent in the DAT arm was clopidogrel in 92%, ticagrelor in 7% and prasugrel in 1%. Mean TAT duration was 66 days (Fig. 1 and Table 1).
Results: At 1 year, there were nonsignificant trends toward lower rates of the primary safety outcome of ISTH major + CRNM bleeding (HR 0.83, 95% CI 0.65–1.05) and of TIMI or ISTH major bleeding with DAT versus TAT. The time in therapeutic range was ~60%, in line with others trials [1], [2], [3], [4], [5]. In a post-hoc analysis, there were numerically more bleeds during the first two weeks, and significantly fewer from 14 days to the end of trial with DAT versus TAT (p for interaction <0.0001). The authors acknowledge ‘a very early numerical increase in ischaemic events in patients without aspirin’, with a nonsignificant trend toward increased risk of MI (HR 1.26, 95% CI 0.72–2.16) and ST (HR 1.32, 0.46–3.79), although the trial was not powered to assess efficacy outcomes (Table 1).
Appraisal: This is the only trial not reporting formal superior safety of DAT with a DOAC versus (short lasting) TAT. The trend towards a higher early bleeding rate in the DAT arm was attributed to the high proportion of patients with an INR <2 (69% in the first week, 42% in the second week) in the comparator TAT arm, although it is not clear whether patients randomised to TAT received ‘bridging’ anticoagulant therapy until INR levels reached the value of 2. Overall, the ENTRUST-AF-PCI trial results indicate: (a) that DAT with edoxaban shows noninferior safety compared to relatively short lasting TAT with VKA; (b) a trend towards an early increased risk of MI and ST in the DAT arm, especially in the first weeks after randomisation.
4. Overall appraisal of trial results
4.1. Safety
-
•
Each of the six trials supports the use of DAT over TAT for patients with ACS or PCI requiring oral anticoagulation on the basis of superior safety, although the latter was defined by different criteria and, in ISAR TRIPLE [3] and ENTRUST-AF-PCI [6], superior safety was limited to landmark analyses [1], [3], [4], [5].
-
•
The well-known safety profile of DOACs versus VKA in AF patients (especially in terms of intracranial haemorrhages) and the results of AUGUSTUS [5] support DOACs over VKA within both DAT and TAT [22]; thus, RCTs involving DAT with DOACs should be especially considered.
-
•
Subgroup analyses of RE-DUAL PCI and AUGUSTUS show safety benefits of DAT versus TAT consistent with the overall trial results, irrespective of elective-PCI or ACS-related PCI, clopidogrel or ticagrelor (accepting limited sample size and the fact that P2Y12 inhibitor choice was at the discretion of the investigator), and dabigatran dose [19], [23].
-
•
In AUGUSTUS [19], patients with AF and ACS not undergoing PCI showed a trend towards enhanced safety and similar efficacy with DAT versus TAT, encouraging initial routine use of DAT in such patients, given the absence of ST risk [24].
4.2. Efficacy
-
•
None of the six trials show significant differences in efficacy outcomes for DAT vs TAT, suggesting that DAT might be considered a reasonable option in the medium-long term management of these patients [12], [25], [26]. However, no trial was powered for efficacy and, in the setting of patients undergoing PCI, a trend towards increased ischaemic events, such as MI and/or ST, is observed in all four trials involving a DOAC (Table 1).
-
•
The signal of increased risk of MI and/or ST in PCI-treated patients with DOAC-DAT vs TAT emerges despite trial design differences, namely: 1) mean time from index event to randomisation ranging from 2 to 6 days, thus admitting initial use of aspirin in both arms and uncertainty on the OAC used in the very early period (Fig. 1 and Table 1); 2) TAT duration ranging from 1 to 12 months; 3) mean follow-up ranging from 6 to 14 months (Table 1).
-
•
A subgroup analysis of RE-DUAL PCI shows a significantly increased risk of ST and MI among AF/ACS-PCI patients treated with clopidogrel plus dabigatran 110 mg bid compared to TAT, that is attenuated in those receiving ticagrelor [23]; although this finding is based on small subgroups and is only hypothesis generating, it suggests the potential relevance of using a powerful P2Y12 inhibitor and of discouraging the use of dabigatran 110 mg in ACS-PCI AF patients treated with DAT involving a DOAC.
-
•
A recent post-hoc analysis of AUGUSTUS [20] describes the timing of stent thrombosis in patients undergoing PCI (n = 3,498); it shows 80% of ST, defined as ‘definite or probable’, occurring within 30 days from PCI. This finding underlines the importance of considering early triple ATT for at least 1 month after PCI.
5. Appraisal of meta-analyses
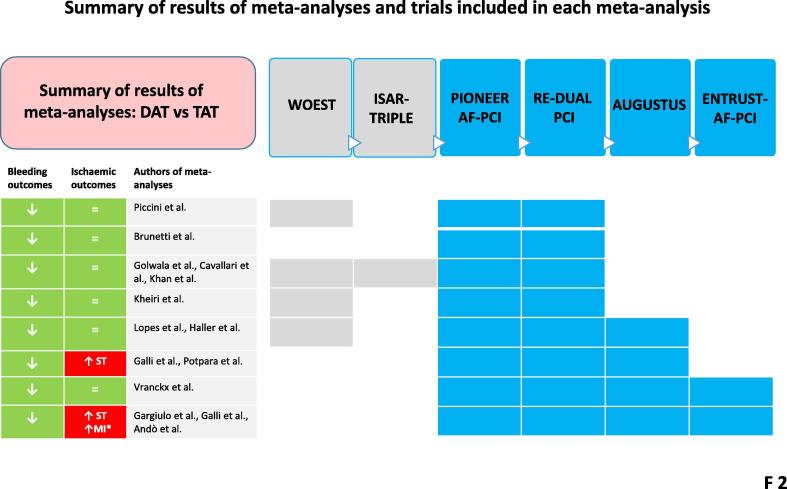
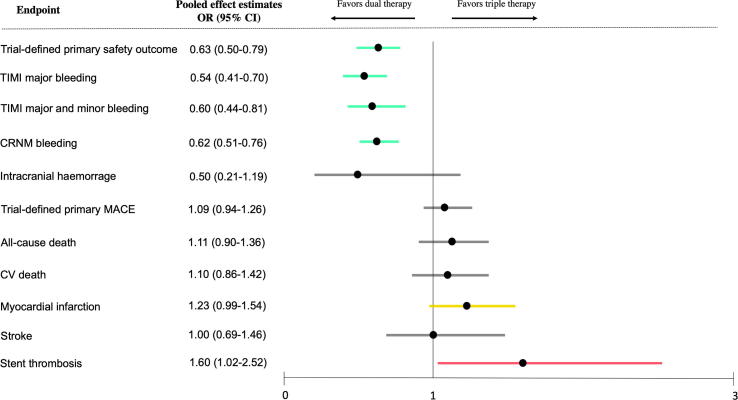
To overcome the limited power of the individual trials, particularly in assessing ischaemic event rates, 14 meta-analyses have now been conducted [6], [9], [10], [11], [12], [13], [14], [15], [25], [26], [27], [28], [29], [30]. They confirm the superior safety and – until very recently – report a comparable ischaemic risk with DAT vs TAT [12], [25], [26], [27], [28], [29], [30] (Fig. 2). As stated above, given the established safer profile of DOACs vs VKA in AF patients, RCTs involving DAT with DOACs merit specific attention. Only 4 meta-analyses to date have analysed all four RCTs with DOACs (Fig. 2). Although some of the latter reported that DAT with DOACs was associated with similar risks of major adverse cardiovascular events, all-cause death, stroke, ST, or MI compared to TAT [6], [26], others [13], [14], [9], [10], [11] showed a significant increase in the risk of ST. Indeed, in one of the most recent analyses [13], the odds of ST with DAT vs TAT increased 60%, with a number needed to treat (NNT) for a harmful outcome (NNTH) of 274 and an associated borderline significant increase of MI (NNTH = 151) (Fig. 2 and Fig. 3).
Fig. 2.
Schematic summary of the results of 14 meta-analyses. Boxes indicate the randomised trials included in each meta-analysis. Of note, the most recent meta-analyses, focused exclusively on double antithrombotic therapy (DAT) with direct oral anticoagulants, show discrepancies for ischaemic outcomes. MI: myocardial infarction; ST: stent thrombosis; TAT: triple antithrombotic therapy.
Fig. 3.
Forest plots of safety and efficacy outcomes for double antithrombotic therapy with direct oral anticoagulants versus triple antithrombotic therapy. From Galli et al, Europace 2020 [13].
Reasons for the above discrepancies can be found in the definition of ST. Indeed, the meta-analyses not reporting increased ST rates with DAT vs TAT [6], [26] included ‘any stent thrombosis’ (i.e., definite, probable and possible) events from AUGUSTUS [26], whereas the other meta-analyses focused only on ‘definite’ or ‘probable’ ST, as recommended by the Academic Research Consortium 2 (ARC 2) [1], [4], [13], [14], [31]. Analyses including ‘possible’ stent thrombosis are discouraged by the ARC 2, given poor specificity [20], [31].
Most meta-analyses did not report outcomes in relation to type of index event (ACS vs non-ACS), type of stent (drug-eluting vs bare-metal), CHA2DS2VASC score, or P2Y12 inhibitor used [14], [25], [9], [10], [11], [12], [27], [28], [29], [30]. Only one meta-analysis [13] performed a subgroup pooled analysis by type of index event (ACS-related PCI vs elective PCI), showing a significant 40% increase in the risk of MI among ACS-PCI AF patients receiving DAT compared to TAT. Despite the subgroup meta-analysis of only two trials, the increased incidence of ST and MI should be carefully taken into account in high ischaemic risk patients, such as those with previous ST, complex PCI (e.g., left main PCI), or ACS presentation [32], inducing caution against premature DAT in ACS/PCI AF patients.
In terms of safety outcomes, the three most recent meta-analyses comparing all available trials on DAT with DOACs vs TAT showed a significant 37% reduction of trial-defined primary safety outcomes (with a NNT of 17) and a significant 46% reduction of TIMI major bleeding (NNT = 76) (Fig. 3). Importantly, the risk-to-benefit ratio of DAT compared to TAT, calculated as NNT to avoid an intracranial haemorrhage (=314) versus NNTH causing a ST event (=274) also discourages early DAT initiation versus early TAT [13].
6. Conclusions
Recent meta-analyses [25], [26], [9], [10], [11], [13], [14], [15], position papers [7], [8], and guidelines [22] report contrasting results and recommendations on antithrombotic therapy for AF patients with ACS or undergoing PCI. Our appraisal of the six original RCTs and subsequent meta-analyses indicates that: 1) DOACs at highest approved dose (with the exception of rivaroxaban at 15 instead of 20 mg daily) should be preferred over VKAs; 2) while early DAT may be used in medically-managed ACS-AF patients, a tailored strategy based on clinical and procedural features should be considered in AF-PCI patients, favouring a TAT strategy with DOACs until discharge, and up to 1 month (or longer) in patients at high thrombotic risk, especially in ACS patients, unless bleeding risk is prohibitively high.
Declaration of Competing Interest
FA reports receiving consultant or speaker fees from Amgen, Bayer, B-I, BMS-Pfizer and Daiichi Sankyo, outside the present work. FC reports receiving personal fees from Biotronic, Amgen, Astra Zeneca, Servier, Menarini, BMS, outside the present work. Other authors have nothing to disclose.
Contributor Information
Felicita Andreotti, Email: felicita.andreotti@unicatt.it.
Domenico D'Amario, Email: domenico.damario@policlinicogemelli.it.
References
- 1.Cannon C.P., Bhatt D.L., Oldgren J., Lip G.Y.H., Ellis S.G., Kimura T. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N. Engl. J. Med. 2017;377(16):1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 2.Dewilde W.J.M., Oirbans T., Verheugt F.W.A., Kelder J.C., De Smet B.J.G.L., Herrman J.-P. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. The Lancet. 2013;381(9872):1107–1115. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 3.Fiedler K.A., Maeng M., Mehilli J., Schulz-Schupke S., Byrne R.A., Sibbing D. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting Stent implantation: The ISAR-TRIPLE trial. J. Am. Coll. Cardiol. 2015;65(16):1619–1629. doi: 10.1016/j.jacc.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 4.Gibson C.M., Mehran R., Bode C., Halperin J., Verheugt F.W., Wildgoose P. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N. Engl. J. Med. 2016;375(25):2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 5.Lopes R.D., Heizer G., Aronson R., Vora A.N., Massaro T., Mehran R. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N. Engl. J. Med. 2019;380(16):1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 6.Vranckx P., Valgimigli M., Eckardt L., Tijssen J., Lewalter T., Gargiulo G. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet (London, England) 2019;394(10206):1335–1343. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- 7.Angiolillo D.J., Goodman S.G., Bhatt D.L., Eikelboom J.W., Price M.J., Moliterno D.J. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: A north American perspective–2018 update. Circulation. 2018;138(5):527–536. doi: 10.1161/CIRCULATIONAHA.118.034722. [DOI] [PubMed] [Google Scholar]
- 8.Lip G.Y.H., Collet J.-P., Haude M., Byrne R., Chung E.H., Fauchier L. Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA) Europace. 2019;21(2):192–193. doi: 10.1093/europace/euy174. euy174-euy. [DOI] [PubMed] [Google Scholar]
- 9.Potpara T.S., Mujovic N., Proietti M., Dagres N., Hindricks G., Collet J.-P. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials. EP Europace. 2019 doi: 10.1093/europace/euz259. [DOI] [PubMed] [Google Scholar]
- 10.Galli M., Andreotti F., D'Amario D., Vergallo R., Montone R.A., Porto I. Dual Therapy with DOACs significantly increases the risk of Stent Thrombosis compared to Triple Therapy. Eur. Heart J. Cardiovasc Pharmacother. 2019 doi: 10.1093/ehjcvp/pvz030. [DOI] [PubMed] [Google Scholar]
- 11.Gargiulo G., Goette A., Tijssen J., Eckardt L., Lewalter T., Vranckx P. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur. Heart J. 2019 doi: 10.1093/eurheartj/ehz732. [DOI] [PubMed] [Google Scholar]
- 12.Haller P.M., Sulzgruber P., Kaufmann C., Geelhoed B., Tamargo J., Wassmann S. Bleeding and ischaemic outcomes in patients treated with dual or triple antithrombotic therapy: systematic review and meta-analysis. Eur. Heart J. – Cardiovascul. Pharmacother. 2019;5(4):226–236. doi: 10.1093/ehjcvp/pvz021. [DOI] [PubMed] [Google Scholar]
- 13.Galli M., Andreotti F., Porto I., Crea F. Intracranial haemorrhages vs. stent thromboses with direct oral anticoagulant plus single antiplatelet agent or triple antithrombotic therapy: a meta-analysis of randomized trials in atrial fibrillation and percutaneous coronary intervention/acute coronary syndrome patients. Europace. 2020;22(4):538–546. doi: 10.1093/europace/euz345. [DOI] [PubMed] [Google Scholar]
- 14.Ando G., Costa F. Double or triple antithrombotic therapy after coronary stenting and atrial fibrillation: A systematic review and meta-analysis of randomized clinical trials. Int. J. Cardiol. 2020;302:95–102. doi: 10.1016/j.ijcard.2019.12.054. [DOI] [PubMed] [Google Scholar]
- 15.Khan S.U., Osman M., Khan M.U., Khan M.S., Zhao D., Mamas M.A. Dual versus triple therapy for atrial fibrillation after percutaneous coronary intervention: A systematic review and meta-analysis. Ann. Intern. Med. 2020;172(7):474–483. doi: 10.7326/M19-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohla M., Vennekate C.K., Tentzeris I., Freynhofer M.K., Farhan S., Egger F. Long-term mortality of patients with atrial fibrillation undergoing percutaneous coronary intervention with stent implantation for acute and stable coronary artery disease. Int. J. Cardiol. 2015;184:108–114. doi: 10.1016/j.ijcard.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Ancedy Y., Lecoq C., Saint Etienne C., Ivanes F., Angoulvant D., Babuty D. Antithrombotic management in patients with atrial fibrillation undergoing coronary stent implantation: What is the impact of guideline adherence? Int. J. Cardiol. 2016;203:987–994. doi: 10.1016/j.ijcard.2015.11.090. [DOI] [PubMed] [Google Scholar]
- 18.Chang K.W., Arbit B., Hsu J.C. Antithrombotic regimens in patients with atrial fibrillation and coronary artery disease after percutaneous coronary intervention: A focused review. Int. J. Cardiol. 2017;243:263–269. doi: 10.1016/j.ijcard.2017.05.119. [DOI] [PubMed] [Google Scholar]
- 19.Windecker S., Lopes R.D., Massaro T., Jones-Burton C., Granger Christopher B., Aronson R. Antithrombotic Therapy in Patients with Atrial Fibrillation and Acute Coronary Syndrome Treated Medically or with Percutaneous Coronary Intervention or Undergoing Elective Percutaneous Coronary Intervention: Insights from the AUGUSTUS Trial. Circulation. 2019;140(23):1921–1932. doi: 10.1161/CIRCULATIONAHA.119.043308. [DOI] [PubMed] [Google Scholar]
- 20.Lopes R.D., Leonardi S., Wojdyla D.M., Vora A.N., Thomas L., Storey R.F. Stent thrombosis in patients with atrial fibrillation undergoing coronary stenting in the AUGUSTUS trial. Circulation. 2019;141(9):781–783. doi: 10.1161/CIRCULATIONAHA.119.044584. [DOI] [PubMed] [Google Scholar]
- 21.Gremmel T., Sulzgruber P., Niessner A. Critical appraisal of the AUGUSTUS trial. Eur. Heart J. – Cardiovascul. Pharmacother. 2019;5(4):187–188. doi: 10.1093/ehjcvp/pvz017. [DOI] [PubMed] [Google Scholar]
- 22.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) Eur. Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 23.Oldgren J., Steg P.G., Hohnloser S.H., Lip G.Y.H., Kimura T., Nordaby M. Dabigatran dual therapy with ticagrelor or clopidogrel after percutaneous coronary intervention in atrial fibrillation patients with or without acute coronary syndrome: a subgroup analysis from the RE-DUAL PCI trial. Eur. Heart J. 2019;40(19):1553–1562. doi: 10.1093/eurheartj/ehz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galli M., Andreotti F., D'Amario D., Vergallo R., Vescovo G.M., Giraldi L. Antithrombotic therapy in the early phase of non-ST-elevation acute coronary syndromes: a systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2020;6(1):43–56. doi: 10.1093/ehjcvp/pvz031. [DOI] [PubMed] [Google Scholar]
- 25.Golwala H.B., Cannon C.P., Steg P.G., Doros G., Qamar A., Ellis S.G. Safety and efficacy of dual vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of randomized clinical trials. Eur. Heart J. 2018;39(19):1726–1735. doi: 10.1093/eurheartj/ehy162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes R.D., Hong H., Harskamp R.E., Bhatt D.L., Mehran R., Cannon C.P. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: A network meta-analysis of randomized controlled trials. JAMA Cardiol. 2019 doi: 10.1001/jamacardio.2019.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccini J.P., Jones W.S. Triple therapy for atrial fibrillation after PCI. N. Engl. J. Med. 2017;377(16):1580–1582. doi: 10.1056/NEJMe1710753. [DOI] [PubMed] [Google Scholar]
- 28.Cavallari I., Patti G. Meta-analysis comparing the safety and efficacy of dual versus triple antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am. J. Cardiol. 2018;121(6):718–724. doi: 10.1016/j.amjcard.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Kheiri B., Osman M., Bakhit A., Radaideh Q., Abdalla A., Barbarawi M., Zayed Y., Ahmed S., Bachuwa G., Hassan M.J. Dual versus triple therapy for patients with atrial fibrillation and acute coronary syndrome: a meta-analysis and trial sequential analysis of randomized controlled trials. J Thromb Thrombolysis. 2019;48(3):511–513. doi: 10.1007/s11239-019-01874-1. [DOI] [PubMed] [Google Scholar]
- 30.Brunetti N.D., Tarantino N., De Gennaro L., Correale M., Santoro F., Di Biase M. Direct oral anticoagulants versus standard triple therapy in atrial fibrillation and PCI: meta-analysis. Open Heart. 2018;5(2):e000785. doi: 10.1136/openhrt-2018-000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galli M., Andreotti F., D'Amario D., Porto I., Crea F. Stent thrombosis with dual antithrombotic therapy in atrial fibrillation-ACS/PCI Trials. J. Am. Coll. Cardiol. 2020;75(14):1727–1728. doi: 10.1016/j.jacc.2020.01.054. [DOI] [PubMed] [Google Scholar]
- 32.Galli M., Andreotti F., Savarese G., D’Amario D., Vergallo R., Della Bona R. Dropping aspirin in patients with atrial fibrillation undergoing percutaneous coronary intervention: a jump with a weak parachute? Eur. Heart J. – Cardiovascul. Pharmacother. 2018;5(1):55–56. doi: 10.1093/ehjcvp/pvy039. [DOI] [PubMed] [Google Scholar]