Highlights
-
•
STEMI patients treated with PiCSO were propensity matched to INFUSE-AMI controls.
-
•
Infarct size at day 5 was significantly lower in the PiCSO group.
-
•
There were no major adverse cardiac events (MACE) related to the PiCSO.
Abbreviations: ACS, acute coronary syndrome; AMI, acute myocardial infarction; BARC, Bleeding Academic Research Consortium; CI, Confidence interval; CMR, Cardiac magnetic resonance; CRT, Cardiac Resynchronization Therapy; IMR, Index of microcirculatory resistance; LAD, left anterior descending artery; LV, Left ventricle; MACE, Major adverse cardiac events; pPCI, Primary percutaneous coronary intervention; PiCSO, Pressure-controlled intermittent coronary sinus occlusion; SD, Standard deviation; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombosis in myocardial infarction
Keywords: Infarct size reduction, Pressure-controlled intermittent coronary sinus occlusion (PICSO), ST-segment elevation myocardial infarction (STEMI)
Abstract
Background
The aim of this clinical research was to investigate the effects of Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) on infarct size at 5 days after primary percutaneous coronary intervention (pPCI) in patients with ST-segment elevation myocardial infarction (STEMI).
Methods and results
This comparative study was carried out in four UK hospitals. Forty-five patients with anterior STEMI presenting within 12 h of symptom onset received pPCI plus PiCSO (initiated after reperfusion; n = 45) and were compared with a propensity score-matched control cohort from INFUSE-AMI (n = 80). Infarct size (% of LV mass, median [interquartile range]) measured by cardiac magnetic resonance (CMR) at day 5 was significantly lower in the PiCSO group (14.3% [95% CI 9.2–19.4%] vs. 21.2% [95% CI 18.0–24.4%]; p = 0.023). There were no major adverse cardiac events (MACE) related to the PiCSO intervention.
Conclusions
PiCSO, as an adjunct to pPCI, was associated with a lower infarct size at 5 days after anterior STEMI in a propensity score-matched population.
1. Introduction
The treatment of choice for patients with acute ST-segment elevation myocardial infarction (STEMI) is primary percutaneous coronary intervention (pPCI), performed as quickly as possible [1], [2]. However, despite substantial improvements in survival among patients with STEMI [3] attributable to increased use of reperfusion therapy and appropriate adjunct pharmacotherapy, 30-day mortality rates among those who have undergone pPCI remain approximately 3–4% [3], [4]. Despite the ability of pPCI to restore epicardial reperfusion in approximately 90% of patients, infarct size is often substantial, leading to increased heart failure and increased mortality [5]. Infarct size determined within 1 month after pPCI has been strongly correlated with all-cause mortality and hospitalization for heart failure.
Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) is a mechanical catheter-based device placed into the coronary sinus after initial pPCI which intermittently increases mean coronary sinus pressure and coronary sinus pulse pressure [6]. Previous experimental and human research have reported potential beneficial effects of PiCSO on cardiac function [7], [8] and infarct size [9], [10]. We therefore evaluated the effects of PiCSO as an adjunct to pPCI on infarct size and myocardial function in patients with anterior STEMI.
2. Methods
2.1. Study design
This study was a prospective, parallel-cohort, comparative study (clinicaltrials.gov identifier NCT02197325) that was carried out between 13/1/2015 and 26/10/2017 in four centers in the UK (Freeman Hospital, Newcastle-upon-Tyne; St. Bartholomew’s Hospital, London; Liverpool Heart and Chest Hospital, Liverpool; and Northern General Hospital, Sheffield). Consecutive patients with anterior STEMI were treated with pPCI plus PiCSO if a PiCSO-trained physician was available. The control group consisted of patients with anterior STEMI at the same centers admitted when a PiCSO-trained physician was not available.
The study protocol was approved by the ethics committee (14/NE/1129) and conducted in accordance with the 2013 declaration of Helsinki, the International Conference on Harmonization guidelines on Good Clinical Practice, and ISO 14155. As treatment was time-critical, initial assent was verbal (witnessed and documented). Within 24 h post treatment, patients were given written information about the study and written informed consent was obtained.
2.2. Patients
To be included in the study all of the following conditions had to be present: first occurrence of STEMI; culprit lesion in the left anterior descending artery; and age ≥ 25 years. Patients were excluded for any of the following: complicated pPCI (i.e. angioplasty followed by stent placement or direct stenting with adverse event(s) that would preclude the use of PiCSO, including major bleeding, perforation, hypotension, pulmonary edema, or clinical instability; symptom onset time > 12 h; previous coronary artery bypass graft surgery; history of stroke, transient ischemic attack, or reversible ischemic neurological disease within the past 6 months; hospitalization with a primary diagnosis of acute myocardial infarction previously or evidence of previous Q-wave infarct; known contraindication for cardiac magnetic resonance imaging (CMR); active or treated malignancies in the previous 12 months; pregnancy; non-cardiac comorbidities and life expectancy <1 year; and use of warfarin.
2.3. PiCSO treatment
The PiCSO Impulse System (Miracor Medical SA, Awans, Belgium) consists of a console which is controlled by a graphical user interface and a single-use balloon catheter. The catheter contains four lumens, a balloon on the distal end, and soft tip and connectors on the proximal end for the shuttle gas supply and coronary sinus pressure measurement. The system saves a log file for each patient from which the device performance can be determined, i.e. increase in coronary sinus pressure during inflation cycles.
In the current study qualifying patients with anterior STEMI were treated with PiCSO in adjunct to standard PCI if a suitably-trained PiCSO operator was present, otherwise the patients have undergone a standard PCI. PiCSO therapy was started immediately after successful flow restoration in the occluded LAD by balloon angioplasty or aspiration, but before stent insertion. PiCSO was then provided during stenting and for at least 20 min thereafter. Treatment was continued until a PiCSO Quantity (sum of modulation of coronary sinus pressure during occlusion phase over the time [9]) of 800 mmHg was reached.
2.4. Assessments and follow-up
Follow-up in STEMI patients was performed at 5 ± 2 and 120 ± 14 days after pPCI for adverse event assessment and performance of CMR. All data were entered in an electronic database (EDC2GO, Genae, Antwerp, Belgium). Blinded analysis of the CMR scans was performed by an independent core laboratory (VU University Medical Center, Amsterdam, the Netherlands). Blinded quantification of pre- and post-pPCI Thrombolysis In Myocardial Infarction (TIMI) flow was performed by an independent angiographic core laboratory (Cardialysis, Rotterdam, Netherlands). An independent clinical events committee adjudicated all safety events. The data were independently monitored by Genae. MACE was defined as a composite of cardiac death, new or worsening hospitalization for heart failure, target vessel revascularisation, stroke, major bleeding (BARC 3–5) or coronary sinus damage requiring intervention. Propensity score matching was performed by independent statisticians at the Cardiovascular Research Foundation (New York, NY, USA).
2.5. Statistical analysis
Descriptive statistics are given as means with standard deviations (SD) or medians (interquartile range) for continuous variables and counts and percentages for categorical variables. PiCSO and control groups were compared using the two-sample t-test or Mann Whitney U test for continuous variables and Fisher’s exact or Pearson’s chi square test for categorical variables. All statistical analyses were performed using SPSS, Version 24.0 (IBM Corp., Armonk, NY, USA).
As the baseline characteristics of the PiCSO and parallel groups varied (Supplementary Table 1), PiCSO-treated patients were also compared with a propensity-matched control cohort of patients from INFUSE-AMI [11], [12] who had CMR data at 5 days and did not receive intracoronary abciximab (control group). PiCSO-treated patients meeting the eligibility criteria were matched on sex, age, diabetes, culprit lesion location (proximal vs. mid left anterior descending artery), symptom-to-balloon time, pre-pPCI TIMI flow (0/1 vs. 2/3), and post-pPCI TIMI flow (2 vs. 3). Each PiCSO-treated patient with available 5-day post-MI infarct size was matched to three control patients from INFUSE-AMI (calliper = 0.5 × SD of propensity score). PiCSO patients who could not be adequately matched were excluded. While the propensity matching has been performed by one statistician blinded to infarct size measurements, the efficacy analysis has been performed by a second statistician. Infarct size at day 5 was chosen as the endpoint for this analysis as it has been widely used in clinical studies [10], [11], [13], [14], [15] and has been strongly associated with all-cause mortality and heart failure hospitalization [5].
As an additional analysis, MACE rates at 30 days were evaluated in PiCSO-treated patients meeting the eligibility criteria in whom at least 23 days of follow-up was present or earlier MACE had occurred. This rate was compared to an objective performance goal for 30-day MACE of 10% based on outcomes from the AMIHOT I/II, CRISP and INFUSE-AMI studies. Based on binomial distribution assessment this objective performance goal would be met if out of 40 patients having valid 30-day follow-up less than 7 patients had a MACE event.
3. Results
3.1. Patients
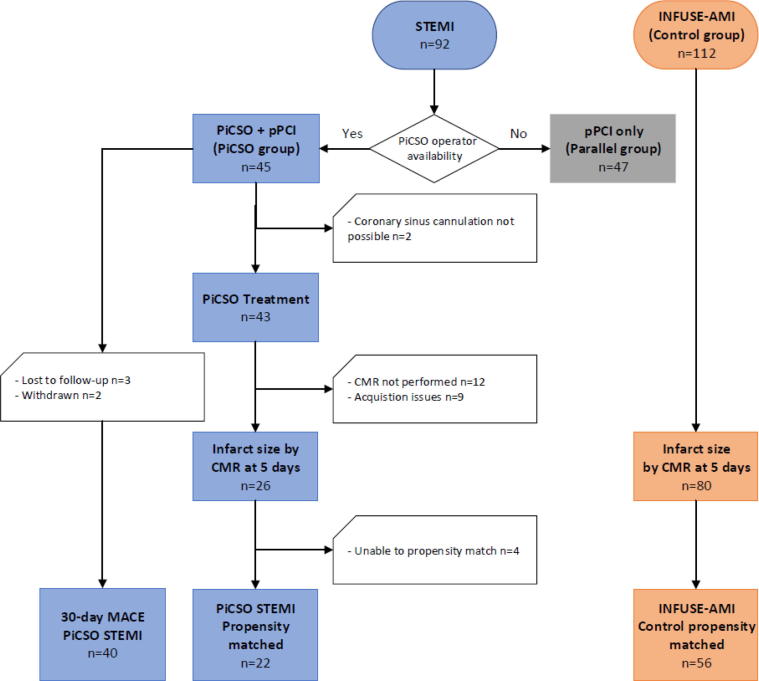
A total of 92 patients were enrolled into the study. Among those, 45 underwent pPCI with PiCSO and 47 underwent pPCI alone (Supplementary Table 1 and Fig. 1).
Fig. 1.
Study flow diagram. CMR: cardiac magnetic resonance imaging; PiCSO: Pressure-controlled intermittent Coronary Sinus Occlusion; pPCI: primary percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
PiCSO was initiated in 43/45 STEMI patients (95.6%); in two STEMI patients difficulty obtaining femoral vein access (n = 1) and the inability to cannulate the coronary sinus (n = 1) precluded PiCSO delivery. PiCSO was started after flow restoration but before stenting (i.e. per the protocol) in 35/43 STEMI patients (81.4%) and after stenting in the remainder of the patients due to longer times needed for cannulating the coronary sinus (12 ± 8 min for pre-stenting start vs 22 ± 15 for post stenting start). Patients have received PiCSO treatment on average for 33 ± 19 min and have reached mean PiCSO quantity of 757 ± 301 mmHg. CMR was performed in 34 PiCSO patients (at mean 5.2, SD 2.4 days) and the infarct size was analyzable in 26 patients.
The 26 PiCSO patients treated were compared with 80 control patients from INFUSE-AMI [11], [12] study. Patients were matched on sex, age, pre-PCI TIMI flow (0/1 vs 2/3), post PCI TIMI flow (2 vs 3), diabetes mellitus, culprit lesion location (proximal vs mid LAD), and symptom to balloon time. After propensity score matching 1 patient from the PiCSO study to a possible 3 patients from INFUSE-AMI with a caliper of ½ the standard deviation of the propensity score, 22 PiCSO patients and 56 INFUSE-AMI remained.
Finally, 40 PiCSO patients were available for the 30-day MACE rate analysis; 5 patients withdrew from the study within 7 days.
3.2. Efficacy results
The initial analysis comparing efficacy results of the PiCSO group to the parallel control group reviewed that though the Area at Risk has been comparable between the 2 groups, the infarct size was consistently lower in the PiCSO vs. parallel control group at 5 days (13.99 ± 12.79 vs. 17.10 ± 11.72). Nevertheless, the differences were not statistically significant (p = 0.242) (Supplementary Table 3). Although the mean microvascular obstruction among all patients was similar, numerically fewer patients in the PiCSO group had microvascular obstruction (26.9% vs. 46.7%; p = 0.17) when compared to the parallel control group. Left ventricular ejection fraction was numerically better in the PiCSO group vs. parallel control group at day 5, but not statistically significantly different (50.5% vs. 46.8%; p = 0.076).
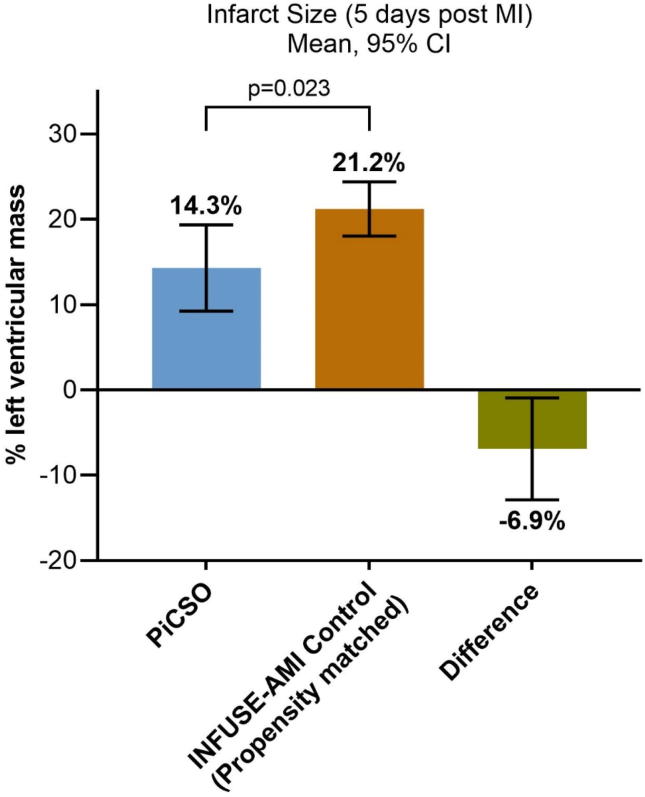
In order to account for the potential selection bias and the differences in baseline characteristics between the PiCSO group and parallel control group, the baseline characteristics of 22 per protocol PiCSO-treated patients were well matched with 56 INFUSE-AMI patients (Table 1). Infarct size (% LV mass) at 5 days was smaller in those anterior STEMI patients treated with PiCSO compared with the propensity-matched controls (mean 14.3% vs. 21.2%; mean difference −6.9% (95% confidence interval [CI] −12.9 to −0.9%; p = 0.023) (Fig. 2). The ejection fraction (50.8 vs. 47.1, p = 0.136), left ventricular end systolic volume (42.5 vs. 48.1, p = 0.183) and left ventricular end diastolic volume (50.8 vs. 47.13, p = 0.136) did not differ in this analysis at 5 days between the PiCSO treated patients and the propensity matched control patients.
Table 1.
Baseline characteristics and CMR timing of PiCSO-treated patients compared with a propensity-matched control group from the INFUSE-AMI trial.
| PiCSO (n = 22) | INFUSE-AMI Control (n = 56) | p-value | |
|---|---|---|---|
| Men | 16 (72.7) | 41 (73.2) | 0.97 |
| Age, years | 58.7 ± 12.2 | 61.2 ± 12.9 | 0.44 |
| Diabetes | 2 (9.1) | 6 (10.7) | 1.00 |
| Culprit location | 0.74 | ||
| Proximal LAD | 15 (68.2) | 36 (64.3) | |
| Mid LAD | 7 (31.8) | 20 (35.7) | |
| Symptom-to-balloon time, minutes | 146.5 (107, 220) | 157.5 (129, 210) | 0.37 |
| Pre-pPCI TIMI flow | 0.66 | ||
| 0/1 | 15 (68.2) | 41 (73.2) | |
| 2/3 | 7 (31.8) | 15 (26.8) | |
| Post-pPCI TIMI flow | 0.49 | ||
| 2 | 4 (18.2) | 7 (12.5) | |
| 3 | 18 (81.8) | 49 (87.5) | |
| Propensity score | 0.71 ± 0.15 | 0.76 ± 0.12 | 0.19 |
| CMR after pPCI, days | 4.6 ± 1.9 | 4.8 ± 1.5 | 0.75 |
Data are shown as n (%), mean ± SD or median (quartile 1, quartile 3). CMR: cardiac magnetic resonance imaging; PiCSO: Pressure-controlled intermittent Coronary Sinus Occlusion; pPCI: primary percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; TIMI: Thrombolysis In Myocardial Infarction.
Fig. 2.
Infarct size in propensity score-matched PiCSO and control patients with STEMI who underwent pPCI. Error bars show 95% confidence intervals. PiCSO: Pressure-controlled intermittent Coronary Sinus Occlusion; pPCI: primary percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
3.3. Safety results
Five MACE events occurred during the course of this study; as listed in Supplementary Table 2. Three were cardiac deaths and one new onset of heart failure in the PiCSO group. In addition, one patient in the parallel control group has developed new onset of heart failure. None of these events were adjudicated as procedure- or device-related. Among PiCSO-treated STEMI patients with 30-day follow-up (n = 40), MACE occurred in 4 patients (10.0%), below the threshold for the objective performance goal (Table 2). In a tipping point analysis, at least 3 of these 5 patients (60%) would need to have a 30-day MACE in order for the study to be considered a failure for safety. Between 30 days and the last follow-up visit (120 days) no additional MACE occurred.
Table 2.
30-day MACE in PiCSO-treated patients.
| MACE/Component | n/N (%) | 95% CI |
|---|---|---|
| 30-Day MACE | 4/40 10.0%) | 2.8%, 23.7% |
| Cardiac death | 3/40 (7.5%) | 1.6%, 20.4% |
| New or worsening heart failure | 1/40 (2.5%) | 0.0%, 13.2% |
| Hospitalization for heart failure | 0/40 (0.0%) | 0.0%, 8.8% |
| Target vessel revascularization | 0/40 (0.0%) | 0.0%, 8.8% |
| Stroke | 0/40 (0.0%) | 0.0%, 8.8% |
| Major bleeding | 0/40 (0.0%) | 0.0%, 8.8% |
| Coronary sinus damage requiring intervention | 0/40 (0.0%) | 0.0%, 8.8% |
Data are shown as n of total N (%) and 95% Confidence Interval.
4. Discussion
4.1. Main findings
Infarct size assessment within the first 30 days post myocardial infarction has been widely used as an efficacy endpoint in clinical studies of reperfusion therapy in STEMI as it is strongly associated with all-cause mortality and heart failure hospitalization (5, 10, 11, 13–15). In the current study qualifying patients with anterior STEMI were treated with PiCSO in adjunct to standard PCI if a suitably-trained PiCSO operator was present, otherwise the patients have undergone a standard PCI. Due to the non-randomized nature of the treatment allocation the baseline characteristics of the two groups (PiCSO group and parallel control group) were quite different, most notably that patients in the PiCSO group had better pre-PCI TIMI flow than patients in the control group. As better pre-PCI TIMI flow has been strongly correlated with smaller infarct size [16], we hypothesized that this may have accounted for the smaller infarct size in the PiCSO group. Therefore, we compared the PiCSO patients with propensity-matched controls from the INFUSE-AMI trial [11], [12]. In this analysis of patients with a first anterior STEMI undergoing pPCI, treatment with PiCSO after initial reperfusion resulted in a significant decrease in infarct size at 5 days compared to a propensity-adjusted control population from the INFUSE-AMI trial.
4.2. Timing of PICSO and its relationship to salvage and the mode of action
Myocardial salvage is the paramount objective of PCI. Despite improvements in the reperfusion techniques and optimized workstreams, the mortality of STEMI patients has plateaued over the past decade [17]. The incomplete recovery post PCI due to underlying pathophysiology of reperfusion and clinical consequences of microcirculatory obstruction might be one of the reasons for such stagnation.
During myocardial infarction the size of the myocardial necrosis extends antegrade from the culprit lesion and stretches to the peripheral border zones. If the Area at Risk is too large to be perfused with collateral or diffusive blood flow, the size of infarction would be greater leading also to a greater area of microvascular obstruction [18].
Today, there is no doubt that the “Time is Muscle” principle positively alters the severity and extent of myocardial ischemic injury. Deferred‐stenting strategy alone when compared with immediate stenting has not been able to reduce the occurrence of no‐ or slow‐reflow during pPCI, nor has it influenced the mortality [19]. Since with PiCSO we are aiming to facilitate the washout first and clear the microcirculation, deferring stenting to post PiCSO start could allow “preparing” the deprived myocardial areas for the next wave injury caused stenting [20].
During reperfusion and revascularization induced injury, the coronary venous drainage system may play an integral part in accessing the obstructed microvasculature, paving the path to new therapies like PiCSO allowing for retro-perfusion of the deprived myocardium as well as simultaneous washout of debris and toxic accumulations [20].
During occlusion of the coronary sinus by PiCSO Impulse Catheter, the venous pressure increases gradually until the systolic peaks reach a plateau (which differs from patient to patient as well as within the procedure based on the physiological state and impairment grade of the coronary flow) resulting in an accumulation of blood in the venous bed and an increase of diastolic coronary sinus pressure. The additional input of blood during systole causes a further distension and thus systolic pressure elevation in the coronary sinus. During this occlusion period (which lasts approximately 5–15 s) venous flow is redistributed from normally perfused areas towards the deprived zones and then reenters the microcirculation [21]. Systolic pressure increase (average maximum during systole of 70 ± 17 mmHg as seen in this study) in the venous compartment pushes the blood plasma though the obstructed microcirculatory bed creating a plasma skimming effect in the venous microcirculation leading to increased perfusion of venules with plasma containing oxygen and metabolites. While plasma skimming seems to be one of the central drivers for microcirculatory clearance upon release of the occlusion [20] the occlusion of the venous outflow also allows re-distribution of the flow to the deprived perfusion border zones due to pressure induced vasodilation and increased collateral flow [21]. Furthermore, aspects of coronary sinus pressure elevation inducing molecular signals have been tested recently in patients with chronic heart failure indicating that, beyond salvage, PiCSO may induce regenerative pathways analogous to other methods using mechano-transduction to stimulate regional molecular pathways [22]. Upon sudden release of the occlusion, a rapid decline in venous pressure (average pressure measured during release phase of 13 ± 7 mmHg, creating a gradient of 57 mmHg; as seen in this study) allows for removal of excessive myocardial water (edema) and leads to washout of debris and toxic accumulations from the occlusion site and the impaired microcirculation, thus improving flow via culprit lesions towards deprived myocardium. This release phase lasts approximately 3–4 s. This novel mechanism of action of the PiCSO Impulse System may addresses the limitations of the current primary PCI approach, by clearing the microcirculation thus reducing both ischemia and reperfusion injury.
4.3. Previous research on confirming the unique mode of action of PiCSO
Various animal studies evaluating the PiCSO system have reported beneficial effects on infarct size [23], microvascular obstruction and hemorrhagic lesions [24], cardiac function [7], [8], and endothelial tissue structure and mechanics, namely increase of hemeoxygenase-1 gene expression in the center of infarction and increase of vascular endothelial growth factor (VEGF) in border zones [21].
In the Prepare RAMSES study [9], 19 patients with anterior STEMI underwent pPCI and PiCSO. Compared to matched historical controls (pPCI alone), there were no significant benefits of PiCSO on infarct size or LV function. However, PiCSO quantity delivered varied widely between patients in this study, and a significant correlation between PiCSO quantity and infarct size reduction between over time (r2 = 0.70; p = 0.008). Among patients with PiCSO quantities above the median of 494 vs. their matched controls, there were significant improvements in infarct size reduction from 5 days to 120 days (41.6% LV mass vs. 27.7% LV mass; p = 0.04).
In the OxAMI-PICSO study [10], 25 patients with anterior STEMI and an index of microcirculatory resistance (IMR) > 40 who underwent pPCI and PiCSO were compared with 50 historical controls with a similar IMR who underwent pPCI alone. PiCSO patients had a smaller median infarct size at 6 months than controls (26% vs. 33% LV mass; p = 0.006) [10]. In addition to the infract size reduction, PiCSO treated patients had on average significantly lower IMR measured after 24–48 h (24.8 vs. 45.0, p < 0.001). The responder analysis showed that 90% of PiCSO treated patients had an IMR less than 40 after 24–48 h while in the control group only 35.5% managed to get below 40.
Outside of the STEMI setting, in a series of 32 patients after successful CRT implantation 8 patients were investigated with PICSO and 24 patients constituted the controls [22]. To test immediate molecular responses, in both patient groups, coronary venous blood samples were taken at baseline and after 20 min, the time required for the PiCSO intervention. As compared with controls, significant differential expression of microRNA patterns associated with cardiac development was observed with PICSO. Co-cultured post-PICSO sera significantly increased cellular proliferation both in fibroblasts and adult cardiomyocytes sampled from a transplant recipient as compared with controls.
The results of these studies, in concert with the present report suggest that PiCSO treatment as an adjunct to pPCI may not only reduce infarct size, but also clear the microcirculation by inducing a mechanochemical feedback loop. The need of such dual pathway was postulated in a recent review on Optimized Treatment of ST-Elevation Myocardial Infarction [18]. Authors conclude that presence and extent of microvascular obstruction represents an important independent predictor of adverse LV remodeling and that simultaneously addressing infarct size and microvascular obstruction may help to translate cardioprotective strategies into improved clinical outcomes post PCI.
4.4. Safety outcomes
The 30-day incidence of MACE in the PiCSO-treated group in the current study was 10%, concordant with the outcomes in the treatment and control groups from the earlier randomized trials [11], [13], [14], [15] (5–11%). Of note, no MACE in the PiCSO group was considered to be related to the PiCSO procedure or device. The seemingly higher (7.5%, 3/40) 30 days death rate reported in the PiCSO group is probably only due to coincidence, since in this intent to treat analysis, one patient did not receive PiCSO treatment due to difficulties cannulating the coronary sinus, one patient has been readmitted 23 days post initial PCI with inferior STEMI followed by cardiogenic shock and one patient developed AF post stenting followed by cardiogenic shock. In the SWEDEHEART registry [17], approximately 2.7% STEMI patients are admitted with cardiogenic shock to the hospital, but these patients have been a priori excluded from the current study. Nevertheless, SWEDEHEART authors still report 7.8% in hospital mortality and 9.2% 30 days mortality post PCI. While none of the above-mentioned deaths has been adjudicated as related to PiCSO device or procedure we conclude that the observed death rate in this study is within the expected range for STEMI patients undergoing PCI.
5. Conclusions
In the present propensity score-matched analysis of patients with anterior STEMI, those who received PiCSO after reperfusion had a significantly smaller infarct size at 5 days than historical controls who underwent pPCI alone. PiCSO therapy was safe, without specific procedural or device-related adverse effects.
The reduction of ischemic injury by re-distributing blood flow and the plasma skimming-induced clearance of microvascular obstruction seen during the PiCSO treatment addresses an unmet need of additional reduction of infarct size and clearance of microvascular obstruction, thus positively influencing long-term cardiac function in STEMI patients. Further adequately powered randomized controlled studies are warranted to evaluate the impact of the unique mechanochemical feedback loop induced by PiCSO treatment.
6. Study limitations
The PiCSO in ACS study was a non-randomized study without a formal powered hypothesis. Due to the lack of randomization, PiCSO treated patients and parallel control patients were not well matched. For this reason, a comparative analysis using propensity score-matched historical controls was also performed. However, this non-randomized post-hoc comparison also has limitations, and residual confounding cannot be excluded.
In INFUSE-AMI all control patients that were used for propensity matching have been treated with bivalirudin According to a subgroup analysis from the HORIZONS-AMI study bivalirudin does not seem to influence the infarct size measured by CMR [25]. Furthermore a recent metanalysis showed that compared with heparin, bivalirudin was associated with a similar incidence of ischemic events following PCI for ACS [26]. A significant association of bivalirudin with decreased risk of bleeding was only found with unbalanced use of GP IIb/IIIa inhibitors in conjunction with heparin. Further bivalirudin compared with heparin monotherapy was associated with a similar incidence of all-cause death and ischemic events after PCI [27].
Since the INFUSE-AMI study has been conducted between 2009 and 2011, 29.6% of patients have been treated with bare-metal stents and 60% of the patients have received clopidogrel. While the use of clopidogrel might lead to increased infarct size when compared to prasugrel or ticagrelor [28], there is no evidence that use of bare-metal stents, despite all their limitation and increased risk or restenosis, would negatively impact the infarct size assessed at 5 days post procedure.
Funding
The study was funded by Miracor Medical SA (formerly known as Miracor Medical Systems GmbH).
CRediT authorship contribution statement
Mohaned Egred: Investigation, Writing - original draft, Writing - review & editing. Alan Bagnall: Investigation. Ioakim Spyridopoulos: Investigation. Ian F. Purcell: Investigation. Rajiv Das: Investigation. Nick Palmer: Investigation. Ever D. Grech: Investigation. Ajay Jain: Investigation. Gregg W. Stone: Methodology, Writing - original draft. Robin Nijveldt: Investigation. Thomas McAndrew: Formal analysis, Writing - original draft. Azfar Zaman: Investigation, Supervision, Writing - original draft.
Declaration of Competing Interest
The authors declare the following conflicts of interests:
-
•
Mohaned Egred reports no relationships that could be construed as a conflict of interest
-
•
Alan Bagnall reports no relationships that could be construed as a conflict of interest
-
•
Ioakim Spyridopoulos reports no relationships that could be construed as a conflict of interest
-
•
Ian F. Purcell reports no relationships that could be construed as a conflict of interest
-
•
Rajiv Das reports no relationships that could be construed as a conflict of interest
-
•
Nick Palmer reports no relationships that could be construed as a conflict of interest
-
•
Ever D. Grech reports no relationships that could be construed as a conflict of interest
-
•
Ajay Jain reports no relationships that could be construed as a conflict of interest
-
•
Gregg W. Stone has received consulting fees from Miracor.
-
•
Robin Nijveldt reports no relationships that could be construed as a conflict of interest
-
•
Thomas McAndrew reports no relationships that could be construed as a conflict of interest
-
•
Azfar Zaman has received consulting fees from Miracor.
Acknowledgements
Medical writing assistance was provided by Jenny Lloyd (MedLink Healthcare Communications Limited) as well as Jozef Tanczos (Miracor Medical SA).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100526.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Neumann F.J., Sousa-Uva M., Ahlsson A., Alfonso F., Banning A.P., Benedetto U. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019;40(2):87–165. [Google Scholar]
- 2.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 3.Puymirat E., Simon T., Steg P.G., Schiele F., Gueret P., Blanchard D. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA. 2012;308(10):998–1006. doi: 10.1001/2012.jama.11348. [DOI] [PubMed] [Google Scholar]
- 4.Doost Hosseiny A., Moloi S., Chandrasekhar J., Farshid A. Mortality pattern and cause of death in a long-term follow-up of patients with STEMI treated with primary PCI. Open Heart. 2016;3(1) doi: 10.1136/openhrt-2016-000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone G.W., Selker H.P., Thiele H., Patel M.R., Udelson J.E., Ohman E.M. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J. Am. Coll. Cardiol. 2016;67(14):1674–1683. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 6.Van de Hoef T.P., Nolte F., Delewi R., Henriques J.P., Spaan J.A., Tijssen J.G. Intracoronary hemodynamic effects of pressure-controlled intermittent coronary sinus occlusion (PICSO): results from the First-In-Man Prepare PICSO Study. J. Interv. Cardiol. 2012;25(6):549–556. doi: 10.1111/j.1540-8183.2012.00768.x. [DOI] [PubMed] [Google Scholar]
- 7.Lazar H.L., Rajaii A., Roberts A.J. Reversal of reperfusion injury after ischemic arrest with pressure-controlled intermittent coronary sinus occlusion. J. Thorac. Cardiovasc. Surg. 1988;95(4):637–642. [PubMed] [Google Scholar]
- 8.Mohl W., Punzengruber C., Moser M., Kenner T., Heimisch W., Haendchen R. Effects of pressure-controlled intermittent coronary sinus occlusion on regional ischemic myocardial function. J. Am. Coll. Cardiol. 1985;5(4):939–947. doi: 10.1016/s0735-1097(85)80437-x. [DOI] [PubMed] [Google Scholar]
- 9.van de Hoef T.P., Nijveldt R., van der Ent M., Neunteufl T., Meuwissen M., Khattab A. Pressure-controlled intermittent coronary sinus occlusion (PICSO) in acute ST-segment elevation myocardial infarction: results of the Prepare RAMSES safety and feasibility study. EuroIntervention. 2015;11(1):37–44. doi: 10.4244/EIJY15M03_10. [DOI] [PubMed] [Google Scholar]
- 10.De Maria G.L., Alkhalil M., Borlotti A., Wolfrum M., Gaughran L., Dall'Armellina E. Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: the Oxford Acute Myocardial Infarction - Pressure-controlled Intermittent Coronary Sinus Occlusion study (OxAMI-PICSO study) EuroIntervention. 2018;14(3):e352–e359. doi: 10.4244/EIJ-D-18-00378. [DOI] [PubMed] [Google Scholar]
- 11.Stone G.W., Maehara A., Witzenbichler B., Godlewski J., Parise H., Dambrink J.H. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. 2012;307(17):1817–1826. doi: 10.1001/jama.2012.421. [DOI] [PubMed] [Google Scholar]
- 12.Brener S.J., Maehara A., Dizon J.M., Fahy M., Witzenbichler B., Parise H. Relationship between myocardial reperfusion, infarct size, and mortality: the INFUSE-AMI (Intracoronary Abciximab and Aspiration Thrombectomy in Patients With Large Anterior Myocardial Infarction) trial. JACC Cardiovasc. Interv. 2013;6(7):718–724. doi: 10.1016/j.jcin.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill W.W., Martin J.L., Dixon S.R., Bartorelli A.L., Trabattoni D., Oemrawsingh P.V. Acute Myocardial Infarction with Hyperoxemic Therapy (AMIHOT): a prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2007;50(5):397–405. doi: 10.1016/j.jacc.2007.01.099. [DOI] [PubMed] [Google Scholar]
- 14.Patel M.R., Smalling R.W., Thiele H., Barnhart H.X., Zhou Y., Chandra P. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011;306(12):1329–1337. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 15.Stone G.W., Martin J.L., de Boer M.J., Margheri M., Bramucci E., Blankenship J.C. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ Cardiovasc Interv. 2009;2(5):366–375. doi: 10.1161/CIRCINTERVENTIONS.108.840066. [DOI] [PubMed] [Google Scholar]
- 16.Schaaf M.J., Mewton N., Rioufol G., Angoulvant D., Cayla G., Delarche N. Pre-PCI angiographic TIMI flow in the culprit coronary artery influences infarct size and microvascular obstruction in STEMI patients. J. Cardiol. 2016;67(3):248–253. doi: 10.1016/j.jjcc.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Szummer K., Wallentin L., Lindhagen L., Alfredsson J., Erlinge D., Held C. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur. Heart J. 2017;38(41):3056–3065. doi: 10.1093/eurheartj/ehx515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niccoli G., Montone R.A., Ibanez B., Thiele H., Crea F., Heusch G. Optimized Treatment of ST-Elevation Myocardial Infarction. Circ. Res. 2019;125(2):245–258. doi: 10.1161/CIRCRESAHA.119.315344. [DOI] [PubMed] [Google Scholar]
- 19.Qiao J., Pan L., Zhang B., Wang J., Zhao Y., Yang R. Deferred Versus Immediate Stenting in Patients With ST-Segment Elevation Myocardial Infarction: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017;6(3) doi: 10.1161/JAHA.116.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohl W., Milasinovic D., Faxon D.P. Amending a dogma. EuroIntervention. 2018;14(12):e1258–e1261. doi: 10.4244/EIJV14I12A227. [DOI] [PubMed] [Google Scholar]
- 21.Mohl W., Gangl C., Jusic A., Aschacher T., De Jonge M., Rattay F. PICSO: from myocardial salvage to tissue regeneration. Cardiovasc. Revasc. Med. 2015;16(1):36–46. doi: 10.1016/j.carrev.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Mohl W., Spitzer E., Mader R.M., Wagh V., Nguemo F., Milasinovic D. Acute molecular effects of pressure-controlled intermittent coronary sinus occlusion in patients with advanced heart failure. ESC Heart Fail. 2018 doi: 10.1002/ehf2.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohl W., Glogar D.H., Mayr H., Losert U., Sochor H., Pachinger O. Reduction of infarct size induced by pressure-controlled intermittent coronary sinus occlusion. Am. J. Cardiol. 1984;53(7):923–928. doi: 10.1016/0002-9149(84)90526-5. [DOI] [PubMed] [Google Scholar]
- 24.Khattab A.A., Stieger S., Kamat P.J., Vandenberghe S., Bongoni A., Stone G.W. Effect of pressure-controlled intermittent coronary sinus occlusion (PICSO) on myocardial ischaemia and reperfusion in a closed-chest porcine model. EuroIntervention. 2013;9(3):398–406. doi: 10.4244/EIJV9I3A63. [DOI] [PubMed] [Google Scholar]
- 25.Wöhrle J., Merkle N., Kunze M., Cristea E., Mehran R., Rottbauer W. Effect of bivalirudin compared with unfractionated heparin plus abciximab on infarct size and myocardial recovery after primary percutaneous coronary intervention: the horizons-AMI CMRI substudy. Catheter Cardiovasc. Interv. 2012;79(7):1083–1089. doi: 10.1002/ccd.23179. [DOI] [PubMed] [Google Scholar]
- 26.Nuhrenberg T.G., Hochholzer W., Mashayekhi K., Ferenc M., Neumann F.J. Efficacy and safety of bivalirudin for percutaneous coronary intervention in acute coronary syndromes: a meta-analysis of randomized-controlled trials. Clin. Res. Cardiol.: Off. J. German Cardiac Soc. 2018;107(9):807–815. doi: 10.1007/s00392-018-1251-1. [DOI] [PubMed] [Google Scholar]
- 27.Erlinge D., Omerovic E., Frobert O., Linder R., Danielewicz M., Hamid M. Bivalirudin versus Heparin Monotherapy in Myocardial Infarction. New Engl. J. Med. 2017;377(12):1132–1142. doi: 10.1056/NEJMoa1706443. [DOI] [PubMed] [Google Scholar]
- 28.Khan J.N., Greenwood J.P., Nazir S.A., Lai F.Y., Dalby M., Curzen N. Infarct Size Following Treatment With Second- Versus Third-Generation P2Y12 Antagonists in Patients With Multivessel Coronary Disease at ST-Segment Elevation Myocardial Infarction in the CvLPRIT Study. J. Am. Heart Assoc. 2016;5(6) doi: 10.1161/JAHA.116.003403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.