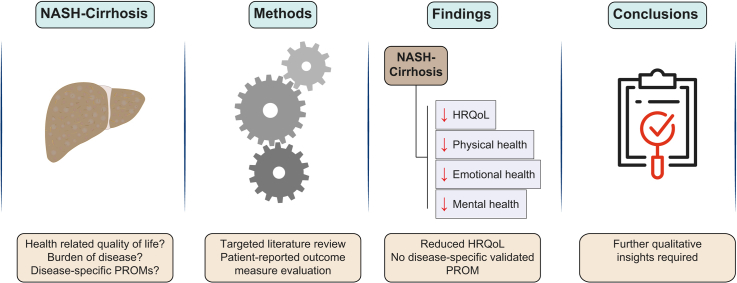
Abstract
Background & Aims
Non-alcoholic steatohepatitis (NASH) is known to have a negative impact on patients' health-related quality of life (HRQoL), even before progression to cirrhosis has occurred. The burden of NASH-related cirrhosis from the patient perspective remains poorly understood. Herein, we aimed to identify the burden of disease and HRQoL impairment among patients with NASH-related compensated cirrhosis.
Methods
This targeted literature review sought first to identify the humanistic burden of disease from the perspective of patients with diagnosed NASH-cirrhosis and, secondly, to identify generic or disease-specific patient-reported outcome measures (PROMs) used to assess the impact of NASH-cirrhosis. Searches were conducted in bibliographical databases, grey or unpublished literature, liver disease websites, support group websites and online blogs. A quality assessment of specific PROMs was conducted.
Results
Patients with NASH-cirrhosis are reported to suffer from lower HRQoL than patients with non-cirrhotic NASH and the general population with respect to physical health/functioning, emotional health and worry, and mental health. Thirteen PROMs were identified, of which 4 were liver-disease specific: CLDQ, CLDQ-NAFLD, LDQoL and LDSI. The most commonly used measures do not comply with current industry or regulatory standards for PROMs and/or are not validated for use in a cirrhotic NASH population.
Conclusions
Patients with NASH-cirrhosis have lower HRQoL and poorer physical health than patients with non-cirrhotic NASH. However, the literature lacked detail of the everyday impact on patients' lives. Currently, a number of PROMs are available to measure the impact of the disease in patients with chronic liver conditions. The lack of studies that include qualitative insights in this population mandates further exploration and research.
Lay summary
It is not well understood how having non-alcoholic fatty liver disease (NAFLD)-related cirrhosis affects a person's everyday wellbeing and quality of life. Some research has been done with patients who have early stages of liver disease but not people with cirrhosis. We found that patients with NAFLD-related cirrhosis tended to have poorer health than patients without cirrhosis. But there was not very much information from patients themselves and there were no tools or questionnaires just for this group of patients.
Keywords: Non-alcoholic steatohepatitis, NAFLD, Cirrhosis, liver, health-related quality of life, patient-reported outcome measures
Abbreviations: CLDQ, chronic liver disease questionnaire; COSMIN, The COnsensus-based Standards for the selection of health Measurement INstruments; EMA, European Medicines Agency; FDA, United States Food and Drug Administration; FIS, fatigue impact scale; HRQoL, health-related quality of life; LDQoL, liver disease quality of life questionnaire; LDSI, liver disease symptom index; MS, multiple sclerosis; NAFL, non-alcoholic fatty liver; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; RI, researcher interpretation; PHAQ, patient-reported outcome measurement information system health assessment questionnaire; PRO, patient-reported outcome; PROM, patient-reported outcome measure; QoL, quality of life; SF-36, short form health profile 36
Graphical abstract
Highlights
-
•
The burden of cirrhotic NASH from the patient perspective remains poorly understood.
-
•
Patients with NASH-related compensated cirrhosis are reported to suffer from lower HRQoL.
-
•
Most commonly used PROMs are not validated for use in a cirrhotic NASH population.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver condition worldwide, affecting approximately a quarter of the adult population.1 NAFLD represents a spectrum of liver disease, ranging from simple steatosis (non-alcoholic fatty liver, NAFL) to the inflammatory form, non-alcoholic steatohepatitis (NASH). NAFLD is strongly associated with obesity, type 2 diabetes mellitus and other features of the metabolic syndrome but, as its name suggests, occurs in the absence of excessive alcohol consumption.2 Although only a subset of patients progress to advanced liver disease,2,3 the transition from NAFL to NASH promotes hepatic fibrosis (scarring) that, unchecked, may lead to cirrhosis and consequently increased morbidity and mortality.2,4
At present, there are no regulatory approved pharmacological treatments for NAFLD and so management focusses on lifestyle modification to effect weight loss and interventions that reduce cardiovascular risk.5,6 Considering this, there is substantial interest in developing pharmacological therapies targeting liver disease in patients with NASH. Much work is underway to define clinically meaningful endpoints for clinical trials in NASH that directly measure how patients feel, function and survive.7,8 The severity of NAFLD can be defined based upon the extent of hepatic fibrosis. Hepatic Fibrosis diagnosed on liver biopsy is measured using a semi-quantitative histological scoring system where fibrosis severity is expressed as F0 (normal) to F4 (cirrhosis).9 Even once F4 is reached, compensated cirrhosis represents a relatively asymptomatic histological condition characterised by diffuse fibrosis and nodule formation but with relatively preserved hepatic function that is physiologically sufficient under non-stressed conditions. In contrast, later decompensated cirrhosis describes a more severe phase of disease during which the function of the liver is severely impaired and patients experience overt complications that may include: jaundice, ascites, hepatic encephalopathy and variceal haemorrhage.10 Whilst primary outcome measures of efficacy in clinical trials are largely focussed on histological changes that serve as surrogates for hard clinical endpoints,11,12 these measures fail to capture the impact of the disease from a patient perspective. There is an increased focus on the patient's subjective perception of the impact of disease and its treatment on his or her daily life, including emotional, social and physical functioning and wellbeing—this is referred to as health-related quality of life (HRQoL).13,14
Patient-reported outcomes (PROs) is an umbrella term describing the measurement of any aspect of a patient's health status that comes directly from the patient without interpretation from anyone else15; this can range from symptom frequency, duration, or severity to activities of daily living or more complex issues of HRQoL. A PRO measure (PROM) has the potential to provide a method with which to assess the impact and burden of NASH from a patient's point of view. Moreover, there is an increasing need to highlight the value and utility of such a PRO among practitioners and specialists as there is uncertainty in how best to assess liver fibrosis.16 In addition, regulatory bodies such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have an expectation that regulatory submissions for new medicines will include information on the impact of interventions from the patient perspective.17 Whilst both the FDA and EMA have released documents to improve the quality of PROMs used in support of product labelling claims, the FDA has established guidance to describe what evidence is required to support the development of PROMs intended for use in label claims.15 For both agencies, the provision of PRO data generated via well-developed, psychometrically robust, standardised PROMs is now an essential component of most regulatory submissions.
Previously, a novel condition-specific PROM was developed by a NASH-PRO Task Force. The “NASH-CHECK” PROM was developed and tested in non-cirrhotic patients with NASH (fibrosis stages F0-F3).18,19 However, as alluded to, the symptom burden of compensated cirrhosis due to NASH (fibrosis stage F4) from the patient perspective is not well understood. Building on the work conducted whilst developing NASH-CHECK, and to support future development of this PROM in NASH-cirrhosis, a literature review was undertaken. The objectives of this review were twofold: a) to identify the humanistic burden of disease and impairment of HRQoL in patients with diagnosed NASH and compensated cirrhosis expressed histologically as fibrosis stage F4 based on NASH Clinical Research Network categorisation9 from the perspective of patients, clinicians and carers; b) to identify which generic and disease-specific PROMs have been used to assess the impact of disease among this target population and critically appraise the validity of these PROMs in accordance with FDA guidance for PROMs intended for use in label claims.
Patients and methods
A review protocol was designed and developed by the authors and in consultation with an information specialist (FB). The protocol included inclusion and exclusion criteria; search strategy; study selection and data extraction; data synthesis and presentation of results and, dissemination plans.
Search strategy and eligibility criteria
A search strategy was designed, incorporating information from searches conducted by the NASH-PRO Task Force as well as published guidance.20 The following elements were addressed in separate searches: (i) the humanistic burden of NASH-cirrhosis, and (ii) PROMs used to assess NASH-cirrhosis. The following were considered:
-
•Population (for both searches):
-
○Adult patients diagnosed with NASH-cirrhosis. The single term “cirrhosis” was explored, but it retrieved a vast amount of literature, which was not related to NAFLD as the causative aetiology so “cirrhosis” was used as a search term only in connection with NASH or NAFLD.
-
○
-
•Outcomes (for the first search):
-
○Patient-reported HRQoL
-
○Patient/carer/clinician-reported symptoms
-
○
-
•Outcomes (for the second search):
-
○Liver-specific PROMs
-
○NASH-specific PROMs
-
○PROMs – generic instruments
-
○
The search strategy was designed in MEDLINE using MeSH headings and keywords, and the thesaurus headings and syntax were translated appropriately to other databases. The following databases were searched from their inception dates to 28th March 2018 for searches addressing objective (a), and 15th June 2018 for searches addressing objective (b).
-
•
MEDLINE (OVID) 1946 to March 2018 week 3 (search 1)/June week 2 (search 2)
-
•
MEDLINE (OVID) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily
-
•
Embase (OVID) 1974 to 2018 Week 13 (search 1)/Week 24 (search 2)
-
•
PsycINFO 1806 to March 2018 Week 3 2018 (search 1)/June week 2 (search 2)
Both searches were restricted to papers published in English. All types of studies including case reports were included. Example search strategies are available in the supplementary information—appendix 1. The reference lists of the final included studies were also searched for additional relevant papers. Search results were downloaded from the databases into Endnote and de-duplicated.
A search of the grey or unpublished literature and resources was carried out. The search terms: ‘adults with NASH F4’ or ‘NASH cirrhosis’ or ‘NAFLD cirrhosis’ were entered into Liver disease websites, liver patient support group websites and online blogs search functions. Relevant extracts from the grey literature sources were copied and saved in a Word document.
Titles and abstracts were screened independently and agreed by 2 members of the study team). Endnote software21 was used for managing the data and Rayyan software22 for the screening process. Irrelevant studies were excluded and full texts were independently screened by 2 reviewers (LM and MB) based on the inclusion criteria, and study search results extracted and recorded. In cases of disagreement, a third reviewer (LV) was consulted to arbitrate. For included studies data extraction forms developed for a similar review for F1-3 disease were used.
Data synthesis
Objective a: humanistic burden of NASH-cirrhosis
In order to provide an overall picture of current knowledge from a heterogeneous sample of studies; a narrative synthesis23 was conducted. The HRQoL impacts—where not definitively reported—were interpreted by and discussed with the research team.
Objective b: PROMs used in NASH-cirrhosis population
The PROMs identified as being used in this patient population were critically assessed to determine their validity for the target population in relation to PROM development guidance (availability and quality of evidence related to qualitative development and psychometric validation). Specifically, the review considered the FDA's evidence requirements for reviewing instruments intended to support a label claim in the USA. A focussed quality assessment was conducted for the PROMs that were most likely to meet the FDA requirements.
Quality assessment
A quality assessment of PROMs identified from the literature was conducted using US FDA guidance15 and COSMIN guidelines24 for reviews of PRO measures. The FDA guideline sets out specific requirements for the development of PROMs and describes in detail how the FDA evaluates existing and newly developed tools that are used in clinical trials or support drug approvals for product labelling. The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) initiative's checklist was presented in 2010 as a comprehensive methodological guideline for systematic review of PROMs.23 The assessment reviewed the PROMs, with particular reference to the quality of the available evidence to support the use of the instruments for inclusion in a label claim. The factors considered — in line with FDA recommendations16 and COSMIN guidelines23 — as properties relevant for the assessment of PROMs are presented in Table S1.
Results
Study selection
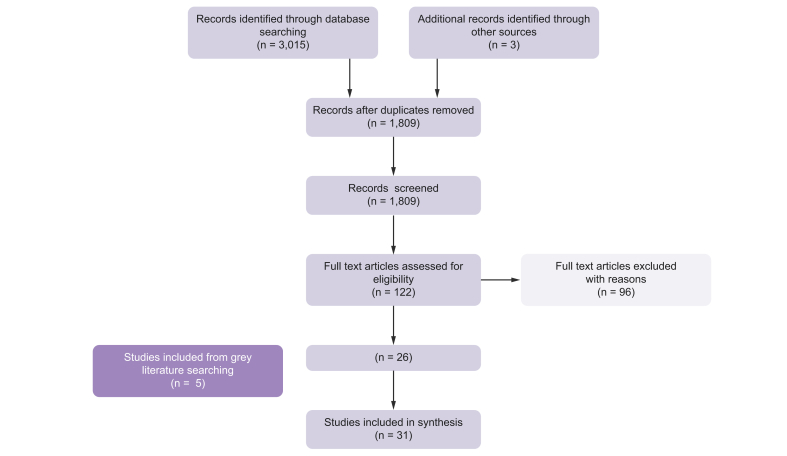
A total of 26 data sources and 5 eligible reports from liver disease websites and patient blogs reporting impact of NASH-cirrhosis and HRQoL impairment were included in this review. The PRISMA diagram in Fig. 1 described the results of the searching and screening process for objective (a).
Fig. 1.
PRISMA flow diagram: for objective (a) — humanistic burden of NASH-cirrhosis.
3,015 records were identified from databases. After removal of duplicates, 1,809 records were screened. Eligibility assessment of texts, plus 5 grey literature studies, resulted in 31 studies being included in the humanistic burden of NASH-cirrhosis synthesis.
Many studies did not distinguish NASH-cirrhosis from other aetiologies of cirrhosis, for example, alcohol-related liver disease. Some studies reported finding no differences in the results between liver diseases of differing aetiologies.14,25,26
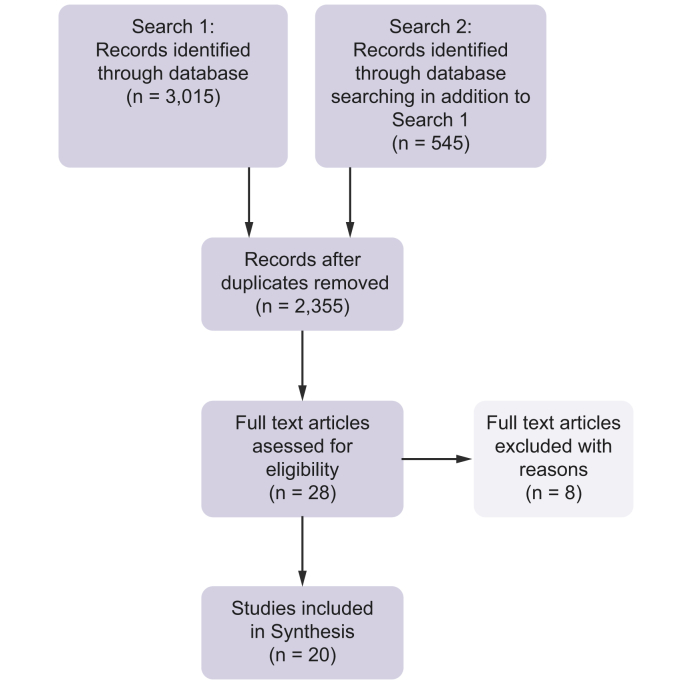
For PROMs used in NASH-cirrhosis patients, 20 relevant data sources were included. Full details of results and screening are shown in Fig. 2.
Fig. 2.
PRISMA flow diagram: for Objective (b) — PROMs F4.
A second database search identified a further 545 studies in addition to search one (objective (a)). After removal of duplicates, 2,355 records were screened. Eligibility assessment of texts resulted in 20 studies being included in the PROMs synthesis.
Study characteristics
Humanistic burden of NASH-cirrhosis
Table S2 reports the characteristics of the identified literature that met the inclusion criteria for this objective. The majority of studies (data sources) were conducted in the USA (n = 18, 58%), no more than 3 studies were conducted in any other country and in total; studies were conducted in 8 different mainly Western countries. Nine studies were specifically aimed at determining HRQoL14,25,[27], [28], [29], [30], [31], [32], [33]; 13 studies were focussed on specific related symptoms such as varices, pain or overall physical/mental symptoms[34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]; 2 were case studies each describing the experience of a single patient45,46; 3 studies were concerned with PROM development and validation.[47], [48], [49] Within the identified studies, 1 was an opinion piece50; 5 were conference abstracts32,33,[42], [43], [44] and 5 were patient blogs/stories.[51], [52], [53], [54], [55]
The majority of the studies (n = 19) specifically identified the inclusion of NASH-cirrhosis patients,14,[28], [29], [30],33,[35], [36], [37], [38], [39], [40], [41],43,45,46,49,51,55,56 4 studies were assumed to refer to NASH-cirrhosis—these studies grouped ‘other’ liver disease aetiologies as 1 group32,34,43,47; the final 7 studies25,42,48,50,[52], [53], [54] only referred to their target group as ‘cirrhosis patients’.
Comorbidities, complications and symptoms
The main comorbidities (where reported in studies, but not included in Table S2) of patients with cirrhosis were type 2 diabetes, obesity, and cardiovascular disease and hypertension. Variceal bleeding and ascites were each reported in 3 studies. The key symptoms (where reported by included reports) were stated to be abdominal symptoms; abdominal pain; lack of energy; tiredness, pain; and sleep symptoms.
HRQoL concepts
Where explicitly reported, the HRQoL concepts are described as reported by the study. Where HRQoL concepts were not reported, or not directly referred to as HRQoL, such as in the grey literature, the possible concepts were discussed within the team and described as ‘researcher interpretation (RI)’. The most commonly reported areas of HRQoL impact related to the following concepts; related to physical health/functioning; emotional health/worry; pain; mental health and general health.
NASH-cirrhosis in relation to cirrhosis of differing aetiology
A finding of the review was that some studies reported symptoms correlating with the severity but not aetiology of liver disease. Therefore, they did not specifically refer to NASH-cirrhosis. An example of this was for gastrointestinal symptoms.28 This did not apply to all symptoms. For example, for the relationship between pain and the cause of disease, 1 study specifically excluded patients with other known causes of pain. This study reported that patients with NASH or hepatitis C-associated cirrhosis were more likely to suffer pain than patients with cirrhosis linked to alcohol-related liver disease.39 In studies focusing on HRQoL, it was found that NASH-cirrhosis patients had worse physical health than other NAFLD groups.27 Patients with NASH and cirrhosis reported “more impairment” on physical and mental status scales compared to non-cirrhotic patients.14 HRQoL has been reported to be significantly lower in patients with cirrhosis compared to a ‘healthy population’ and this was significantly worse in patients with increased clinical severity of the disease measured by Child-Pugh class.25 Excessive daytime sleepiness was found to be a burden for 40% of cirrhotic patients in a study by Sobhonslidsuk et al. (2011).42
Compensated and decompensated patients
In the studies that considered patients with compensated/decompensated cirrhosis, using the EQ-5D-3L, it was reported that patients with decompensated cirrhosis had a higher risk of having problems with mobility, self-care and usual activities compared to ‘healthy subjects’.32 Edula et al. (2014), in their case study of a patient with NASH-cirrhosis, asserted that bleeding from gastro-oesophageal varices ‘can often present as a first decompensating event’57 for patients with previously identified compensated disease. Patients with NASH-cirrhosis were also reported to be at risk of bleeding from varices (30/47 patients) in the Japanese study by Nakamura et al.58
Grey literature and blogs
Findings from the grey literature blogs highlighted perspectives both from patients with NASH-cirrhosis (n = 3) and their spouse/family members (n = 2). With the exception of 1 report, where a patient had not suffered any symptoms before diagnosis,54 symptoms were often described as progressing quickly or in multitude. Reported symptoms included loss of appetite, weight loss, fatigue, pain, itchy skin and confusion. Two of the patients52,53 were forced to give up work. Not being able to perform routine daily tasks impacted considerably on the patients' everyday quality of life.[51], [52], [53] The overall impressions garnered from the blogs were of shock, disbelief and of a life being put on hold. It was reported that, 1 patient had died,51 1 had received a living donor transplant,53 1 was on the waiting list for a transplant,52 and the remaining 254,55 were ‘living’ with NASH-cirrhosis. Also apparent was the lack of knowledge surrounding NASH liver disease; for their families this appeared to be a key issue.
In summary, patients with cirrhosis are reported to suffer from a lower HRQoL than the ‘healthy’ population and non-cirrhotic patients with NAFLD, particularly with respect to physical health/functioning, emotional health and worry, and mental health.
PROMs identified
Thirteen PROMs were identified in the literature as used to assess burden of disease in patients with NASH-cirrhosis: short form health profile 36 (SF-36), Nottingham health profile, chronic liver disease questionnaire (CLDQ), liver disease quality of life questionnaire (LDQoL), fatigue impact scale (FIS), Epworth sleepiness scale, patient-reported outcome measurement information system health assessment questionnaire (PHAQ), chronic liver disease questionnaire-NAFLD (CLDQ_NAFLD), liver disease symptom index (LDSI-2.0), World Health Organisation quality of life, self-rating anxiety scale, self-rating depression scale and EQ-5D-3L. Four of these were liver disease specific: CLDQ,47 CLDQ-NAFLD,59 LDQoL60 and LDSI 2.0.61 No PROM specifically developed for NASH and cirrhosis was identified. PROMs were selected for the quality assessment that were most likely to meet FDA requirements. A key requirement of the FDA guidance is that instruments should be developed with input from the target population. As the generic PROMs were not developed based on input from patients with liver disease these were not included in the quality assessment. A critical appraisal is reported for the following selected PROMs: CLDQ-NAFLD, LDSI 2.0, LDQoL as used in studies across the spectrum of NASH; and 1 symptom-specific PROM, FIS.62 The FIS was included as it assesses fatigue, which was identified during the review as being a prominent symptom in this patient population and its psychometric properties have been evaluated extensively.[63], [64], [65]
Quality assessment of PROMs
CLDQ-NAFLD
The CLDQ47 is a liver disease-specific PROM, developed in 1999, comprising 29 items across 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms and worry. It was developed in a group of patients with an established diagnosis of chronic liver disease and various aetiologies included hepatocellular liver disease, chronic hepatitis C, chronic hepatitis B, primary biliary cholangitis, primary sclerosing cholangitis, viral hepatitis B and C-related cirrhosis, alcohol-related cirrhosis and other types of liver disease, with no specific mentioning of NAFLD or NASH.45 The initial development took place before the publication of FDA guidance for PROMs and was completed by experts with some subsequent patient review, rather than through traditional concept elicitation processes. A list of health-related problems likely to be relevant to patients with chronic liver disease was developed by hepatologists and subsequently presented to 60 patients with chronic liver disease, which resulted in the final version of the questionnaire.
The CLDQ-NAFLD,59 developed in 2017, is an extended version of the CLDQ with an additional 7 questions that focus the PRO more towards NAFLD. The development work for these 7 additional questions involved presenting 75 items to a sample of 25 patients with NAFLD. Of these 25, 20 had NASH and 5 were diagnosed with histological cirrhosis. Further validation involved a psychometric evaluation in a sample of 104 patients with biopsy or imaging-proven diagnosis of NAFLD. Of these 104 patients, 50% were diagnosed with histological NASH and 15% with compensated cirrhosis. The 7 additional questions were added to existing CLDQ questions following an item reduction step. Domain scores and an overall score are presented on a 1–7 Likert scale with higher values representing better quality of life (QoL).
The development process followed for the CLDQ-NAFLD was not clearly described in the study report and so it does not meet the most recent standards for PROM development. In particular, the concept elicitation stage involving patients is not described and the rationale for the selection of 75 original items is not explained. Construct validity was however reported to be adequate; worse scores being correlated with increased disease severity in all scales. Correlations were reported between the activity, emotional, fatigue and systemic symptoms domains of CLDQ-NAFLD and similar domains of SF-36, providing evidence of convergent validity of the measure. In patients with NAFLD, the CLDQ-NAFLD was able to discriminate between those with obesity, type 2 diabetes and metabolic syndrome, but no evidence was presented of a difference between cirrhotic and non-cirrhotic patients.59 The Cronbach's alpha coefficient was reported to be between 0.74 and 0.9 suggesting good to excellent internal consistency of the domains. Test-retest reliability data came from a small subgroup of patients (n = 27) and so may lack sufficient statistical power to detect differences. Furthermore, no formal evidence of content validity for a NASH population has been published to date.
LDQOL 1.0
The LDQoL 1.060 consists of a total of 111 questions: 36 generic questions taken from the SF-3666 and 75 questions grouped into 12 liver disease-specific multi-item scales. The recall period is 4 weeks and all questions are scored on a 0–100 visual analogue scale with higher scores representing better QoL.
The tool was developed in a group of 15 patients with chronic liver disease (aetiologies included hepatitis C, hepatitis B, alcohol-related liver disease, autoimmune hepatitis, primary sclerosing cholangitis, cryptogenic cirrhosis, and biliary atresia) awaiting liver transplantation and psychometric properties established in a cohort of 221 individuals with advanced chronic liver disease (hepatitis C, hepatitis B, alcoholic liver disease, primary biliary cholangitis, primary sclerosing cholangitis, cryptogenic cirrhosis etc.).60 Content validity was tested by organising focus groups of patients with chronic liver disease, consulting hepatologists and gastroenterologists, as well as reviewing the literature on HRQoL in general.60
The authors reported high internal consistency coefficients (Cronbach's α) between 0.62 and 0.95, suggesting potential item redundancy for some domains, with 19 scales having >0.70, except for the quality of social interaction scale. Multi-trait scaling analysis is reported to provide strong support for item discrimination across scales. Correlations among SF-36 items and liver-specific items ranged from 0.14 to 0.78. Authors report 17 out of 20 scales statistically associated with worse scores, the scales that did not reach this level of significance included hopelessness, loneliness and the quality of social interaction. Worse scores were associated with higher severity of self-reported symptoms and higher number of disability days in the previous month.
There was evidence of associations between physical role limitation, pain, fatigue scales and worse self-reported liver disease symptoms. This suggests that either the tool is able to capture liver disease-specific QoL information that a generic PRO measure would not be able to obtain in the CLD population or it could also show major item redundancy. The main limitation of the tool is the length of the scale as a 111-item questionnaire may be impractical to employ. Additionally, the 4-week recall period reduces the suitability of the instrument for use in product label claims. Furthermore, given that this instrument was originally developed in patients with advanced chronic liver disease, it could be argued that the main value of this instrument may be in a decompensated cirrhotic population.
LDSI 2.0
The LDSI 2.061 is a liver disease-specific questionnaire consisting of 24 items with a recall period of 1 week. The tool was developed in 2004 before the publication of the FDA guidance and psychometrically tested in a large cohort of 1,175 patients with chronic liver disease. The tool is a modified version of the original LDSI67 measure with “jaundice”, “depression” and “worry about family situation” added to the list of items after consultations with a liver patients' organisation.
Nine items measure severity of itch, joint pain, pain in the right upper abdomen, sleepiness during the day, worry about family situation, decreased appetite, depression, fear of complications and jaundice experienced within the previous week. Nine other items measure the impact of these symptoms on person's daily activities. Six additional items concern memory problems, change of personality, financial affairs, change in use of time, reduced sexual activity and reduced sexual interest. All items are individually scored on a 0–5-point scale ranging from “not at all” to “to a high extent”.
The assessment of content validity is not described in the original paper. Authors report adequate feasibility, test-retest reliability and construct validity in a population of patients with chronic liver disease.61 High correlations were detected between the “Joint pain”, “Sleepiness during the day” and “Depression” symptoms severity and corresponding hindrance items. However, authors demonstrated larger impact of hindrance items on overall QoL score than the symptom severity items, which leads to the conclusion that different items of the instrument measure different aspects of QoL.
Internal consistency is reported as high with Cronbach's α >0.79. Spearman correlations suggested strong convergent relations between symptom severity items and their accompanying symptom hindrance items (0.52–0.80).61
Fatigue impact scale
The FIS62 was developed in 1994 in a group of patients with multiple sclerosis (MS). Rather than measuring the level of fatigue itself, the instrument reflects a patient's perception of the functional limitation due to fatigue experienced within the previous month. The tool is composed of 40 items grouped into 3 scales: cognitive, physical and psychosocial functioning. All items are scored on a 0-4 scale ranging from “no problem” to “extreme problem”.
Internal consistency is reported to be high for overall scores and the 3 subscales (Cronbach's α ≥0.87), as well as the test-retest reliability (0.72–0.83).62 The tool was able to discriminate between the 3 patient groups (patients with MS; with chronic fatigue and mild hypertension groups) based on both overall score and specific FIS items. Further validity of the tool has been established in patients with chronic fatigue syndrome, primary biliary sclerosis, chronic hepatitis C and MS,68 but no validation in NASH populations has ever been performed. Table 1 presents a summary comparison of the PRO measurement tools included in the assessment using both FDA and COSMIN criteria.
Table 1.
Comparison of selected PRO measurement properties to FDA and COSMIN recommended standards.
| PRO | Content/item source | Items/domains | Recall period | Construct validity and other validity | Reliability | Instrument modification | Main limitation |
|---|---|---|---|---|---|---|---|
| CLDQ-NAFLD | Items were developed using a variety of sources (HRQoL tools, focus groups, patient interviews — 25 patients with NAFLD, among whom 20% had histological cirrhosis. Validated in 104 patients with NAFLD, among whom 15% had compensated cirrhosis |
6 domains (36 items): abdominal symptoms, activity, emotional, fatigue, systemic symptoms, worry | 2 weeks | Construct validity: domains highly correlated with SF-36: activity, emotional, fatigue and systemic symptoms psychometrically evaluated in a group of patients with NAFLD (n = 104). Known-groups validity: worse scores correlated with increased disease severity in all scales; unable to discriminate between cirrhotic and non-cirrhotic patients. Not validated in a cohort of patients with advanced liver cirrhosis; not validated in NASH |
Tested and retested in a small subgroup of patients with NAFLD (n = 27; 5-19 weeks apart) — non-statistically significant. Internal consistency: Cronbach's α 0.74-0.90 |
Modified version of CLDQ | Lack of evidence of content validity within a NASH population |
| LDQoL 1.0 | Developed after conducting focus group interviews with 15 patients awaiting liver transplantation and literature search on HRQoL in liver disease Input from patient focus groups combined with views of gastroenterologists and hepatologists and literature search of HRQoL |
SF-36 + 12 disease-targeted scales (75 items): symptoms of liver disease, effects of liver disease, concentration, memory, quality of social interaction, health distress, sleep problems, loneliness, hopelessness, stigma of Liver disease, sexual functioning, sexual problems | 4 weeks | Psychometrically evaluated in a group of end-stage liver disease patients. Known-groups validity: worse scores correlated with worse Child-Pugh class, worse self-rated liver disease severity and higher number of disability days |
Internal consistency: Cronbach's α 0.62–0.95 | n.a. | The tool is impractical to use given its length; uses maximum recall period recommended for QoL tools |
| LDSI 2.0 | Items were developed in a large group of patients with liver diseases (patient interviews) | 24 items: 9 items measure severity of symptoms (itch, joint pain, pain in the right upper abdomen, sleepiness during the day, worry about family situation, decreased appetite, depression, fear of complication, jaundice); 9 items measure the impact of these symptoms on person's daily activities; 6 items evaluate memory problems, change of personality, financial affairs, change in use of time, reduced sexual activity and reduced sexual interest | 1 week | Construct validity: tested in comparison with SF-36 and the multidimensional fatigue index-20, showed low to moderate correlations indicating a slight to moderate overlap between the information given by the LDSI and the other 2 questionnaires Spearman correlations reported between symptom severity and related hindrance items ranged between 0.52-0.80. Psychometrically evaluated in a general population of patients with chronic liver disease (n = 1,175) |
Test-retest reliability in a small group of patients (n = 34) only 3 days apart. Internal consistency: Cronbach's α>0.79 |
Modified version of LDSI | Lack of evidence of content validity |
| FIS | Developed on the basis of existing fatigue questionnaires in a group of patients with multiple sclerosis (n = 30) (patient interviews) Designed in accordance with the taxonomy on quality of life in clinical trials73 |
3 domains: cognitive functioning, physical functioning, and psychosocial functioning (40 items) | 1 month | Construct validity: statistically significant correlations between the total FIS and sickness impact profile (generic HRQoL measure) score Evaluated in a group of patients referred to the Infectious Disease Unit for investigation of fatigue |
Internal consistency: Cronbach's α 0.98 | n.a. | Not validated in the NASH patient population; maximum recall period recommended for QoL tools |
The table presents the relevant PROs which were evaluated for the review. The PROs have limitations relating to population group, tool practicality, evidence or validation. CLDQ, chronic liver disease questionnaire; FDA, United States Food and Drug Administration; FIS, fatigue impact scale; HRQoL, health-related quality of life; LDQoL, liver disease quality of life questionnaire; LDSI, liver disease symptom index; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PROs, patient-reported outcomes.
Discussion
NAFLD is now recognised to affect approximately 25% of the global adult population, and so is a leading cause of liver dysfunction and cirrhosis.1 At present, there are no licenced pharmacological therapies for NASH and so management focusses on lifestyle modification to achieve weight loss by diet and exercise. Although prior to hepatic decompensation, NAFLD/NASH is generally considered asymptomatic, this dogma is increasingly being brought into question.69,70 A holistic approach to patient care implies a need for clinicians to appreciate the wider consequences of a NASH diagnosis, including how its psychosocial and symptom burden impacts on HRQoL and the ability of patients to make substantive lifestyle changes, even prior to hepatic decompensation. Such knowledge, objectively measured, not only informs our understanding of the lived experience of the disease but allows the impact of novel therapies on HRQoL to be measured; addressing the first tenant of the FDA's patient-focussed drug development, that treatment must benefit how patients “feel, function and survive”.11 The aim of this review was firstly, to identify humanistic burden of disease in patients with diagnosed NASH and compensated cirrhosis, and secondly, to identify which instruments, generic or disease-specific PROMs have been used to assess the impact of NASH-cirrhosis and have sufficient evidence to render them suitable for use in support of regulatory label claims.
The limited heterogeneous literature meant that the level of detail in the reporting varied greatly between studies, which made synthesis difficult. Studies were included even if they did not refer solely to NASH-cirrhosis. This is because several studies reported findings correlating with the severity but not the aetiology of liver disease.
From the included studies, several common themes can be identified. Where reported, type 2 diabetes was cited as the main comorbidity for patients with liver disease, this was followed by obesity. Obesity and insulin resistance are well documented risk factors for developing NAFLD and approximately 85% of NAFLD patients will also have one of these comorbidities.71 Several symptoms were reported as being problematic for patients with liver disease, these included: abdominal pain, general pain, lack of energy, tiredness and sleep symptoms. Frequent comorbid conditions raise the possibility that patient burden is impacted by an illness other than NASH. However, due to associated conditions (e.g. overweight and diabetes) and the high prevalence of other comorbidities, it is challenging to assert that effects are solely down to NASH.
Although it could be argued that the differentiation and identification of symptoms between different comorbidities, i.e. liver disease and diabetes could be problematic72; cirrhotic patients were reported as having worse physical health/functioning,27 emotional health, mental health,50 and were reported to experience more pain than non-cirrhotic patients with NAFLD.39 Indeed, in 1 study aiming to determine the factors related to disability in cirrhotic outpatients, pain-related disability was reported to be ‘nearly universal’.44
Nonetheless, despite many studies reporting the key HRQoL concepts, details of the impact of these findings on patients' everyday work and family life are lacking. Only 9 of the data sources specifically aimed to determine HRQoL. Findings from the patient blogs provided a more detailed insight into the personal impact of living with NASH-cirrhosis such as ‘shock’, ‘disbelief’ and ‘life being put on hold’. The importance of HRQoL concepts being from a patient or clinical perspective has been highlighted.13 This suggests that further studies, especially those incorporating a qualitative60 element, would be valuable in determining the full humanistic burden of living with NASH-cirrhosis. Also lacking are studies which focus specifically on NASH-cirrhosis or which differentiate between compensated and decompensated patients; whether this is due to lack of studies within this population or the way in which findings are presented in identified studies is unclear. Additionally, most studies meeting the inclusion criteria were conducted in the USA and it is known that cultural factors have the potential to influence the burden of disease; this raises the need for further studies in other geographical and cultural settings.
PROMs
The presented PRO instruments (apart from LDSI 2.0) have all been used in clinical trials for several years. However, they all have limitations in terms of their development and psychometric properties. In part, this may be because they were developed prior to the publication of the FDA guidance for PRO instruments for use in product labelling. Hence, published documentation supporting the development, use, content validity and interpretation of results of LDQoL, FIS and LDSI 2.0 instruments lacks the detail required by current FDA guidance. CLDQ-NAFLD is a relatively new PROM and it is the only PRO instrument that has been specifically developed to be used in the NAFLD population. However, the lack of evidence of the tool's content validity within a NASH population is a significant limitation of the CLDQ-NAFLD and means that the appropriateness of its use amongst a cirrhotic population is not known. As a result, none of the PRO measures described meet the current FDA guidance, therefore they cannot be considered suitable for use as a PRO in cirrhotic NASH clinical trials, where the intention is to seek a PRO-based product label claim.
Strengths and limitations
This targeted review, focusing on patients with NASH-cirrhosis, made use of standard review processes which added rigour to the scoping process. Two reviewers worked on each stage of the review process and the search strategy was developed in consultation with an information specialist. The heterogeneous nature of the literature and the different ways in which data were reported made synthesis challenging. Based on the available literature, it was also not possible to evaluate the relative impact of common comorbid health conditions in patients with NASH-cirrhosis e.g. type 2 diabetes. Furthermore, only English language studies were retrieved and reviewed. A quality assessment of the PRO instruments identified was also conducted. However, it is acknowledged that some relevant studies may have been missed by our search strategy.
Conclusions
The objective of this study was to describe the humanistic burden of NASH in the cirrhotic population and to determine which PROMs (if any) were used with this group. Patients with cirrhosis reportedly have lower HRQoL and cirrhosis is associated with poorer physical health. However, the findings lacked detail of everyday impact on patients' lives. Some additional symptoms were identified, namely: abdominal symptoms, muscle cramps and depression/anxiety. Further qualitative studies with this patient population would be of benefit to understand how this disease affects patient's daily lives and if the experience of this group differs from the non-cirrhotic NASH population. Currently, a number of HRQoL tools are available to measure the impact of the disease in patients with chronic liver conditions, however, the most commonly used measures do not comply with the most recent standards of the US FDA and/or are not validated to meet the needs of the population of patients with NASH-cirrhosis. It is important to assess the impact of the disease-specific symptoms and complications, particularly in a population of patients with NASH, as non-specific PRO tools will likely miss those particular symptoms experienced by the NASH-cirrhosis population. Therefore, there is a clear need for a fully validated disease-specific PROM for use in patients with NASH that can also be used in the population with compensated cirrhosis.
Financial support
LM, MB, GF, YO, LV, LT, MMB, CAB, FB and QMA are members of the LITMUS consortium, which has received funding from the Innovative Medicines Initiative 2 (IMI2) Joint Undertaking under grant agreement No. 777377 www.imi.europa.eu. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA. QMA is a Newcastle NIHR Biomedical Research Centre investigator. The funding body were not involved in the study design, collection, analysis and interpretation of data. LD is an employee of RTI-HS, which has received funding from Novartis Pharma AG. AS is President of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect and Galmed. He has served as a consultant to Astra Zeneca, Nitto Denko, Enyo, Ardelyx, Conatus, Nimbus, Amarin, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer-Ingelheim, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, Surrozen and Bristol Myers Squibb. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland and Novartis. He receives royalties from Elsevier and UptoDate. QMA is coordinator of the LITMUS IMI2 Consortium funded by the European Commission through grant agreement 777377. Consultancy: 89Bio, Abbott Laboratories, Acuitas Medical, Allergan/Tobira, Altimmune, AstraZeneca, Axcella, Blade, BMS, BNN Cardio, Celgene, Cirius, CymaBay, EcoR1, E3Bio, Eli Lilly & Company Ltd., Galmed, Genentech, Genfit SA, Gilead, Grunthal, HistoIndex, Indalo, Imperial Innovations, Intercept Pharma Europe Ltd., Inventiva, IQVIA, Janssen, Madrigal, MedImmune, Metacrine, NewGene, NGMBio, North Sea Therapeutics, Novartis, Novo Nordisk A/S, Pfizer Ltd., Poxel, ProSciento, Raptor Pharma, Servier, Terns, Viking Therapeutics. Research Grant Funding: Abbvie, Allergan/Tobira, AstraZeneca, GlaxoSmithKline, Glympse Bio, Novartis Pharma AG, Pfizer Ltd., Vertex.
Authors' contributions
LM and MB completed the data screening, extracted, and recorded the results. LM and GF analysed, interpreted the data and drafted the manuscript. MB contributed to the revision of the manuscript. YO, LV, LT, MMB, LD, CB, AS and QMA made substantial contributions to the conception or design of the work and contributed to the revision of the manuscript. FB contributed to the study design, completed the database searches and contributed to the revision of the manuscript. All authors approved the final version.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
The study team would like to thank Dr Magdalena Harrington, Director, Patient-Centered Outcomes Assessment. Pfizer Inc. for her technical help on the PROM analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100099.
Supplementary data
References
- 1.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 3.McPherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 7.Temple R.J. A regulatory authority's opinion about surrogate endpoints. In: Nimmo W.S., Tucker G.T., editors. Clinical Measurement in Drug Evaluation. J. Wiley; New York: 1995. [Google Scholar]
- 8.Group. F-NBW . Food and Drug Administration (US) and National Institutes of Health (US); Bethesda (MD): 2016. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet] Available at: https://www.ncbi.nlm.nih.gov/books/NBK326791/ [Google Scholar]
- 9.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 10.Dooley J.S., Lok A.S., Burroughs A.K., Heathcote E.J., editors. Sherlock's Diseases or the Liver & Biliary System. 12th ed. Wiley-Blackwell; Oxford: 2011. [Google Scholar]
- 11.Sanyal A.J., Friedman S.L., McCullough A.J., Dimick-Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American association for the study of liver diseases–U.S. Food and Drug Administration joint Workshop. Hepatology. 2015;61(4):1392–1405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui M.S., Harrison S.A., Abdelmalek M.F., Anstee Q.M., Bedossa P., Castera L. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. Hepatology. 2018;67(5):2001–2012. doi: 10.1002/hep.29607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutteling J.J., de Man R.A., Busschbach J.J.V., Darlington A.-S.E. Overview of research on health-related quality of life in patients with chronic liver disease. Neth J Med. 2007;65(7):227–234. [PubMed] [Google Scholar]
- 14.Afendy A., Kallman J.B., Stepanova M., Younoszai Z., Aquino R.D., Bianchi G. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther. 2009;30(5):469–476. doi: 10.1111/j.1365-2036.2009.04061.x. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological health . Labeling Claims FDA; Rockville: 2009. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support. [Google Scholar]
- 16.Mahady S.E., Adams L.A. Burden of non-alcoholic fatty liver disease in Australia. J Gastroenterol Hepatol. 2018;33(S1):1–11. doi: 10.1111/jgh.14270. [DOI] [PubMed] [Google Scholar]
- 17.McKenna S.P. Measuring patient-reported outcomes: moving beyond misplaced common sense to hard science. BMC Med. 2011;9:86. doi: 10.1186/1741-7015-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Twiss J., Balp L., Doward L., Slota C., Cryer D., Langford A. Development OF a new patient-reported outcome measure for nonalcoholic steatohepatitis: NASH-check. Value Health. 2017;20(9):A638–A. [Google Scholar]
- 19.Doward L.C., Balp M.M., Stewart K.E., Cryer D., Langford A., Twiss J. Exploring the patient perceived impact of non-alcoholic steatohepatitis. J Hepatol. 2017;66(1):S422–S423. [Google Scholar]
- 20.Paisley S., Booth A., Mensinkai S. Chapter 12: Health-related Quality of Life Studies. U.S National Library of Medicine; 2005. Etext on Health Technology Assessment (HTA) Information Resources.https://www.nlm.nih.gov/archive/20060905/nichsr/ehta/chapter12.html#search [updated 19/01/2018 9 May 2005]. Available at: [Google Scholar]
- 21.Endnote, [Internet] 2017. http://endnote.com/ [cited 08/02/18]. Available at: [Accessed 4 May 2018]
- 22.Rayyan a web and mobile app for systematic reviews [Internet] 2016. https://rayyan.qcri.org/welcome Available at: [Accessed 4 May 2018] [DOI] [PMC free article] [PubMed]
- 23.Popay J., Roberts H., Sowden A., Petticrew M., Arai L., Rodgers M. Lancaster University; Lancaster: 2006. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product of the ESRC Methods Programme. [Google Scholar]
- 24.Mokkink L.B., Prinsen C.A.C., Bouter L.M., Vet HCWd, Terwee C.B. The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) and how to select an outcome measurement instrument. Braz J Phys Ther. 2016;20(2):105–113. doi: 10.1590/bjpt-rbf.2014.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sumskiene J., Sumskas L., Petrauskas D., Kupcinskas L. Disease-specific health-related quality of life and its determinants in liver cirrhosis patients in Lithuania. World J Gastroenterol. 2006;12(48):7792–7797. doi: 10.3748/wjg.v12.i48.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teixeira M.C.D., de Fatima Gomes de Sa Ribero M., Strauss E. A new insight into the differences among non-cirrhotic and cirrhotic patients using the liver disease quality of life instrument (LDQOL) Ann Hepatol. 2005;4(4):264–271. [PubMed] [Google Scholar]
- 27.David K., Kowdley K.V., Unalp A., Kanwal F., Brunt E.M., Schwimmer J.B. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(6):1904–1912. doi: 10.1002/hep.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalaitzakis E., Simrén M., Olsson R., Henfridsson P., Hugosson I., Bengtsson M. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol. 2006;41(12):1464–1472. doi: 10.1080/00365520600825117. [DOI] [PubMed] [Google Scholar]
- 29.Sayiner M., Stepanova M., Pham H., Noor B., Walters M., Younossi Z.M. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106. doi: 10.1136/bmjgast-2016-000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dan A.A., Kallman J.B., Wheeler A., Younoszai Z., Collantes R., Bondini S. Health-related quality of life in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;26(6):815–820. doi: 10.1111/j.1365-2036.2007.03426.x. [DOI] [PubMed] [Google Scholar]
- 31.Younossi Z.M., Henry L. Economic and quality-of-life implications of non-alcoholic fatty liver disease. Pharmacoeconomics. 2015;33(12):1245–1253. doi: 10.1007/s40273-015-0316-5. [DOI] [PubMed] [Google Scholar]
- 32.Cortesi P.A., Rota M., Scalone L., Cozzolino P., Cesana G., Mantovani L. A comparison between the health-related quality of life reported by the general population and by patients with major liver diseases. Value Health. 2014;17:A369. doi: 10.1016/j.jval.2014.08.835. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy-Martin T., Bae J., Paczkowski R., Freeman E.C. A review of the quality of life burden of non-alcoholic steatohepatitis. ISPOR 22nd Annual International Meeting. 2017 [Google Scholar]
- 34.Marchesini G., Bianchi G., Amodio P., Salerno F., Merli M., Panella C. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120(1):170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 35.Chatrath H., Liangpunsakul S., Ghabril M., Otte J., Chalasani N., Vuppalanchi R. Prevalence and morbidity associated with muscle cramps in patients with cirrhosis. Am J Med. 2012;125(10):1019–1025. doi: 10.1016/j.amjmed.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura S., Konishi H., Kishino M., Yatsuji S., Tokushige K., Hashimoto E. Prevalence of esophagogastric varices in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008;38(6):572–579. doi: 10.1111/j.1872-034X.2008.00318.x. [DOI] [PubMed] [Google Scholar]
- 37.Stine J.G., Niccum B.A., Zimmet A.N., Intagliata N., Caldwell S.H., Argo C.K. Increased risk of venous thromboembolism in hospitalized patients with cirrhosis due to non-alcoholic steatohepatitis. Clin Transl Gastroenterol. 2018;9(3):140. doi: 10.1038/s41424-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott C., Frith J., Day C.P., Jones D.E., Newton J.L. Functional impairment in alcoholic liver disease and non-alcoholic fatty liver disease is significant and persists over 3 years of follow-up. Dig Dis Sci. 2013;58(8):2383–2391. doi: 10.1007/s10620-013-2657-2. [DOI] [PubMed] [Google Scholar]
- 39.Rogal S.S., Bielefeldt K., Wasan A.D., Lotrich F.E., Zickmund S., Szigethy E. Inflammation, psychiatric symptoms, and opioid use are associated with pain and disability in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(5):1009–1016. doi: 10.1016/j.cgh.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogal S.S., Winger D., Bielefeldt K., Szigethy E. Pain and opioid use in chronic liver disease. Dig Dis Sci. 2013;58(10):2976–2985. doi: 10.1007/s10620-013-2638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madan A., Barth K.S., Balliet W.E., Hernandez-Tejada M.A., Borckardt J.J., Malcolm R. Chronic pain among liver transplant candidates. Prog Transplant. 2012;22(4):379–384. doi: 10.7182/pit2012535. [DOI] [PubMed] [Google Scholar]
- 42.Sobhonslidsuk A., Satitpornkul P., Sornmayura P. Excessive daytime sleep disorder and fatigue in non-alcoholic fatty liver disease in comparison to cirrhosis. J Gastroenterol Hepatol. 2011;26(Suppl 5):164. [Google Scholar]
- 43.Rogal S.S., Bielefeldt K., Zickmund S., Di Martini A. Healthcare utilization among patients with cirrhosis: the role of inflammation, medications, pain, and depression. Hepatology. 2013;1:950A–951A. [Google Scholar]
- 44.Rogal S.S., Bielefeldt K., Szigethy E., DiMartini A. Inflammation and pain-related disability in patients with cirrhosis. Gastroenterology. 2014;1:S909–S910. doi: 10.1016/j.cgh.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edula R.G., Qureshi K., Khallafi H. Hemorrhagic ascites from spontaneous ectopic mesenteric varices rupture in NASH induced cirrhosis and successful outcome: a case report. World J Gastroenterol. 2014;20(25):8292–8297. doi: 10.3748/wjg.v20.i25.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burgos A., Bermejo P.E., Calleja J.L., Vaquero A., Abreu L.E. Acquired chronic hepatocerebral degeneration due to cirrhosis from non-alcoholic steatohepatitis. Rev Esp Enferm Dig. 2009;101(11):806–811. doi: 10.4321/s1130-01082009001100009. [DOI] [PubMed] [Google Scholar]
- 47.Younossi Z.M., Guyatt G., Kiwi M., Boparai N., King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45(2):295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Younossi Z.M., Stepanova M., Henry L., Racila A., Lam B., Pham H.T. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37(8):1209–1218. doi: 10.1111/liv.13391. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira M.C.D., de Fátima Gomes de Sá Ribeiro M., Strauss E. A new insight into the differences among non-cirrhotic and cirrhotic patients using the liver disease quality of life instrument (LDQOL) Ann Hepatol. 2005;4(4):264–271. [PubMed] [Google Scholar]
- 50.Marchesini G., Bianchi G. Nonalcoholic fatty liver disease: disease and comorbidity-effects on quality of life. Nat Rev Gastroenterol Hepatol. 2009;6(9):504–506. doi: 10.1038/nrgastro.2009.142. [DOI] [PubMed] [Google Scholar]
- 51.Veronica's Story [Internet] https://www.britishlivertrust.org.uk/stories/veronicas-story/ Available at:
- 52.Bronagh's Story [Internet] https://www.britishlivertrust.org.uk/stories/bronaghs-story/ Available at:
- 53.Carol S [Internet] 2017. https://liverfoundation.org/carol-s/ Available at: [Accessed 22 October 2018]
- 54.The beast in my belly: Living with a chronic liver disease [Internet] 2016. https://scopeblog.stanford.edu/2016/12/20/the-beast-in-my-belly-living-with-a-chronic-liver-disease/ Available at: [Accessed 22 October 2018]
- 55.My Sick Liver. http://mysickliver.weebly.com/gs-story.html Available at:
- 56.Simmons D., Lillis S., Swan J., Haar J. Discordance in perceptions of barriers to diabetes care between patients and primary care and secondary care. Diabetes Care. 2007;30(3):490–495. doi: 10.2337/dc06-2338. [DOI] [PubMed] [Google Scholar]
- 57.Edula R.G.R., Qureshi K., Khallafi H. Hemorrhagic ascites from spontaneous ectopic mesenteric varices rupture in NASH induced cirrhosis and successful outcome: a case report. World J Gastroenterol. 2014;20(25):8292–8297. doi: 10.3748/wjg.v20.i25.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura S., Konishi H., Kishino M., Yatsuji S., Tokushige K., Hashimoto E. Prevalence of esophagogastric varices in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008;38(6):572–579. doi: 10.1111/j.1872-034X.2008.00318.x. [DOI] [PubMed] [Google Scholar]
- 59.Younossi Z.M., Younossi I., Pham H.T., Stepanova M., Lam B.P., Hunt S. Development and validation of a disease-specific health-related quality (HRQL) instrument for patients with non-alcoholic fatty liver disease (NAFLD) and Non-alcoholic Steatohepatitis (NASH): the CLDQ-NAFLD. Hepatology. 2017;64(Suppl 1):118A–119A. [Google Scholar]
- 60.Gralnek I.M., Hays R.D., Kilbourne A., Rosen H.R., Keeffe E.B., Artinian L. Development and evaluation of the liver disease quality of life instrument in persons with advanced, chronic liver disease—the LDQOL 1.0. Am J Gastroenterol. 2000;95(12):3552–3565. doi: 10.1111/j.1572-0241.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 61.van der Plas S.M., Hansen B.E., de Boer J.B., Stijnen T., Passchier J., de Man R.A. The liver disease symptom index 2.0; validation of a disease-specific questionnaire. Qual Life Res. 2004;13(8):1469–1481. doi: 10.1023/B:QURE.0000040797.17449.c0. [DOI] [PubMed] [Google Scholar]
- 62.Fisk J.D., Ritvo P.G., Ross L., Haase D.A., Marrie T.J., Schlech W.F. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;Suppl 1:S79–S83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 63.Newton J.L., Jones D.E.J., Henderson E., Kane L., Wilton K., Burt A.D. Fatigue in non-alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut. 2008;57(6):807–813. doi: 10.1136/gut.2007.139303. [DOI] [PubMed] [Google Scholar]
- 64.Newton J.L., Pairman J., Wilton K., Jones D.E.J., Day C. Fatigue and autonomic dysfunction in non-alcoholic fatty liver disease. Clin Auton Res. 2009;19(6):319. doi: 10.1007/s10286-009-0031-4. [DOI] [PubMed] [Google Scholar]
- 65.Amtmann D., Bamer A.M., Noonan V., Lang N., Kim J., Cook K.F. Comparison of the psychometric properties of two fatigue scales in multiple sclerosis. Rehabil Psychol. 2012;57(2):159. doi: 10.1037/a0027890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ware J.E., Kosinkski M., Keller S.D. Health Assessment Lab NEMC; Boston: 1994. Physical and Mental Health Summary Scales: A User's Manual. [Google Scholar]
- 67.Unal G., de Boer J.B., Borsboom G.J., Brouwer J.T., Essink-Bot M., de Man R.A. A psychometric comparison of health-related quality of life measures in chronic liver disease. J Clin Epidemiol. 2001;54(6):587–596. doi: 10.1016/s0895-4356(00)00372-3. [DOI] [PubMed] [Google Scholar]
- 68.Frith J., Newton J. Fatigue impact scale. Occup Med. 2010;60(2):159. doi: 10.1093/occmed/kqp180. [DOI] [PubMed] [Google Scholar]
- 69.Huber Y., Boyle M., Hallsworth K., Tiniakos D., Straub B.K., Labenz C. Health-related quality of life in nonalcoholic fatty liver disease associates with hepatic inflammation. Clin Gastroenterol Hepatol. 2019;17(10):2085–2092.e1. doi: 10.1016/j.cgh.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 70.Younossi Z.M., Stepanova M., Henry L., Racila A., Lam B., Pham H.T. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37(8):1209–1218. doi: 10.1111/liv.13391. [DOI] [PubMed] [Google Scholar]
- 71.Abd El-Kader S.M., El-Den Ashmawy E.M.S. Non-alcoholic fatty liver disease: the diagnosis and management. World J Hepatol. 2015;7(6):846–858. doi: 10.4254/wjh.v7.i6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kennedy-Martin T., Bae J., Paczkowski R., Freeman E.C. Health-related quality of life burden of nonalcoholic steatohepatitis: a robust pragmatic literature review. J Patient Rep Outcomes. 2018;2:28. doi: 10.1186/s41687-018-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guyatt G.H., Veldhuyzen Van Zanten S.J., Feeny D.H., Patrick D.L. Measuring quality of life in clinical trials: a taxonomy and review. CMAJ. 1989;140(12):1441–1448. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.