Abstract
Objective(s):
Salsola collina is widely distributed along the Bohai coast and consumed as an edible plant by native residents. We have found surprisingly that S. collina extracts promoted gastrointestinal motility in mice previously. In the present study, effects of S. collina on gastrointestinal motility in rats and its underlying mechanism were explored.
Materials and Methods:
In vivo, different fraction extracts from S. collina were prepared and the effects on gastric emptying and small intestinal propulsion in normal rats were measured. Plasma ghrelin (GRL), motilin (MTL), gastrin (GAS) and vasoactive intestinal peptide (VIP) and expressions of GRL receptor (GHSR), MTL receptor (MTLR), VIP receptor 2 (VIPR2) in the duodenum were also detected. In vitro, gastric antrum strips were prepared and activities of different extracts on gastric smooth muscle contractions were evaluated.
Results:
Results showed that the ethyl acetate extract (EAE) was the most effective fraction to promote gastric emptying and intestinal propulsion, showing a dose-dependent manner. EAE increased plasma GRL and GAS, elevated GHSR expression and restrained VIPR2 expression in the duodenum. In vitro, EAE promoted contraction of normal gastric antrum strips as well as relaxed strips induced by atropine.
Conclusion:
These data indicate that EAE has a significant prokinetic activity via a mechanism that mainly involves in modulating plasma GRL and GAS, expressions of GSHR and VIPR2 in the duodenum and activating M-cholinergic receptor. Our study provides a pharmacological basis for the use of S. collina extract in treating gastrointestinal motility disorders.
Key Words: Atropine, Epinephrine, Gastrointestinal motility, Gastrointestinal hormones, Rat
Introduction
Gastrointestinal diseases, the major causes of human ill-health, are common public health problems throughout the world (1). Indigestion and constipation, in particular, are commonly prevailing disorders. Indigestion is known to affect approximately 11-29.2% of the population, while the prevalence of constipation is up to 27% (2, 3). Dietary and lifestyle measures are first-line treatment for gut motility disorders world widely (4). The second choice of therapy is the prokinetics and laxatives which would become less efficient with long-term use (5). Recently, medicinal plants have attracted attention to treat gastrointestinal disorders such as indigestion and constipation, since there is an increasing evidence that multiple constituents found in medicinal plants have the potential synergies (6, 7). Medicinal plants are considered relatively safe and effective in prolonged use, especially in patients with chronic gut motility disorders.
Salsola collina, a perennial herb of the Chenopodiaceae, is widely distributed in China, and the fresh S. collina is consumed as an herbal drink or medicine to treat diseases including hypertension, headache and vertigo (8, 9). It has been reported that the ethanol extract of S. collina has antioxidative, anti-cancer and antihypertensive activities (10, 11). In addition, an aqueous extract from S. collina is an effective means for the prophylaxis of cholelithiasis (12). Although the use of S. collina has a long history in traditional Chinese medicine, there are few studies on its pharmacological effect involving in gastrointestinal motility as well as the possible underlying mechanism.
The aim of the present study was to investigate the activities of different extracts from S. collina on gastrointestinal motility both in vivo and in vitro. At the same time, the underlying mechanisms were explored here.
Materials and Methods
Reagents and chemicals
Standards for ferulic acid and vanillic acid (98.0%) were purchased from Chengdu Derick Biotenhnology Co., Ltd. (Chengdu, China). Atropine and epinephrine were purchased from Sigma Chemical Co., Ltd. (St. Louis, MO, USA). The Krebs solution contained (mM, pH 7.4): NaCl, 118; KCl, 4.8; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25; glucose, 11 (13). All other reagents were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Plant material and preparation of S. collina
S. collina was purchased from Qizhou medicine Hall (Hebei, China) and identified as the over-ground part of S. collina by Professor Hong Jin (Qingdao University of Science and Technology, China). S. collina was powdered and extracted with 70% ethanol by heating reflux method for 2 hr and 3 times. The extract was evaporated under reduced pressure to remove ethanol at a temperature below 60 °C, then suspended in water and partitioned successively with petroleum ether, ethyl acetate and n-butanol. The extracts obtained from each step was evaporated to dryness separately and redissolved in saline before use. Four extracts from S. collina were petroleum ether extract (PEE), ethyl acetate extract (EAE), n-butanol extract (NBE) and remainder after extraction (RAE), respectively.
Animals
Male Sprague Dawley rats (250-270 g) were provided by Qingdao Daren Fortune Animal Technology Co., Ltd. (approval number: SCXK (Jing) 2016-0002) housed in cages in a temperature-controlled room (22±2 °C) and on a 12-hr light-dark cycle, with free access to standard animal chow and tap water. All the protocols were approved by the Animal Care and Use Committee at Qingdao University of Science and Technology (approval number: ACQUST-2017-025).
HPLC analysis of EAE
HPLC was performed on an Agilent 1220 Infinity LC System (Agilent Technologies, USA), and agilent Eclipse XDB C18 (4.6×250 mm, 5 μm; Agilent, USA) at 30 °C. Elution solvents were 0.01% (v/v) phosphoric acid in water (A) and acetonitrile (B), with a gradient elution program was set as follows: 5% (B) for 0-9 min, 5-10% (B) for 9-15 min, 10-15% (B) for 15-20 min, 15-20 (B) for 20-30 min, 20-30 (B) for 30-40 min, 30-20 (B) for 40-50 min, 20-15 (B) for 50-60 min. The flow rate was set at 1.0 ml/min. Chromatograms were acquired with a UV detector at 290 nm.
In vivo experimental design
Different extracts of S. collina in rats
The effect of different extracts on gastrointestinal motility in rats was observed. Rats were randomly divided into six groups (n=8): control group, PEE group, EAE group, NBE group, RAE group, domperidone group. The rats in PEE, EAE, NBE and RAE groups, PEE, EAE, NBE, RAE (40 mg/kg) were given by intragastric (IG) administration once a day for 7 days. In the positive group, the rats were given 3 mg/kg of domperidone for 7 days. In the control group, the rats were given 0.9% saline solution for 7 days. The gastric emptying rate and intestinal transit rate of each rat was measured at 30 min after the last administration.
Different doses of EAE in rats
Rats were divided into five groups (n=8) randomly. The control and Dom groups were treated as mentioned above. The rats in low-dose-EAE group (L-EAE), middle-dose-EAE group (M-EAE), and high-dose-EAE group (H-EAE) were administered EAE for 7 days at doses of 20, 40, 80 mg/kg (IG), respectively. The gastric emptying rate and intestinal transit rate was measured at 30 min after the last treating.
Test of gastric emptying rate and intestinal transit rate
Rats were fasted for 24 hr before the experiments. 1.5 ml of 0.05% (w/v) phenol red suspended in 1.5% aqueous methylcellulose solution was administered to rats (IG). Twenty min later, the blood was obtained from abdominal aorta. Immediately, the rats were sacrificed and the whole stomach and intestine tissues were taken out carefully. The stomachs were cut into several pieces and homogenized in 100 ml of 0.1M NaOH. The suspension was settled for 1 hr at room temperature. And then 5 ml of the supernatant was precipitated with 0.5 ml of 20% trichloroacetic acid and centrifuged at 3000 rpm for 10 min. The supernatant was mixed with 4 ml of 0.5M NaOH, and the absorbance was measured at 560 nm. Gastric emptying (%)=(1-amount of phenol red recovered from test stomach/ average amount of phenol red recovered from standard stomachs)×100 (14). The small intestine was stretched on a white paper. The whole length and the pushing distance of phenol red solution were measured accurately. The rate of intestinal transit was expressed as the ratio between the distance travelled by phenol red solution and the total length of intestine (15). Meanwhile, the duodenal was washed with saline on the ice and put into liquid nitrogen immediately for Western blot assays.
Mearsurement of gastrointestinal hormone in plasma
Blood samples were collected in tubes containing EDTA, and centrifuged at 2500 rpm for 15 min at 4 °C. All prepared plasma was stored at -80 °C to determine GRL, MTL, GAS and VIP using an ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China.)
Detection on expressions of the relevant gastrointestinal hormone receptors
After homogenized at 4 °C with 1 mM EDTA and 2.5 ml cell lysate, the proteins (70 μg) were separated from the duodenum by SDA-PAGE and transferred to positively charged nylon membranes (Pall corporation, NY, USA). The blots were probed with rabbit antibodies of anti-GHSR, anti-MTLR, anti-VIPR2 (dilution 1:200) overnight at 4 °C. The secondary antibodies were goat anti-rabbit IgG-HRP (1:5000; Nanjing Jiancheng Biotechnology institute, Nanjing, China) and the blots were washed with Tris-Buffered Saline and Tween 20 (TBST). The bands were observed using an enhanced chemiluminescence substrate (Amersham Bioscience, Buckinghamshire, UK) and quantified with an image analysis program (Image Gauge v3.12 software, Fujifilm, Tokyo, Japan). Protein concentration was determined using the BCA Protein Assay Kit (16).
In vitro experimental design
Preparation of gastric antrum strips
The rats were deprived from food but not water 24 hr prior to experiments, and then stunned by head-striking. The whole stomach was quickly removed and placed in Krebs solution. After removing the mucosa by blunt dissection, gastric antrum strips with approximately 2 cm length were prepared by cutting parallel to the long axis of the tissue and each end was attached by a thread. Then the strips were suspended in tissue chambers filled with Krebs solution at 37±0.5 °C, bubbled with a mixture of oxygen (95%) and carbon dioxide (5%) (17). The strips were mounted under an initial load of 1.0 g, and equilibrate for 1 hr with washout every 20 min before starting the experiment (18).
Test of the spontaneous contraction on normal gastric antrum muscle strips
After equilibration for 1 hr, PEE, EAE, NBE and RAE (0.1, 0.2, 0.4 mg/ml) were added to the Krebs solution individually and muscle contractions were recorded. The amplitude of contractions occurring after administration of each concentration of PEE, EAE, NBE, and RAE were determined for 10 min. Relative changes in contractile responses induced by the different concentrations of PEE, EAE, NBE, and RAE relative to the basal levels (before treatment with those extract) were calculated as percentages.
Test of the spontaneous contraction in relaxed gastric antrum muscle strips
To investigate the underlying mechanisms of the EAE induced effects on spontaneous contractions, atropine (10-6 mol/l) or epinephrine (10-6 mol/l) solution was appended into the bath and the contraction curves were recorded. Then the EAE was also added to the bath to 0.4 mg/ml and the amplitude changes of spontaneous contraction were recorded.
Statistical analysis
Data analysis was performed using SPSS 23.0 system. The data of each group were expressed in means±SD and analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s test. P-value< 0.05 was considered statistically significant.
Results
HPLC analysis of EAE
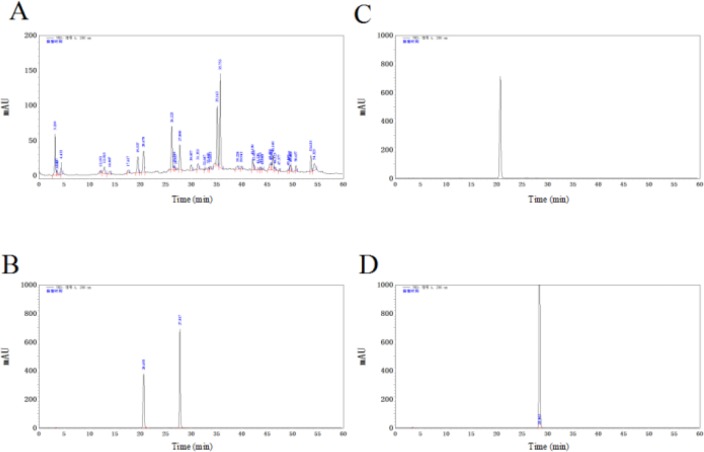
HPLC analysis of EAE was illustrated in Figure 1. The peak of vanillic acid and ferulic acid in the extract (Figure 1A) were identified according to the retention times of their reference samples (Figure 1C-D) in HPLC.
Figure 1.
HPLC chromatogram of EAE. HPLC chromatogram of the EAE extract (A); HPLC chromatogram of the mixture of reference samples (B); HPLC chromatogram of vanillic acid (C); HPLC chromatogram of ferulic acid (D). EAE: ethyl acetate extract, HPLC: high-performance liquid chromatography
Effects of different extracts on gastric emptying and small intestinal motility in rats
To investigate the effects of PEE, EAE, NBE, RAE, we examined the effects of gastric emptying and small intestinal motility in rats at the same dose of 40 mg/kg. EAE, NBE increased the gastric emptying with the ratio 67.63±11.53%, 63.27±10.17%, respectively (versus 52.39±9.76% in control), while PEE and RAE had no effect (Figure 2). Apparently, EAE had the most conspicuous effect compared to the control group (P-value<0.01, Figure 2) and similar to domperidone (73.30±12.04%) (P-value>0.05, Figure 2).
Figure 2.
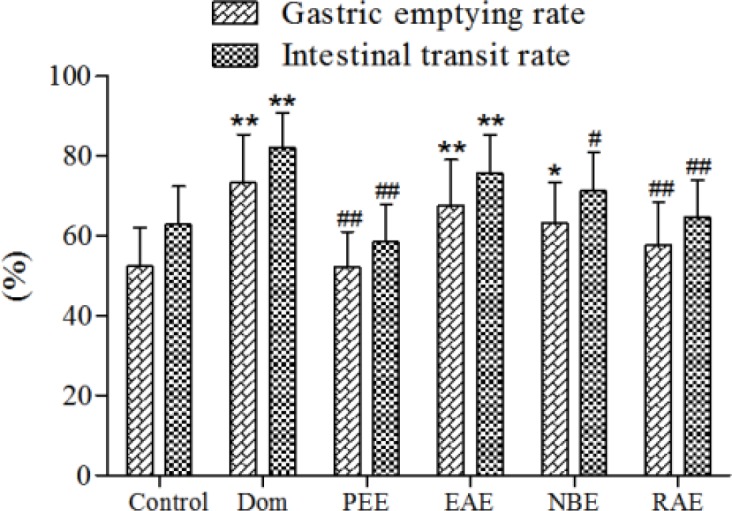
Effects of different extracts on gastric emptying and small intestinal motility in rats. PEE, EAE, NBE, RAE (40 mg/kg) were given by i.g. administration once a day for 7 days, Dom (3 mg/kg) was positive group and the rats were given 0.9% saline solution in the control group. Data are represented as the mean±SD. **Compared with control, P-value<0.01; *compared with control, P-value< 0.05.##Compared with Dom, P-value<0.01; #compared with Dom, P-value<0.05. Dom: domperidone group; PEE:petroleum ether extract; EAE: ethyl acetate extract; NBE: n-butanol extract ; RAE: remainder after extraction
EAE significantly enhanced the intestinal propulsion with the ratio of 75.77±9.57% (P-value<0.05, Figure 2), and showing no difference with Dom group. In addition, NBE, RAE and PEE had no significant difference with the saline control group (versus 62.90±9.48% in control group, P-value > 0.05, Figure 2).
Effects of EAE on gastric emptying and small intestinal motility in rats
Compared to the control group, M-EAE and H-EAE significantly increased the gastric emptying rate (P-value<0.01, Figure 3) and intestinal propulsion (P-value<0.05 and P-value<0.01 respectively, Figure 3). However, neither of them are as effective as domperidone (P-value > 0.05, Figure 3).
Figure 3.
Effects of different doses of EAE on gastric emptying and small intestinal motility in rats. Data are represented as the mean±SD. **Compared with control, P-value<0.01; *compared with control, P-value<0.05. ##Compared with Dom, P-value<0.01; #compared with Dom, P-value<0.05
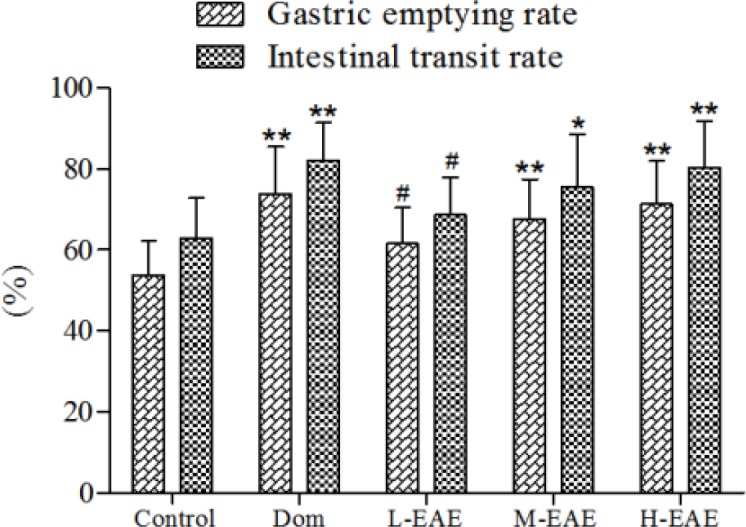
EAE: ethyl acetate extract
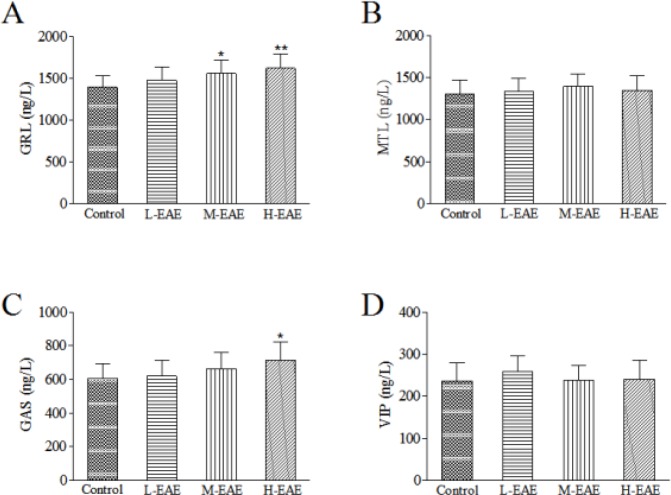
Effects of EAE on gastrointestinal hormone in plasma
Compared to the control group, plasma GRL and GAS increased in the H-EAE group (P-value<0.01 and P-value<0.05, respectively, Figure 4), while MTL and VIP had no significant change (P-value>0.05, Figure 4).
Figure 4.
Effect of EAE on gastrointestinal hormone level in plasma. The concentrations of GRL (A), MTL (B), GAS (C) and VIP (D) were measured by ELISA. Data are represented as the mean±SD. **Compared with control, P-value<0.01; *compared with control, P-value<0.05. GRL: ghrelin, MTL: motilin, GAS: gastrin, VIP: vasoactive intestinal peptide, ELISA: enzymelinked immunosorbent assay
EAE: ethyl acetate extract
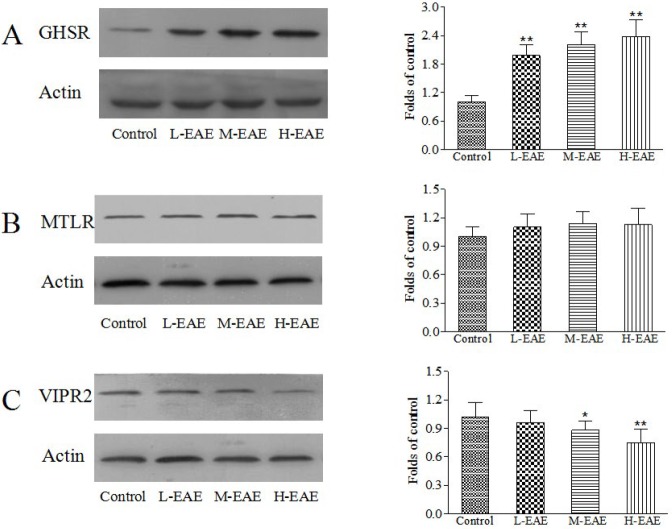
Effect of EAE on expressions of the relevant gastrointestinal hormone receptors
Compared to control group, the expressions of GHSR in the duodenum in different doses of EAE group increased while MTLR had no change (P-value>0.05, Figure 5). Besides, the expression of VIPR2 decreased both in M-EAE and H-EAE group (P-value<0.05, Figure 5).
Figure 5.
Effect of EAE on the expressions of the relevant gastrointestinal hormone receptors. The expressions for GHSR (A), MTLR (B) and VIPR2 (C) in the duodenum were determined by western blot. Data are represented as the mean±SD. **Compared with control, P-value<0.01; *compared with control, P-value<0.05
EAE: ethyl acetate extract
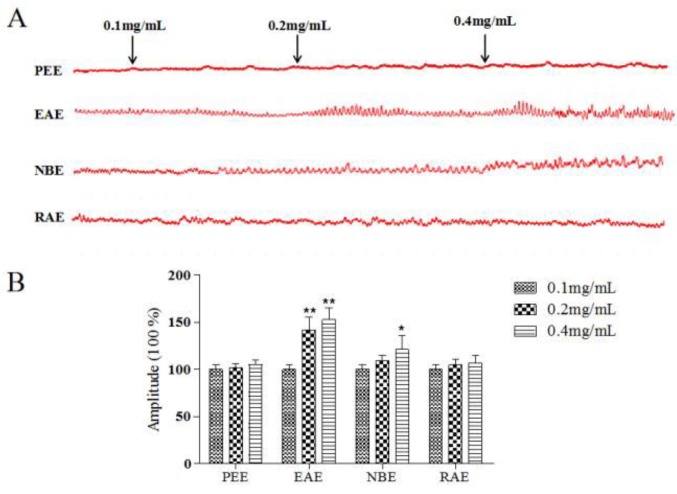
Effect of different extracts on the contractile responses in isolated rat gastric antrum muscle strips
The effects of PEE, EAE, NBE and RAE on isolated rat gastric antrum muscle motility at doses of 0.1, 0.2, 0.4 mg/ml was observed. EAE showed the strongest effects (P-value<0.01, Figure 6) and was tested in the subsequent experiments. NBE (0.4 mg/ml) also had effect (P-value<0.05), while PEE and RAE had no effect on isolated rat gastric antrum muscle motility (P-value>0.05). EAE increased the strips contraction with a dose-dependent manner in range of 0.1-0.4 mg/ml.
Figure 6.
Effects of different extracts on the spontaneous contractility of rat gastric antrum strips. The spontaneous contractility of gastric antrum strips treated with different extracts (A). Amplitude changes in every group (B). The mean contractile amplitude of rat gastric atrum strips in normal contractile state is set as 100% (control). Data are represented as the mean±SD. **Compared with control, P-value<0.01; *compared with control, P-value<0.05
EAE: ethyl acetate extract
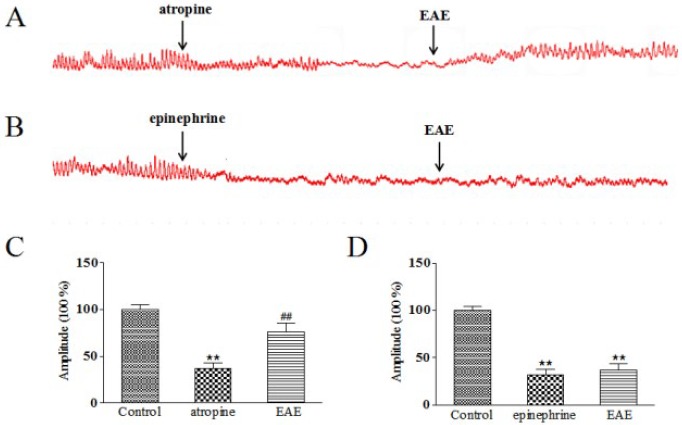
Effect of EAE on the relaxed gastric antrum muscle strips treated by atropine and epinephrine
The atropine significantly induced relaxation of gastric strips, indicating that atropine inhibited M-cholinergic receptor in gastric antrum. Atropine’s blocking effect was reversed by 0.4 mg/ml of EAE with marked increase of contracting amplitude (P-value<0.01, Figure 7), which demonstrated that EAE’s improving function on gastric antrum muscle strips contract was likely involved in activating M-cholinergic receptor. However, the relaxed strips treated by epinephrine had no remarkably change when given with 0.4 mg/ml of EAE (P-value>0.05), suggesting that EAE’s exciting effect might had no relation with the adrenergic receptor (Figure 7).
Figure 7.
Effects of EAE on atropine and epinephrine induced relaxation of gastric antrum strips. The spontaneous contractility treated with EAE (0.4 mg/ml) after 10 min pretreatment with atropine (10-6 mol/l) and epinephrine (10-6 mol/l), respectively (A, B). Amplitude changes in atropine (C) and epinephrine (D) group. The mean contractile amplitude of rat gastric antrum strips in normal contractile state is set as 100% (control). Data are represented as the mean±SD. **Compared with control, P-value<0.01; *compared with control, P-value<0.05
EAE: ethyl acetate extract
Discussion
S. collina is a pioneer halophyte distributed along the Bohai coast where the soil salt is up to 3%. It is consumed as an edible plant by native residents as it is rich in proteins, microelements, vitamins and antioxidant components (19). Furthermore, its virtues in phyto-remediation for heavy metals and oil in saline soil has been demonstrated in recent years (20, 21). In our previous study, we have found surprisingly that S. collina extracts promoted gastrointestinal motility in mice and this discovery drew our great interest to further this research. In the present experiment, the effects of S. collina on gastrointestinal motility in rats and its possible underlying mechanism were explored. The results indicate that EAE has a significant prokinetic activity via a mechanism that mainly involves in modulating plasma GRL and GAS, the expressions of GSHR and VIPR2 in the duodenum and activating M-cholinergic receptor.
Delayed gastric emptying and reduced gastrointestinal motility might resulted in functional gastrointestinal disorders such as indigestion and constipation (22). Natural plants and Chinese herbals are one of the routine treatments for gastrointestinal disorders in China and are gaining much more popularity in other countries (23, 24). Fortunately, we have obtained S. collina from plenty of plant resources through extensive screening work. In vivo, S. collina was confirmed to promote gastric emptying and intestinal propulsion and the ethyl acetate extract was the most effective fraction. Gastrointestinal motility is internally regulated by gastrointestinal smooth muscle electrical activity, nervous system and gastrointestinal hormones. In particularly, gastrointestinal hormones, including GAS, MTL, GRL and VIP, play an important role (25). GAS, synthesized and secreted from G cells locally distributed in the gastric antrum, accelerated gastric emptying and gastrointestinal motility (26). EAE from S. collina significantly elevated plasma GAS level in rats. MTL and GRL, synthesized in the upper gastrointestinal tract, are similar in structure and promote gastrointestinal muscle contraction (27). EAE increased plasma GRL and GRL expression in the duodenum while had no significant effect on MTL. Contrary to the above peptides, VIP results in smooth muscles relaxation and gastrointestinal motility inhibition via its specific receptors (28). There are two types of VIP receptor including VIP receptor-1 (VIPR1) and VIP receptor-2 (VIPR2). However, only VIPR2 is abundantly expressed in the gastrointestinal tract (29). In the present study, we revealed that EAE restrained the expression of VIPR2 in the duodenum although plasma VIP had no significant change. Therefore, EAE might increase plasma GRL and GAS, elevate the expression of GHSR and restrain the expression of VIPR2 in the duodenum, which resulted in the acceleration of gastric emptying and intestinal propulsion in vivo.
In order to explore the effects and underlying mechanisms of S. collina in vitro, gastric antrum strips were prepared to test the effects of different fractions from S. collina. It revealed that different fractions from S. collina stimulated the contraction of gastric antrum strips and the intensity was as follows: EAE>NBE>RAE>PEE. The stimulation of EAE was the strongest, which was consistent with the in vivo results. Furthermore, EAE reversed the blocking effect of atropine rather than epinephrine. Atropine, a non-selective muscarinic antagonist, causes an obvious relaxation of gastric antrum strips via blocking the M-muscarinic receptor. Simultaneously, epinephrine activates β2-adrenegic receptor in the gastric smooth muscles and results in a relaxation effect. Integratively, the stomach receives a dense parasympathetic vagal innervations arised from the dorsal motor nucleus of the vagus (DMV) in the brainstem (30). However, no reverse effect of EAE on that of epinephrine was observed. Taken together, the stimulation of EAE on gastric antrum strips might likely involve in activating M-cholinergic receptor.
Conclusion
Our results suggest that EAE from S. collina has a significant prokinetic activity via a mechanism that mainly involves in modulating plasma GRL and GAS, the expressions of GSHR and VIPR2 in the duodenum and activating M-cholinergic receptor. These findings provide a pharmacological basis for the use of an extract of S. collina in the intervention of gastrointestinal motility disorders. The potential application value remains for further exploration.
Acknowledgment
This study was supported by Key Research and Development Project of Shandong Province (Project No: 2018GSF119005), National Training Program of Innovation and Entrepreneurship for Undergraduates (Project No: 201810426025), Eco-chemical Engineering Cooperative Innovation Center of Shandong, and Shandong Key Lab of Multiphase fluid reaction & separation engineering.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Mehmood MH, Aziz N, Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of psyllium husk (Ispaghula) in constipation and diarrhea. Dig Dis Sci. 2011;56:1460–1471. doi: 10.1007/s10620-010-1466-0. [DOI] [PubMed] [Google Scholar]
- 2.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: A global perspective. World J Gastroenterol. 2006;12:2660–2666. doi: 10.3748/wjg.v12.i17.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng , CW , Bian , ZX , Wu , TX Systematic review of Chinese herbal medicine for functional constipation. World J Gastroenterol. 2009;15:4886–4895. doi: 10.3748/wjg.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100:936–971. doi: 10.1111/j.1572-0241.2005.40925.x. [DOI] [PubMed] [Google Scholar]
- 5.Jan T, Stefan ML. Treatment of chronic constipation: current pharmacologic approaches and future directions. Clin Gastroenterol Hepatol. 2009;7:502–508. doi: 10.1016/j.cgh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Sangiovanni E, Di LC, Colombo E, Colombo F, Fumagalli M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. leaves. Food Funct. 2015;6:2453–2463. doi: 10.1039/c5fo00410a. [DOI] [PubMed] [Google Scholar]
- 7.Rtibi K, Selmi S, Jabri MA, Mamadou G, Nzouzi NL, Sebai H, et al. Effects of aqueous extracts from Ceratonia siliqua L pods on small intestinal motility in rats and jejunal permeability in mice. Rsc Adv. 2016;6:44345–44353. [Google Scholar]
- 8.Liu DF, Dong ZM, Zhang ZX, Zhu XW, Wang HY, Wang N. Development of fermented milk drink containing Salsola collina juice. Beverage industry. 2009;12:24–28. [Google Scholar]
- 9.Wang J. Salsola collina fruit-vinegar beverage. China Patent. 201110331107,July 17,2013. [Google Scholar]
- 10.You NO, Jin S, Park HJ, Kwon HJ, Kim BW. Anti-oxidative and anti-cancer activities by cell cycle regulation of Salsola collina extract. Korean J Microbiol Biotechnol. 2014;42:73–81. [Google Scholar]
- 11.Meng XP, Liu JX. Antihypertensive effects of alcoholic extracts from Salsola. Mod Food Sci Technol. 2007;23:17–19. [Google Scholar]
- 12.Nikiforov SB, Semenov AA, Syrchina AI. Effect of an aqueous extract of Salsola collina on the course of experimental cholelithiasis in rabbits. Pharm Chem J. 2002;36:496–499. [Google Scholar]
- 13.Qiu XJ, Huang X, Chen ZQ, Ren P, Huang W, Qin F, et al. Pharmacokinetic study of the prokinetic compounds meranzin hydrate and ferulic acid following oral administration of Chaihu-Shugan-San to patients with functional dyspepsia. J Ethnopharmacol. 2011;137:205–213. doi: 10.1016/j.jep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Ruan M, Yu B, Xu L, Zhang L, Long J, Shen XC. Attenuation of stress-induced gastrointestinal motility disorder by gentiopicroside, from Gentiana macrophylla Pall. Fitoterapia. 2015;103:265–276. doi: 10.1016/j.fitote.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Guo H, Zhang JZ, Gao WY, Qu Z, Liu CX. Anti-diarrhoeal activity of methanol extract of Santalum album L in mice and gastrointestinal effect on the contraction of isolated jejunum in rats. J Ethnopharmacol. 2014;154:704–710. doi: 10.1016/j.jep.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 16.Huang S, Liu HF, Meng N, Li B, Wang J. Hypolipidemic and antioxidant effects of Malus toringoides (Rehd) hughes leaves in high-fat-diet-induced hyperlipidemic rats. J Med Food. 2017;20:258–264. doi: 10.1089/jmf.2016.3778. [DOI] [PubMed] [Google Scholar]
- 17.Mendel M, Skalicka-Woźniak K, Chłopecka M, Dziekan N. Effect of Imperatorin on the Spontaneous Motor Activity of Rat Isolated Jejunum Strips. Evid-Based Complement Altern Med. 2015;2015:614849. doi: 10.1155/2015/614849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimoyama T, Takahashi R, Kimura M, Fukuda Y. Study of the mechanisms of a Japanese traditional fermented medicine in the improvement of constipation. J Gastroenterol Hepatol. 2015;30:53–59. doi: 10.1111/jgh.12741. [DOI] [PubMed] [Google Scholar]
- 19.Zhao HL, Ma YZ, Li JX, Piao L. Study on edible value of Suaeda salsa (L) Pall. J Anhui Agric Sci. 2010;38:14350–14351. [Google Scholar]
- 20.Liu XL, Yang CY, Zhang LB, Li LZ, Liu SJ, Yu JB, et al. Metabolic profiling of cadmium-induced effects in one pioneer intertidal halophyte Suaeda salsa by NMR-based metabolomics. Ecotoxicology. 2011;20:1422–1431. doi: 10.1007/s10646-011-0699-9. [DOI] [PubMed] [Google Scholar]
- 21.Liu XL, Wu HF, Ji CL, Wei L, Zhao JM, Yu JB. An integrated proteomic and metabolomic study on the chronic effects of mercury in Suaeda salsa under an environmentally relevant salinity. PLoS One. 2013;8:e64041. doi: 10.1371/journal.pone.0064041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talley NJ, Stanghellini V, Heading RC, Holtmann G, Hu PJ, Malagelada J, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Kim I, LeeMC , Kim HJ, Lee GS, Kim H, et al. Effects of Hwangryunhaedok-tang on gastrointestinal motility function in mice. World J Gastroenterol. 2017;23:2705–2715. doi: 10.3748/wjg.v23.i15.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan WX, Li Y, Wang Y, Zhang Z, Wang T, Zhou Q, et al. Anti-coagulative and gastrointestinal motility regulative activities of Fructus Aurantii Immaturus and its effective fractions. Biomed Pharmacother. 2017;90:244–252. doi: 10.1016/j.biopha.2017.03.060. [DOI] [PubMed] [Google Scholar]
- 25.Sanger GJ, Furness JB. Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2016;13:38–48. doi: 10.1038/nrgastro.2015.163. [DOI] [PubMed] [Google Scholar]
- 26.Fourmy D, Gigoux V, Reubi JC. Gastrin in gastrointestinal diseases. Gastroenterology. 2011;141:814–818. doi: 10.1053/j.gastro.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Ohno T, Mochiki E, Kuwano H. The roles of motilin and ghrelin in gastrointestinal motility. Int J Pept. 2010;2010:466–469. doi: 10.1155/2010/820794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimiya M, Inui A. Peptidergic regulation of gastrointestinal motility in rodents. Peptides. 2000;21:1565–1582. doi: 10.1016/s0196-9781(00)00313-2. [DOI] [PubMed] [Google Scholar]
- 29.Gao RM, Xu L, Meng XY, Wang Q. Expression of motilin and vasoactive intestinal peptide in gastric mucosa of patients with primary bile reflux gastritis. World Chin J Digestol. 2010;18:722–725. [Google Scholar]
- 30.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4:1339–1368. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]