Abstract
Background
Low-dose methotrexate (LD-MTX) is the most commonly used drug for systemic rheumatic diseases worldwide and the recommended first-line agent for rheumatoid arthritis. Despite extensive clinical use for over 40 years, few data on adverse event (AE) rates derive from randomized placebo-controlled trials, where both causality and magnitude of risk can be inferred.
Objective
To investigate AE rates, risk, and risk differences, comparing LD-MTX to placebo.
Design
Pre-specified secondary analyses of a double-blind placebo-controlled randomized trial.
Setting
North America.
Participants
Adults with known cardiovascular disease and diabetes or metabolic syndrome.
Intervention
Random allocation to LD-MTX (maximum of 20mg/week) or placebo. All subjects received folic acid 1mg per day for six days/week.
Measurements
The risk of specific AEs of interest as well as all AEs were compared across treatment arms, after blinded adjudication.
Results
6,158 patients were enrolled and 4,786 randomized after an active run-in period; median follow-up was 23 months and median weekly dosage 16mg. Of the randomized subjects, 81.2% were male with a median age of 65.0 years and a median body mass index of 31.5 kg/m2. Of 2,391 subjects randomized to LD-MTX, 2080 (87.0%) experienced an AE of interest compared to 1951 of 2,395 (81.5%) randomized to placebo (HR 1.17, 95% CI 1.10 – 1.25). The relative hazards of gastrointestinal (HR 1.91, 95% CI 1.75 – 2.10), pulmonary (HR 1.52, 95% CI 1.16 – 1.98), infectious (HR 1.15, 95% CI 1.01 – 1.30), and hematologic (HR 1.15, 95% CI 1.07 – 1.23) AEs were elevated, comparing LD-MTX to placebo. With the exception of an increased risk of skin cancers (HR 2.05 (1.28, 3.28)), there was no difference between treatment arms for the risk of other malignancies, mucocutaneous, neuro-psychiatric, or musculoskeletal AEs. Renal AEs were reduced in subjects randomized to LD-MTX (HR 0.86, 95% CI 0.78 – 0.94).
Limitations
The trial was conducted in non-rheumatic disease patients who tolerated LD-MTX during an active run-in.
Conclusion
Use of LD-MTX was associated with small to moderate elevations in risks for skin cancer, gastrointestinal, infectious, pulmonary, and hematologic AEs, while renal AEs were decreased.
INTRODUCTION
Low-dose methotrexate (LD-MTX) serves as the cornerstone treatment and first-line medication in rheumatoid arthritis, used by millions of people worldwide (1). Dosages used for rheumatoid arthritis are typically 10–25mg per week, much lower than those used for cancer. LD-MTX has been used in rheumatoid arthritis for over 30 years with studies demonstrating reduced symptoms, less joint damage, synergistic benefits with biologic treatments, and possible mortality benefits (2–7). LD-MTX is also widely used in other systemic rheumatic diseases, such as psoriatic arthritis, dermatomyositis, and systemic lupus erythematosus (8–10).
With decades of clinical use, LD-MTX has been associated with many toxicities, including hematologic (11–13), malignant, gastrointestinal (hepatic, nausea, and others) (14–22), pulmonary (23, 24), infectious (25–27), mucocutaneous (28, 29), renal (30), neuro-psychiatric (31), and musculoskeletal (32). For example, anemia, thrombocytopenia, and leukopenia were reported in 3 – 10% of patients during the 1980s and 1990s (11, 13, 33). Folic acid supplementation became routine in the late 1990s, but its impact on bone marrow toxicity and cytopenias is not clear (34). The impact of LD-MTX on cancer risk is unknown. High-dose MTX is widely used to treat various cancers, but observational data in patients with rheumatoid arthritis using LD-MTX demonstrate elevated standardized incidence ratios for any cancer, melanoma, lung cancer, and non-Hodgkins lymphoma (35). As well, several other epidemiologic studies observed an association between LD-MTX use and an increased risk of skin cancers (35, 36).
Liver toxicity associated with LD-MTX is a major concern for clinicians and patients and can result in cirrhosis, prompting monitoring recommendations to conduct liver testing approximately every 8 to 12 weeks (37). These recommendations are based on the observation that persistent liver test abnormalities are almost always seen prior to major hepatic toxicity, such as cirrhosis (38). While abnormal liver tests may be observed as the first evidence of a potential problem, the vast majority of abnormal liver tests represent innocuous fluctuations and normalize with little or no manipulation of the LD-MTX dosage. There are no trials that performed blinded adjudication of hepatic-related adverse events. In contrast to the relatively uncommon outcome of cirrhosis, gastrointestinal intolerance is considered common with LD-MTX use, even with contemporary folic acid supplementation. A recent systematic review described that 52 of 174 (30%) patients using LD-MTX experienced nausea or gastrointestinal upset (39).
LD-MTX may induce pulmonary AEs such as cough and dyspnea (40). Hypersensitivity pneumonitis due to LD-MTX is thought to be a relatively rare syndrome characterized by fever, shortness of breath, eosinophilia, and interstitial lung abnormalities on chest imaging; some cases progress to permanent lung damage (41, 42). Prior studies suggest pneumonitis occurs in 0.1–1% of patients using LD-MTX (43). However, systemic rheumatic diseases may have pulmonary manifestations such as interstitial lung disease unrelated to LD-MTX, posing a challenge for determining causality (44). In addition, LD-MTX may increase the risk for infection due to its immunosuppressive effects (26).
Prior studies of LD-MTX toxicity have been based on patients followed in rheumatic disease cohorts in which all subjects receive treatment; thus, previous observational studies have been limited by lack of a placebo control group. In addition, most prior placebo-controlled trials had small sample size with short duration, and routine folic acid supplementation was uncommon (45). Prior studies have also grouped all adverse events (AEs) to increase statistical power, but this limits interpretation.
To avoid these issues, we blindly adjudicated AEs based on standardized clinical criteria and record review and formally evaluated rates associated with LD-MTX use in the setting of a large contemporary randomized double-blind placebo-controlled trial that enrolled men and women with cardiovascular disease and either type 2 diabetes or metabolic syndrome. We pre-specified the current analyses and the LD-MTX toxicity endpoints assessed.
METHODS
Study Population and Design
The Cardiovascular Inflammation Reduction Trial (CIRT) was a randomized double-blind placebo-controlled trial with enrollment goals based on a target number of cardiovascular outcomes. CIRT was prematurely halted because of a lack of efficacy in the primary outcome of MI, stroke, or death due to cardiovascular events (46). The trial enrolled patients starting in April 2013 with study drug terminated in April 2018 and final safety visits in December 2018. Alongside CIRT, we performed the current prospective study of pre-specified and adjudicated AEs (1). The primary study population was the randomized CIRT trial cohort (see Supplemental Figure 1, but we also included subjects from the active LD-MTX run-in phase as part of sensitivity analyses. Patients with known systemic rheumatic disease were excluded from CIRT. All potentially eligible patients were required to have had a known myocardial infarction or clearly demonstrated multi-vessel obstructive coronary artery disease at entry, as well as type 2 diabetes or metabolic syndrome. A full list of selection criteria is reported in Supplemental Table 1.
All subjects meeting selection criteria and consenting were entered into a 5–8 week active run-in period during which all were given LD-MTX. The dosage was up-titrated from 5mg to 15mg weekly over the run-in period; patients were seen after 2–3 weeks to assess for possible adverse events and laboratory assessment occurred at the end of the run-in period to assist in determining tolerability of LD-MTX. Subjects who successfully completed the active 5–8 week run-in phase with minimal side effects and sufficient compliance were randomized 1:1 to receive either oral LD-MTX or placebo; no subcutaneous dosing was used. The initial weekly dosage post-randomization was 15mg on one day per week and 1mg of folic acid on the six other days. After 16 weeks, if all safety criteria were met, the study drug (LD-MTX or placebo) was increased to the maximum dose of 20mg weekly. During follow-up, subjects who experienced an AE were eligible to remain on study drug if the event was deemed reversible and did not put the subject at undue risk for future LD-MTX toxicity. However, subjects were withdrawn from study drug with continued follow-up if they experienced significant and unexplained chronic liver test abnormalities, myelosuppression, chronic infections, a malignancy (except for non-melanoma skin cancers and localized prostate cancer), interstitial lung abnormalities, and newly diagnosed chronic kidney disease stage 3 or 4. Study drug was discontinued for patients who developed systemic rheumatic disease (n = 10) where LD-MTX or other immunosuppressants may be indicated. Study drug was also discontinued for subjects who developed a confirmed primary cardiovascular endpoint.
Using a central blinded computerized drug-dosing algorithm, subjects in both the active and placebo groups had their dosage of study drug reduced or folic acid increased in response to certain mild toxicities, as commonly done in routine practice. For example, subjects who noted mouth pain or alopecia would have their study drug dosage reduced (e.g., 20mg to 15mg weekly) and folic acid increased (e.g., from 1mg to 2mg per day for six days each week). Laboratory assessments were conducted at study visits every 4–12 weeks.
Identification and Adjudication of Adverse Events
We identified all potential AEs of interest (see Supplemental Figure 2 for adjudication forms and below for a list), regardless of whether the site investigator thought that the AE was related to the study drug or not. Potential AEs were identified from multiple sources, such as routine visit and AE case report forms, reasons given by the site for a temporary or permanent study drug discontinuation, and central laboratory monitoring values.
We pre-specified AEs of interest because prior literature suggested an association with LD-MTX; these AEs were adjudicated by study physicians blinded to randomization group using standardized forms based on previously published criteria (47–50). Details of the adjudication process and a full list of AEs can be found in Supplementary Figure 2. The list of these events included gastrointestinal (hepatic and other), pulmonary, infectious, hematologic (hemorrhage and cytopenias), malignant, mucocutaneous (skin and oral), renal (acute kidney injury or nephrolithiasis), neuro-psychiatric, and musculoskeletal. Identification of potential AEs of interest that required adjudication triggered a process of record requests from the subject’s site. Information was requested before adjudication moved forward. If initial adjudication revealed the need for specific information that had not been provided by the site, up to three additional requests were made.
The malignant AEs included all malignancies, with pre-malignant diagnoses not included. A hematology-oncology sub-specialist (NB) supervised the blinded adjudication based on medical records using a standardized form (see Supplementary Figure 2). Cancer outcomes were considered definite if pathology reports noted malignancy, probable if treatment for cancer was documented but no definite pathology was available, and possible if cancer was mentioned but no pathology or cancer treatment was described in the medical record.
A hepatologist (AR) guided the blinded adjudication of gastrointestinal and liver AEs using a standardized form (see Supplementary Figure 2). Liver test abnormalities were categorized based on the upper limits of normal (ULN) from national reference laboratories. The ULN for AST was 35 (men and women); for ALT, it was 29 for women and 46 for men. Mild elevations in the liver tests were defined as >1 to 2x ULN, moderate elevations, >2 to 3x ULN, and severe >3x ULN. We also examined the frequency of repeated abnormal liver tests (i.e., elevations in 5 of 9 consecutive AST determinations); this has been suggested as a risk factor for cirrhosis in prior work (20). The other gastrointestinal AEs included the following sub-types: nausea, vomiting, or diarrhea (all categorized together); abdominal pain without nausea, vomiting or diarrhea; gall stones or cholecystitis; and other. The other category included small bowel obstruction, constipation, pancreatitis, and other less common gastrointestinal conditions.
Infectious AEs underwent adjudication under the guidance of an infectious diseases specialist (SPH). At each study visit, current and recent infections and infectious symptoms were queried. If positively reported, medical records were obtained to classify the presence, dates of onset/resolution, type, likelihood, and severity of infection. Possible infections were defined by symptoms of infection such as fever that were not treated with systemic antimicrobials. Probable infections were defined by symptoms of infection that were treated with systemic (non-topical) antimicrobials. Definite infections were defined by the combination of symptoms of infection with microbiological evidence and/or infectious disease consultant with impression of infection and/or radiographic evidence without other more likely causes. Infections were broadly grouped into categories based on the predominant clinical manifestations: upper respiratory/viral syndrome, skin/soft tissue, pneumonia, ears/eyes/nose/throat and dental, genitourinary, gastrointestinal, shingles, bone/joint, candidiasis, sepsis, and not otherwise specified (NOS).
Possible pulmonary AEs underwent blinded adjudication using a standardized form (see Supplementary Figure 2) under the guidance of a rheumatology/critical care medicine specialist (PFD). Possible pulmonary AEs were elicited from subjects at every study visit and medical records were obtained to classify the presence, dates of onset/resolution, type, likelihood, and severity of events. Possible pulmonary AEs were defined by new respiratory symptoms with normal or no diagnostic testing obtained. Probable pulmonary AEs had new symptoms with abnormalities on diagnostic testing. Definite pulmonary AEs had new symptoms, diagnostic test abnormalities, and symptoms improved off study drug. Pulmonary AEs were broadly grouped into categories: bronchitis, shortness of breath, cough, chronic obstructive pulmonary disease/asthma flare, pneumonitis, and NOS.
Any pulmonary AE with mention of pulmonary fibrosis, interstitial lung disease, and/or ground-glass opacities was additionally reviewed for the possibility of pneumonitis. We used previously published criteria to classify the probability of pneumonitis due to LD-MTX (47). Possible pneumonitis required at least four of the following criteria: fever, tachypnea or cough, radiologic evidence of interstitial/alveoloar abnormalities, leukocytosis, and negative blood or sputum cultures. Probable cases additionally had clinical suspicion of being induced by LD-MTX. Definite cases of pneumonitis were additionally strongly suspected of being induced by LD-MTX and symptoms improved after study drug discontinuation.
For AEs that are primarily subjective complaints, such as nausea, abdominal pain, and alopecia, further records were not pursued, and the description given on the case report form was used for adjudication. After reviewing available medical records and information from the sites, AEs of interest were classified by likelihood: possible, probable, or definite events; as well as by severity: mild, moderate, or severe. Some AEs of interest were not categorized as possible, probable or definite but rather “reported” by patients or “confirmed” by medical records; the reported events were considered possible, and the confirmed events considered definite. The primary outcome included probable or definite (or those confirmed by medical records) and did not include possible. Secondary outcomes examined AEs by likelihood and by severity. Severity of the AEs was defined as mild, moderate or severe based on pre-specified thresholds (see Supplementary Table 2). Laboratory assessments, including complete blood counts (anemia, thrombocytopenia, and leukopenia), liver tests (elevated AST or ALT), and serum creatinine (translated into the estimated glomerular filtration rate, eGFR, using the MDRD equation), were performed at central laboratories approximately every eight weeks during follow-up. We specified thresholds for the abnormal laboratory values according to standard definitions (46).
We also defined a secondary outcome using not only the AEs of interest, but all AEs noted on the case report forms; this improved comparability to the main CIRT trial analyses (46). For this secondary analysis, AEs were categorized according to MedDRA rather than the adjudicated categories (51). While the adjudication process described above provides a more sensitive and specific measure for the events of interest, we chose to use the MedDRA standardized method across all event types for this secondary outcome to give numbers comparable to the original trial report.
Statistical Analyses
As per the pre-specified analysis plan, the primary analysis examined the occurrence of any of the pre-specified adjudicated AEs of interest and laboratory abnormalities in the randomized study cohort. Thus, the primary analysis excluded subjects who did not complete the active run-in phase. The primary analyses considered all post-randomization first events within each category by severity and/or sub-type of event, depending on the analysis, occurring after randomization; thus, only the first AE of a given type was included and subjects were able to contribute AEs in multiple categories. The primary analysis of the frequency and relative rates of adverse events followed a modified intention-to-treat strategy (sometimes referred to as an “as treated” approach). Since approximately 19% of subjects prematurely discontinued treatment after randomization and may have been followed for many months after treatment discontinuation, we followed subjects from the time of randomization through 180 days after the known date of treatment discontinuation, as noted by the study site. This modified intention-to-treat strategy (sometimes referred to as an “as-treated” approach) is commonly used for safety analyses. A secondary analysis used an intention-to-treat approach, without censoring at study drug discontinuation.
We constructed cumulative incidence curves for the AE endpoints. These curves examined the time to first AE for the LD-MTX and placebo arms separately. Statistical significance was judged using a likelihood ratio test based on proportional hazard regression models, adjusted for the stratifying variables: time since qualifying CV event (≥ 6 months versus < 6 months), type of prior cardiovascular event (myocardial infarction versus multivessel coronary artery disease), and diabetes mellitus versus metabolic syndrome alone at baseline. The three-year cumulative incidence percent risks were estimated from the Kaplan-Meier survival curves, and proportional hazard models were fit separately for any AE and then for each AE of interest. The null hypothesis being tested was no association between assignment to LD-MTX versus placebo, and the exact method was used to handle ties. The proportional hazard assumption was tested using Schoenfeld residuals (52). The differences in the three-year cumulative incidence risk between LD-MTX and placebo were calculated.
Several secondary analyses were pre-specified, but we did not correct p-values for multiple comparisons; instead, we show the 95% confidence intervals. First, analysis of the primary outcome was conducted using an intention-to-treat approach, including all time from randomization until the first of any of the following: AE, death, end of trial, withdrawal of consent, or termination of study. Second, the modified intention-to-treat analysis was conducted using 0- and 90-days after termination of treatment as alternative censoring dates. Third, possible adverse events were included along with the probable and definite events. Fourth, the hazard of specific types of AEs of interest were estimated using the same proportional hazards models described above for the modified intention-to-treat analysis. Fifth, the frequency and relative rates of mild, moderate, and severe events were estimated separately. Sixth, the frequency and relative risk of any AE, including the MedDRA site-reported but not adjudicated events, were also estimated. Finally, the frequency of AEs occurring during pre-randomization period was assessed and logistic regression was used to examine factors associated with subjects not randomized.
All analyses were conducted in SAS (Cary NC, version 9.4).
RESULTS
9,321 subjects were screened for the CIRT trial and 6,158 entered the pre-randomization phase (see Supplemental Figure 1). 4,786 (77.7%) of those who participated in the active run-in were eventually randomized and followed for a median of 23 months on study drug, with similar follow-up in both arms. The characteristics of the randomized groups are displayed in Table 1. Age, sex, body mass index, tobacco use, alcohol use, frequency of diabetes, eGFR, and use of medications were similar across treatment arms. People not randomized were more likely to be black, non-users of statins and aspirin and had lower self-reported general health.
Table 1:
Baseline Characteristics of Randomized and Non-Randomized Populations in CIRT Trial
| Randomized population | Non-randomized population* (n = 1,372) | ||
|---|---|---|---|
| Low dose methotrexate (n = 2,391) | Placebo (n = 2,395) | ||
| Median (IQR) or n (%) unless noted | |||
| Female sex | 461 (19.3) | 437 (18.2) | 321 (23.4) |
| Age, (age at enrollment date) | 65.5 (59.5, 71.6) | 65.9 (59.7, 71.6) | 65.6 (58.6, 72.0) |
| Race | |||
| White | 2008 (84.0) | 2059 (86.0) | 1118 (81.5) |
| Black or African American | 194 (8.1) | 156 (6.5) | 135 (9.8) |
| Asian | 89 (3.7) | 92 (3.8) | 54 (3.9) |
| American Indian or Alaska Native | 6 (0.25) | 7 (0.29) | 4 (0.29) |
| Native Hawaiian or other Pacific Islander | 4 (0.17) | 6 (0.25) | 5 (0.36) |
| Multiple | 15 (0.6) | 9 (0.38) | 8 (0.58) |
| Other | 75 (3.1) | 66 (2.8) | 48 (3.5) |
| Diabetes | 1620 (67.8) | 1615 (67.4) | 957 (69.8) |
| Metabolic syndrome | 1603 (67.0) | 1572 (65.6) | 900 (65.6) |
| Hyperlipidemia | 2053 (85.9) | 2050 (85.6) | 962 (70.1) |
| Hypertension | 2153 (90.0) | 2169 (90.6) | … |
| Current cigarette use | 267 (11.2) | 270 (11.3) | … |
| Alcohol use | |||
| Rarely or never | 1487 (62.2) | 1473 (61.50) | … |
| ≤ 1 drink/week | 514 (21.5) | 520 (21.71) | … |
| >1 drink/week | 390 (16.3) | 402 (16.8) | … |
| eGFR, ml/min/1.73m2 | 81.2 (67.8, 95.3) | 80.9 (66.4, 95.5) | … |
| Body mass index, kg/m2 | 31.6 (28.1, 35.7) | 31.3 (28.0, 35.5) | 31.2 (27.8, 35.3) |
| Weekly study drug dosage (mean, SD)** | 14.9 (4.5) | 15.3 (4.3) | - |
| Statin use | 2062 (86.2) | 2052 (85.7) | 976 (71.1) |
| Aspirin use | 1861 (77.8) | 1807 (75.4) | 875 (63.8) |
| Insulin use | 515 (21.5) | 535 (22.3) | 297 (21.7) |
| Oral corticosteroid use | 27 (1.1) | 22 (0.9) | 7 (0.5) |
| Respiratory medication use ǂ | 273 (11.4) | 291 (12.2) | 164 (12.0) |
| Non-corticosteroid inhalers | 186 (7.8) | 212 (8.9) | 120 (8.8) |
| Corticosteroid inhalers | 42 (1.8) | 55 (2.3) | 22 (1.6) |
| Combination inhaler | 136 (5.7) | 135 (5.6) | 88 (6.4) |
| Oral and intravenous medications | 48 (2.0) | 54 (2.3) | 19 (1.4) |
| SF36 General Health | 60 (45, 75) | 60 (45, 75) | 55 (35, 70) |
| CES-D 10 | 5 (2, 9) | 5 (2, 8) | 6 (3, 10) |
Abbreviations: eGFR, estimated glomerular filtration rate; SF36, short-form 36; CES-D 10, Center for Epidemiologic Studies – Depression Scale, 10-item version.
Includes subjects that took a dosage of open-label methotrexate during the pre-randomization run-in phase but was not ultimately randomized. The ellipsis (…) signifies that over 90% of that variable was missing for non-randomized subjects since these were primarily collected at the time of randomization.
Mean weekly dosage refers to the post-randomization period.
Respiratory medications include non-corticosteroid, corticosteroid and combination inhalers, as well as oral and intravenous medications used for respiratory conditions.
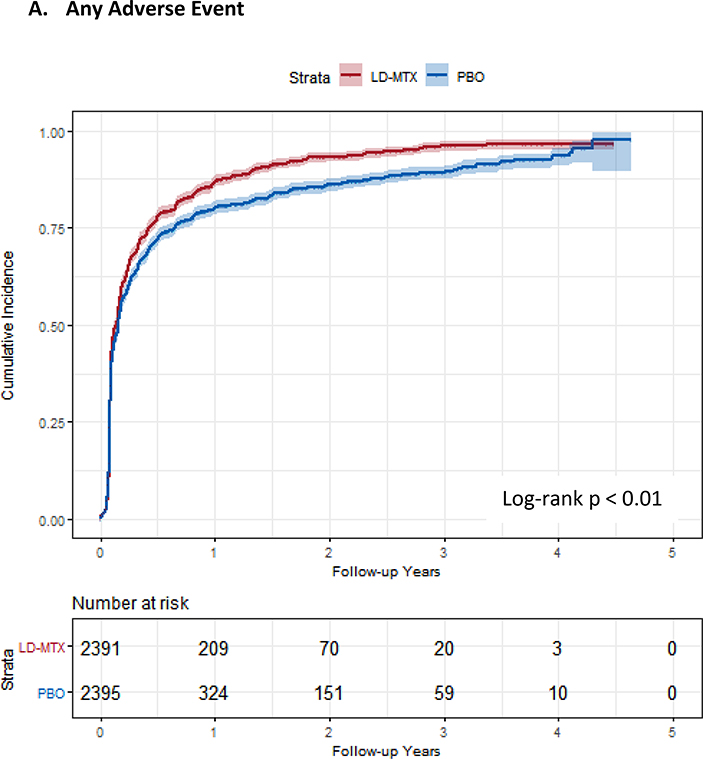
Of the 2,391 subjects randomized to LD-MTX, 2,156 (90.20%) experienced any AE and 2080 (87.0%) experienced an adjudicated AE of interest. Of the 2,395 randomized to placebo, 2,076 (86.7%) experienced any AE and 1951 (81.5%) experienced an adjudicated AE of interest. The relative rate of an AE of interest was 17% higher for those randomized to LD-MTX (HR 1.17, 95% CI 1.10 – 1.25) compared to placebo. The cumulative incidence curves for AEs are shown in Figure 1 and demonstrate an increased risk of overall AEs (log-rank p < 0.001). The frequency, relative rates, cumulative risk and risk differences of mild, moderate, and severe events were estimated for both treatment arms (see Table 2). We found similar increases in relative rates for LD-MTX compared to placebo across all severity ratings.
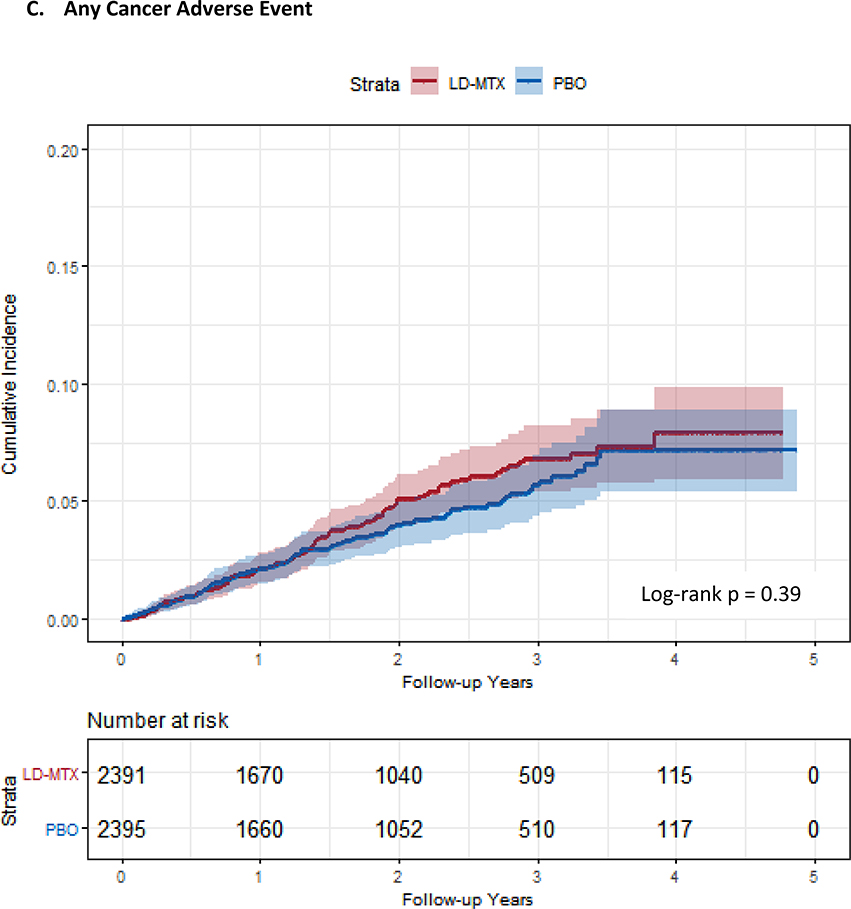
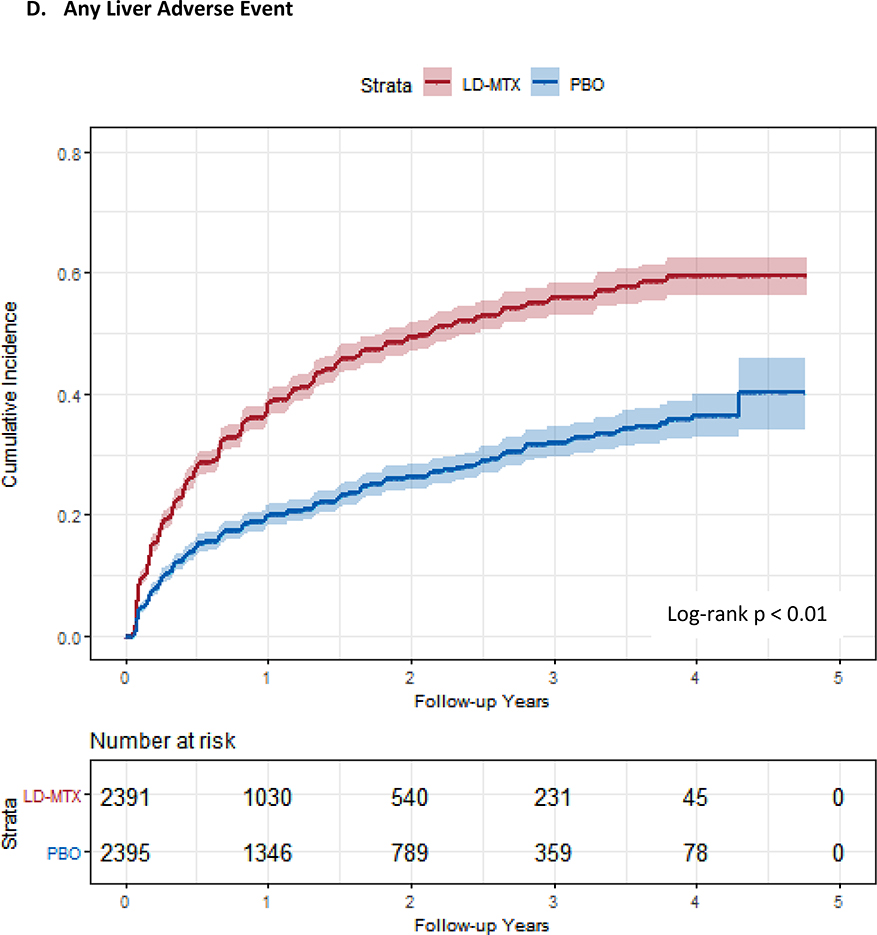
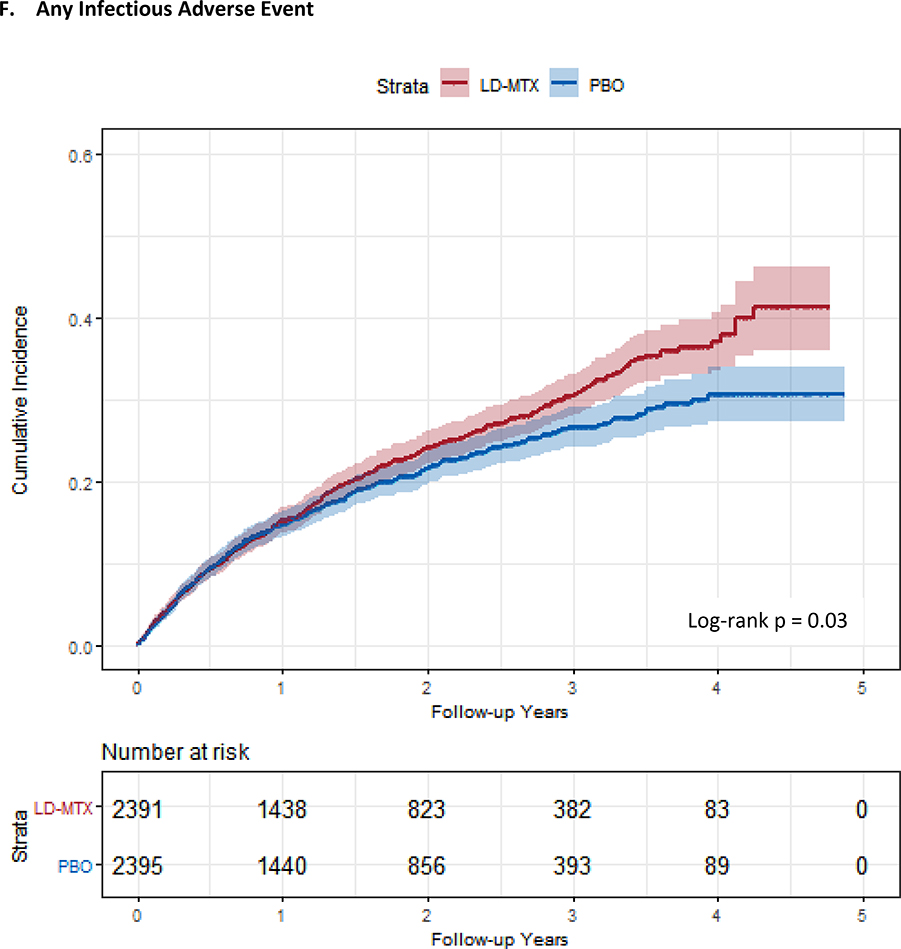
Figure 1:
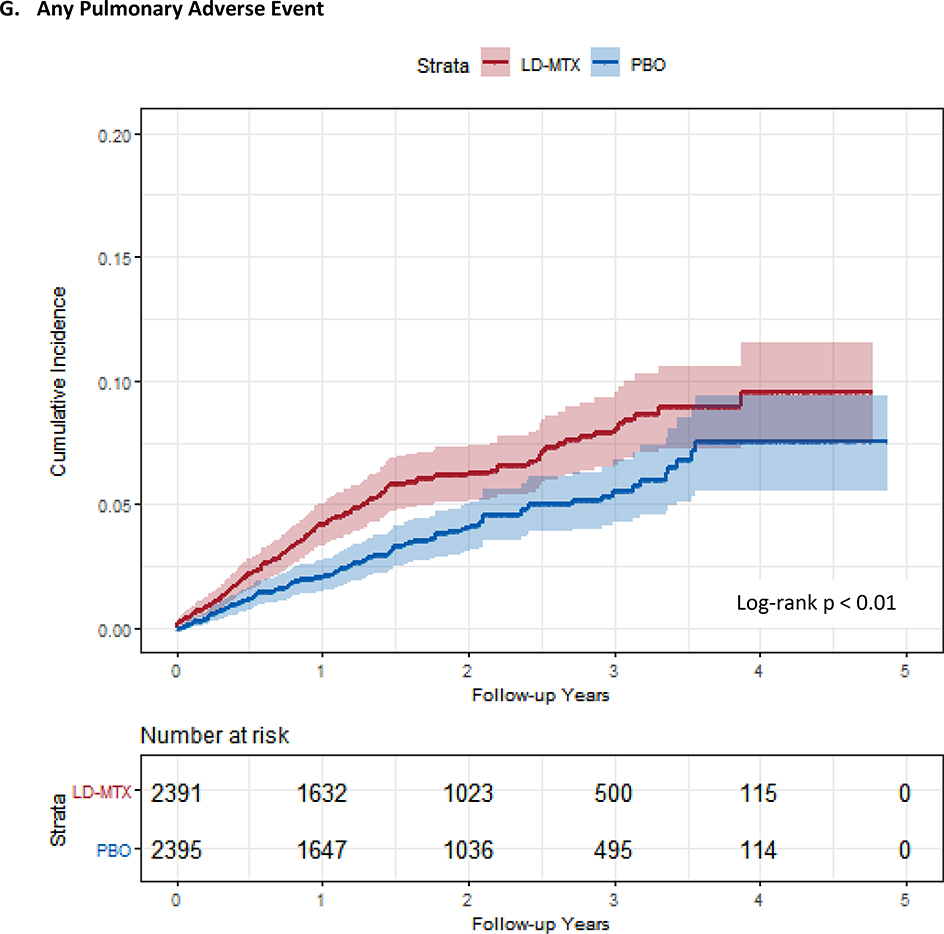
Cumulative incidence plots with 95% confidence intervals for the modified intention to treat analyses, censoring 180 days after the last dosage of study drug. Log-rank test was used to estimate the p-value. Abbreviations: LD-MTX, low dose methotrexate; PBO, placebo. Panel A: All adverse events of interest with laboratory abnormalities included. Panel B: Any hematologic adverse events with laboratory abnormalities included. Panel C: All cancer adverse events. Panel D: All liver adverse events with laboratory abnormalities included. Panel E: All gastrointestinal adverse events, excluding liver and liver test abnormalities. Panel F: All infectious adverse events. Panel G: All pulmonary adverse events.
Table 2:
Risks Comparing Adverse Events for Methotrexate to Placebo During the Randomized Phase of CIRT
| Low dose methotrexate (n=2391) | Placebo (n=2395) | Risk Difference* | Hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| N (%)* | Rate* | 3-year* | N (%)* | Rate* | 3-year* | |||
| Adverse events of interest, including labs* | 2080 (87.0) | 241 (230, 251) | 0.96 (0.95, 0.97) | 1951 (81.5) | 172 (164, 179) | 0.89 (0.88, 0.91) | 0.07 (0.05, 0.09) | 1.17 (1.10, 1.25) |
| Mild | 2023 (84.7) | 212 (203, 221) | 0.95 (0.93, 0.96) | 1890 (78.9) | 153 (146, 160) | 0.87 (0.85, 0.89) | 0.08 (0.05, 0.10) | 1.18 (1.11, 1.26) |
| Moderate | 772 (32.3) | 22.0 (20.4, 23.5) | 0.44 (0.41, 0.47) | 661 (27.6) | 18.2 (16.8, 19.5) | 0.37 (0.35, 0.40) | 0.07 (0.03, 0.10) | 1.19 (1.08, 1.33) |
| Severe | 222 (9.3) | 5.1 (4.4, 5.8) | 0.13 (0.12, 0.15) | 155 (6.5) | 3.5 (2.9, 4.1) | 0.10 (0.08, 0.11) | 0.04 (0.01, 0.06) | 1.46 (1.19, 1.79) |
| Adverse events of interest, excluding labs | 1012 (42.3) | 33.1 (31.0, 35.1) | 0.54 (0.52, 0.57) | 901 (37.6) | 28.1 (26.3, 29.9) | 0.49 (0.46, 0.52) | 0.05 (0.02, 0.09) | 1.16 (1.06, 1.27) |
| Mild | 612 (25.6) | 16.5 (15.2, 17.8) | 0.35 (0.33, 0.38) | 512 (21.3) | 13.5 (12.3, 14.7) | 0.28 (0.26, 0.31) | 0.07 (0.04, 0.10) | 1.21 (1.08, 1.37) |
| Moderate | 530 (22.2) | 13.7 (12.6, 14.9) | 0.30 (0.28, 0.33) | 475 (19.8) | 12.1 (11.0, 13.2) | 0.27 (0.25, 0.30) | 0.03 (0.00, 0.06) | 1.13 (1.00, 1.28) |
| Severe | 152 (6.4) | 3.4 (2.9, 4.0) | 0.09 (0.08, 0.11) | 125 (5.2) | 2.8 (2.3, 3.3) | 0.08 (0.07, 0.10) | 0.01 (−0.01, 0.03) | 1.22 (0.97, 1.55) |
| Coded adverse events** | 2156 (90.9) | 305 (292, 318) | 0.97 (0.96, 0.98) | 2076 (86.7) | 226 (217, 236) | 0.94 (0.92, 0.95) | 0.04 (0.02, 0.06) | 1.14 (1.08, 1.22) |
| MedDRA events, with labs | 2155 (90.1) | 289 (277, 301) | 0.97 (0.95, 0.98) | 2071 (86.5) | 214 (205, 224) | 0.93 (0.91, 0.94) | 0.04 (0.02, 0.06) | 1.15 (1.08, 1.22) |
| MedDRA events, no labs | 1483 (62.0) | 65.8 (62.5, 69.1) | 0.74 (0.72, 0.77) | 1432 (59.8) | 62 (58, 65) | 0.73 (0.71, 0.75) | 0.02 (−0.02, 0.05) | 1.06 (0.98, 1.14) |
Notes: These numbers are based on the modified intention to treat analyses (see text for details).
N includes first events of a given type and the percentages refer to the number of subjects with a given adverse event divided by all subjects in the respective treatment arm. The first mild, moderate, and severe are all included. As well, the first of each type of adverse event was included. Rates are per 100 person-years. 3-year refers to the cumulative incidence percent risk. Risk difference is based on the 3-year cumulative incidence percent risk.
The outcome labeled “coded adverse events” includes MedDRA coded events, not just the adjudicated events of interest.
The relative rates of gastrointestinal (HR 1.91, 95% CI 1.75–2.10), pulmonary (HR 1.52, 95% CI 1.16–1.98), infectious (HR 1.15, 95% CI 1.01–1.30), and hematologic (HR 1.15, 95% CI 1.07–1.23) AEs were elevated for LD-MTX (see Tables 3–5) compared to placebo. There were no differences between treatment arms for the risk of overall malignancies (see Table 3), but subjects in the LD-MTX arm experienced 53 (2.2%) skin cancers and placebo 26 (1.1%), for an HR 2.05 (95% CI 1.28 – 3.28). The risk for squamous cell skin cancers reached statistical significance, HR 3.31 (95% CI 1.63 – 6.71). As well, the risk of anemia (HR 1.36, 95% CI 1.22–1.52) and leukopenia (HR 1.46, 1.19–1.80) were elevated, but no increase was observed for thrombocytopenia (HR 0.78, 95% CI 0.65–0.93).
Table 3:
Frequency and Relative Risks Comparing Hematologic and Cancer Adverse Events for Methotrexate to Placebo During the Randomized Phase of CIRT
| Low dose methotrexate (n=2391) | Placebo (n=2395) | Risk Difference* | Hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| N (%)* | Rate* | 3-year* | N (%)* | Rate* | 3-year* | |||
| Anemia | 722 (30.2) | 20.9 (19.4, 22.4) | 0.37 (0.35, 0.40) | 555 (23.2) | 15 (13.8, 16.3) | 0.30 (0.28, 0.33) | 0.07 (0.04, 0.11) | 1.36 (1.22, 1.52) |
| Mild | 630 (26.4) | 17.7 (16.3, 19.1) | 0.32 (0.30, 0.35) | 485 (20.3) | 12.8 (11.6, 13.9) | 0.26 (0.24, 0.29) | 0.06 (0.03, 0.09) | 1.36 (1.21, 1.53) |
| Moderate | 110 (4.6) | 2.4 (2.0, 2.9) | 0.07 (0.05, 0.08) | 76 (3.2) | 1.7 (1.3, 2.1) | 0.04 (0.03, 0.06) | 0.02 (0.01, 0.04) | 1.45 (1.08, 1.94) |
| Severe | 10 (0.4) | 0.2 (0.1, 0.3) | 0.01 (0.00, 0.01) | 9 (0.4) | 0.2 (0.1, 0.3) | 0.01 (0.00, 0.01) | 0.00 (0.00, 0.00) | 1.10 (0.45, 2.71) |
| Thrombocytopenia | 204 (8.5) | 4.7 (4.1, 5.4) | 0.12 (0.10, 0.14) | 256 (10.7) | 6.1 (5.4, 6.9) | 0.14 (0.12, 0.16) | −0.02 (−0.04, 0.01) | 0.78 (0.65, 0.93) |
| Mild | 202 (8.5) | 4.7 (4.0, 5.3) | 0.12 (0.10, 0.14) | 255 (10.7) | 6.1 (5.3, 6.8) | 0.14 (0.12, 0.16) | −0.02 (−0.04, 0.01) | 0.77 (0.64, 0.93) |
| Moderate | 2 (0.1) | 0.0 (0.0, 0.1) | 0.00 (0.00, 0.00) | 3 (0.1) | 0.1 (0.0, 0.1) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) | 0.66 (0.11, 3.96) |
| Severe | 0 (0.0) | 0.0 (0.0, 0.0) | 0.00 (0.00, 0.00) | 0 (0.0) | 0.0 (0.0, 0.0) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | - |
| Leukopenia | 220 (9.2) | 5.1 (4.4, 5.8) | 0.12 (0.11, 0.14) | 152 (6.4) | 3.5 (2.9, 4.0) | 0.08 (0.07, 0.10) | 0.04 (0.02, 0.06) | 1.46 (1.19, 1.80) |
| Mild | 204 (8.5) | 4.7 (4.1, 5.4) | 0.12 (0.10, 0.13) | 139 (5.8) | 3.2 (2.7, 3.7) | 0.08 (0.06, 0.09) | 0.04 (0.02, 0.06) | 1.48 (1.19, 1.84) |
| Moderate | 28 (1.2) | 0.6 (0.4, 0.8) | 0.02 (0.01, 0.03) | 19 (0.8) | 0.4 (0.2, 0.6) | 0.01 (0.01, 0.02) | 0.00 (0.00, 0.01) | 1.47 (0.82, 2.62) |
| Cancer Type | ||||||||
| Any cancer | 101 (4.2) | 2.2 (1.8, 2.7) | 0.07 (0.06, 0.08) | 89 (3.7) | 2.0 (1.6, 2.4) | 0.06 (0.05, 0.07) | 0.01 (−0.01, 0.03) | 1.13 (0.85, 1.51) |
| Skin | 53 (2.2) | 1.2 (0.8, 1.5) | 0.04 (0.03, 0.05) | 26 (1.1) | 0.6 (0.3, 0.8) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.03) | 2.04 (1.28, 3.26) |
| Solid tumor | 39 (1.6) | 0.8 (0.6, 1.1) | 0.02 (0.02, 0.03) | 49 (2.1) | 1.1 (0.8, 1.4) | 0.03 (0.02, 0.04) | −0.01 (−0.02, 0.00) | 0.79 (0.52, 1.20) |
| Lung | 10 (0.4) | 0.2 (0.1, 0.3) | 0.01 (0.00, 0.01) | 8 (0.3) | 0.2 (0.1, 0.3) | 0.01 (0.00, 0.01) | 0.00 (0.00, 0.00) | 1.24 (0.49, 3.13) |
| Head and neck | 0 (0.0) | 0.0 (0.0, 0.0) | 0.00 (0.00, 0.00) | 1 (0.0) | 0.0 (0.0, 0.1) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | - |
| Prostate | 10 (0.4) | 0.2 (0.1, 0.3) | 0.01 (0.00, 0.02) | 16 (0.7) | 0.3 (0.2, 0.5) | 0.01 (0.01, 0.02) | 0.00 (−0.01, 0.00) | 0.62 (0.28, 1.36) |
| Bladder | 7 (0.3) | 0.2 (0.0, 0.3) | 0.00 (0.00, 0.01) | 5 (0.2) | 0.1 (0.0, 0.2) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) | 1.39 (0.44, 4.40) |
| Breast | 2 (0.1) | 0.0 (0.0, 0.1) | 0.00 (0.00, 0.00) | 5 (0.2) | 0.1 (0.0, 0.2) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) | 0.40 (0.08, 2.06) |
| Kidney | 2 (0.1) | 0.0 (0.0, 0.1) | 0.00 (0.00, 0.01) | 4 (0.2) | 0.1 (0.0, 0.2) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) | 0.49 (0.09, 2.72) |
| Intestinal/colon | 8 (0.3) | 0.2 (0.1, 0.3) | 0.00 (0.00, 0.01) | 10 (0.4) | 0.2 (0.1, 0.4) | 0.01 (0.00, 0.01) | 0.00 (−0.01, 0.00) | 0.80 (0.32, 2.01) |
| Hematologic | 6 (0.3) | 0.1 (0.0, 0.2) | 0.00 (0.00, 0.01) | 7 (0.3) | 0.2 (0.0, 0.3) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) | 0.85 (0.29, 2.51) |
| Other | 3 (0.1) | 0.1 (0.0, 0.1) | 0.00 (0.00, 0.01) | 8 (0.3) | 0.2 (0.1, 0.3) | 0.01 (0.00, 0.01) | 0.00 (−0.01, 0.00) | 0.37 (0.01, 1.39) |
Notes: These numbers are based on the modified intention to treat analyses (see text for details).
N includes first events of a given type and the percentages refer to the number of subjects with a given adverse event divided by all subjects in the respective treatment arm. The first mild, moderate, and severe are all included. As well, the first of each type of adverse event was included. Rates are per 100 person-years. 3-year refers to the cumulative incidence percent risk. Risk difference is based on the 3-year cumulative incidence percent risk.
Table 5:
Frequency and Relative Risks Comparing Infection and Pulmonary Adverse Events for Methotrexate to Placebo During the Randomized Phase of CIRT
| Low dose methotrexate (n=2391) | Placebo (n=2395) | Risk Difference | Hazard ratio(95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| N (%)* | Rate | 3-year | N (%)* | Rate | 3-year | |||
| Any infection | 531 (22.2) | 13.6 (12.4, 14.8) | 0.31 (0.28, 0.34) | 466 (19.5) | 11.8 (10.8, 12.9) | 0.27 (0.25, 0.30) | 0.04 (0.00, 0.07) | 1.15 (1.01, 1.30) |
| Mild | 340 (14.2) | 8.2 (7.4, 9.1) | 0.20 (0.18, 0.23) | 282 (11.8) | 6.8 (6.0, 7.6) | 0.16 (0.14, 0.18) | 0.04 (0.01, 0.07) | 1.21 (1.03, 1.42) |
| Moderate | 241 (10.1) | 5.6 (4.9, 6.3) | 0.14 (0.12, 0.16) | 219 (9.1) | 5.1 (4.4, 5.7) | 0.13 (0.12, 0.16) | 0.01 (−0.02, 0.03) | 1.10 (0.91, 1.32) |
| Severe | 51 (2.1) | 1.1 (0.8, 1.4) | 0.03 (0.02, 0.04) | 50 (2.1) | 1.1 (0.8, 1.4) | 0.03 (0.03, 0.05) | 0.00 (−0.02, 0.01) | 1.02 (0.69, 1.50) |
| Type of infection | ||||||||
| URI/flu syndrome | 125 (5.2) | 2.8 (2.3, 3.3) | 0.08 (0.07, 0.10) | 105 (4.4) | 2.4 (1.9, 2.8) | 0.06 (0.05, 0.08) | 0.01 (0.00, 0.03) | 1.19 (0.92, 1.54) |
| Skin and soft tissue | 129 (5.4) | 2.9 (2.4, 3.4) | 0.08 (0.07, 0.09) | 125 (5.2) | 2.8 (2.3, 3.3) | 0.08 (0.07, 0.10) | 0.00 (−0.02, 0.02) | 1.03 (0.80, 1.31) |
| Pneumonia | 67 (2.8) | 1.5 (1.1, 1.8) | 0.04 (0.03, 0.06) | 52 (2.2) | 1.1 (0.8, 1.4) | 0.03 (0.02, 0.04) | 0.01 (0.00, 0.02) | 1.28 (0.89, 1.83) |
| EENT and dental | 182 (7.6) | 4.1 (3.5, 4.7) | 0.11 (0.09, 0.13) | 149 (6.2) | 3.4 (2.9, 4.0) | 0.08 (0.07, 0.10) | 0.03 (0.01, 0.05) | 1.21 (0.98, 1.51) |
| Genitourinary | 111 (4.6) | 2.5 (2.0, 2.9) | 0.06 (0.05, 0.08) | 86 (3.6) | 1.9 (1.5, 2.3) | 0.05 (0.04, 0.07) | 0.01 (−0.01, 0.03) | 1.30 (0.98, 1.72) |
| Gastrointestinal | 37 (1.6) | 0.8 (0.5, 1.1) | 0.02 (0.02, 0.03) | 35 (1.5) | 0.8 (0.5, 1.0) | 0.02 (0.02, 0.03) | 0.00 (−0.01, 0.01) | 1.05 (0.66, 1.67) |
| Shingles | 23 (1.0) | 0.5 (0.3, 0.7) | 0.01 (0.01, 0.02) | 17 (0.7) | 0.4 (0.2, 0.5) | 0.01 (0.01, 0.02) | 0.00 (0.00, 0.01) | 1.34 (0.72, 2.51) |
| Bone and Joint | 21 (0.9) | 0.5 (0.3, 0.6) | 0.01 (0.01, 0.02) | 11 (0.5) | 0.2 (0.1, 0.4) | 0.01 (0.01, 0.02) | 0.00 (0.00, 0.01) | 1.91 (0.92, 3.96) |
| Yeast | 6 (0.3) | 0.1 (0.0, 0.2) | 0.00 (0.00, 0.01) | 9 (0.4) | 0.2 (0.1, 0.3) | 0.01 (0.00, 0.01) | 0.00 (0.00, 0.00) | 0.66 (0.24, 1.83) |
| Sepsis | 12 (0.5) | 0.3 (0.1, 0.4) | 0.01 (0.00, 0.01) | 14 (0.6) | 0.3 (0.1, 0.5) | 0.01 (0.00, 0.01) | 0.00 (−0.01, 0.00) | 0.85 (0.39, 1.82) |
| NOS | 22 (0.9) | 0.5 (0.3, 0.7) | 0.01 (0.01, 0.03) | 22 (0.9) | 0.5 (0.3, 0.7) | 0.01 (0.01, 0.02) | 0.00 (−0.01, 0.01) | 0.99 (0.55, 1.79) |
| Pulmonary events | 137 (5.7) | 3.1 (2.6, 3.6) | 0.08 (0.07, 0.10) | 135 (5.6) | 3.1 (2.5, 3.6) | 0.06 (0.05, 0.07) | 0.03 (0.01, 0.04) | 1.42 (1.14, 1.77) |
| Mild | 77 (3.2) | 1.7 (1.3, 2.1) | 0.05 (0.04, 0.06) | 66 (2.8) | 1.5 (1.1, 1.8) | 0.03 (0.02, 0.05) | 0.01 (0.00, 0.03) | 1.40 (1.02, 1.93) |
| Moderate | 50 (2.1) | 1.1 (0.8, 1.4) | 0.03 (0.02, 0.04) | 61 (2.6) | 1.3 (1.0, 1.7) | 0.02 (0.01, 0.03) | 0.01 (0.00, 0.02) | 1.41 (1.02, 1.96) |
| Severe | 13 (0.5) | 0.3 (0.1, 0.4) | 0.01 (0.00, 0.02) | 8 (0.3) | 0.2 (0.1, 0.3) | 0.00 (0.00, 0.00) | 0.01 (0.00, 0.01) | 2.99 (1.34, 6.65) |
| Type of pulmonary event | ||||||||
| Bronchitis | 116 (4.9) | 2.6 (2.1, 3.1) | 0.07 (0.06, 0.08) | 73 (3.1) | 1.6 (1.3, 2.0) | 0.04 (0.03, 0.06) | 0.02 (0.01, 0.04) | 1.60 (1.19, 2.14) |
| Cough | 4 (0.2) | 0.1 (0.0, 0.2) | 0.00 (0.00, 0.01) | 5 (0.2) | 0.1 (0.0, 0.2) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) | 0.80 (0.21, 2.96) |
| Short of breath | 11 (0.5) | 0.2 (0.1, 0.4) | 0.04 (0.03, 0.06) | 8 (0.3) | 0.2 (0.1, 0.3) | 0.03 (0.02, 0.04) | 0.01 (0.00, 0.02) | 1.36 (0.55, 3.38) |
| COPD/asthma flare | 3 (0.1) | 0.1 (0.0, 0.1) | 0.01 (0.00, 0.01) | 6 (0.3) | 0.1 (0.0, 0.2) | 0.01 (0.00, 0.02) | 0.00 (−0.01, 0.00) | 0.50 (0.13, 1.99) |
| Pneumonitis | 7 (0.3) | 0.2 (0.0, 0.3) | 0.00 (0.00, 0.01) | 1 (0.04) | 0.0 (0.0, 0.1) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.00) | 6.94 (0.85, 56.0) |
| NOS | 1 (0.0) | 0 (0.0, 0.1) | 0.00 (0.00, 0.01) | 2 (0.1) | 0.0 (0.0, 0.1) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.01) | 0.48 (0.04, 5.37) |
Notes: These numbers are based on the modified intention to treat analyses (see text for details).
N includes first events of a given type and the percentages refer to the number of subjects with a given adverse event divided by all subjects in the respective treatment arm. The first mild, moderate, and severe are all included. As well, the first of each type of adverse event was included. Rates are per 100 person-years. 3-year refers to the cumulative incidence percent risk. Risk difference is based on the 3-year cumulative incidence percent risk. CI, confidence interval; HR, hazard ratio; EENT, eye, ear, nose, throat, and dental; COPD, chronic obstructive pulmonary disease; NOS, not otherwise specified; URI, upper respiratory infection.
Hepatic pathology was more commonly observed in the LD-MTX arm (see Table 4). Five cases of cirrhosis were observed in the primary modified intention-to-treat analysis (rate 0.11, 95% CI 0.01 – 0.20, per 100 person-years) versus none in the placebo arm (p-value 0.03, exact test). Of note, none of the cirrhosis cases reported ever using alcohol at the baseline visit. The body mass indices (BMIs) were all in the obese range, except for one; however, the mean BMI for the trial was also in the obese range. All subjects with cirrhosis had diabetes (versus 68% overall in the trial), three had metabolic syndrome, and none reported tobacco use. The duration of LD-MTX use was only several months three subjects and several years for the others. All subjects had one or more liver test abnormalities prior to the diagnosis of cirrhosis, but none had severe abnormalities. Three diagnosed with cirrhosis had repeated elevations in the AST, defined as 5 elevations out of a consecutive 9 assessments; this is from a total of 139 subjects who were randomized to LD-MTX and experienced repeated elevations in AST. No cases of cirrhosis developed in the 72 subjects in the placebo arm with this same abnormal pattern. One of the subjects in the LD-MTX arm with cirrhosis died during follow-up, and cirrhosis was part of his cause of death. In addition to liver pathology, gastrointestinal AEs were also increased (HR 1.23, 95% CI 1.03–1.47).
Table 4:
Frequency and Relative Risks Comparing Liver and Other Gastrointestinal Adverse Events for Methotrexate to Placebo During the Randomized Phase of CIRT
| Low dose methotrexate (n=2391) | Placebo (n=2395) | Risk Difference | Hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| N (%)* | Rate | 3-year | N (%)* | Rate | 3-year | |||
| Liver test elevation ǂ | 1042 (43.6%) | 36.1 (33.9, 38.3) | 0.56 (0.53, 0.58) | 576 (24.1%) | 15.6 (14.4, 16.9) | 0.32 (0.29, 0.34) | 0.24 (0.20, 0.27) | 2.14 (1.93, 2.37) |
| Mild | 1010 (42.2%) | 34.4 (32.3, 36.5) | 0.54 (0.51, 0.57) | 559 (23.3%) | 15.1 (13.8, 16.3) | 0.31 (0.28, 0.33) | 0.23 (0.20, 0.27) | 2.12 (1.91, 2.35) |
| Moderate | 187 (7.8%) | 4.2 (3.6, 4.9) | 0.12 (0.10, 0.14) | 49 (2.1%) | 1.1 (0.8, 1.4) | 0.03 (0.02, 0.04) | 0.09 (0.07, 0.11) | 3.94 (2.87, 5.39) |
| Severe | 69 (2.9%) | 1.5 (1.2, 1.9) | 0.04 (0.03, 0.06) | 26 (1.1%) | 0.6 (0.4, 0.8) | 0.01 (0.01, 0.02) | 0.03 (0.02, 0.04) | 2.67 (1.70, 4.19) |
| Any liver pathology | 13 (0.5%) | 0.3 (0.1, 0.4) | 0.01 (0.01, 0.02) | 7 (0.3%) | 0.2 (0.0, 0.3) | 0.00 (0.00, 0.01) | 0.01 (0.00, 0.01) | 1.83 (0.73, 4.55) |
| Cirrhosis | 5 (0.2%) | 0.1 (0.0, 0.2) | 0.00 (0.00, 0.01) | 0 (0.0%) | 0.0 (0.0, 0.0) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | … |
| NASH | 8 (0.3%) | 0.2 (0.1, 0.3) | 0.01 (0.00, 0.02) | 7 (0.3%) | 0.2 (0, 0.3) | 0.00 (0.00, 0.01) | 0.00 (0.00, 0.01) | 1.13 (0.41, 3.08) |
| Gastrointestinal | ||||||||
| Any | 273 (11.4) | 6.4 (5.7, 7.2) | 0.16 (0.14, 0.18) | 224 (9.4) | 5.2 (4.5, 5.9) | 0.13 (0.12, 0.15) | 0.03 (0.00, 0.05) | 1.23 (1.03, 1.47) |
| Mild | 118 (4.9) | 2.6 (2.2, 3.1) | 0.07 (0.06, 0.08) | 101 (4.2) | 2.3 (1.8, 2.7) | 0.06 (0.05, 0.07) | 0.01 (−0.01, 0.02) | 1.17 (0.90, 1.52) |
| Moderate | 132 (5.5) | 3.0 (2.4, 3.5) | 0.08 (0.07, 0.10) | 109 (4.6) | 2.4 (2.0, 2.9) | 0.07 (0.05, 0.08) | 0.01 (0.00, 0.03) | 1.21 (0.94, 1.56) |
| Severe | 53 (2.2) | 1.2 (0.8, 1.5) | 0.03 (0.02, 0.04) | 44 (1.8) | 1.0 (0.7, 1.3) | 0.03 (0.02, 0.04) | 0.00 (−0.01, 0.01) | 1.20 (0.81, 1.79) |
| By sub category | ||||||||
| N/V/D | 174 (7.3) | 3.9 (3.4, 4.5) | 0.10 (0.09, 0.12) | 135 (5.6) | 3.1 (2.5, 3.6) | 0.07 (0.06, 0.09) | 0.03 (0.01, 0.05) | 1.30 (1.03, 1.63) |
| Abdominal pain | 74 (3.1) | 1.6 (1.3, 2.0) | 0.05 (0.04, 0.06) | 60 (2.5) | 1.3 (1.0, 1.7) | 0.04 (0.03, 0.05) | 0.01 (−0.01, 0.02) | 1.23 (0.87, 1.73) |
| GS or cholecystitis | 22 (0.9) | 0.5 (0.3, 0.7) | 0.01 (0.01, 0.02) | 15 (0.6) | 0.3 (0.2, 0.5) | 0.01 (0.00, 0.01) | 0.01 (0.00, 0.01) | 1.45 (0.74, 2.83) |
| Other ** | 42 (1.8) | 0.9 (0.6, 1.2) | 0.02 (0.02, 0.03) | 47 (2.0) | 1.0 (0.7, 1.3) | 0.03 (0.02, 0.04) | 0.00 (−0.02, 0.01) | 0.89 (0.57, 1.38) |
Notes: N/V/D: nausea, vomiting, or diarrhea; GS, gall stones. These numbers are based on the modified intention to treat analyses (see text for details).
N includes first events of a given type and the percentages refer to the number of subjects with a given adverse event divided by all subjects in the respective treatment arm. The first mild, moderate, and severe are all included. As well, the first of each type of adverse event was included. Rates are per 100 person-years. 3-year refers to the cumulative incidence percent risk. Risk difference is based on the 3-year cumulative incidence percent risk.
Other includes to constipation, pancreatitis, small bowel obstruction, weight loss and NOS.
Six (0.3%) cases of possible pneumonitis occurred in LD-MTX and 1 (0.04%) in placebo (HR 6.94, 95% CI 0.85–56.0; p-value 0.04 by exact test); no cases could be considered probable or definite as too little information was provided. The cases in the LD-MTX arm occurred between 2–27 months after randomization. All subjects were men, none reported any history of tobacco use, and two died. In addition, when considering any pulmonary AE, we found an increased risk with LD-MTX (HR 1.42, 95% CI 1.14–1.77). Finally, the risk of any infectious AE, pulmonary or otherwise, was increased (HR 1.15, 95% CI 1.01–1.30).
There was a reduction in renal AEs among LD-MTX users (HR 0.85, 95% CI 0.78 – 0.93). This was driven primarily by different trends in eGFR (ml/min/m2): LD-MTX users final median eGFR was 79.1 (IQR 65.7 – 93.4) versus final placebo eGFR 77.2 (63.4 – 91.8) (Kruskal-Wallis p = 0.001).
Results of the sensitivity analyses for any AE were very similar to the primary analyses. Varying the censoring date from zero to 90 days produced similar risks (zero days: HR 1.18, 95% CI 1.11 – 1.25; and 90 days: HR 1.17, 95% CI 1.10 – 1.25). Furthermore, broadening the outcome to include possible events also did not change the risk (HR 1.17, 95% CI 1.10 – 1.24), neither did including MedDRA coded events (see Table 2).
Finally, we examined AEs during the pre-randomization period (see Supplementary Table 3) and baseline correlates of subjects who were not randomized. The types of AEs observed during the 5–8 week active run-in were similar to those observed during the post-randomization period. Many of the renal and/or hematologic events that are noted in Supplementary Table 3 were abnormal laboratory values.
DISCUSSION
LD-MTX serves as the most commonly used drug for systemic rheumatic diseases worldwide and is the recommended first-line agent for rheumatoid arthritis. Yet, despite extensive clinical use for over 30 years, few data on AE rates derive from randomized double-blind placebo-controlled trials where both causality and magnitude of risk can be inferred. Here, in a contemporary clinical trial that included recommended use of folic acid to reduce drug associated toxicities, we observed statistically significant increases in the risks for any adjudicated AE of interest, as well as infectious, pulmonary, gastrointestinal, and hematologic AEs. We also observed an increase in skin cancers and an unanticipated reduction in renal AEs, mostly related to differences in the final eGFR.
Existing data for LD-MTX toxicity derive almost exclusively from case reports, case series, and observational studies which have been both inconclusive and contradictory. For example, while at least three prior studies found significant increases in infection risk among patients with RA using LD-MTX compared with patients on no immunosuppressive agents (22, 23, 40), other studies have found no increase in the risk of pneumonia and objectively confirmed infections (41, 42). We believe the current data provide what is perhaps the first objective measures of true toxicity associated with use of LD-MTX, risks that need to be balanced with the clear clinical benefits that derive from the use of this drug in the setting of rheumatoid arthritis or psoriasis. For many of the toxicities noted here, there are specific clinical situations and predictors of risk that prescribers of LD-MTX should be aware of; some of these predictors have been described (22, 25), and others will be examined in future analyses.
The reduction in the risk of renal AEs was unanticipated and small. High dose MTX used for oncology patients is known to be associated with renal toxicity (53). MTX is renally cleared, and thus kidney function needs to be monitored during treatment, even with LD-MTX. While review articles describe renal toxicity of LD-MTX in rheumatoid arthritis (54), it is difficult to find cases of renal toxicity during use of LD-MTX when other drugs (e.g., NSAIDs or cylcosporine) or other causes (e.g., renal amyloid) may not have contributed. There are almost no data on a potential renal protective effect of LD-MTX, except in rodents with collagen induced arthritis (55). However, there is a robust literature suggesting that inflammation accelerates chronic kidney disease (56) and diabetic kidney disease (57). It is possible that LD-MTX could have improved eGFR through its impact on inflammation.
In addition to our ability to conduct the current analyses in the setting of a randomized placebo-controlled trial, two other aspects of the methods should be highlighted. First, active run-in facilitates improved compliance after randomization since subjects who do not tolerate the study drug and/or comply with follow-up procedures can be identified and excluded. However, use of an active run-in would also reduce AE rates following randomization..
Second, we pre-specified AEs of interest and prospectively adjudicated them, using structured procedures with clinical criteria based on prior work or developed for this study (47–50). It is unusual for a trial designed to examine potential benefits to examine and adjudicate AEs with this level of rigor. This step allows us to better characterize the nature and severity of the AEs. As well, the adjudication procedures were blinded to treatment arm assignment. Severity of the AEs was based on the clinical context (see Supplementary Table 2) and not on the typical MedDRA criteria for a “serious AE”, defined simply as life-threatening or causing hospitalization.
The results of the current study need to be viewed in light of potential limitations. The CIRT trial was not performed in patients with systemic rheumatic diseases. It is possible that the absolute risks of AEs differ between the CIRT population and typical patients with systemic rheumatic disease who have higher levels of systemic inflammation and often use concomitant immunosuppressants. Patients with systemic rheumatic disease may metabolize LD-MTX differently for a variety of reasons, including differences in the gut microbiota (58). However, it is probable that the relative risks do not differ; this is not testable in our study cohort. The collection of AEs was conducted systemically at routine study visits every 4–12 weeks. It is possible that patients may have experienced AEs between study visits and failed to report them. The under-reporting would affect both LD-MTX and placebo arms similarly and thus unlikely to introduce systematic bias. It is also possible that intensive monitoring of patients for AEs may have resulted in increased reporting. The median follow-up of nearly 2 years was much longer than the vast majority of LD-MTX trials, but still may be too short to observe some AEs that require long-term exposure. Finally, patients in the placebo arm (and LD-MTX) took folate six days each week; folate may have impacted the rates of adverse events in both arms.
In conclusion, we found that LD-MTX users experienced an increased risk of any AE, as well as several specific types of AEs: hematologic, skin cancers, gastrointestinal, liver, infections, and pulmonary. The risk of AEs that we observed among patients in the LD-MTX arm, such as cirrhosis and pneumonitis, generally agrees with prior reports (19, 40). The fact that median dosages of LD-MTX and placebo were very similar suggests relatively good tolerability of LD-MTX when given with folate. Moreover, there are strong data that LD-MTX is efficacious for many patients with rheumatoid arthritis and is recommended as first-line treatment. The data presented herein provide an important source of new evidence to improve the monitoring guidelines and safe prescribing of LD-MTX.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by the National Institutes of Health (NIH R01 HL119718, NIH U01 HL101422, and U01 HL101389).
Disclosures: DHS receives research support unrelated to the present study from Abbvie, Amgen, Corrona, Genentech, Janssen, and Pfizer. BME receives research support unrelated to the present study from Novartis, and has served as a consultant to Amgen, Amarin, Merck and Roche. ADP receives research support unrelated to the present study from Kowa, Denka-Seiken, and Optum Health. SPH received research support unrelated to the present study from AiCuris and Merck. DAR receives research support unrelated to the present study from Merck and Celgene, and has served as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Pfizer, and Scipher Medicine. JAS has received research support unrelated to the present study from Amgen and Bristol-Myers Squibb and served as a consultant for Gilead, Janssen, and Optum. PMR receives research support unrelated to the present study from Kowa, Novartis, and Amarin, and has served as a consultant to Corvidia, Inflazome, and CiviBioPharm.
Footnotes
Clinical Trial Registration: NCT 01594333
Contributor Information
DH Solomon, Division of Rheumatology.
RJ Glynn, Division of Preventive Medicine.
EW Karlson, Division of Rheumatology.
F Lu, Division of Rheumatology.
C Corrigan, Division of Rheumatology.
J Colls, Division of Rheumatology.
C Xu, Division of Rheumatology.
J MacFadyen, Division of Preventive Medicine.
M Barbhaiya, Brigham and Women’s Hospital; Hospital for Special Surgery.
N Berliner, Division of Hematology.
PF Dellaripa, Division of Rheumatology.
BM Everett, Division of Preventive Medicine; Division of Cardiovascular Medicine.
AD Pradhan, Division of Preventive Medicine.
SP Hammond, Division of Infectious Diseases.
M Murray, Division of Rheumatology.
DA Rao, Division of Rheumatology.
S Ritter, Division of Rheumatology.
A Rutherford, Division of Gastrointestinal Diseases.
JA Sparks, Division of Rheumatology.
J Stratton, Division of Rheumatology.
DH Suh, Division of Rheumatology.
SK Tedeschi, Division of Rheumatology.
KMM Vanni, Division of Rheumatology.
NP Paynter, Division of Preventive Medicine.
PM Ridker, Division of Preventive Medicine; Division of Cardiovascular Medicine.
REFERENCES
- 1.Sparks JA, Barbhaiya M, Karlson EW, Ritter SY, Raychaudhuri S, Corrigan CC, et al. Investigating methotrexate toxicity within a randomized double-blinded, placebo-controlled trial: Rationale and design of the Cardiovascular Inflammation Reduction Trial-Adverse Events (CIRT-AE) Study. Semin Arthritis Rheum. 2017;47(1):133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benucci M, Saviola G, Manfredi M, Sarzi-Puttini P, Atzeni F. Cost effectiveness analysis of disease-modifying antirheumatic drugs in rheumatoid arthritis. A systematic review literature. Int J Rheumatol. 2011;2011:845496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta-analysis. Arthritis Rheum. 2006;55(6):864–72. [DOI] [PubMed] [Google Scholar]
- 4.Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985;312(13):818–22. [DOI] [PubMed] [Google Scholar]
- 5.Weinblatt ME, Maier AL, Fraser PA, Coblyn JS. Longterm prospective study of methotrexate in rheumatoid arthritis: conclusion after 132 months of therapy. J Rheumatol. 1998;25(2):238–42. [PubMed] [Google Scholar]
- 6.Wasko MC, Dasgupta A, Hubert H, Fries JF, Ward MM. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65(2):334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359(9313):1173–7. [DOI] [PubMed] [Google Scholar]
- 8.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(2):199–207 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato EI. Methotrexate therapy in systemic lupus erythematosus. Lupus. 2001;10(3):162–4. [DOI] [PubMed] [Google Scholar]
- 10.Ash Z, Gaujoux-Viala C, Gossec L, Hensor EM, FitzGerald O, Winthrop K, et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2012;71(3):319–26. [DOI] [PubMed] [Google Scholar]
- 11.Hanrahan PS, Scrivens GA, Russell AS. Prospective long term follow-up of methotrexate therapy in rheumatoid arthritis: toxicity, efficacy and radiological progression. Br J Rheumatol. 1989;28(2):147–53. [DOI] [PubMed] [Google Scholar]
- 12.Buchbinder R, Hall S, Sambrook PN, Champion GD, Harkness A, Lewis D, et al. Methotrexate therapy in rheumatoid arthritis: a life table review of 587 patients treated in community practice. J Rheumatol. 1993;20(4):639–44. [PubMed] [Google Scholar]
- 13.Weinblatt ME. Toxicity of low dose methotrexate in rheumatoid arthritis. J Rheumatol Suppl. 1985;12 Suppl 12:35–9. [PubMed] [Google Scholar]
- 14.Kremer JM, Phelps CT. Long-term prospective study of the use of methotrexate in the treatment of rheumatoid arthritis. Update after a mean of 90 months. Arthritis Rheum. 1992;35(2):138–45. [DOI] [PubMed] [Google Scholar]
- 15.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68(7):1100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albrecht K, Muller-Ladner U. Side effects and management of side effects of methotrexate in rheumatoid arthritis. Clin Exp Rheumatol. 2010;28(5 Suppl 61):S95–101. [PubMed] [Google Scholar]
- 17.Verstappen SM, Jacobs JW, van der Veen MJ, Heurkens AH, Schenk Y, ter Borg EJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis. 2007;66(11):1443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazici Y, Sokka T, Kautiainen H, Swearingen C, Kulman I, Pincus T. Long term safety of methotrexate in routine clinical care: discontinuation is unusual and rarely the result of laboratory abnormalities. Ann Rheum Dis. 2005;64(2):207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visser K, van der Heijde DM. Risk and management of liver toxicity during methotrexate treatment in rheumatoid and psoriatic arthritis: a systematic review of the literature. Clin Exp Rheumatol. 2009;27(6):1017–25. [PubMed] [Google Scholar]
- 20.Kremer JM, Alarcon GS, Lightfoot RW Jr., Willkens RF, Furst DE, Williams HJ, et al. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology.[see comment]. Arthritis & Rheumatism. 1994;37(3):316–28. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JR, Beukelman T, Onofrei A, Cassell S, Greenberg JD, Kavanaugh A, et al. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis. 2010;69(1):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent PD, Luthra HS, Michet C Jr. Risk factors for methotrexate-induced abnormal laboratory monitoring results in patients with rheumatoid arthritis. J Rheumatol. 2004;31(9):1727–31. [PubMed] [Google Scholar]
- 23.Hargreaves MR, Mowat AG, Benson MK. Acute pneumonitis associated with low dose methotrexate treatment for rheumatoid arthritis: report of five cases and review of published reports. Thorax. 1992;47(8):628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden MR, Katz RS, Balk RA, Golden HE. The relationship of preexisting lung disease to the development of methotrexate pneumonitis in patients with rheumatoid arthritis. J Rheumatol. 1995;22(6):1043–7. [PubMed] [Google Scholar]
- 25.Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology (Oxford). 2007;46(7):1157–60. [DOI] [PubMed] [Google Scholar]
- 26.van der Veen MJ, van der Heide A, Kruize AA, Bijlsma JW. Infection rate and use of antibiotics in patients with rheumatoid arthritis treated with methotrexate. Ann Rheum Dis. 1994;53(4):224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards CJ, Cooper C, Fisher D, Field M, van Staa TP, Arden NK. The importance of the disease process and disease-modifying antirheumatic drug treatment in the development of septic arthritis in patients with rheumatoid arthritis. Arthritis Rheum. 2007;57(7):1151–7. [DOI] [PubMed] [Google Scholar]
- 28.Kalantzis A, Marshman Z, Falconer DT, Morgan PR, Odell EW. Oral effects of low-dose methotrexate treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(1):52–62. [DOI] [PubMed] [Google Scholar]
- 29.Varatharajan N, Lim IG, Anandacoomarasamy A, Russo R, Byth K, Spencer DG, et al. Methotrexate: long-term safety and efficacy in an Australian consultant rheumatology practice. Intern Med J. 2009;39(4):228–36. [DOI] [PubMed] [Google Scholar]
- 30.Gilani ST, Khan DA, Khan FA, Ahmed M. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. J Coll Physicians Surg Pak. 2012;22(2):101–4. [PubMed] [Google Scholar]
- 31.Wernick R, Smith DL. Central nervous system toxicity associated with weekly low-dose methotrexate treatment. Arthritis Rheum. 1989;32(6):770–5. [DOI] [PubMed] [Google Scholar]
- 32.Preston SJ, Diamond T, Scott A, Laurent MR. Methotrexate osteopathy in rheumatic disease. Ann Rheum Dis. 1993;52(8):582–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alarcon GS, Tracy IC, Blackburn WD Jr. Methotrexate in rheumatoid arthritis. Toxic effects as the major factor in limiting long-term treatment. Arthritis Rheum. 1989;32(6):671–6. [DOI] [PubMed] [Google Scholar]
- 34.van Ede AE, Laan RF, Rood MJ, Huizinga TW, van de Laar MA, van Denderen CJ, et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2001;44(7):1515–24. [DOI] [PubMed] [Google Scholar]
- 35.Solomon DH, Kremer JM, Fisher M, Curtis JR, Furer V, Harrold LR, et al. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. 2014;43(4):489–97. [DOI] [PubMed] [Google Scholar]
- 36.Scott FI, Mamtani R, Brensinger CM, Haynes K, Chiesa-Fuxench ZC, Zhang J, et al. Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA Dermatol. 2016;152(2):164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh JA, Saag KG, Bridges SL Jr., Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(1):1–26. [DOI] [PubMed] [Google Scholar]
- 38.Kremer JM, Lee RG, Tolman KG. Liver histology in rheumatoid arthritis patients receiving long-term methotrexate therapy. A prospective study with baseline and sequential biopsy samples. Arthritis Rheum. 1989;32(2):121–7. [DOI] [PubMed] [Google Scholar]
- 39.Shea B, Swinden MV, Ghogomu ET, Ortiz Z, Katchamart W, Rader T, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. J Rheumatol. 2014;41(6):1049–60. [DOI] [PubMed] [Google Scholar]
- 40.Conway R, Low C, Coughlan RJ, O’Donnell MJ, Carey JJ. Methotrexate use and risk of lung disease in psoriasis, psoriatic arthritis, and inflammatory bowel disease: systematic literature review and meta-analysis of randomised controlled trials. BMJ. 2015;350:h1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imokawa S, Colby TV, Leslie KO, Helmers RA. Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. Eur Respir J. 2000;15(2):373–81. [DOI] [PubMed] [Google Scholar]
- 42.Kremer JM, Alarcon GS, Weinblatt ME, Kaymakcian MV, Macaluso M, Cannon GW, et al. Clinical, laboratory, radiographic, and histopathologic features of methotrexate-associated lung injury in patients with rheumatoid arthritis: a multicenter study with literature review. Arthritis Rheum. 1997;40(10):1829–37. [DOI] [PubMed] [Google Scholar]
- 43.Sathi N, Chikura B, Kaushik VV, Wiswell R, Dawson JK. How common is methotrexate pneumonitis? A large prospective study investigates. Clin Rheumatol. 2012;31(1):79–83. [DOI] [PubMed] [Google Scholar]
- 44.Fischer A, Strek ME, Cottin V, Dellaripa PF, Bernstein EJ, Brown KK, et al. Proceedings of the American College of Rheumatology/Association of Physicians of Great Britain and Ireland Connective Tissue Disease-Associated Interstitial Lung Disease Summit: A Multidisciplinary Approach to Address Challenges and Opportunities. Arthritis Rheumatol. 2019;71(2):182–95. [DOI] [PubMed] [Google Scholar]
- 45.Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford). 2012;51(8):1368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380(8):752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Searles G, McKendry RJ. Methotrexate pneumonitis in rheumatoid arthritis: potential risk factors. Four case reports and a review of the literature. J Rheumatol. 1987;14(6):1164–71. [PubMed] [Google Scholar]
- 48.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–47. [DOI] [PubMed] [Google Scholar]
- 49.Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109–17. [DOI] [PubMed] [Google Scholar]
- 52.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometika. 1982;69:239–41. [Google Scholar]
- 53.Darmon M, Ciroldi M, Thiery G, Schlemmer B, Azoulay E. Clinical review: specific aspects of acute renal failure in cancer patients. Crit Care. 2006;10(2):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schiff MH, Whelton A. Renal toxicity associated with disease-modifying antirheumatic drugs used for the treatment of rheumatoid arthritis. Semin Arthritis Rheum. 2000;30(3):196–208. [DOI] [PubMed] [Google Scholar]
- 55.Budancamanak M, Kanter M, Demirel A, Ocakci A, Uysal H, Karakaya C. Protective effects of thymoquinone and methotrexate on the renal injury in collagen-induced arthritis. Arch Toxicol. 2006;80(11):768–76. [DOI] [PubMed] [Google Scholar]
- 56.Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M, et al. Inflammation and Progression of CKD: The CRIC Study. Clin J Am Soc Nephrol. 2016;11(9):1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuttle KR. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol. 2005;16(6):1537–8. [DOI] [PubMed] [Google Scholar]
- 58.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7(10):569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.