Abstract
The rise in consumption of energy-dense foods has resulted in the displacement of several essential dietary gaps, causing numerous long-lasting diseases, including obesity, stroke, hypertension, and several forms of cancer. Epidemiological studies encourage more fruit consumption to prevent these diseases. The defensive mechanisms provided by these fruits against illness are due to the existence of several antioxidants. Recent studies proved that (poly) phenolic compounds are ideally the core phytochemicals with both functional and health-promoting properties found in the plant's kingdom, and low intake could result in the risk of certain diseases. Phytonutrients are powerful antioxidants that can modify metabolic activation and detoxification of carcinogens. The ideal motive of this review is to provide an overview as well as illuminate the polyphenolic merits of fruits in general. Fruits have several merits, including weight maintenance, proper health development, and satiety. There are many analytical methods for determining and measuring the phenolic content of different products. Phenolic compounds are of nutritional interest since they aid in the retardation and inhibition of lipids by acting as scavengers that prevent and protect the proliferation of oxidative chains. Future studies are required to help identify the physiological metabolic activities as well as to improve human health.
1. Introduction
The growth, urbanization, and economic development of Africa and the world at large have resulted in a dietary transition and evolution from a traditional to a modernized diet, where the quality of food has been affected. This transition in today's society has resulted in a high consumption of processed bottled and canned foods with a high content of calories. The rise in the ingestion of these energy-dense foods has caused a high amount of essential nutrients to be displaced in our diets [1]. The consumption of unhealthy diets generates a high nutritional gap, and these are the leading causes of several chronic diseases, including overweight (obesity), cardiovascular diseases, diabetes, stroke, hypertension, and a few types of cancer. Hence, it remains necessary to explore the nutritional merits as well as the constituents of these foodstuffs. In avoidance of these, epidemiological education encourages the intake of more fruits, vegetables, and leguminous plants [2, 3].
For the past years, the consumption rate of fruits has attracted attention since many biochemical and epidemiological studies have focused on the merits associated with the regular intake of these natural diets and the reduction rate of several ailments such as cancer and chronic and heart-related diseases. The defensive mechanisms that these fruits provide against illnesses are associated with the existence of several antioxidants, particularly vitamins (vitamins A, C, E, etc.) and provitamins. Recent studies also indicate plant polyphenolic compounds as the core phytochemicals due to their antioxidant properties [4–7].
The term “phenolic” or “polyphenol” is chemically defined as substances that encompass an aromatic ring bearing one or more hydroxyl substituents or groups, with functional by-products (esters, methyl ethers, glycosides, etc.), and their simple molecular structures may differ from composite high-molecular-mass polymer. Thus, “phenol” is a term explaining a phenyl ring having single or extra hydroxyl substituents whiles “polyphenol” is used in expressing natural products having not less than double phenyl rings bearing single or additional hydroxyl substituents. Usually, phenols have double or more hydroxyl groups, which are the biologically active substance that occurs in food plants mostly consumed by a considerable number of people. It is represented chemically as C6H5OH, with a structure of hydroxyl group (-OH) fused to a phenyl ring. Phenolic compounds comprised of an aromatic benzene ring with single or double hydroxyl groups (e.g., polyphenols) [8–10]. The structure is in Figure 1.
Figure 1.
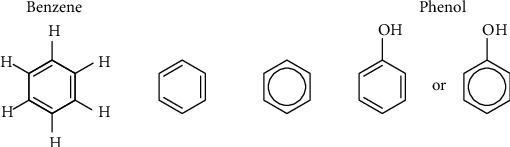
Chemical representation of a phenolic structure.
2. Phenolic Profile of Fruits and Products
2.1. Composition and Classification of Secondary Metabolites
Phenolic compounds are considered one of the abundant classes of phytochemicals with health-promoting qualities and functions. Phenolic compounds are classified as secondary metabolites commonly distributed in the kingdom of plants, with immense structures and functions. It is regarded as the most significant and abundant class of compounds in the plant's kingdom. The metabolism of plants is categorized into primary and secondary metabolic forms [11–14]. The primary metabolites include lipids, carbohydrates, proteins, and nucleic acids, as well as all essential elements required for cell growth and development. Secondary metabolites are compounds in specific cells that are indirectly essential for simple respiratory photosynthesis or metabolism but are understood to be vital for a plant's survival. The metabolic flows in plants and detail classification of polyphenol are presented in Figures 2 and 3, respectively [15–20].
Figure 2.
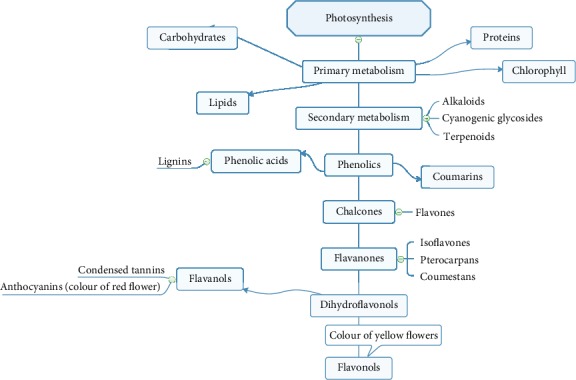
Metabolic flows of polyphenols in plants, adapted from Giada et al. [19].
Figure 3.
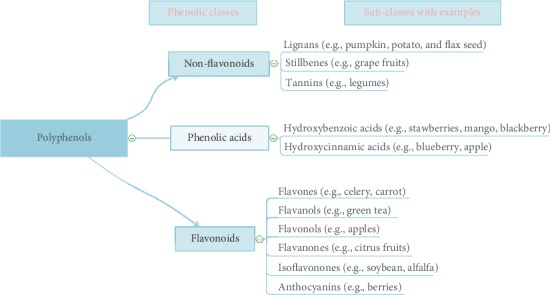
The detail classification of polyphenols, adapted from Basheer and Kerem [20].
Phenolic compounds can be further grouped into water-soluble compounds (phenolic acids, flavonoids, phenylpropanoids, and quinones) and water-insoluble compounds (condensed tannins, lignins, and cell-wall bound hydroxycinnamic acids). This classification is significant due to the nutritional composition or constituents since its solubility and digestibility are needed most for effective utilization within the gastrointestinal tract and some physiological operations. When insoluble phenolic compounds are unable to digest, they will be passed out in the feces wholly or partially, while the soluble compounds can be absorbed through the intestines into the bloodstreams as metabolites. It is subdivided into several forms, e.g., phenolic acids (hydroxybenzoic acids and hydroxycinnamic acids), flavonoids (flavones, flavanols, flavanones, and isoflavones), tannins, stilbenes, and lignans [21–25]. Comparatively, among subphenolic classes, flavonoids are the group with the highest researches due to their supposed health-promoting qualities [26]. Previous studies on cardiovascular diseases were focused mainly on flavones and flavanols, but recent studies have been on nonflavonoid as well as other flavonoid forms [27].
3. The Role of Phenolic Compounds in Fruits
Fruits remain a good source of compounds with high phenolic functions, which are leading ingredients in our daily diets [28]. Phenolic compounds, particularly those in fruits, have caught the attention of scientists across the globe, resulting in increased research and reviews in that area [29]. Most of the reports confirm that constant consumption of fruits contributes significantly to a healthy diet, and less consumption could result in the danger of certain chronic diseases like cancer, heart disease, and stroke [30]. Fruits and fruit products play a significant role in human health and diet, predominantly as bases of thiamine, vitamins, niacin, pyridoxine, minerals, folic acid, iron, dietary fiber, magnesium, malic acid, tartaric acid calorie, and citric acids. The phytochemical components (phytonutrients) of some fruits and its products contain high antioxidants, which can alter metabolic activities and cleansing of carcinogens with the potentials of even influencing the factors causing cell tumor [31–33]. There is variation in the phytochemical components of all fruits, thereby resulting in different antioxidant capacity in fruits. Therefore, high consumption leads to high antioxidant capacity. Phyto is a known Greek word denoting plant. Therefore, it is in plants and plant products (vegetables, seeds, grains, legumes, roots, fruits, herbs, nuts, and leaves). Several reports recommend the consumption of at least five serving fruits with vegetables in a day. The nutrients that are possessed by fruits have several merits, including weight maintenance, proper health development, and satiety, as well as supplies a variety of tastes [34, 35].
3.1. Phytochemical Components of Some Fruits
Table 1 displays the mean content of some fruits with their phenolic compounds, respectively (mg/100 g of sample) [19].
Table 1.
Phenolic contents of some fruits.
| Fruits | Total phenolics (mg%) |
|---|---|
| Green apple | 118 |
| Red apple | 125 |
| Yellow apple | 100 |
| Blueberry [36] | 327 |
| Sour cherry | 156 |
| Sweet cherry | 79 |
| Black grape | 213 |
| White grape | 184 |
| Grape | 893 |
| Pink guava | 247 |
| White guava | 145 |
| Kiwi | 791 |
| Lemon | 843 |
| Lime | 751 |
| Litchi | 60 |
| White nectarine | 38 |
| Yellow nectarine | 25 |
| White peach | 53 |
| Yellow peach | 35 |
| Pear | 125 |
| Pineapple | 94 |
| Black plum | 88 |
| Red plum | 73 |
| Pomegranate | 147 |
| Pomelo | 57 |
| Raspberry, black | 670 |
| Raspberry, red | 342 |
| Raspberry, yellow | 426 |
| Strawberry | 199 |
Adapted from Giada et al. [19].
As displayed in Table 1, phenolic compounds remain generally spread in plant foods (fruits). Blueberry and citrus fruits are predominantly rich with phenolic compounds. Phenolic compounds are the key determinant of the antioxidant potentials in plant foods and thereby represent the fundamental basis of antioxidants.
4. Ways of Identifying the Content of Phenolic Compounds in Fruits
Table 1 shows that fruits differ in the amount and even forms of phenolic antioxidants. Therefore, there are many analytical methods available for measuring and determining the sum of phenols in food products, starting with sample preparation methods and extraction per the products (fruits) nature and the phenolic structure [28, 37]. The most vital procedure in the phenolic analysis is both the preparation of samples and the methods used in extraction. Due to the compositional structure of phenolic compounds, it is difficult choosing the best methods for both sample preparation and extractions. Fresh samples (fruits) do not always give better results, even though fresh fruits are the ideal requirement for polyphenolic extraction. This is due to perishability, shelf life, quality, and the season of research. For efficient extraction and preparation of phenolic samples in some situations, it requires either air, shade, or oven drying, lyophilization as well as nitrogen pulverization [38–40], because it solely depends on the type of the medium and the organic composition of samples involved. It includes polarity, molecular structure, concentration, hydroxyl groups, and the number of the aromatic rings involved. Therefore, it is difficult to single out a universal method for the preparation and extraction of phenols for many plant products. Lyophilization is the process used in removing water from materials like food and organic samples. It is regarded as one of the best techniques for prolonging shelf life and product stability [41]. After both preparation of samples and phenolic extraction, it is then followed by classification and quantification with the aid of spectrophotometry, gas chromatography (GC), near-infrared spectroscopy, high-performance liquid chromatography (HPLC), or capillary electrophoresis (CE) methods [42]. The quantification and classification of analytical methods for phenolic compounds are summarized and presented in Figure 4.
Figure 4.
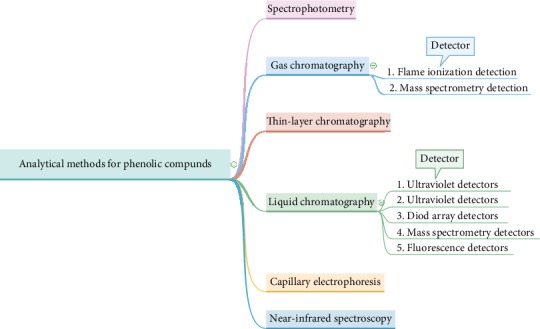
Quantification and classification of analytical methods for phenolic compounds, adapted from Kafkas et al. [37].
4.1. Other Tests for Quantification and Separation of Phenolic Compounds
There are other simple but less utilized methods compared to high-performance liquid chromatography and gas chromatography, which can as well aid in the identification of phenolic compounds. These include paper chromatography (PC), high-speed countercurrent chromatography (HSCCC), and supercritical fluid chromatography (SFC) [43].
The SFC is environmentally friendly, with short analysis duration and high separation efficiency, as well as high-resolution power with varied types of detectors. There are a variety of phenolic research done with the aid of SFC techniques which includes polyhydroxy flavonoid identification and extraction of polyphenols in grape seed [43–47]. Paper chromatography (PC) is well used in other studies for the identification of phenolic acids, flavonoids, glycoflavones, and phenolic acids from tea leaf using acetic acid/water/butanol as the mobile phase [48, 49]. High-speed countercurrent chromatography (HSCC) is also known as a biphasic liquid-liquid partitioning method, which is usually used for separation, isolation, and purification of natural compounds [24]. It isolates mixed components or solvent phase due to their partition coefficiency and hydrophobicity. This method uses only liquid samples to ensure the permanent adsorption of the compounds. An efficient and successful separation was achieved using high-speed countercurrent chromatography (HSCC) on a two-phase solvent system consisting of ethyl acetate/methanol/n-hexane/water (15 : 4 : 5 : 7, v/v) [43].
Several factors influence phenolic extraction, and to achieve successful results requires critical consideration of the following: extraction time, the ratio of both the solute and the solvent, and the size of the sample particles [50].
5. The Potential Health Content of (Poly) Phenols in Fruits and Products
5.1. What Makes Fruit Consumption Much More Important?
The study on phenolic compounds for the past decades has significantly progressed. The presence of fruit antioxidants is all linked to the health and medicinal merits. Fruits were regarded as high dietary fiber sources and are recently confirmed scientifically to possess essential phytochemicals that are useful and beneficial to human health. Therefore, people who consume fruits as part of their main diet are with the possibility of having a reduced risk of certain chronic diseases [51, 52].
5.1.1. Nutrition, Health Content, and Antioxidant Potentials of Fruits
According to the United States Department of Agriculture (USDA), the most vital nutrients, e.g., potassium, dietary fiber, vitamin C, and foliate, could be obtained from fruits and yet are underconsumed. Naturally, fruits are of low fat, calories, sodium, and zero percent cholesterol. Additionally, diets rich in potassium, folic acid, and vitamin C aid in healthy blood pressure conservation, the formation of red blood cells, and both growth and maintenance of body tissues, respectively. Dietary fiber from fruits helps reduce heart disease risk and blood cholesterol levels [53]. Several fruits have been studied to help identify the hidden molecular mechanisms that will aid in illuminating their health merits. Among all known mechanisms, antioxidant activities possess most phytochemicals [54–62]. Research by Southgate [63] on nature and variability of human food consumption summarized the compositional features of most plant foods, which include fruits, legumes, and vegetables. Table 2 shows the average compositional features of fruits. Fruits generally contain sugar and fibers, such as pectin, with a high-water content and less amount of fat and protein [63, 64].
Table 2.
The average compositional features of fruits.
| Composition | g/100 g edible matter |
|---|---|
| Water | 61.0–89.1 |
| Protein | 0.5–1.1 |
| Fat | Trace–4.4 |
| Sugar | 4.4–34.8 |
| Starch | Trace–3.0 |
| Dietary fiber | 2.0–14.8 |
| Energy (kcal) | 90–646 |
| Micronutrient | Vitamin C, K, Mg, carotenoids |
| Toxic constituents | Cyanogenic glycosides in seeds |
Adapted from Slavin and Lloyd [64].
Some fruits were studied separately due to their phytochemical contents, which include antioxidants, polyphenols, and phytoestrogens [64]. Phytochemicals can be well-defined as compounds obtained from plants and are considered not to possess any essential nutritional values but have abundant bioactive properties that give environmental protection and encouraging beneficial human health [65, 66]. An antioxidant is any substance that, when available in small quantity or concentration comparatively to an oxidizing substrate, can significantly impede that substrate's oxidation [67]. Therefore, since oxidizing species and other radicals are accountable for oxidative stress resulting in several long-lasting diseases, such as cardiovascular disease, cancer, and diabetes, antioxidant capacity of phytochemicals is the primary mechanism in the prevention of such diseases and promotion of human health [68, 69]. The consumption of this substance or compound is good for human health since it aids in the retardation and inhibition of lipids by acting as scavengers that prevent and protect the proliferation of oxidative chains. Several studies on both humans and animals reveal how polyphenols aid in the inhibition and prevention of cardiovascular diseases as well as cancers when taken on a day-to-day basis. Also, in some cases, they are used purposely due to their pharmaceutical properties [70–74]. For example, polyphenols may intermingle with responsive intermediates and trigger both mutagens and carcinogens, which will aid modulate the actions of the vital proteins which are involved in regulating cell progression cycle as well as influence the activities of various cancer-associated genes [75–78]. It is discovered in Panama that flavanol intake daily is one of the fundamental reasons for recording a decreased incidence of hypertension and cardiovascular diseases. Also, recent analysis supports the fact that polyphenols such as flavanol-rich products reduce blood pressure [79–81]. Further research shows the connections between flavonol, flavanol, and flavone intake and their effect on coronary disease reduction as well as the roles of flavanone and anthocyanin in decreasing cardiovascular disease and mortality [82, 83].
When free radicals are generated within a living organism, several antioxidants provide protection from oxidative damage. This serves as the initial or first line of defense, protective antioxidants, for example, metal-chelating proteins and peroxidases overpower the action and generation of free radicals. Subsequently, the antioxidant radical scavengers, e.g., vitamins C and E, hunt radicals, in order to obstruct the development of oxidative chains and to avoid the spread of chains by serving as the next or second line of defense. It may also include the destroying of chains by the reaction of two radicals. A new enzyme (e.g., proteases, lipases, and transferases) assists by fixing and reconstructing damaged membranes as another line of defense [68, 84].
6. Conclusions
In conclusion, plant-based phenolic compounds are one of the vital phytochemicals with a positive response to several disease preventions. Most fruits contain a high number of active antioxidants, and high consumption promotes good health. Primarily, due to its significance and the relationship between consumption and health benefits, there have been vast increments in polyphenol studies. Fruits and beverages, for example, coffee, tea, and red wine, stand as the best and excellent sources of polyphenols, but leguminous plants, vegetables, and cereals are also good sources; therefore, the search for new or improved methodologies for both phenolic extraction and isolation as well as separation, identification, and quantification for various fruits is essential for a clear understanding as well as identifying an excellent source of these phytochemicals. The content of polyphenol in food varies due to genetic variation and technological and environmental factors, which, when altered, will end up affecting the phenolic concentration of food. There is the need to identify plants that are rich in polyphenol, to enhance growing methods and minimize losses during cooking and industrial processing. The mechanism of phenolic absorption is still not well known; therefore, further studies should focus on the potential interactions (metabolism) between polyphenols and the microorganisms within the gastrointestinal tract as well as their physiological activities after administration and upon reaching the site of absorption. Naturally, the phenolic content of fruits, in general, differs thereby resulting in antioxidant variation. Detailed research is needed to help discover the physiological metabolic activities within the living organism as well as to help improve human health by scavenging against human chronic disease-causing organisms.
Acknowledgments
The authors would like to thank the Earmarked Fund for Modern Agro-industry Technology Research System (CARS-04) for the support.
Conflicts of Interest
The authors have declared no conflict of interest.
References
- 1.Grimm G. C., Harnack L., Story M. Factors associated with soft drink consumption in school-aged children. Journal of the American Dietetic Association. 2004;104(8):1244–1249. doi: 10.1016/j.jada.2004.05.206. [DOI] [PubMed] [Google Scholar]
- 2.Mertz C., Gancel A. L., Gunata Z., et al. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. Journal of Food Composition and Analysis. 2009;22(5):381–387. doi: 10.1016/j.jfca.2008.06.008. [DOI] [Google Scholar]
- 3.Espinosa-Alonso L. G., Lygin A., Widholm J. M., Valverde M. E., Paredes-Lopez O. Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.) Journal of Agricultural and Food Chemistry. 2006;54(12):4436–4444. doi: 10.1021/jf060185e. [DOI] [PubMed] [Google Scholar]
- 4.Quirantes-Piné R., Funes L., Micol V., Segura-Carretero A., Fernández-Gutiérrez A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. Journal of Chromatography. A. 2009;1216(28):5391–5397. doi: 10.1016/j.chroma.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 5.Liazid A., Palma M., Brigui J., Barroso C. G. Investigation on phenolic compounds stability during microwave-assisted extraction. Journal of Chromatography A. 2007;1140(1-2):29–34. doi: 10.1016/j.chroma.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Magalhães A. S., Silva B. M., Pereira J. A., Andrade P. B., Valentão P., Carvalho M. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food and Chemical Toxicology. 2009;47(6):1372–1377. doi: 10.1016/j.fct.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Salas P., Morales-Soto A., Segura-Carretero A., Fernández-Gutiérrez A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules. 2010;15(12):8813–8826. doi: 10.3390/molecules15128813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harborne J. B. In Methods in plant biochemistry. In: Harborne J. B., editor. Plant Phenolics. London, UK: Academic Press; 1989. pp. 1–28. Vol. 1. [Google Scholar]
- 9.Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chemistry. 2006;99(1):191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 10.Ghasemzadeh A., Jaafar H. Z. E. Optimization of reflux conditions for total flavonoid and total phenolic extraction and enhanced antioxidant capacity in pandan (Pandanus amaryllifolius Roxb.) using response surface methodology. The Scientific World Journal. 2014;2014:10. doi: 10.1155/2014/523120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ornston L. N., Yeh W. K. Origins of metabolic diversity: evolutionary divergence by sequence repetition. Proceedings of the National Academy of Sciences. 1979;76(8):3996–4000. doi: 10.1073/pnas.76.8.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lattanzio V. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Berlin, Heidelberg: Springer; 2013. Phenolic compounds: introduction; pp. 1543–1580. [DOI] [Google Scholar]
- 13.Lehfeldt C., Shirley A. M., Meyer K., et al. Cloning of the SNG1 gene of Arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. The Plant Cell. 2000;12(8):1295–1306. doi: 10.1105/tpc.12.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vickery M. L., Vickery B. Secondary Plant Metabolism. Macmillan Press; 1981. [Google Scholar]
- 15.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. The Journal of Nutrition. 2000;130(8):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 16.Rosato E., Tauber E., Kyriacou C. P. Molecular genetics of the fruit-fly circadian clock. European Journal of Human Genetics. 2006;14(6):729–738. doi: 10.1038/sj.ejhg.5201547. [DOI] [PubMed] [Google Scholar]
- 17.Broun P. Transcriptional control of flavonoid biosynthesis: a complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Current Opinion in Plant Biology. 2005;8(3):272–279. doi: 10.1016/j.pbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 18.do Nascimento N. C., Fett-Neto A. G. Plant Secondary Metabolism Engineering. Totowa, NJ: Humana Press; 2010. Plant secondary metabolism and challenges in modifying its operation: an overview; pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 19.de Lourdes Reis Giada M. Oxidative stress and chronic degenerative diseases—A role for antioxidants. InTech; 2013. Food phenolic compounds: main classes, sources, and their antioxidant power; pp. 87–112. [DOI] [Google Scholar]
- 20.Basheer L., Kerem Z. Interactions between CYP3A4 and Dietary Polyphenols. Oxidative medicine and cellular longevity. 2015;2015:15. doi: 10.1155/2015/854015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. The American Journal of Clinical Nutrition. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 22.Aguilera Y., Martin-Cabrejas M. A., González de Mejia E. Phenolic compounds in fruits and beverages consumed as part of the Mediterranean diet: their role in prevention of chronic diseases. Phytochemistry Reviews. 2016;15(3):405–423. doi: 10.1007/s11101-015-9443-z. [DOI] [Google Scholar]
- 23.Stougaard J., Udvardi M., Parniske M., et al. Lotus japonicus Handbook. Springer Science & Business Media; 2006. [Google Scholar]
- 24.Naczk M., Shahidi F. Extraction and analysis of phenolics in food. Journal of Chromatography. A. 2004;1054(1-2):95–111. [PubMed] [Google Scholar]
- 25.González C. S. M. Compuestos polifenólicos: Estructura y clasificación. Presencia en alimentos y consumo. Biodisponibilidad y metabolismo. Alimentaria: Revista de tecnología e higiene de los alimentos. 2002;329:19–28. [Google Scholar]
- 26.Halliwell B., Rafter J., Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? The American Journal of Clinical Nutrition. 2005;81(1 Suppl):268S–276S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Mateos A., Heiss C., Borges G., Crozier A. Berry (poly)phenols and cardiovascular health. Journal of Agricultural and Food Chemistry. 2014;62(18):3842–3851. doi: 10.1021/jf403757g. [DOI] [PubMed] [Google Scholar]
- 28.Xu C.-C., Wang B., Pu Y.-Q., Tao J.-S., Zhang T. Advances in extraction and analysis of phenolic compounds from plant materials. Chinese Journal of Natural Medicines. 2017;15(10):721–731. doi: 10.1016/s1875-5364(17)30103-6. [DOI] [PubMed] [Google Scholar]
- 29.Ignat I., Volf I., Popa V. I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chemistry. 2011;126(4):1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Van Duyn M. A. S., Pivonka E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: selected literature. Journal of the American Dietetic Association. 2000;100(12):1511–1521. doi: 10.1016/S0002-8223(00)00420-X. [DOI] [PubMed] [Google Scholar]
- 31.Liu S., Manson J. E., Lee I. M., et al. Fruit, and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. The American Journal of Clinical Nutrition. 2000;72(4):922–928. doi: 10.1093/ajcn/72.4.922. [DOI] [PubMed] [Google Scholar]
- 32.Wargovich M. J. Anticancer properties of fruits and vegetables. Hort Science. 2000;35(4):573–575. doi: 10.21273/HORTSCI.35.4.573. [DOI] [Google Scholar]
- 33.Bozhuyuk M. R., Pehluvan M., Kaya T., Dogru B. Organic acid composition of selected mulberry genotypes from Aras Valley. Ataturk Universitesi Ziraat Fakultesi Dergisi- Journals for Free. 2016;46(2):74–89. [Google Scholar]
- 34.Oguntibeju O. O., Truter E. J., Esterhuyse A. J. The role of fruit and vegetable consumption in human health and disease prevention. Diabetes mellitus–insights and perspectives. InTech Publishers; 2013. [Google Scholar]
- 35.Sheehan J. The functions of fruits & vegetables. Healthy Eating |SF Gate. July 2019, http://healthyeating.sfgate.com/functions-fruits-vegetables-4125.html.
- 36.Colak A. M., Küpe M., Bozhuyuk M. R., Ercisli S., Gündoğdu M. Identification of some fruit characteristics in wild bilberry (Vaccinium myrtillus L.) accessions from eastern Anatolia. Gesunde Pflanzen. 2018;70(1):31–38. doi: 10.1007/s10343-017-0410-z. [DOI] [Google Scholar]
- 37.Kafkas N. E., Kosar M., Öz A. T., Mitchell A. E. Advanced analytical methods for phenolics in fruits. Journal of Food Quality. 2018;2018:6. doi: 10.1155/2018/3836064. [DOI] [Google Scholar]
- 38.Gião M. S., Pereira C. I., Fonseca S. C., Pintado M. E., Malcata F. X. Effect of particle size upon the extent of extraction of antioxidant power from the plants Agrimonia eupatoria, Salvia sp. and Satureja montana. Food Chemistry. 2009;117(3):412–416. doi: 10.1016/j.foodchem.2009.04.020. [DOI] [Google Scholar]
- 39.Sejali S. N. F., Anuar M. S. Effect of drying methods on phenolic contents of neem (Azadirachta indica) leaf powder. Journal of Herbs Spices & Medicinal Plants. 2011;17(2):119–131. doi: 10.1080/10496475.2011.584293. [DOI] [Google Scholar]
- 40.Weidner S., Powałka A., Karamać M., Amarowicz R. Extracts of phenolic compounds from seeds of three wild grapevines—comparison of their antioxidant activities and the content of phenolic compounds. International Journal of Molecular Sciences. 2012;13(3):3444–3457. doi: 10.3390/ijms13033444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasper J. C., Friess W. The freezing step in lyophilization: physico-chemical fundamentals, freezing methods, and consequences on process performance and quality attributes of biopharmaceuticals. European Journal of Pharmaceutics and Biopharmaceutics. 2011;78(2):248–263. doi: 10.1016/j.ejpb.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Kelebek H., Selli S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. Journal of the Science of Food and Agriculture. 2011;91(10):1855–1862. doi: 10.1002/jsfa.4396. [DOI] [PubMed] [Google Scholar]
- 43.Khoddami A., Wilkes M. A., Roberts T. H. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18(2):2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chester T. L., Pinkston J. D., Raynie D. E. Supercritical fluid chromatography, and extraction. Analytical Chemistry. 1994;66(12):106–130. doi: 10.1021/ac00084a006. [DOI] [PubMed] [Google Scholar]
- 45.Bamba T. Application of supercritical fluid chromatography to the analysis of hydrophobic metabolites. Journal of Separation Science. 2008;31(8):1274–1278. doi: 10.1002/jssc.200700499. [DOI] [PubMed] [Google Scholar]
- 46.Kamangerpour A., Ashraf-Khorassani M., Taylor L. T., McNair H. M., Chorida L. Supercritical fluid chromatography of polyphenolic compounds in grape seed extract. Chromatographia. 2002;55(7-8):417–421. doi: 10.1007/BF02492270. [DOI] [Google Scholar]
- 47.Miller J. M. Chromatography: Concepts and Contrasts. 2nd ed. Hoboken, NJ, USA: John Wiley and Sons; 2004. [Google Scholar]
- 48.Nambiar V. S., Daniel M., Guin P. Characterization of polyphenols from Coriander leaves (Coriandrum sativum), red Amaranthus (A. paniculatus) and green Amaranthus (A. frumetaceus) using paper chromatography: and their health implications. Journal of Herbal Medicine and Toxicology. 2010;4:173–177. [Google Scholar]
- 49.Roberts E. A. H., Wood D. J. A study of the polyphenols in tea leaf by paper chromatography. The Biochemical Journal. 1951;49(4):414–422. doi: 10.1042/bj0490414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haminiuk C. W. I., Plata-Oviedo M. S. V., Guedes A. R., Stafussa A. P., Bona E., Carpes S. T. Chemical, antioxidant and antibacterial study of Brazilian fruits. International Journal of Food Science & Technology. 2011;46(7):1529–1537. doi: 10.1111/j.1365-2621.2011.02653.x. [DOI] [Google Scholar]
- 51.Yahia E. M. The contribution of fruit and vegetable consumption to human health. In: Rosa L. A., Alvarez‐Parrilla E., González‐Aguilar G. A., editors. Fruit and vegetable phytochemicals. John Wiley & Sons, Inc.; 2010. pp. 3–51. [DOI] [Google Scholar]
- 52.Fu L., Xu B. T., Xu X. R., et al. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chemistry. 2011;129(2):345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 53.Nutrients and health benefits. July 2019, https://www.choosemyplate.gov/fruits-nutrients-health.
- 54.Liu R. H. Potential synergy of phytochemicals in cancer prevention: mechanism of action. The Journal of Nutrition. 2004;134(12):3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 55.Mackinnon E. S., Rao A. V., Josse R. G., Rao L. G. Supplementation with the antioxidant lycopene significantly decreases oxidative stress parameters and the bone resorption marker N-telopeptide of type I collagen in postmenopausal women. Osteoporosis International. 2011;22(4):1091–1101. doi: 10.1007/s00198-010-1308-0. [DOI] [PubMed] [Google Scholar]
- 56.Vauzour D., Rodriguez-Mateos A., Corona G., Oruna-Concha M. J., Spencer J. P. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2(11):1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fraga C. G., Galleano M., Verstraeten S. V., Oteiza P. I. Basic biochemical mechanisms behind the health benefits of polyphenols. Molecular Aspects of Medicine. 2010;31(6):435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Wang L. S., Stoner G. D. Anthocyanins and their role in cancer prevention. Cancer Letters. 2008;269(2):281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayes J. D., Kelleher M. O., Eggleston I. M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. European Journal of Nutrition. 2008;47(S2):73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- 60.Pratheeshkumar P., Sreekala C., Zhang Z., et al. Cancer prevention with promising natural products: mechanisms of action and molecular targets. Anti-Cancer Agents in Medicinal Chemistry. 2012;12(10):1159–1184. doi: 10.2174/187152012803833035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bishayee A., Haskell Y., Do C., et al. Potential benefits of edible berries in the management of aerodigestive and gastrointestinal tract cancers: preclinical and clinical evidence. Critical Reviews in Food Science and Nutrition. 2016;56(10):1753–1775. doi: 10.1080/10408398.2014.982243. [DOI] [PubMed] [Google Scholar]
- 62.Darvesh A. S., Bishayee A. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutrition and Cancer. 2013;65(3):329–344. doi: 10.1080/01635581.2013.767367. [DOI] [PubMed] [Google Scholar]
- 63.Southgate D. A. Nature and variability of human food consumption. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1991;334(1270):281–288. doi: 10.1098/rstb.1991.0117. [DOI] [PubMed] [Google Scholar]
- 64.Slavin J. L., Lloyd B. Health benefits of fruits and vegetables. Advances in Nutrition. 2012;3(4):506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basu A., Rhone M., Lyons T. J. Berries: emerging impact on cardiovascular health. Nutrition Reviews. 2010;68(3):168–177. doi: 10.1111/j.1753-4887.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scalbert A., Andres-Lacueva C., Arita M., et al. Databases on food phytochemicals and their health-promoting effects. Journal of Agricultural and Food Chemistry. 2011;59(9):4331–4348. doi: 10.1021/jf200591d. [DOI] [PubMed] [Google Scholar]
- 67.van Breda S. G. J., de Kok T. M. C. M. Smart combinations of bioactive compounds in fruits and vegetables may guide new strategies for personalized prevention of chronic diseases. Molecular Nutrition & Food Research. 2018;62(1):p. 1700597. doi: 10.1002/mnfr.201700597. [DOI] [PubMed] [Google Scholar]
- 68.Halliwell B., Gutteridge J. M. C. Free Radicals in Biology and Medicine. Oxford: Clarendon Press; 1991. [Google Scholar]
- 69.Duthie S. J., Dobson V. L. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. European Journal of Nutrition. 1999;38(1):28–34. doi: 10.1007/s003940050043. [DOI] [PubMed] [Google Scholar]
- 70.Calomme M., Pieters L., Vlietinck A., Vanden Berghe D. Inhibition of bacterial mutagenesis by Citrus flavonoids. Planta Medica. 1996;62(3):222–226. doi: 10.1055/s-2006-957864. [DOI] [PubMed] [Google Scholar]
- 71.Plaumann B., Fritsche M., Rimpler H., Brandner G., Hess R. D. Flavonoids activate wild-type p 53. Oncogene. 1996;13(8):1605–1614. [PubMed] [Google Scholar]
- 72.van Erk M. J., Roepman P., van der Lende T. R., et al. Integrated assessment by multiple gene expression analysis of quercetin bioactivity on anticancer-related mechanisms in colon cancer cells in vitro. European Journal of Nutrition. 2005;44(3):143–156. doi: 10.1007/s00394-004-0503-1. [DOI] [PubMed] [Google Scholar]
- 73.Willcox J. K., Ash S. L., Catignani G. L. Antioxidants and prevention of chronic disease. Critical Reviews in Food Science and Nutrition. 2004;44(4):275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 74.Lampe J. W. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. The American Journal of Clinical Nutrition. 1999;70(3):475s–490s. doi: 10.1093/ajcn/70.3.475s. [DOI] [PubMed] [Google Scholar]
- 75.Navarro J., Flores P., Garrido C., Martinez V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chemistry. 2006;96(1):66–73. doi: 10.1016/j.foodchem.2005.01.057. [DOI] [Google Scholar]
- 76.Wijngaard H. H., Rößle C., Brunton N. A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chemistry. 2009;116(1):202–207. doi: 10.1016/j.foodchem.2009.02.033. [DOI] [Google Scholar]
- 77.Percival M. Antioxidants clinical nutrition insights. Advanced Nutrition Publications, Inc.; 1998. [Google Scholar]
- 78.Bazzano L. A., Li T. Y., Joshipura K. J., Hu F. B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31(7):1311–1317. doi: 10.2337/dc08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Desch S., Schmidt J., Kobler D., et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. American Journal of Hypertension. 2010;23(1):97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 80.Taubert D., Roesen R., Schomig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Archives of Internal Medicine. 2007;167(7):626–634. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- 81.Arts I. C., Hollman P. C. Polyphenols and disease risk in epidemiologic studies. The American Journal of Clinical Nutrition. 2005;81(1 Suppl):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 82.Mink P. J., Scrafford C. G., Barraj L. M., et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. The American Journal of Clinical Nutrition. 2007;85(3):895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 83.Wang L., Manson J. E., Gaziano J. M., Buring J. E., Sesso H. D. Fruit, and vegetable intake and the risk of hypertension in middle-aged and older women. American Journal of Hypertension. 2012;25(2):180–189. doi: 10.1038/ajh.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niki E. In Food and Free Radicals. New York: Plenum Press; 1997. Free radicals in chemistry and biochemistry; p. p. 2. [Google Scholar]


