How a new wave of research on venoms from an array of creatures could seed future pharma development.
Pediatric neurosurgeon Amy Lee works by the small, bright light of a microscope, her gaze focused on the opened skull of a child. Lee moves her hands calmly and confidently over the exposed brain, plucking out as much tumor as she safely can.
A handful of promising new drug candidates are derived from peptides in the venom of scorpions and other animals. Image credit: Shutterstock/Bens_Hikes.
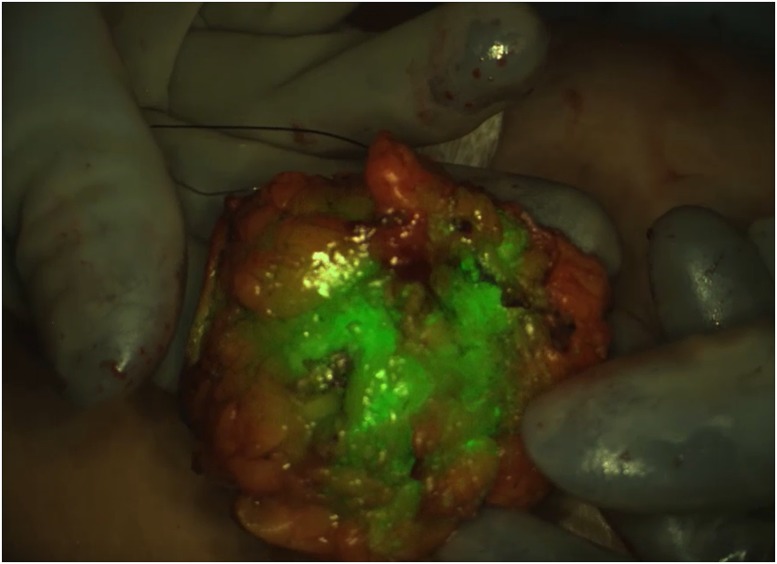
But there are some surgeries, and some parts of the brain, where tumor tissue and healthy tissue look very much alike. In those cases, Lee, based at Seattle Children’s Hospital in Washington, looks to a computer monitor beside the operating table, where a view of the brain shows tumor, illuminated in fluorescent green, nestled in otherwise white, healthy tissue. This new diagnostic tool helps surgeons gauge, in real time, how much tumor they’ve removed, and how much is left behind.
That green glow comes from tozuleristide, a new diagnostic drug in phase two clinical trials. But the drug’s novelty stems not only from its potential to highlight troublesome childhood tumors, but also from the compound’s source: the potent venom of the Israeli deathstalker scorpion (Leiurus quinquestriatus).
Tozuleristide uses a peptide from the venom and an infrared dye to seek out and illuminate tumors of all kinds, including in the breast, colon, and skin. The drug has gone through safety testing and early clinical trials to image brain tumors in children, and today stands about two years from possible US Food and Drug Administration (FDA) approval. The key ingredient, the scorpion venom peptide chlorotoxin, is just one of many untapped, and potentially lifesaving toxins in the venom of snakes, scorpions, spiders, and other creatures, honed through millions of years of evolution to immobilize prey or fend off predators.
Now, the pharmaceutical industry has a growing interest in venom, as some companies opt to return to drug discovery inspired by natural compounds, a trend that fell out of fashion about 40 years ago. Excitement about drugs such as viper venom-inspired captopril in the early 1980s waned a few years later in favor of less complex synthetic small molecules that chemists could dream up in a lab. The industry sank billions of dollars into designing small molecules, often inspired by the molecular structure of existing medicines, but otherwise lacking biological context, “and none of it worked,” says chemical biologist Mandë Holford, who studies terebrid snail toxins at Hunter College, the American Museum of Natural History, and Weill Cornell Medicine in New York City.
“Over the last few years it has felt like there’s a push back to natural products,” adds biologist and biochemist Helena Safavi at the University of Copenhagen in Denmark. Biting, stinging organisms have had millions of years to evolve an array of toxins that act on specific physiological pathways, she notes. Often these toxins have clear potential to treat human disease. She calls them a “treasure trove for biomedical explorations.”
Yvonne Angell, the head of peptide chemistry at ChemPartner in South San Francisco, CA, also attributes the trend to advances in affordably mass-producing peptide-based drugs, and in slowing the peptide’s breakdown in the gut or bloodstream, allowing these compounds more time than ever to act as therapeutics in the body. Better tools to characterize small volumes of venoms also let researchers study never-before-investigated peptide toxins, from tiny critters such as centipedes that produce a miniscule amount of poison (1).
Drug candidate tozuleristide, derived from scorpion venom, glows green in breast tissue removed during a lumpectomy surgery. Image credit: Blaze Bioscience.
Slow Burn
People have used venoms as medicines for thousands of years. In India, needles dipped in snake venom feature in a fiery kind of Ayurvedic acupuncture to treat joint pain and inflammation (2, 3). In China, dried venoms from the lumpy brown skins of toads are traditional anticancer treatments (4). In Southeastern Mexico, a beverage of mashed tarantula, alcohol, and herbs traditionally treats chest pain and asthma (5). Western medicine’s interest in venom grew from traditional uses and blossomed in the early 1980s with the advent of the first venom-derived drug, captopril.
Captopril was conceived as a specific enzyme inhibitor, according to the drug’s codevelopers (6). The researchers collaborated at the Squibb Institute for Medical Research in New Brunswick, NJ, beginning in the 1960s. Captopril’s key ingredient, inspired by a peptide in the Brazilian jararaca pit viper’s venom, binds and blocks the active site of angiotensin-converting enzyme (ACE), preventing release of a peptide hormone that causes blood vessels to constrict. When taken orally, captopril lowers high blood pressure by relaxing the circulatory system, explains Glenn King, a biochemist at the University of Queensland outside Brisbane, Australia. Having studied venom toxins since 1995, he’s seen the FDA approve nine more venom-derived drugs, with the most recent approval in 2012. At least one more venom-derived drug, batroxobin, is available outside of the United States (7).
All 10 FDA-approved medications are based on venom peptides or larger proteins, the main ingredients in the cocktail of toxins delivered by a bite or sting. Peptides and proteins are stable in the body, and highly specific, often acting on just one or several kinds of membrane protein, ion channel, enzyme, or other binding site.
Take spiders, for example: They’ve had 400 million years to evolve hundreds of venom peptides, many of which act on nervous system ion channels. Some tarantula venom peptides that King is now studying selectively bind and inactivate one ion channel involved in pain signaling to paralyze their prey, by binding voltage sensors outside of the pore, which can selectively shut off the channel.
Pharmaceutical companies have tried with limited success to develop a drug inhibiting the same kind of ion channel—using drugs to block the channel’s pore, “like a cork in a wine bottle,” King says. The trouble with that approach: Pore-blocking synthetic small-molecule drugs can’t distinguish between the pores. King wanted a more selective molecule than a pore binder, and found that the tarantula venom peptides bind much more specific voltage sensors. Now, he is exploring the tarantula venom peptides as highly specific channel inhibitors that could one day be new painkillers. In a 2017 study on the venom of the giant blue bloom tarantula, King and collaborators found one such peptide, at least 40 times more selective for the pain channel than for any other channels (8). However, there’s plenty of work to be done—the same study found that the peptide may not completely inhibit the channel in vivo.
Painkillers could also come from the toxins of scorpions, wasps, bees, and ants, all of which use venoms that primarily target the nervous system. Toxins from snakes have more potential as therapeutics for heart attack and stroke, because they affect the cardiovascular system.
And one recently discovered ingredient in marine cone snail venom could offer a promising avenue for diabetes treatments. The coral reef-dwelling geographer cone has a wicked means of attack: The cone releases a cloud of insulin to incapacitate small prey fish by crashing their blood sugar. In work carried out at the University of Utah in Salt Lake City since the mid-2010s, Safavi has shown that the snail insulin acts in seconds on the human insulin receptor (9), likely because the snail’s insulin lacks a clunky hinge-like structure that other insulins require for binding. In a study published earlier this year, Safavi discovered two more fish-hunting cone snails that also have fast-acting insulins (10). Although these venoms may bring instant death to little fish, Safavi thinks they could offer fast-acting new medicines to diabetics, whose therapies typically take 15 to 90 minutes. Safavi says she and collaborators recently licensed a compound inspired by snail insulin to a small San Francisco, California‐based biotech company called Monolog, which is leading the compound’s drug development in the lead-up to clinical trials.
Feeling the Heat
Despite the growing interest, only 10 drugs have received FDA approval for venoms since 1981, and the last one was more than eight years ago. “Certainly the techniques and processes are much better,” to develop venom-based drugs, King says. “So you’d think we’d be doing better, not worse.”
He sees no smoking gun, but suspects that the drug drought comes partly “because we’re going for harder things.” Many of the earliest venom-based drugs acted on enzymes in the cardiovascular system, such as clotting enzymes, he notes, which tend to have just one or several subtypes for a drug to target. Now drug development is looking to target diseases of the nervous system, which entails designing drugs that can act selectively on ion channels that can have dozens of hard-to-distinguish subtypes. Designing drugs to target such diseases in the brain is harder still, because the therapeutic compounds must cross the highly selective blood–brain barrier.
The consequences of a drug binding the wrong target are also more dangerous in the nervous system, King notes, where side effects can include paralysis or even death. Mistakes in the cardiovascular system are usually more fixable, because existing drugs can reverse excessive clotting or bleeding. This confluence of challenges has waylaid new toxin drug development.
A few drug candidate contenders could break the dry spell. Tozuleristide is one of them. So far it’s the only fluorescent brain tumor-imaging agent for kids. The drug completed phase one clinical trials in pediatric brain cancer patients in 2018, demonstrating that it’s safe to use in surgery. Neurosurgeon Lee at Seattle Children’s is now leading a phase two trial, in collaboration with surgeons at nine hospitals around the country, to determine how well tozuleristide illuminates tumor tissue rather than healthy brain.
Lee uses tozuleristide to complement her expert judgment, to cross-check how much tumor she has already removed, and how much tumor remains. If, during surgery, there’s no green glow left on the monitor beside her, “then I know I’ve gotten all of it,” Lee explains. “Or if it’s not safe to remove, we’re aware we left tumor there,” she adds. The troublesome tissue could then be treated with radiation or chemotherapy.
A few miles away from Lee’s operating room, Blaze Bioscience of Seattle, WA, is working to develop tozuleristide. President and CEO Heather Franklin estimates that the drug, if approved for pediatric brain cancer and eventually for most solid tumor cancer surgeries, could be useful to about two million patients per year in the United States and Europe.
What makes tozuleristide unique, says Blaze cofounder Jim Olson, is that its venom peptide chlorotoxin is retained in tumors and can cross the blood–brain barrier. Exactly how the toxin does this, and how it invades tumor cells—not only in the brain, but throughout the body—remains unclear. Olson, whose lab at the nearby Fred Hutchinson Cancer Research Center in Seattle created the molecular proof of concept for the drug, suspects that chlorotoxin’s tight, knot-like shape protects it from enzymes that would degrade the peptide, and may help it slip across the blood–brain barrier.
Insulin in the venom of the geographer cone snail (Left) acts quickly on the human insulin receptor. Autoimmune disease drug candidate dalazatide was inspired by a venom peptide from the sun anemone (Right). Image credit: (Left) Science Source/David Hall and (Right) Shutterstock/Damsea.
A Wide Web
Several other toxins are also snaking through clinical trials, including a drug candidate called dalazatide, inspired by the venom of the Caribbean sun sea anemone. From above, the anemone’s soft bed of yellow-green tentacles look spongy and inviting, but they pack a nasty sting, strong enough to kill little prey fish.
One component of the anemone’s sting, a peptide called ShK, shows promise to treat autoimmune diseases, including lupus, type 1 diabetes, inflammatory bowel disease, psoriasis, and multiple sclerosis (11). The peptide’s power comes from its ability to bind and block a potassium channel involved in autoimmune disorders. Blocking this channel, which is common on the surface of certain T cells, stops the cells from triggering the hallmark inflammation of autoimmune diseases, explains immunologist Michael Cahalan at the University of California, Irvine.
“We will see an explosion of information about venoms and venomous animals in the next 10 years or so.“
—Glenn King
One caveat is that ShK isn’t highly selective. It can bind and block several different kinds of potassium channels, not just the targeted one. Potential side effects in humans are unknown. “Rats injected with ShK seem perfectly fine,” Cahalan says. “They run about and sniff each other and engage in normal rat behavior.”
To get around potential side effects, Cahalan collaborated with George Chandy for more than 30 years at UC Irvine to develop dalazatide, a synthetic mimic of ShK that’s more selective, because it has an extra phosphorylated amino acid. The addition makes the peptide a little bigger, so it doesn’t bind as well to the unintended channels (12). Dalazatide is now gearing up for phase 1b clinical trials, according to the start-up TEKv Therapeutics in Columbus, OH, which is leading the dalazatide program.
And this March, a study reported that another scorpion venom-derived peptide, CDP-11R, rapidly concentrates in the cartilage of mice (13), the first venom peptide known to do so. Researchers used the peptide to selectively target cartilage to deftly deliver antiinflammatory steroids to arthritic joints. Steroids are used to treat arthritis, but they often have undesirable side effects when applied systemically. In the new study, Olson and a team from the Fred Hutchinson Cancer Research Center and Blaze Bioscience showed that a conjugate of the venom peptide and antiinflammatory steroids could much more selectively direct steroids to reverse inflammation in cartilage. The project is now poised for the preclinical safety studies, Olson says.
According to King, the pace of venom research is accelerating, and the number of candidates in the pipeline is growing. Some 220,000 animal species are known to be venomous, of which few have been studied in depth (1). New tools, such as transcriptomics, can sequence a droplet of liquid in a tiny venom gland, revealing the hundreds of peptides that an organism might use as toxins.
With ready tools revealing thousands of new peptides, “we will see an explosion of information about venoms and venomous animals in the next 10 years or so,” King predicts. Whether those venoms will contain peptides that treat major diseases is more difficult to divine.
But even if the venoms themselves are not medicinal, understanding which receptors they hit in the body could offer new treatment targets, he adds. The itchy, stinging hairs of the Amazonian Pararama caterpillar, for instance, contain a toxin that temporarily causes pain, swelling, and other arthritis-like symptoms that can eventually damage cartilage and joints (14). Researchers at Brazil’s biomedical Instituto Butantan in São Paulo are now studying the toxins in the hairs to identify new molecules the caterpillar uses to induce inflammation. Identifying the molecules involved may point to new antiinflammatory drug targets for arthritis treatments. The question in that case, King says, is not “what are the venom molecules, but what are they hitting?”
Whether the venom drug drought ends with candidates like tozuleristide, the field of venom drug development is starting a new chapter. “We’re going to find new molecules,” King says. But he sounds a cautious note. “It will be a long time until we understand the function of those molecules, and whether any of them might be therapeutically useful.”
References
- 1.Holford M., Daly M., King G. F., Norton R. S., Venoms to the rescue. Science 361, 842–844 (2018). [DOI] [PubMed] [Google Scholar]
- 2.King G. F., Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 11, 1469–1484 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Gomes A., et al. , Ethno biological usage of zoo products in rheumatoid arthritis. Indian J. Exp. Biol. 49, 565–573 (2011). [PubMed] [Google Scholar]
- 4.Meng Z., et al. , Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer 115, 5309–5318 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machkour-M’Rabet S., Hénaut Y., Winterton P., Rojo R., A case of zootherapy with the tarantula Brachypelma vagans Ausserer, 1875 in traditional medicine of the Chol Mayan ethnic group in Mexico. J. Ethnobiol. Ethnomed. 7, 12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cushman D. W., Ondetti M. A., History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension 17, 589–592 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Takacs Z., Medicines from animal venom toxins: Therapies. Available at http://www.zoltantakacs.com/venom_medicines_snake_toxin_drugs_zoltan_takacs.shtml. Accessed December 6, 2019.
- 8.Deuis J. R., et al. , Pharmacological characterisation of the highly NaV1.7 selective spider venom peptide Pn3a. Sci. Rep. 7, 40883 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safavi-Hemami H., et al. , Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proc. Natl. Acad. Sci. U.S.A. 112, 1743–1748 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahorukomeye P., et al. , Fish-hunting cone snail venoms are a rich source of minimized ligands of the vertebrate insulin receptor. eLife 8, e41574 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida M. J., Dalazatide (Formerly ShK-186) for Multiple Sclerosis. Multiple Sclerosis News Today. Available at https://multiplesclerosisnewstoday.com/dalazatide-formerly-shk-186-multiple-sclerosis/. Accessed December 6, 2019.
- 12.Tarcha E. J., et al. , Safety and pharmacodynamics of dalazatide, a Kv1.3 channel inhibitor, in the treatment of plaque psoriasis: A randomized phase 1b trial. PLoS One 12, e0180762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook Sangar M. L., et al. , A potent peptide-steroid conjugate accumulates in cartilage and reverses arthritis without evidence of systemic corticosteroid exposure. Sci. Transl. Med. 12, eaay1041 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Villas-Boas I. M., et al. , Premolis semirufa (Walker, 1856) envenomation, disease affecting rubber tappers of the Amazon: Searching for caterpillar-bristles toxic components. PLoS Negl. Trop. Dis. 6, e1531 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]