Significance
The increase in human population has fueled demand for pollination services; the resulting intensification and globalization of honey bee management has coincided with increased pathogen pressure. We hypothesized that Israeli acute paralysis virus (IAPV) can alter host social behavior, predicting different behavioral changes depending on social context. Using automated and manual behavioral monitoring, we find that honey bees have social immune mechanisms that may keep IAPV from spreading within a colony, but IAPV infection results in behavioral and physiological changes that could increase transmission between colonies. These results show how IAPV could take advantage of modern apiculture to increase its virulence and highlight the critical need to understand how human manipulation of managed species can lead to increased pathogen pressure.
Keywords: honey bee, virus, host–pathogen evolution, pathogen manipulation
Abstract
Anthropogenic changes create evolutionarily novel environments that present opportunities for emerging diseases, potentially changing the balance between host and pathogen. Honey bees provide essential pollination services, but intensification and globalization of honey bee management has coincided with increased pathogen pressure, primarily due to a parasitic mite/virus complex. Here, we investigated how honey bee individual and group phenotypes are altered by a virus of concern, Israeli acute paralysis virus (IAPV). Using automated and manual behavioral monitoring of IAPV-inoculated individuals, we find evidence for pathogen manipulation of worker behavior by IAPV, and reveal that this effect depends on social context; that is, within versus between colony interactions. Experimental inoculation reduced social contacts between honey bee colony members, suggesting an adaptive host social immune response to diminish transmission. Parallel analyses with double-stranded RNA (dsRNA)-immunostimulated bees revealed these behaviors are part of a generalized social immune defensive response. Conversely, inoculated bees presented to groups of bees from other colonies experienced reduced aggression compared with dsRNA-immunostimulated bees, facilitating entry into susceptible colonies. This reduction was associated with a shift in cuticular hydrocarbons, the chemical signatures used by bees to discriminate colony members from intruders. These responses were specific to IAPV infection, suggestive of pathogen manipulation of the host. Emerging bee pathogens may thus shape host phenotypes to increase transmission, a strategy especially well-suited to the unnaturally high colony densities of modern apiculture. These findings demonstrate how anthropogenic changes could affect arms races between human-managed hosts and their pathogens to potentially affect global food security.
Anthropogenic changes create evolutionarily novel environments that present opportunities for emerging diseases (1, 2), potentially changing the balance of host–pathogen coevolutionary arms races (3, 4). This problem is likely to worsen as humans continue to move domesticated species around the world and adopt increasingly intense management practices. The western honey bee (Apis mellifera), one of the world’s most important pollinators, has experienced this shift to increasingly industrial-scale apicultural practices over the past several decades (5–7), which involve moving honey bee colonies extensively and maintaining them at unnaturally high densities (6, 8, 9) for pollination of many important food and fiber crops (10). Increased pollination demand has also coincided with the worldwide spread of the parasitic mite Varroa destructor and several mite-vectored pathogenic viruses in the last 30 y, causing devastating losses to managed and wild honey bees (11).
Although there is evidence for both individual-level and group-level behavioral immune responses to combat other pathogens in honey bees (12, 13), responses to the highly detrimental Varroa-vectored viruses (11) are not well understood. In addition, it is not known whether pathogen manipulation of host behavior is involved in disease transmission. Honey bees and other highly social insects present exceptional opportunities for parasites and pathogens, as tens of thousands of highly related individuals live together in densely populated colonies (12). To combat the challenges posed by these factors, many social insects exhibit adaptive behaviors that reduce pathogen spread within their colonies (12). Transmission between colonies is also made difficult for pathogens by sophisticated recognition systems that help prevent foreign individuals from entering the colony (14). However, anthropogenic changes that increase the density of colonies can disrupt these pathogen defense systems and increase pest and pathogen movement (8), and have been hypothesized to lead to increased intercolony virulence (6, 8, 15). Unnaturally high apiary densities may create new opportunities for selection on traits related to increased pathogen transmission (6, 12).
Here, we combined automated and manual behavioral monitoring with chemical analysis to test how honey bees respond to infection with Israeli acute paralysis virus (IAPV). IAPV is a Varroa-vectored (11) virus with the potential to evolve rapidly (16). It is frequently found in honey bee colonies and is associated with increased colony mortality (11, 17, 18). Because many honey bee pathogens, including IAPV (17), are also spread horizontally through physical contact, we hypothesized that if honey bees have evolved adaptive behavioral responses to minimize disease impact, experimental inoculation will elicit behaviors that decrease social interactions among colony members (19).
To test this prediction, we employed a recently developed automated behavior monitoring system in which all colony members are individually barcoded and tracked continuously (20). This system features computer vision algorithms for detecting specific behaviors and allows for monitoring and quantification of movement, survivorship, and social food-sharing (trophallaxis), which may also lead to the transfer of pathogens (21, 22). In addition to monitoring these in-hive behaviors, a separate entrance monitoring system provided simultaneous quantification of flight activity as a proxy for foraging. Following these whole-colony monitoring experiments, we used more targeted manual observational assays of small groups of bees in the laboratory, using both IAPV inoculation and a pathogen-free double-stranded RNA (dsRNA)-immunostimulation to determine whether behavioral differences were part of a generalized antiviral response or were specific to IAPV infection per se.
We further hypothesized that, while honey bees may exhibit adaptive responses to generalized infection within a colony, IAPV could have evolved mechanisms to manipulate host behavior to increase its transmission between colonies. Specifically, we predicted that this phenomenon will manifest in behaviors that would increase or facilitate movement of infected individuals into susceptible colonies. Under normal conditions, honey bees from one colony can mistakenly join another. This phenomenon, called drifting, begins with errors during orientation or foraging flights, leading to incorrect orientation to the home colony (23). Pathogens or parasites, including other viruses, can affect cognition, likely reducing the bees’ ability to orient correctly (15, 24, 25). A variety of stressors (26, 27), including deformed wing virus infection (28), is associated with other foraging perturbations that could also be linked to intercolony movement, and the energetic stress of parasitism could also affect drifting (29). Therefore, it has been proposed that higher drifting rates could be a mechanism of pest (8, 15) and pathogen (6) transmission between colonies, although this has not been clearly shown.
Further, even if foragers do drift toward a foreign colony, they are not guaranteed access. Most social insect species have adaptations, like aggression toward nonnestmates, to prevent entrance of foreign individuals into their nest (14, 30). Honey bee colonies are able to prevent foreign individuals from entering the nest with specialized guard bees that stop and investigate individuals at the colony entrance; the decision to allow entrance is influenced by the profile of surface chemicals, most notably cuticular hydrocarbons (CHCs), that encode identifying information (14, 31). The CHC profiles of bees are linked to their colony and are determined by both genetics and environment; however, they are also highly dynamic, changing with age and behavioral status (14, 30, 32). Using our automatically monitored colonies, we tested how IAPV infection affected the incidence of bees leaving the colony. We then used multiple approaches to investigate the response of guard bees from other colonies and characterized how virus treatment affects CHC profiles. We aimed to understand whether changes in these behaviors and physiological factors have the potential to allow pathogen exploitation of the overcrowded conditions of human-managed bee colonies.
Results and Discussion
In experiments with three colonies that were each continuously observed for 5 d, we found that inoculated bees engaged in significantly fewer trophallaxis interactions with nestmates than did controls (33) (Fig. 1A; general linear mixed model [GLMM], P < 0.0004). Reduced social interactions were not driven by an overall reduction in activity, as inoculated bees moved more (Fig. 1B; GLMM, P < 0.003). These results highlight the value of an automated monitoring system that measures specific behaviors in addition to general movement (Fig. 1C). We suggest that the observed reduction in trophallaxis serves as an adaptive social immunity mechanism to reduce pathogen transmission via physical contact within colonies, as has been hypothesized in other systems (12, 34). Honey bee viruses (35), and IAPV in particular (36), have been shown to be transmissible through interindividual contact, with IAPV appearing to move through both contact and trophallaxis from infected workers (36). Further, even small changes in contact behaviors, as seen here, are predicted to have more substantial effects on the spread of a pathogen (20). Therefore, even modest reductions in contact and food sharing (independent of movement) have the potential to decrease virus transmission. However, the increase in movement we observed may be increasing disease spread through the hive, and thus be part of a viral counterattack. Alternatively, IAPV-infected bees may move more as they unsuccessfully search for trophallaxis partners.
Fig. 1.
Viral infection decreases honey bee social interactions but not overall movement in automatically monitored colonies. Violin plots with inset box-plots (horizontal line within rectangle indicates median; upper and lower hinges denote first and third quartiles, respectively, upper (Lower) whiskers extend from the hinge to the largest (smallest) value no further than 1.5 * interquartile range from the hinge). (A) Number of trophallaxis interactions per hour. IAPV-inoculated bees had significantly fewer trophallaxis interactions than sucrose-only control bees (GLMM for gamma distributed response variables, fixed effect: treatment, random effect: trial; likelihood ratio test against null model, χ2 = 12.69, 1 d.f., P < 0.0004). (B) Distance moved (in millimeters) per hour. IAPV-inoculated bees covered significantly greater distances than sucrose-only bees (GLMM for gamma distributed response variables, fixed effect: treatment; random effect: trial; likelihood ratio test against null model, χ2 = 9.50, 1 d.f., P < 0.003). Both measures were adjusted for lifespan on an individual bee basis. (C) Typical image obtained from the automatic system for identifying honey bees and detecting trophallaxis, showing barcoded bees inside the observation hive. The hive entrance is in the lower-right corner. (Inset) Close-up of two bees that were automatically detected performing trophallaxis. For these two bees, the results of applying our computer vision algorithms for head and trophallaxis detection are visualized in magenta and green, respectively.
Is reduction in social contacts an IAPV-specific response? While previous studies have found that bacterial immunostimulation without a live pathogen can adaptively affect honey bee behavior (12, 19), there are no comparable studies for bee viruses, which elicit a very different immune response primarily triggered by production of viral dsRNA (37). To understand IAPV–honey bee coevolution, it is critical to determine whether observed responses are specific to IAPV infection or due to general activation of the antiviral immune system. To accomplish this, we used targeted behavioral observations of small groups of bees in the laboratory infected with IAPV and groups of bees immunostimulated with dsRNA matching viral genome sequences, which has been previously shown to elicit an antiviral immune response in honey bees (38, 39). These manual behavioral observations revealed that both dsRNA-immunostimulated and IAPV-inoculated bees engaged in less trophallaxis than controls, but did not differ from each other (33) (Fig. 2A; negative binomial GLMM on estimated marginal means [EMM] with a Tukey post hoc test, control-dsRNA: P = 0.007; control-IAPV: P = 0.003; dsRNA-IAPV: P = 0.95), consistent with the findings from the colony-level experiments. We found no differences in overall activity (Fig. 2B; negative binomial GLMM, EMM, Tukey post hoc test; control-dsRNA: P = 0.76; control-IAPV: P = 0.92; dsRNA-IAPV: P = 0.52), indicating that reduction in trophallaxis is not caused by generalized sickness behavior such as a general reduction in activity. IAPV-inoculated and dsRNA-immunostimulated bees were antennated approximately as often as controls (Fig. 2C; negative binomial GLMM, EMM, Tukey post hoc test: control-dsRNA: P = 0.89; control-IAPV: P = 0.25; dsRNA-IAPV: P = 0.11). These findings suggest that, while trophallaxis is reduced, treated bees are still antennated and thus not generally ignored, raising the possibility that bees may be able to discriminate dsRNA-immunostimulated and IAPV-infected individuals from normal bees via antennation. Antennation is the main method used by honey bees for nestmate recognition, primarily through the detection and evaluation of chemical cues on the cuticle (30, 31). In doing so, uninfected bees may perceive CHC signals of illness and avoid engaging in trophallaxis with infected or immunostimulated bees. Overall, both laboratory and colony-based observations indicated that honey bees change their behavior to reduce social contact when the antiviral immune system of at least one participant is triggered.
Fig. 2.
Intracolonial reduction in social interactions is caused by a general immune response. Violin plots with inset box-plots (as in Fig. 1) showing counts of select behaviors, and results from negative binomial GLMM followed by estimated marginal means (EMM) with a Tukey post hoc test. (A) Honey bees treated with IAPV or dsRNA were about half as likely to engage in trophallaxis as untreated focal bees [Control-dsRNA: P = 0.007, Z = −3.028 exp(β) = 2.00; Control-IAPV: P = 0.003, Z = 3.275, exp(β) = 2.186; IAPV-dsRNA: P = 0.947, Z = 0.316]. (B) Measurement of movement did not differ significantly between treatment groups. (C) Antennation of treatment bees by partners did not significantly differ between treatment groups.
We also hypothesized that IAPV has evolved mechanisms to increase its virulence, such as triggering host behaviors that could increase intercolony transmission. This hypothesis is especially relevant in modern apiaries, where unnaturally high colony densities could pose increased transmission risk (6, 8, 15, 40). Automated monitoring of the bees’ flight activity in whole-colony experiments showed that flight activity was not diminished in IAPV-inoculated individuals (SI Appendix, Fig. S1 A–C), and inoculated bees left the colony normally, consistent with previous research (41). This suggests that infected foragers could thus transmit viruses when they leave the hive, particularly during drifting (6, 23). In considering intercolony transmission, however, exiting the host hive is only the first step. To enter a colony, foreign bees must negotiate guard bees that aggressively exclude nonnestmates, which they accomplish in part through evaluation of CHC profiles (14, 30, 31).
To test the hypothesis that IAPV is driving intercolonial host movement, we first used a laboratory nestmate recognition assay that recapitulates the guard behaviors in which honey bees aggressively exclude intruders (14, 42). With this approach, we found that IAPV-inoculated bees experienced significantly reduced aggression from foreign bees relative to dsRNA-immunostimulated bees, with controls being intermediate (33) (Fig. 3A and SI Appendix, Fig. S2; negative binomial GLM, EMM, Tukey honestly significant difference (HSD); dsRNA-IAPV: P = 0.0143; dsRNA-control: P = 0.44; control-IAPV: P = 0.23). Furthermore, these IAPV-inoculated bees elicited more grooming (SI Appendix, Fig. S2D; negative binomial GLM, EMM, and Tukey HSD; dsRNA-IAPV: P = 0.006; control-IAPV: P = 0.0823; dsRNA-control: P = 0.61) and trophallaxis (Fig. 3B; negative binomial GLM, EMM, and Tukey HSD; dsRNA-IAPV: P < 0.0001; control-IAPV: P = 0.0592; control-dsRNA: P = 0.0148) than immunostimulated bees, potentially increasing transmission of IAPV through social feeding or other physical contacts that take place during trophallaxis (17). Importantly, grooming and trophallaxis have previously been associated with appeasement and acceptance into foreign social insect colonies (43), thus increasing such responses contributes to a behavioral mechanism by which infected bees are better able to bypass guards. In contrast, dsRNA-immunostimulated bees experienced significantly greater aggression than did inoculated bees (Fig. 3A and SI Appendix, Fig. S2 A–C; negative binomial, EMM, Tukey HSD; dsRNA-IAPV: P = 0.0143). These results suggest that immunostimulation reduces acceptance by increasing aggressive responses, and that the responses to IAPV-inoculated bees (which would also exhibit immune activation) are likely IAPV-specific.
Fig. 3.
Intercolonial reduction in aggression is IAPV-specific. Violin plots with inset box-plots (as in Fig. 1) showing counts of select behaviors and results of negative binomial GLMM followed by EMM with a Tukey post hoc test: (A) IAPV-inoculated bees experienced significantly reduced aggression from putative guards compared to immunostimulated bees, with controls intermediate [dsRNA-IAPV: P = 0.0143, exp(β) = 5.601, Z = 2.796; Control-IAPV: P = 0.2345; dsRNA-Control: P = 0.4397]. (B) IAPV-inoculated bees elicited significantly more trophallaxis behaviors between treatment bees and putative guards compared to immunostimulated bees [dsRNA-IAPV: P < 0.0001, exp(β) = 0.38, Z = −5.012; Control-IAPV: P = 0.0592, exp(β) = 0.66, Z = −2.276; dsRNA-Control: P = 0.0148, exp(β) = 1.747, Z = 2.784]. (C) A significantly greater proportion of IAPV-treated intruder bees were accepted at foreign colony entrances than control or dsRNA-immunostimulated bees (Pearson’s Chi-squared test, IAPV-Control, χ2 = 5.9665, d.f. = 1, P = 0.0218; IAPV-dsRNA, χ2 = 19.13, d.f. = 1, Benjamini–Hochberg-corrected P < 0.0001; Control-dsRNA, χ2 = 1.099, d.f. = 1, P = 0.294; Ncontrol = 42, NdsRNA = 31, and NIAPV = 30; sample sizes indicated inside bars).
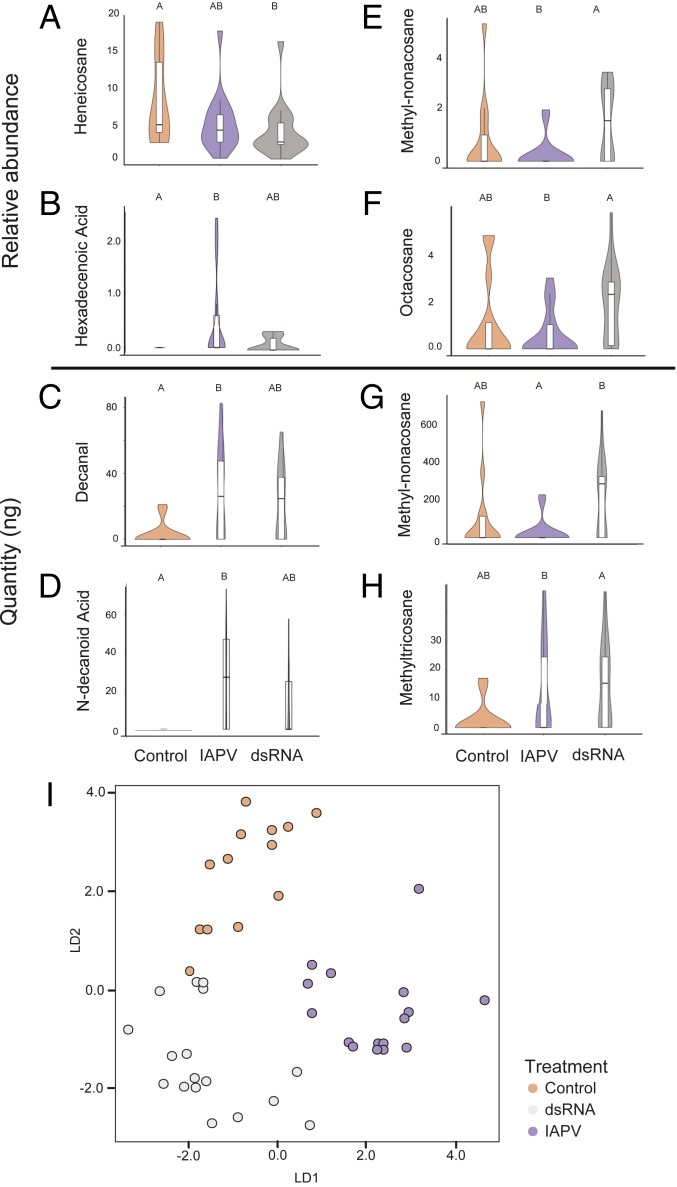
Analysis of honey bee CHCs, which mediate nestmate recognition (31), revealed how IAPV may achieve this remarkable reversal (33). A survey of 52 CHCs from bees from the laboratory nestmate recognition assay identified significant virus- or dsRNA-induced differences in the relative or total abundance of 8 identified compounds, with similar trends in several others (Fig. 4 A–H and SI Appendix, Table S1). Changes in CHC profiles were complex, but differences in individual compound totals or proportions can increase (44) or decrease (45) aggression toward foreign intruders. For example, octacosane was a relatively small component of the CHC profile in IAPV-inoculated bees (Fig. 4F), and lower levels of this CHC have been previously associated with increased acceptance (32). Visualization of overall CHC profiles suggests IAPV infection resulted in a profile that was distinct from either dsRNA-treatments or controls, but with characteristics of both (Fig. 4I and SI Appendix, Fig. S3). Some CHCs were likely associated only with immune stimulation, as they were similar in both IAPV and dsRNA-treated bees, but not controls (Fig. 4 A–D). However, other compounds were changed by dsRNA treatment, but not by IAPV (Fig. 4 E–G), and may therefore be more specific to IAPV infection.
Fig. 4.
Viral infection changes honey bee cuticular hydrocarbon profile. Violin plots with inset box-plots (as in Fig. 1) of cuticular hydrocarbon relative abundance (A, B, E, and F) and nanogram (C, D, G, and H) values found to be significantly different between groups (statistics are reported in SI Appendix, Table S1). Comparisons were made with a standard GLMM followed by an EMM analysis with a Tukey post hoc test. Letters denote significant differences (P < 0.05). (I) Separation of CHC profiles (relative abundance) visualized by using linear discriminant analysis shows distinct profile patterns of IAPV-inoculated, dsRNA-treated, and control bees. Variance explained by each LD is as follows: LD1: 0.522, LD2: 0.478.
To further test the hypothesis that IAPV is driving intercolonial host movement, it is critical to also determine how the virus affects successful acceptance into a susceptible colony. We conducted field-based assays at the entrances of three normally managed honey bee colonies. This approach, well-established as a proxy for nestmate recognition (46, 47), presents treated workers to guard bees at the entrances of their colonies and assesses whether they are accepted or rejected (32). We found that IAPV-inoculated bees were significantly more likely to be accepted by the guards at the colony entrance than were either controls or dsRNA-immunostimulated bees, with IAPV-inoculated bees less likely to elicit aggression from guards and/or successfully entering the colony (Fig. 3C; Pearson’s Chi-squared test, IAPV-control, χ2 = 5.9665 [degrees of freedom (d.f.) = 1; P = 0.0218]; IAPV-dsRNA, χ2 = 19.13 [d.f. = 1; Benjamini–Hochberg-corrected P < 0.0001]). There was also a nonstatistically significant trend toward reduced acceptance of dsRNA-immunostimulated bees compared with controls (Pearson’s Chi-squared test, control-dsRNA, χ2 = 1.099; d.f. = 1; P = 0.294). More than 30% of the IAPV-inoculated bees were accepted, more than twice as many as the control or immunostimulated bees. This enhanced ability of infected bees to gain entrance to a colony likely increases the probability of spreading infection between colonies.
Together, our findings suggest the following scenario of intercolony transmission: IAPV infection produces a CHC profile distinct from that caused by generalized antiviral immunostimulation, resulting in changes in the responses of guard bees to these odors and increased acceptance of infected individuals by foreign colonies, thereby facilitating the transmission of IAPV from colony to colony. Behavioral changes in infected bees at the colony entrance, such as a higher rate of trophallaxis, may also function to increase acceptance of foreign bees and at the same time facilitate virus transmission. Combined with the intracolony findings showing adaptive behaviors that could reduce pathogen transmission between nestmates, this study suggests that there might be an evolutionary “arms race” between IAPV and honey bees, with context-specific modulation of behavior by both parties. IAPV, like all pathogens, has been under selection to increase transmission, but the behavioral and physiological changes it engenders in infected honey bees are likely to be particularly successful in the stressful, overcrowded environments of modern apiculture.
Conclusions
Pathogen-induced behavioral changes affecting intercolony transmission represent a major cause for concern in controlling honey bee disease, especially in the setting of a modern apiary. Honey bee colonies in natural settings occur at a comparatively low density, approximately one colony per square kilometer (8, 40). However, in modern apiaries, colonies are often kept at substantially higher densities, less than 1 m apart (8); commercial beekeepers typically maintain dozens of colonies at a single apiary (48), and can even place hundreds of colonies in orchards and fields for pollination (10). Such high colony densities have been associated with higher Varroa mite pressure (8), and are predicted to exacerbate pathogen transmission (6, 15), but never before has a mechanism by which a viral pathogen could actively exploit these practices been shown. We demonstrated that IAPV infection, independent from generalized antiviral immunostimulation, results in behavioral and physiological changes in host bees that would aid the virus in moving between nearby colonies. While we hypothesize that selection in favor of these responses would be stronger at modern, high apiary densities, and less likely in natural settings, boundaries between managed and wild honey bee colonies are fluid, with colonies of both types sharing habitat, and thus pathogen populations (8, 40, 49, 50). As such, these effects are likely to be found in both managed bees and natural or feral populations.
Our findings also suggest that infection by IAPV may increase the spread of other viruses and Varroa mites (51). Because Varroa vectors IAPV (17) and is known to change the honey bee viral landscape (52), enhanced intercolony movement (15, 51) could fuel an evolving mutualism between IAPV and Varroa, speeding the selection for behavioral manipulation and implicating a complex three-way coevolutionary system among mites, pathogens, and their common host. While humans have kept honey bees for millennia, only in the last several decades has human management of honey bees moved toward industrial-scale beekeeping, bringing about worldwide distribution of pathogens and unnaturally crowded conditions that create evolutionary novel environments that can select for virulence or exacerbate pathogen transmission mechanisms (6, 7). With increased numbers of honey bee colonies used for pollination (5, 7), the demands for modern food security may play a role in selecting for behavioral manipulation by IAPV. As honey bees and other managed species live under increasingly crowded conditions due to anthropogenic change, and are subject to worldwide dispersal of emerging diseases (1, 2), our work highlights that it is critical to build a better understanding of how humans are influencing host–pathogen coevolution.
Methods
Experiments with Automatically Monitored Colonies.
Automated colony monitoring was performed at the University of Illinois Bee Research Facility in Urbana, IL. All individuals in each colony were outfitted with a bCode barcode following the tagging procedure described previously (20). In short, we automatically monitored movement, flight activity, and trophallaxis of orally IAPV-inoculated and sucrose-only control honey bees in three colonies for 5 d. To standardize these colonies, they were each established with ∼900 barcoded, 2-d-old, untreated adult worker bees (SI Appendix, Table S3) and a barcoded queen, and provided with the same amounts of honey and artificial bee bread (a protein source made from pollen mixed with honey and water). Such colonies have been shown to develop the basic elements of colony social organization despite an atypical age demography (53). Treatment and control group size was initially the same within each colony and similar across colonies, but ranged from ∼90 to 150 bees (10% to 15% of the colony population) across trials (SI Appendix, Fig. Table S3). Movement and trophallaxis were monitored at a resolution of 1 Hz throughout the experiment. Trophallaxis detection was performed using the same trophallaxis detector described previously (20); this detector uses computer vision to identify trophallaxis and has been validated to correctly identify ∼50% of trophallaxis interactions, of which 81% are true positives (20). This detector was used identically on treatment and control bees. Flight activity was monitored at 2 Hz from the first day when the hive entrance was open (day 2 of the experiment) for 5 d. The observation hive and entrance monitor windows were changed daily to ensure a high detection rate throughout the experiment; otherwise, colonies were left undisturbed. To verify successful inoculation, a subset of IAPV-inoculated and sucrose-only fed control bees were frozen at −80 °C 24 h post treatment, and virus titers were quantified via RT-qPCR. In the colonies, we compared the relationships among survival, trophallaxis rate, movement rate, flight rate, fraction of time spent outside, proportion of bees flying, and treatment type. Full experimental details and statistical analyses are reported in the SI Appendix.
Manual Behavioral Observations in the Laboratory.
Preparation of treated bees.
All bees were derived from the research apiary housed at the Iowa State University Horticulture Research Station, Ames, IA, in midsummer, 2016. As with the automatically monitored colonies, these source colonies were derived from naturally mated queens, primarily from commercial stock of Apis mellifera ligustica; all colonies were independent from those used in barcoded colony experiments. For each treatment, 30 one-day-old (0- to 24-h-old) bees each were placed into one of three 70 × 80 × 90 mm ventilated plastic cages; these treated bees were marked to indicate treatment group. Treatment identification color was randomized for each trial to minimize potential observer bias during observations. Each cage received a dish with 600 µL treatment: a sublethal viral dose in 30% sucrose (∼2 × 105 IAPV genome equivalents per bee), 0.05 µg/µL dsRNA in 30% sucrose solution (∼1 µg per bee) (38), or sterile 30% sucrose-solution. 24 h after this first feeding, the cages were provided ad libitum 30% sucrose-solution. From the same large group of 1-d-old bees, six cages of 30 bees were designated to be untreated partner bees; these bees were given 600 µL of 30% sucrose solution on the first day, then ad libitum 30% sucrose solution on the second day (identical to control treatment bees). Partner bees were differentiated from control bees by paint mark. Behavioral observations were conducted 2 d later, when virus titers were most likely to peak (41, 54). Successful inoculation was verified via RT-qPCR (SI Appendix, Table S2 and Fig. S4), described in detail in SI Appendix, Methods.
Intracolony context: Laboratory observations of trophallaxis.
For pairwise observations, a treated bee of a randomly selected treatment was paired with a partner bee in an experimental arena comprised of a Petri dish with a thin sheet of beeswax foundation pressed to the floor, mounted in an upright fashion to mimic the colony environment (55). Each pair of bees was observed via scan sampling for 10 instances of 10 s each, with behaviors noted for each 10-s interval. Bees were then left to incubate with ad libitum 30% sucrose solution for 2 additional days (to allow potential viral infections to manifest). All bees were then collected on dry ice. This process was performed for 80 pairs with a dsRNA-treated bee, 81 with a sucrose-only control, and 77 with a IAPV-inoculated bee. Behavioral data were collected for both the treatment bee and her partner in each pair, recording counts of nine different behaviors (listed and described in SI Appendix, Table S6). Statistical analysis is described in detail in the SI Appendix.
Intercolony context: Laboratory-based intruder assays.
For intruder assay observations, performed in summer 2017, the same preparation and virus/dsRNA treatments as described earlier were used. This assay is a controlled laboratory approach that provides substantial logistical advantages over field-based studies of drifting behavior, as it allows greater replication and better resolution in behavioral observations. Intruder assays have been used extensively and have been shown to recapitulate field results (14, 42, 56–58). To obtain resident (putative guard) bees, we blocked the entrance of normal, unmanipulated hives (i.e., hives unused for previous experiments and not the source of treated bees), and waited 15 min; the hives were then gently jostled, after which the block was removed. Using a small portable vacuum device (BioQuip), we collected bees rushing out of the hives and those returning, selecting for those returning with pollen (e.g., older foragers). These bees thus represented a pool of older, more aggressive bees comprised of both guards and foragers. After collection, these bees were briefly sedated on wet ice, and then transferred to observation arenas (10 residents/arena), then given 15 min to acclimate. We then introduced a treated bee (IAPV-inoculated, dsRNA-treated, or sucrose-only control) and counted the behavioral responses of the putative guard bees (SI Appendix, Table S7) to the treated bee for 10 min. After observations, all bees were humanely euthanized on dry ice and collected in glass vials with polytetrafluoroethylene-lined screw caps. Over the course of five experimental rounds, we observed and collected samples for 164 arena matches (NdsRNA = 55, Ncontrol = 55, and NIAPV = 54). Statistical analysis is described in detail in the SI Appendix.
Cuticular hydrocarbon analysis.
A randomly selected subset of treated bees from the intruder (intercolony) assays was selected for CHC extraction and characterization by GC/MS (Ncontrol = 13, NdsRNA = 17, and NIAPV = 16). Each bee was immersed in high-performance liquid chromatography (HPLC)-grade hexanes for 5 min in her original collection vial. This extract was dried completely with a N2 stream and resuspended in 20 µL HPLC-grade hexanes. A 2-µL subsample of this concentrate was splitlessly injected into an Agilent HP-5MS (30 m × 0.25 mm, film width of 0.25 µm; cat. no. 19091s-433) nonpolar capillary column, with an Agilent 7890B gas chromatograph paired with an Agilent 5977A mass selective detector, held at 60 °C for 1 min, and exposed to incrementally higher temperatures (up to 300 °C) at a rate of 10 °C/min, then held at 300 °C for 15 min (inlet temperature 250 °C, pressure 8.2317 psi, septum purge flow 3 mL/min, purge flow to split vent 15 mL/min at 0.75 min of run). Peaks were identified by library search (59) and manually verified using both GC retention time and MS spectra (SI Appendix, Table S1D). Identified compounds were subsequently checked against compounds identified in honey bees in several studies: a nestmate and caste-recognition study (60), an LPS-inoculation study (19), and two studies observing pathogen-induced CHC changes (61, 62). Nanogram quantification of each compound was performed by comparing peak area of compound in question with that of a standard spike-in (Hexadecane, 100 ng/sample). Statistical analysis is described in detail in the SI Appendix.
Intercolony context: Field-based intruder assays.
Field observation intruder assays were performed in summer 2019 and followed the same preparation and virus/dsRNA treatments as described earlier. Briefly, 30 one-day-old bees (<24 h old) were each paint marked and placed into six acrylic cages, where they received either an IAPV, dsRNA, or control treatment in 30% sucrose solution. The identity of the treatment was hidden from future observers to ensure assays were performed blind with respect to treatment. As with laboratory experiments, intruder assays were performed 60 h after treatment and followed a previously established protocol (32). Three colonies maintained according to standard commercial beekeeping techniques in standard Langstroth hives were fixed with modified hive entrances, which consisted of a square transparent tunnel measuring ∼10 cm wide × 5 cm high. The modified entrances were affixed 48 h before intruder assays to allow the colony’s guards and foragers to acclimate to the novel entrance configuration. During assays, treated intruders were transferred from their plastic cages onto the modified hive entrance using soft forceps, and a barrier was placed on the entrance to prevent intruders from flying away. Over a 5-min trial, intruders were scored as rejected if they were bitten, stung, or chased by hive guards and accepted if they entered the hive or were not attacked by the guards. The order of presenting intruders and hives was randomized, and all intruders that entered hives were removed from these hives after the completion of all trials. For each intruder, data collection began 30 s after introduction to minimize the effect of handling with forceps on behavioral response. In total we assayed Ncontrol = 42, NdsRNA = 31, and NIAPV = 30 intruders. Statistical analysis is described in detail in the SI Appendix.
Data, Materials, and Code Availability.
All datasets are available in the Dryad repository https://datadryad.org/stash/dataset/doi:10.5061/dryad.m63xsj3z8. Computer code for automatically locating and identifying bees in digital images, quantifying movement, detecting trophallaxis, and detecting flight activity is publicly available at https://github.com/gernat/btools. To detect trophallaxis, we used v0.12.0 of the trophallaxis detector available at that location.
Supplementary Material
Acknowledgments
This material is based upon work supported by US Department of Agriculture grant 2019-67013-29300 (A.G.D., B.C.B., and G.E.R.), a grant from the Christopher Family Foundation (G.E.R.), the National Academies Keck Futures Initiatives CB4 (T.G.), NIH grant R01GM117467 (G.E.R. and Nigel Goldenfeld), and the North American Pollinator Protection Campaign (A.L.T. and A.G.D.). We thank T. L. Harrison and A. L. Sankey for bee management in Illinois; N. Howell and the Iowa State University Horticulture Research Station for apiary support in Iowa; C. R. Pace for assistance developing intruder assay methodology; C. Vernier for advice on colony entrance assays; and J. Cullum, S. Bransley, A. Zhang, A. Ray, L. Block, and V. Bagchi for assistance with field work. We are grateful to A. Cash-Ahmed and L. Block for determining virus titers; Bayer for providing Kashmir bee virus dsRNA. We thank Reliance Label Solutions for printing bCodes; the University of Illinois School of Life Sciences Machine shop for constructing bee tracking equipment; K. Wilk, M. A. L. Seyller, and J. Falk for annotating images; members of the University of Illinois Carl R. Woese Institute for Genomic Biology Computer Network Resource Group for assistance with data storage; and L. Hanks, J. Mongold-Diers, and R. Jurenka for assistance with cuticular hydrocarbon profiling. We thank K. Dolezal for manuscript editing advice; Nina Fefferman (Emory University) and members of the A.L.T., G.E.R., and A.G.D. labs for discussions; and E. Hadley for assistance in figure production.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All data have been deposited in the Dryad repository at https://doi.org/10.5061/dryad.m63xsj3z8.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002268117/-/DCSupplemental.
References
- 1.Daszak P., Cunningham A. A., Hyatt A. D., Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 78, 103–116 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Perry B. D., Grace D., Sones K., Current drivers and future directions of global livestock disease dynamics. Proc. Natl. Acad. Sci. U.S.A. 110, 20871–20877 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamo S. A., Parasites: evolution’s neurobiologists. J. Exp. Biol. 216, 3–10 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Aubert A., Sickness and behaviour in animals: A motivational perspective. Neurosci. Biobehav. Rev. 23, 1029–1036 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Daberkow S., Korb P., Hoff F., Structure of the U.S. Beekeeping industry: 1982-2002. J. Econ. Entomol. 102, 868–886 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Fries I., Camazine S., Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 32, 199–214 (2001). [Google Scholar]
- 7.Owen R., Role of human action in the spread of honey bee (Hymenoptera: Apidae) pathogens. J. Econ. Entomol. 110, 797–801 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Seeley T. D., Smith M. L., Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie 46, 716–727 (2015). [Google Scholar]
- 9.Simone-Finstrom M., et al. , Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 6, 32023 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rucker R. R., Thurman W. N., Burgett M., Honey bee pollination markets and the internalization of reciprocal benefits. Am. J. Agric. Econ. 94, 956–977 (2012). [Google Scholar]
- 11.Grozinger C. M., Flenniken M. L., Bee viruses: Ecology, pathogenicity, and impacts. Annu. Rev. Entomol. 64, 205–226 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Cremer S., Pull C. D., Fürst M. A., Social immunity: Emergence and evolution of colony-level disease protection. Annu. Rev. Entomol. 63, 105–123 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Van Meyel S., Körner M., Meunier J., Social immunity: Why we should study its nature, evolution and functions across all social systems. Curr. Opin. Insect Sci. 28, 1–7 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Nouvian M., Reinhard J., Giurfa M., The defensive response of the honeybee Apis mellifera. J. Exp. Biol. 219, 3505–3517 (2016). [DOI] [PubMed] [Google Scholar]
- 15.DeGrandi-Hoffman G., Ahumada F., Graham H., Are dispersal mechanisms changing the host-parasite relationship and increasing the virulence of Varroa destructor (Mesostigmata: Varroidae) in managed honey bee (Hymenoptera: Apidae) colonies? Environ. Entomol. 46, 737–746 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Steinhauer D. A., Holland J. J., Rapid evolution of RNA viruses. Annu. Rev. Microbiol. 41, 409–433 (1987). [DOI] [PubMed] [Google Scholar]
- 17.Chen Y. P., et al. , Israeli acute paralysis virus: Epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog. 10, e1004261 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y. P., Evans J. D., Historical presence of Israeli acute paralysis virus in the United States. Am. Bee J. 147, 1027–1028 (2007). [Google Scholar]
- 19.Richard F. J., Aubert A., Grozinger C. M., Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC Biol. 6, 50 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gernat T., et al. , Automated monitoring of behavior reveals bursty interaction patterns and rapid spreading dynamics in honeybee social networks. Proc. Natl. Acad. Sci. U.S.A. 115, 1433–1438 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribière M., Olivier V., Blanchard P., Chronic bee paralysis: A disease and a virus like no other? J. Invertebr. Pathol. 103 (suppl. 1), S120–S131 (2010). [DOI] [PubMed] [Google Scholar]
- 22.de Miranda J. R., Genersch E., Deformed wing virus. J. Invertebr. Pathol. 103 (suppl. 1), S48–S61 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer K. J., Crailsheim K., Drifting of honeybees. Insectes Soc. 45, 151–167 (1998). [Google Scholar]
- 24.Iqbal J., Mueller U., Virus infection causes specific learning deficits in honeybee foragers. Proc. Biol. Sci. 274, 1517–1521 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kralj J., Brockmann A., Fuchs S., Tautz J., The parasitic mite Varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 193, 363–370 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Perry C. J., Søvik E., Myerscough M. R., Barron A. B., Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc. Natl. Acad. Sci. U.S.A. 112, 3427–3432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rueppell O., Hayworth M. K., Ross N. P., Altruistic self-removal of health-compromised honey bee workers from their hive. J. Evol. Biol. 23, 1538–1546 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Benaets K., et al. , Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc. Biol. Sci. 284 20162149 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordier C., Pioz M., Crauser D., Le Conte Y., Alaux C., Should I stay or should I go: Honeybee drifting behaviour as a function of parasitism. Apidologie 48, 286–297 (2017). [Google Scholar]
- 30.Hölldobler B., Wilson E. O., The Superorganism: The Beauty, Elegance, and Strangeness of Insect Societies (W.W. Norton, New York, ed. 1, 2009), pp. xxi. [Google Scholar]
- 31.Breed M. D., Cook C. N., McCreery H. F., Rodriguez M., “Nestmate recognition in eusocial insects: The honeybee as a model system” in Social Recognition in Invertebrates: The Knowns and the Unknowns, Aquiloni L., Tricarico E., Eds. (Springer International Publishing, Cham, 2015), pp. 147–164. [Google Scholar]
- 32.Vernier C. L., et al. , The cuticular hydrocarbon profiles of honey bee workers develop via a socially-modulated innate process. eLife 8, e41855 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolezal A., et al. , Honey bee virus causes context-dependent changes in host social behavior. Dryad. 10.5061/dryad.m63xsj3z8. Deposited 6 March 2020. [DOI] [PMC free article] [PubMed]
- 34.Stroeymeyt N., et al. , Social network plasticity decreases disease transmission in a eusocial insect. Science 362, 941–945 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Evans J., Feldlaufer M., Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 92, 152–159 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Amiri E., et al. , Israeli acute paralysis virus: Honey bee queen−worker interaction and potential virus transmission pathways. Insects 10, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vijayendran D., Airs P. M., Dolezal K., Bonning B. C., Arthropod viruses and small RNAs. J. Invertebr. Pathol. 114, 186–195 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Maori E., et al. , IAPV, a bee-affecting virus associated with Colony Collapse Disorder can be silenced by dsRNA ingestion. Insect Mol. Biol. 18, 55–60 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Hunter W., et al. , Large-scale field application of RNAi technology reducing Israeli acute paralysis virus disease in honey bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathog. 6, e1001160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seeley T. D., Honey bees of the Arnot Forest: A population of feral colonies persisting with Varroa destructor in the northeastern United States. Apidologie 38, 19–29 (2007). [Google Scholar]
- 41.Dolezal A. G., et al. , Interacting stressors matter: Diet quality and virus infection in honeybee health. R. Soc. Open Sci. 6, 181803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breed M. D., Nestmate recognition in honey bees. Anim. Behav. 31, 86–91 (1983). [Google Scholar]
- 43.Wilson E. O., Social insects. Science 172, 406 (1971). [DOI] [PubMed] [Google Scholar]
- 44.Dani F. R., et al. , Nestmate recognition cues in the honey bee: Differential importance of cuticular alkanes and alkenes. Chem. Senses 30, 477–489 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Cappa F., et al. , Natural biocide disrupts nestmate recognition in honeybees. Sci. Rep. 9, 3171 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Downs S. G., Ratnieks F. L. W., Adaptive shifts in honey bee (Apis mellifera L.) guarding behavior support predictions of the acceptance threshold model. Behav. Ecol. 11, 326–333 (2000). [Google Scholar]
- 47.D’Ettorre P., et al. , Wax combs mediate nestmate recognition by guard honeybees. Anim. Behav. 71, 773–779 (2006). [Google Scholar]
- 48.Smart M. D., Pettis J. S., Euliss N., Spivak M. S., Land use in the Northern Great Plains region of the US influences the survival and productivity of honey bee colonies. Agric. Ecosyst. Environ. 230, 139–149 (2016). [Google Scholar]
- 49.Youngsteadt E., Appler R. H., López-Uribe M. M., Tarpy D. R., Frank S. D., Urbanization increases pathogen pressure on feral and managed honey bees. PLoS One 10, e0142031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seeley T. D., Tarpy D. R., Griffin S. R., Carcione A., Delaney D. A., A survivor population of wild colonies of European honeybees in the northeastern United States: Investigating its genetic structure. Apidologie 46, 654–666 (2015). [Google Scholar]
- 51.Forfert N., et al. , Parasites and pathogens of the honeybee (Apis mellifera) and their influence on inter-colonial transmission. PLoS One 10, e0140337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin S. J., et al. , Global honey bee viral landscape altered by a parasitic mite. Science 336, 1304–1306 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Robinson G. E., Page R. E. Jr, Strambi C., Strambi A., Hormonal and genetic control of behavioral integration in honey bee colonies. Science 246, 109–112 (1989). [DOI] [PubMed] [Google Scholar]
- 54.Carrillo-Tripp J., et al. , In vivo and in vitro infection dynamics of honey bee viruses. Sci. Rep. 6, 22265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shpigler H. Y., Robinson G. E., Laboratory assay of brood care for quantitative analyses of individual differences in honey bee (Apis mellifera) affiliative behavior. PLoS One 10, e0143183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li-Byarlay H., Rittschof C. C., Massey J. H., Pittendrigh B. R., Robinson G. E., Socially responsive effects of brain oxidative metabolism on aggression. Proc. Natl. Acad. Sci. U.S.A. 111, 12533–12537 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rittschof C. C., et al. , Neuromolecular responses to social challenge: Common mechanisms across mouse, stickleback fish, and honey bee. Proc. Natl. Acad. Sci. U.S.A. 111, 17929–17934 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rittschof C. C., Coombs C. B., Frazier M., Grozinger C. M., Robinson G. E., Early-life experience affects honey bee aggression and resilience to immune challenge. Sci. Rep. 5, 15572 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.National Institute of Standards and Technology , Data from "NIST Chemistry WebBook." NIST Standard Reference Database Number 69. 10.18434/T4D303. Accessed 19 April 2018. [DOI]
- 60.Kather R., Drijfhout F. P., Martin S. J., Task group differences in cuticular lipids in the honey bee Apis mellifera. J. Chem. Ecol. 37, 205–212 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Baracchi D., Fadda A., Turillazzi S., Evidence for antiseptic behaviour towards sick adult bees in honey bee colonies. J. Insect Physiol. 58, 1589–1596 (2012). [DOI] [PubMed] [Google Scholar]
- 62.McDonnell C. M., et al. , Ecto- and endoparasite induce similar chemical and brain neurogenomic responses in the honey bee (Apis mellifera). BMC Ecol. 13, 25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.