Significance
Neurodegenerative diseases, including Alzheimer’s and Parkinson’s diseases, are major public health problems. Analysis of gene mutations that cause these diseases points to an important role of membrane trafficking within cells in disease development, but how such processes participate in the pathology is unclear. Here, we analyze a murine model of compromised membrane trafficking (due to mutation of a protein complex at the Golgi apparatus) and find metabolites of sphingolipids, molecules enriched particularly in the brain, accumulate in cells and tissues of this model. Preventing this buildup pharmacologically improved the symptoms of neurodegeneration and survival in these mice. Thus, our data provide evidence that modulating sphingolipid metabolism will provide a therapeutic avenue to treat some forms of neurodegenerative disease.
Keywords: sphingolipid, neurodegeneration, myriocin, amyotrophic lateral sclerosis, wobbler mice
Abstract
Numerous mutations that impair retrograde membrane trafficking between endosomes and the Golgi apparatus lead to neurodegenerative diseases. For example, mutations in the endosomal retromer complex are implicated in Alzheimer’s and Parkinson’s diseases, and mutations of the Golgi-associated retrograde protein (GARP) complex cause progressive cerebello-cerebral atrophy type 2 (PCCA2). However, how these mutations cause neurodegeneration is unknown. GARP mutations in yeast, including one causing PCCA2, result in sphingolipid abnormalities and impaired cell growth that are corrected by treatment with myriocin, a sphingolipid synthesis inhibitor, suggesting that alterations in sphingolipid metabolism contribute to cell dysfunction and death. Here we tested this hypothesis in wobbler mice, a murine model with a homozygous partial loss-of-function mutation in Vps54 (GARP protein) that causes motor neuron disease. Cytotoxic sphingoid long-chain bases accumulated in embryonic fibroblasts and spinal cords from wobbler mice. Remarkably, chronic treatment of wobbler mice with myriocin markedly improved their wellness scores, grip strength, neuropathology, and survival. Proteomic analyses of wobbler fibroblasts revealed extensive missorting of lysosomal proteins, including sphingolipid catabolism enzymes, to the Golgi compartment, which may contribute to the sphingolipid abnormalities. Our findings establish that altered sphingolipid metabolism due to GARP mutations contributes to neurodegeneration and suggest that inhibiting sphingolipid synthesis might provide a useful strategy for treating these disorders.
Neurodegenerative diseases are a major health challenge in aging populations (1, 2). Despite intensive investigation, effective therapies for these diseases are lacking, in part because the most proximate causes of neurodegeneration are not well understood. Genetic evidence from humans or animal models has implicated impaired trafficking and cargo sorting by retromer or the Golgi-associated retrograde protein (GARP) complex as causes of neurodegenerative disease (3, 4). This trafficking pathway involves the retromer complex at endosomes for sorting endocytosed proteins into membrane carriers and the GARP complex for tethering retrograde carriers at the Golgi apparatus to receive those proteins. Defects in endosome-to-Golgi retrograde trafficking caused by a mutation in the retromer component VPS35 (D620N) have been reported as causes of late- and early-onset Parkinson’s disease (5). Reduced expression of retromer proteins has also been linked to Alzheimer’s disease (AD) (6). At the Golgi complex, mutations of GARP proteins (e.g., VPS51 and VPS53) have been identified as causes of severe childhood neurological diseases, including progressive cerebello-cerebral atrophy type 2 (PCCA2) in the case of VPS53 (7, 8). In wobbler mice, a partial loss-of-function mutation (L967Q) in the Vps54-encoded subunit of GARP is responsible for motor neuron loss with features similar to amyotrophic lateral sclerosis (ALS) (9–13).
Previously, we and others showed that mutations of GARP complex proteins result in abnormalities in sterol and sphingolipid metabolism, as well as vacuolar or lysosomal morphology, in cultured yeast or human HeLa cells (14, 15). In particular, GARP mutations result in accompanying increases in the sphingoid long-chain bases, sphinganine and sphingosine, that are intermediates in sphingolipid metabolism. The accumulation of these lipids can lead to cell dysfunction or cell death (16, 17), and sphingoid base accumulation in GARP mutant cells correlates with impaired growth (14). Further, treatment of GARP-deficient yeast or HeLa cells with myriocin, an inhibitor of the first step in sphingolipid synthesis catalyzed by serine palmitoyl transferase, corrects impaired cell growth, cell dysfunction, and cell death (14). Treatment with myriocin similarly alleviates lysosomal dysfunction and sphingolipid accumulation in Drosophila melanogaster mutated for the homolog of Parkinsonism-associated PARK14 that exhibit impaired retromer function (e.g., decreased punctae of VPS26) (18). Additionally, myriocin treatment suppresses behavioral abnormalities associated with aberrant synapses in the PARK14 mutants (18). These findings suggest that mutations in retrograde trafficking and cargo significantly perturb sphingolipid metabolism, and sphingolipid accumulation may contribute to cellular dysfunction and neurodegenerative phenotypes.
In this study, we tested the hypothesis that inhibiting the accumulation of sphingolipid intermediates alleviates cellular abnormalities and slows neurodegeneration that is caused by defects in retrograde trafficking in wobbler mice. We examined the cellular phenotypes in embryonic fibroblasts derived from the mice with respect to sphingolipid metabolism and to sorting of lysosomal proteins, including enzymes of sphingolipid catabolism. We also tested whether treating the mice with myriocin would slow the progression of motor neuron disease and improve their health and lifespan.
Results
Missorting of Lysosomal Enzymes of Sphingolipid Degradation to Golgi-Enriched Fraction in wobbler Fibroblasts.
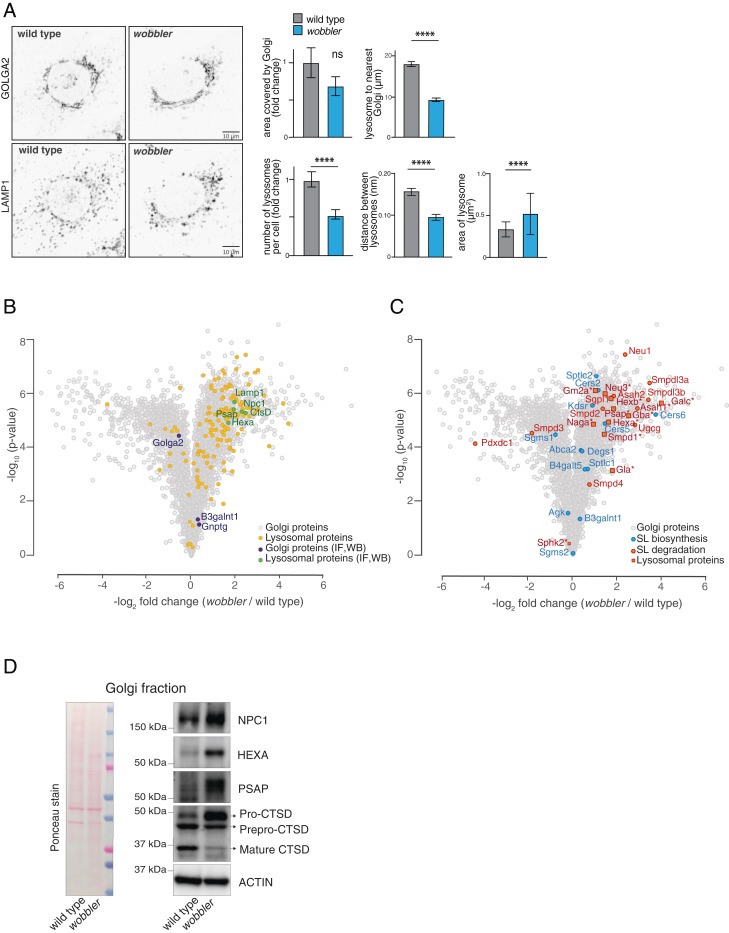
Because deletions of components of the GARP complex can perturb cell morphology and function (14), we first assessed whether the Vps54 mutation L967Q affects morphology of organelles in murine embryonic fibroblasts (MEFs) derived from wobbler mice. Whereas the Golgi apparatus (detected by Golga2 staining) appeared normal in wobbler MEFs, the lysosomes (detected by lysosome-associated membrane protein [LAMP1]) appeared larger and were reduced in numbers (Fig. 1A). Further, lysosomes appeared to be more concentrated near the nucleus of cells and less dispersed to the periphery. Upon analyzing cells stained for both lysosomes and Golgi, we found that lysosomes were in closer proximity to the Golgi in wobbler cells than in control cells. Other organelles, such as mitochondria (SI Appendix, Fig. S1A), appeared to be unaffected in wobbler MEFs, and there was no evidence of activation of the ER stress response (SI Appendix, Fig. S1B).
Fig. 1.
The wobbler mutation of the GARP complex leads to missorting of proteins. (A) GOLGA2 staining in MEFs reveals no significant (ns) differences in the size of the Golgi compartment, whereas LAMP1 staining in wild-type and wobbler MEFs, followed by quantification of LAMP1 per cell (n ∼ 250 cells per condition), shows reduced numbers and larger size of lysosomes. Mean, SD ****P < 0.0001 by Mann–Whitney test. (Scale bar, 10 μm.) Analysis also revealed altered distribution of lysosomes depicted as distance from LAMP1 to GOLGA2 and between LAMP1 particles per cell area (n ∼ 20 cells per genotype) Mean, 95% CI ****P < 0.0001 by Mann–Whitney test. (B) TMT-based quantitative proteomics shows the enrichment of lysosomal proteins (orange) (P value = 2.98 × 10−35) and (C) enrichment of sphingolipid metabolism enzymes (P value = 6.85 × 10−11) in the Golgi-enriched fractions from wobbler cells, by Wilcoxon rank sum test. Lysosomal proteins (orange and green) are enriched in wobbler MEFs while Golgi proteins (purple) were relatively unchanged. Proteins labeled for imaging by immunofluorescence (A) or for Western blots (D and SI Appendix, Fig. S1F) are labeled (purple and green). Sphingolipid biosynthetic (blue) and catabolic (red) enzymes in the Golgi-enriched fraction shows many catabolic enzymes normally residing at lysosome (asterisk). (D) Western blot and Ponceau analysis of lysosomal proteins in Golgi-enriched fractions from wild-type and wobbler cells verifies enrichment of lysosomal proteins and reveals defects in CTSD processing. Actin is shown as a loading control in these fractions with enriched, but not purified, Golgi membranes.
As GARP is an important mediator of protein sorting in the endosomal system (7, 19, 20), we next tested whether the Vps54 wobbler mutation affects protein homeostasis. For this, we first compared the abundance of proteins in wild-type and wobbler MEFs by mass spectrometry of stable isotope labeling with amino acids in cell culture (SILAC)-labeled cells (SI Appendix, Fig. S1C and Dataset S1). Proteins were reproducibly measured in both genotypes (SI Appendix, Fig. S1D). MEFs from wobbler mice had reduced levels of numerous proteins of the retromer complex (SI Appendix, Fig. S1C). The levels of proteins of the Wiskott-Aldrich syndrome protein and scar homolog (WASH) and GARP complexes were mostly unchanged.
We next examined the subcellular proteomes of Golgi-enriched fractions from wild-type and wobbler MEFs (lysosome-enriched fractions could not be purified reliably from wobbler MEFs). The enrichment scheme is shown in SI Appendix, Fig. S1E. We immunoblotted for Golgi proteins and found levels of GNPTG and B3GALNT were comparable between wild-type and wobbler MEFs (Fig. 1F). However, we found differences in processing of B3GALNT in the postnuclear supernatant. After tandem mass tag (TMT) labeling of peptides and proteomic analyses by mass spectrometry, we found that wobbler Golgi-enriched fractions contained normal levels of Golgi markers (e.g., GOLGA1, GNPTG, and GOLGA2) but had a striking enrichment of numerous lysosomal proteins (Fig. 1B and Dataset S2). Of 254 proteins annotated as lysosomal (gene ontology term, cellular component “lysosome”), 143 were enriched in the Golgi-enriched fraction of wobbler MEFs. The wobbler Golgi-enriched fraction also contained many lysosomal enzymes involved in sphingolipid degradation, including GBA, NEU3, ASAH1, ASAH2, and GALC (Fig. 1C). The levels of many of the sphingolipid biosynthetic enzymes, such as KDSR, DEGS1, and SPTLC1, which are normally found in the Golgi, were found at levels close to those of wild-type controls. However, the levels of several enzymes of sphingolipid biosynthesis normally found in the ER, such as serine palmitoyl transferases (SPTCLC1 and SPTLC2) and several ceramide synthases (CERS2, CERS5, and CERS6) were relatively more abundant in the Golgi-enriched fraction of wobbler MEFs.
To confirm the mislocalization of lysosomal proteins to Golgi-enriched fractions in wobbler MEFs, we performed immunoblotting of these fractions. Indeed, the levels of Niemann-Pick disease, type C1 (NPC1), a protein critical for intracellular cholesterol trafficking, β-hexsoaminidase subunits (HEXA), an enzyme critical for degradation of gangliosides, and prosaposin (PSAP), a crucial factor in glycosphingolipid hydrolysis, all appeared to be enriched in wobbler Golgi fractions (Fig. 1B and D). Also, blotting for cathepsin D (CtsTSD) revealed impaired processing of the prepro-CTSD and pro-CTSD forms to its mature form, which normally occurs in the lysosome.
Taken together, these results indicate that the Vps54 mutation in wobbler fibroblasts results in a profound missorting of lysosomal proteins to the Golgi apparatus, including many enzymes of sphingolipid degradation. These data are consistent with impaired trafficking of these lysosomal proteins from the Golgi to the lysosome.
Accumulation of Sphingoid Long-Chain Bases in Embryonic Fibroblasts and Spinal Cords of wobbler Mice.
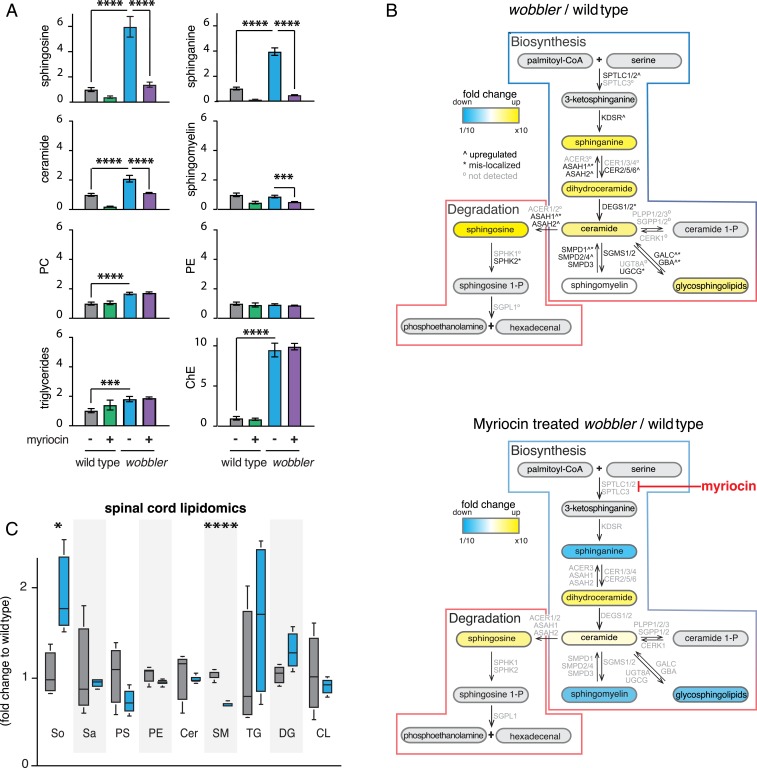
Given the mislocalization of many sphingolipid metabolism enzymes in wobbler MEFs, we next examined the effects of the wobbler mutation on the lipidome. We found that levels of the long-chain bases, sphingosine and sphinganine, and ceramides were increased in wobbler MEFs (Fig. 2 A and B, Top). Sphinganine is part of the biosynthetic pathway, whereas sphingosine is an intermediate in sphingolipid degradation. Ceramide is an intermediate of both synthetic and degradative pathways. In addition, wobbler MEFs exhibited increased levels of cholesterol esters, triglycerides, and phosphatidylcholine (Fig. 2A).
Fig. 2.
The wobbler mutation of the GARP complex leads to accumulation of sphingolipid intermediate species in wobbler MEFs and spinal cords. (A) Lipidomics analysis of different classes of lipids shows a strong accumulation of sphingolipid species in wobbler MEF, that is rescued by myriocin treatment (n = 4 biological replicates). ***P < 0.001 and ****P < 0.0001 by one-way ANOVA with Bonferroni post hoc test. ChE: cholesterol esters, PE: phosphatidylethanolamine, PC: phosphatidylcholine. (B) Schematic representations of sphingolipid metabolism pathway, illustrating the effect of the GARP mutation, enzymes up-regulated and/or mislocalized from lysosome to Golgi and the changes in lipids upon myriocin treatment. Sphingolipid enzymes in the biosynthesis and degradation pathway but not identified by mass spectrometry in those experiments are shown. (C) Lipidomics analysis of different classes of lipids shows a strong accumulation in sphingolipid species in the spinal cord of wobbler mice (n = 4 to 6 biological replicates). *P < 0.05 and ****P < 0.0001 by unpaired t test. So: sphingosine, Sa: sphinganine, SM: sphingomyelin, CL: cardiolipin. No changes in phospholipids, PS and PE, ceramide (Cer) triglyceride (TG) or diacylglycerol (DG).
Previously, we showed that inhibition of sphingolipid synthesis by treatment with myriocin, a specific and potent inhibitor of serine palmitoyl transferase (21), reduced sphingolipid accumulation in yeast or HeLa cells depleted of GARP (14). Consistent with this result, the sphingolipid abnormalities in wobbler MEFs were suppressed when wobbler MEFs were cultured for 16 h in the presence of myriocin (Fig. 2 A and B, Bottom). In particular, the levels of sphingosine, sphinganine, ceramides, sphingomyelin, and glycosphingolipids were all reduced with myriocin treatment. In contrast, myriocin treatment had no effect on the elevated levels of glycerolipids or neutral lipids in wobbler MEFs (Fig. 2A). Thus, MEFs from wobbler mice have increased levels of multiple sphingolipids, similar to what was found with GARP mutant yeast or HeLa cells, and these abnormalities could be lessened by blocking sphingolipid synthesis.
Inasmuch as wobbler mice develop progressive loss of large motor neurons in the spinal cord (12), we also analyzed the lipid content of the spinal cords of 4-mo-old wild-type and wobbler mice (Fig. 2C). Similar to the findings in MEF cells, the levels of sphingosine were nearly twofold higher in spinal cord samples from wobbler mice. The levels of sphingomyelin, which is synthesized from ceramide, were reduced in the spinal cord. In contrast, ceramide and sphinganine levels appeared to be unchanged in spinal cords of wobbler mice (Fig. 2C).
Treatment of wobbler Mice with an Inhibitor of Sphingolipid Synthesis Markedly Improves Outcomes and Survival.
We next tested the hypothesis that accumulation of toxic sphingolipid intermediates leads to neurodegeneration in wobbler mice by treating these mice with the sphingolipid synthesis inhibitor myriocin. After weaning, we fed wild-type and wobbler mice a diet containing 2.2 mg/kg of myriocin, a concentration previously used to effectively inhibit sphingolipid synthesis in vivo (22). At the administered dose, we found no evidence of hepatotoxicity due to myriocin, as assessed by measuring plasma levels of the liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) after 10 or 50 wk of myriocin treatment (SI Appendix, Fig. S2A), although ALT levels trended higher at 50 wk.
In an initial study, we treated wobbler mice with myriocin for 10 wk to study disease-related outcomes and neuropathology. The motor neuron loss in wobbler mice results in forelimb paralysis and impaired mobility and grip strength (23). We assessed mobility in untreated and myriocin-treated mice by monitoring home-cage locomotor activity for 6 h (the study time was restricted from 24 to 6 h owing to the inability of wobbler mice to feed and drink in the metabolic cages). The locomotor activity of wild-type mice was unaffected by myriocin (Fig. 3A). Locomotor activity was reduced by ∼50% in wobbler mice versus wild-type mice, and myriocin treatment restored activity levels to normal (Fig. 3A). Corroborating this result, video recordings showed greater mobility for myriocin-treated wobbler mice than untreated wobbler controls (Movies S1–S4).
Fig. 3.
Inhibiting sphingolipid synthesis improves locomotor activity and grip strength in wobbler mouse. (A) Locomotion activity was measured in metabolic cages equipped with laser beams. Total activity over 6 h shown as area under the curve (AUC). ****P < 0.0001 by one-way ANOVA and Tukey’s multiple comparison (n = 4 to 8 per group) (B, C, and D) Boxplots representing the weekly monitoring of weight, wellness score, and grip strength for the untreated and myriocin-treated wobbler mice (n = 14 to 15 per genotype). Blue regions highlight the interquartile range for untreated wobbler controls at week 10. Scores for wild type marked by gray line (C and D). (E) After 10 wk of myriocin treatment, wobbler mice exhibit improved wellness index and grip strength. **P = 0.002 and P = 0.006 by Mann–Whitney test, for wellness index and grip strength, respectively. (n = 8 and 12 for untreated and myriocin-treated wobbler, respectively.)
We also assessed neurodegeneration-related outcomes, including body weight, a wellness index, and grip strength, in treated wobbler mice over 10 wk. Myriocin-treated wild-type mice showed a modest reduction in weight gain (∼4 g) over the study period (SI Appendix, Fig. S2B), suggesting some effect of the diet in wild-type mice. The wobbler mice gained weight much more slowly than wild-type mice, but untreated- and myriocin-treated wobbler mice gained weight at similar rates (Fig. 3B). At each week of the study period, a wellness index [a combined score of activity, status of fur, eyes, tremor, and posture (ref. 24 and SI Appendix, Fig. S2C)] was assessed for each mouse in the different groups. Remarkably, myriocin treatment improved the wellness index of wobbler mice, and these outcomes were easily visible by inspection of the mice (SI Appendix, Fig. S2D). As previously reported (25), wobbler mice had greatly reduced grip strength in their forelimbs starting at 3 wk of age. Therefore we measured the time mice were able to remain on a vertical grid (Fig. 3D). Grip strength of wobbler mice was markedly improved with myriocin treatment (Fig. 3D and Movies S5–S7).
Given that wobbler phenotypes result from degeneration of motor neurons (12, 26), we analyzed spinal cords of 3-mo-old untreated or myriocin-treated wobbler and wild-type mice for histological signs of neurodegeneration (Fig. 4). In the gray matter of the spinal cord of wobbler mice but not in the wild-type mice, we found numerous dying motor neurons, as shown by their swollen or vacuolated appearance (Fig. 4A). Dying motor neurons were identified in both untreated and myriocin-treated wobbler mice. Astrocytes have been implicated as key cell types in modulating neurodegeneration due to lipid abnormalities (26), possibly by buffering the accumulation of specific lipids. To assess the role of astrocytes in neurodegeneration of wobbler mice, we determined the amount of associated astrogliosis by immunohistochemical staining of the astrocyte marker GFAP (glial fibrillary acidic protein). As reported (27), wobbler mice showed increased astrogliosis in the cervical region of their spinal cords (Fig. 4 B and C). Importantly, we found that GFAP staining in the spinal cord of mice treated with myriocin was markedly decreased (Fig. 4 B and C), consistent with reduced degenerative processes in their neurons. Levels of choline acyltransferase-positive neurons were similar among the study groups (SI Appendix, Fig. S3A). Of interest, the amounts of IBA1-positive cells were increased in myriocin-treated spinal cords of either genotype (SI Appendix, Fig. S3B), suggesting that inhibition of serine palmitoyl transferase increases microglia density independently of genotype, for unclear reasons. In parallel, we also attempted to analyze the fate of neuromuscular junctions in the forelimbs of wobbler mice. As expected with the death of motor neurons and the muscular atrophy, staining of the nicotinic acetylcholine receptor (using α-bungarotoxin) revealed damage to the neuromuscular junctions in wobbler mice, which appeared to be improved with myriocin treatment (SI Appendix, Fig. S3A and C).
Fig. 4.
Inhibiting sphingolipid synthesis improves neuropathology outcomes and increases the survival rate of wobbler mouse. (A) Hematoxylin and eosin staining of the ventral horn in the gray matter from lower cervical spinal cord shows the presence of different stages of dying motor neurons in wobbler mice, compared to wild-type mice, as illustrated in the Insets. (B) Reduced astrocyte gliosis in wobbler mice as revealed by GFAP staining in the ventral horn of the lower cervical spinal cord (black arrowheads show astrogliosis). (Scale bar, 50 μm.) (C) Quantification of the average number of astrocytes (GFAP-positive cells) in the ventral horn of the lower cervical spinal cord (one-way ANOVA P = 0.003, Mann–Whitney between untreated and treated wobbler **P = 0.0047). (D) Myriocin treatment improved the survival rate of wobbler mice during a 1-y treatment (log-rank test: P = 0.003) (n = 17 to 18 mice per group).
Mice harboring the wobbler mutation exhibit marked reductions in lifespan (13, 28). Indeed, in our study, we found that 50% of wobbler mice did not survive to 100 d, whereas all of the wild-type mice survived for over a year (Fig. 4D). To test whether sphingolipids were causally involved in mediating wobbler mortality, we treated a separate cohort of wobbler mice with and without myriocin in their diets and tested whether myriocin treatment improved survival. Remarkably, myriocin treatment markedly improved the survival rate at 100 d from 50% to about 90% and at 1 y, half the cohort were still alive. Myriocin treatment extended maximal lifespan of wobbler mice to well over a year (Fig. 4D, P = 0.003 versus untreated wobbler controls).
Discussion
In this study of the wobbler murine model, we show that defects in retrograde trafficking due to a Vps54 mutation of the GARP complex result in sphingolipid abnormalities that are associated with missorting of lysosomal sphingolipid degradation enzymes. Moreover, we show that treatment of wobbler mice with myriocin, an inhibitor of the first step of sphingolipid synthesis, markedly improved many outcomes for wobbler-associated neurodegeneration, including grip strength, neuropathology, and survival. These findings provide support for the hypothesis that neurodegeneration from defects in retrograde trafficking are due, at least in part, to abnormalities in sphingolipid metabolism that result from protein sorting defects.
Consistent with our previous findings for GARP mutations in yeast and HeLa cells (14), we found here that the sphingoid long-chain bases, primarily sphinganine and sphingosine, accumulated in MEF cells derived from wobbler mice, and sphingosine accumulated in spinal cords. The accumulation of sphingoid bases to excess levels in cells has long been known to be cytotoxic (14, 16, 29, 30). The mechanisms of this cytotoxicity remain unclear, but might involve permeabilization of cellular organelles or interference with normal lipid metabolism (14, 31, 32). Accumulation of long-chain bases in lysosomes is associated with lysosome dysfunction, including changes in calcium signaling or lysosomal leakage (31, 33).
The reasons for accumulation of long-chain bases (and other sphingolipids) in GARP deficiency is unclear. Increased sphinganine levels reflect an increase of a sphingolipid synthesis intermediate, and increased sphingosine reflects a degradation pathway intermediate. This suggests that the abnormalities of sphingolipid metabolism are complex and likely due to derangement of multiple steps in sphingolipid metabolism. Consistent with this, we found mislocalization of both sphingolipid biosynthetic and degradative enzymes to the Golgi-enriched fraction in wobbler MEFs. One possibility to explain these results is that there is increased flux through sphingolipid biosynthesis in the ER and Golgi in wobbler MEFs, combined with mislocalized activity of sphingolipid degradation enzymes to the Golgi apparatus. Although these enzymes are likely not optimally active in the less acidic pH of the Golgi versus the lysosome, they may still possess some activities. In agreement with our observations, mutations impairing retromer function also lead to the cellular accumulation of the sphingolipid ceramide and the long-chain bases sphinganine and, particularly, sphingosine (18). Possibly, the increased levels of the neutral ceramidases Asah1 and Asah2 that we found in wobbler cells could aggravate this metabolic dysregulation.
It is unclear why lysosomal enzymes are mistargeted to the Golgi apparatus if the primary defect in GARP mutations is in retrograde trafficking from the endosome to the Golgi. A possible explanation is that mistrafficking is a secondary consequence of failure to recycle trafficking receptors, such as the mannose-6-phosphate receptor, sortilins, or other proteins that are required for bringing lysosomal proteins from the secretory pathway to the lysosome (19, 20). Indeed, we found doubled amounts of both cation-dependent and -independent mannose-6-phosphate receptors in the Golgi fraction of wobbler MEFs, similar to what was recently reported for VPS51 mutations as well as a fivefold increase of the sortilin-related receptor (Fig. 2 and Datasets S2–S4).
Remarkably, treatment of wobbler mice with myriocin was sufficient to ameliorate many of the phenotypes found in these mice. The wobbler mutant was identified in 1956 and exhibits features of ALS, including progressive degenerative loss of motor neurons and markedly diminished survival (9). We now show that these mice have alterations in sphingolipid metabolism and, importantly, that the neurological disease phenotypes and compromised survival of these mice can be significantly ameliorated by inhibition of sphingolipid synthesis. We note that our study may underestimate the treatment effect of myriocin, since the weakness in wobbler mice leads to poor feeding, thereby likely reducing the amount of drug that was administered. Although myriocin is not clinically utilized, other inhibitors of the first step in sphingolipid synthesis catalyzed by serine palmitoyl transferase have been developed for other diseases (for examples see refs. 34, 35). Thus, a path toward development of therapeutically useful agents seems feasible.
Our data are consistent with accumulating evidence that implicates sphingolipid abnormalities as a cause of neurodegeneration. For instance, in ALS and AD substantial changes of sphingolipids, among other lipid species, have been reported and suggested to cause neurodegeneration (29, 30, 36). Furthermore, genetic defects of lysosomal lipid degradation enzymes have long been recognized as causing inborn errors of metabolism that result in severe neurological diseases (e.g., Gaucher’s, Sandhoff’s, and Tay-Sach’s diseases), and genes causing Parkinson’s disease include sphingolipid catabolic enzymes, such as Gba1 and Gba2 (37–39). In addition, other Parkinsonism genes might affect retrograde trafficking to alter sphingolipid metabolism, as recently shown for PLA2G6 (18). Mutations in (dihydro)ceramide synthase 1 (CERS1) in mice lead to early-onset cerebellar ataxia and Purkinje cell degeneration, and similar to our findings, long-chain sphingoid bases have been implicated as the cause for the neuropathology (16, 40). These findings suggest that the accumulation of intermediates of sphingolipid metabolism can lead to neurodegeneration. Importantly, the current study shows that inhibiting the accumulation of toxic sphingolipid species may provide a therapeutic avenue for treating such neurodegenerative diseases.
Methods
Cell Culture.
MEFs were generated from wobbler mice as described (41) using a heterozygous breeding pair with point mutation L697Q at exon 23. MEFs were collected at embryonic day 13 and genotyped for L597Q mutation using primer sequences 5′-TTTTTACACTGGAAATCTTCAAGCCTTAAAAGGCCTTAAAAATCTGGATC-3′ and 5′-GATGAACGACCTGGGTCTCCAGTCTGTCATCACCTCTTCTGTTCCCAGATTTCGGCCATA-3′ and restriction digestion using BstYI (NEB) as described by the Mutant Mouse Resource and Research Center (MMRRC). To immortalize MEFs, cultures were passaged at least 20 times at a 1:3 dilution every 3 d or until cells were 80 to 90% confluent. Wild-type and wobbler MEFs were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with glutamine, 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin supplement (all from Invitrogen) and maintained at 37 °C in a 5% CO2 incubator. MEFs were grown on a 15-cm culture dish treated with myriocin or dimethyl sulfoxide (DMSO) until cells reached ∼80% confluency and collected for subsequent lipid or protein analysis.
Lipidomics.
Lipids were extracted from cells or tissue according to Folch’s method (42). The organic phase of each cell culture sample was normalized by total protein (using bicinchoninic acid [BCA] assay), whereas tissue samples were normalized according to phosphatidylcholine due to variations in the protein measurements. Samples were routinely subjected to two rounds of extraction. The high-performance liquid chromatography-mass specrometry (HPLC-MS) method was adopted from refs. 43, 44. Briefly, HPLC analysis employed a C30 reverse-phase column (Thermo Acclaim C30, 2.1 × 250 mm, 3 μm, operated at 55 °C; Thermo Fisher Scientific) connected to a Dionex UltiMate 3000 HPLC system and a QExactive orbitrap mass spectrometer (Thermo Fisher Scientific) equipped with a heated electrospray ionization (HESI) probe and performed with run conditions previously described (45). Samples were injected in positive and negative ionization modes and two technical replicates, respectively. Processing of raw data was performed using LipidSearch software (Thermo Fisher Scientific/Mitsui Knowledge Industries) (46, 47). The assembled results were exported to R-Studio where all identified lipids were included for subsequent analyses if they fulfilled the following LipidSearch-based criteria: 1) reject equal to zero, 2) main grade A or main grade B and APValue < 0.01 for at least half of the samples, and 3) no missing values across all samples. Based on these filters, 666 out of 2,211 lipids were present in all samples and displayed high accuracy of identification (Dataset S5). Further quality controls used pairwise correlations between replicates and principal component analysis (PCA) (FactomineR package) (48), comparing sample groups (SI Appendix, Fig. S4). Main areas of the included lipids were summarized according to their class, and the total class abundance was compared between sample groups using one-way ANOVA, followed by a Bonferroni post hoc test.
Immunofluorescence and Organelle Analysis in Cells.
For standard immunofluorescence experiments, cells were grown on glass-bottom 24-well plates and fixed with 4% formaldehyde in phosphate-buffered saline (PBS). Cells were permeabilized in 0.1% saponin and 0.1% bovine serum albumin (BSA). Blocking was performed with 3% BSA and 0.1% Triton X-100 or 0.1% saponin and 5% normal goat serum. Primary and secondary antibody dilutions were performed in the same solution used for blocking. Alexa 488-, Alexa 555-, and Alexa 647-conjugated secondary antibodies were obtained from Invitrogen. Nuclei were stained with 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) during one of the postsecondary antibody washes. Mitochondria and nuclei were identified in live-cell imaging experiments with MitoTracker (Thermo Fisher Scientific) and Hoechst 33342 (1 μg/mL for 10 min; Cell Signaling Technology) staining, respectively. Postacquisition image analysis was performed with ImageJ (NIH), ImageJ plug in, DiAna (49), and Cell Profiler software (50) to measure the average LAMP1, GGOLGA2, or MitoTracker staining per cell. Using the ImageJ plugin DiAna, LAMP1 particles were segmented using spot thresholding at default settings and a Gaussian thresholding method, using a 2-pixel radius for Golgi particles. Distances were measured from the center of LAMP1 particles to the edge of the Golga2 signal in cells costained for lysosomes and the Golgi. The distance from the center of two LAMP1 particles was normalized to total cell area. Lysosomal area was analyzed using auto local threshold Phansalkar method radius 15 and the analyze particle function on ImageJ. Hematoxylin and eosin (H&E) and GFAP staining were performed on the spinal cords by the Histopathology Core and the Rodent Histopathology Core of Dana-Farber/Harvard Cancer Center in Boston, MA. GFAP-positive sections were quantified by blinded investigators.
Antibodies and Immunoblotting.
The following primary antibodies were used in our experiments: tubulin (Sigma-Aldrich), LAMP1 (1D4B) (Developmental Studies Hybridoma Bank), Golga2/Gm130 (BD Transduction Laboratories), Hexa (Novus Biologicals), CtsD (Novus Biologicals), Psap (Proteintech), Npc1 (Proteintech), Actin (Cell Signaling), α-bungarotoxin (Abcam), and Neurofilament-H (Cell Signaling).
qRT-PCR Analysis.
Total RNA was extracted from cells using Qiazol and RNeasy Mini kit (Qiagen). From total RNA, cDNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad). Power SYBR Green Master Mix (Thermo) with the following primers was used in qRT-PCR analysis: mTfeb: 5′-CCACCCCAGCCATCAACAC-3′ (forward primer), 5′-CAGACAGATACTCCCGAACCTT-3′ (reverse primer); mHexa: 5′-TGGCCCCAGTACATCCAAAC-3′ (forward primer), 5′-GGTTACGGTAGCGTCGAAAGG-3′ (reverse primer); mGba: 5′-GCCAGGCTCATCGGATTCTTC-3′ (forward primer), 5′-CACGGGGTCAAGAGAGTCAC-3′ (reverse primer); mLAMP1: 5′-CAGCACTCTTTGAGGTGAAAAAC-3′ (forward primer), 5′-ACGATCTGAGAACCATTCGCA-3′ (reverse primer); mCtsd: 5′-GCTTCCGGTCTTTGACAACCT-3′ (forward primer), 5′-CACCAAGCATTAGTTCTCCTCC-3′ (reverse primer); mCyclophilin: 5′-TGGAAGAGCACCAAGACAACA-3′ (forward primer), 5′-TGCCGGAGTCGACAATGAT-3′ (reverse primer); mXbp1s: 5′-GGTCTGCTGAGTCCGCAGCAGG-3′ (forward primer), 5′-AGGCTTGGTGTATACATGG -3′ (reverse primer); mXbp1t: 5′-TTGTCACCTCCCCAGAACATC-3′ (forward primer), 5′-TCCAGAATGCCCAAAAGGAT-3′ (reverse primer); mBip: 5′-GCCAGGCTCATCGGATTCTTC-3′ (forward primer), 5′-CACGGGGTCAAGAGAGTCAC-3′ (reverse primer); and mChop: 5′-CAGCACTCTTTGAGGTGAAAAAC-3′ (forward primer), 5′-ACGATCTGAGAACCATTCGCA-3′ (reverse primer). Expression was normalized to the average of cyclophilin levels.
Mouse Experiments.
All animal studies followed guidelines issued by Harvard University’s institutional animal care and use committees. The mouse strain (NFR/N genetic background) used for this research project, NFR.B-Vps54wr/Mmmh, RRID:MMRRC_030340-MU, was obtained from the MMRRC at the University of Missouri, a NIH-funded strain repository, and was donated to the MMRRC by Beth A. Bauer, DVM, University of Missouri. Mice were housed at a 12-h light/12-h dark cycle with ad libitum access to food and water. Mice were weaned at 3 to 4 wk of age and put on either a myriocin-supplemented diet (2.2 mg/kg of diet) or the same diet without myriocin (ResearchDiet, New Brunswick, NJ) ad libitum for 10 wk. wobbler and littermate wild types were weighed regularly and observed. During observation, the mice were scored for parameters of wellness, as depicted in supplementary data. This score consists of parameters adapted from a published wellness score (24). Grip strength was recorded weekly by placing mice on a vertical grid for 30 s. For locomotion studies, mice were placed in cages equipped with laser beams for a total of 6 h (3 h with light and 3 h in the dark). Analysis between these groups showed no differences due to gender. At the end of the 10-wk study, mice were anesthetized with isoflurane, blood was collected, and then the mice were perfused with PBS. Brain, liver, and spinal cord were collected for further analysis. AST and ALT measurements were performed by the Analytical Core at the University of Massachusetts Mouse Metabolic Phenotyping Center using plasma samples from 3- or 12-mo-old male and female mice.
SILAC Proteomics of Total Cell Proteins.
For SILAC experiments, cells were cultured with heavy or light lysine and arginine (Cambridge Isotope) and dialyzed FBS (Thermo Fisher Scientific). The cells were incubated in medium supplemented with 1 µM myriocin (MilliporeSigma) for the indicated times (or DMSO as a negative control).
SILAC-labeled cell lysates were reduced using 5 mM dithiothreitol (DTT) (MilliporeSigma) at 37 °C for 1 h, followed by alkylation of cysteine residues using 15 mM iodoacetamide (MilliporeSigma) in the dark at room temperature for 1 h. Excessive iodoacetamide was quenched with 10 mM DTT. Proteins were precipitated by the addition of nine volumes of ice-cold acetone and one volume of methanol and incubated at −80 °C for 2 h. Precipitated proteins were centrifuged for 1 h at 4500 × g and 4 °C. After washing with methanol, proteins were resolubilized in 100 mM NaOH aided by sonication at 4 °C, and the solution was brought to pH 7.5 with 200 mM Hepes-free acid. Protein concentrations were determined using a bicinchoninic acid assay kit (Pierce), followed by equal mixing of proteins at 1:1 ratio (light:heavy labels). Proteins were trypsinized using sequencing grade trypsin (Promega) at 37 °C for 16 h. Digested peptides were subsequently desalted using self-packed C18 STAGE tips (3M Empore) (51), and analyzed on an Orbitrap Q-Exactive HF (Thermo Scientific) mass spectrometer coupled to an Easy nanoLC 1000 (Thermo Scientific) with a flow rate of 300 nl/min. Stationary phase buffer was 0.5% formic acid, and mobile phase buffer was 0.5% (vol/vol) formic acid in acetonitrile. A gradient of increasing organic proportion was used for peptide separation (5 to 40% [vol/vol] acetonitrile over 265 min). Peptides were separated on self-packed analytical column using PicoTip emitter (New Objective) using Reprosil Gold 120 C18 (Dr. Maisch) 1.9-μm particle size resin. The mass spectrometer operated in data-dependent acquisition mode with a top 10 method at a mass range of 300 to 2,000 Da.
Mass spectrometry files were analyzed by MaxQuant (52) version 1.5.2.8 with the Uniprot mouse database downloaded on November 2016, counting 52,026 protein entries. MaxQuant analysis included an initial search with a precursor mass tolerance of 20 ppm for mass recalibration. In the main Andromeda search, precursor mass and fragment mass were searched with initial mass tolerance of 4.5 ppm. The search included variable modifications of methionine oxidation and N-terminal acetylation and fixed modification of carbamidomethyl cysteine. Minimum peptide length was set to six amino acids, and up to two missed cleavages were allowed. The false discovery rate was set to 0.05 for peptide and protein identifications; however, proteins that were independently identified in at least three replicates (n ≥ 3) were considered in the final analysis. For comparisons between samples, we used a labeling scheme based on multiplicity 2: Arg0/Lys0 (light label) and Lys8/Arg10 (heavy label), respectively. A minimum of two ratio counts was used to determine the normalized protein intensity. Protein table was filtered to eliminate the identifications from the reverse database and common contaminants.
Partial Purification of Golgi Membranes.
All steps were carried out at 4 °C. For each genotype, cells grown to 80 to 90% confluency in 15 cm × 2.5 cm (D × H) plates (10 each × 3 replicates = 30 plates) were washed twice with PBS (Corning). The cells were carefully detached in PBS containing protease inhibitors (MilliporeSigma) (2 to 3 mL/plate) using a cell lifter and transferred to 50-mL tubes. Plastic Pasteur pipettes were used to handle cells and cell fractions. The cells were centrifuged at 500 × g for 5 min, supernatants were discarded, and the cells were washed in homogenization buffer (HB) (0.5 M sucrose, 5 mM MgCl2, 0.1 M KH2PO4/K2HPO4 buffer, pH 6.7, protease inhibitors). The cells were centrifuged at 500 × g for 5 min, supernatants were discarded, and cells were resuspended in 4 to 5 mL of HB. The cells were homogenized with 15 to 20 strokes in a Dounce-type homogenizer/Teflon pestle (Thomas Scientific). The homogenate was centrifuged at 1,500 × g for 10 min, and the resulting postnuclear supernatant (PNS) collected. The PNS samples were filtered using 100-μm cell strainers (Corning), normalized based on their concentration (Bradford assay), and each PNS was loaded in the middle of a discontinuous sucrose step gradient. The gradients were assembled in ultra-clear centrifuge tubes (14 × 95 mm/Beckman Coulter) with decreasing sucrose buffer (containing 5 mM MgCl2, 0.1 M KH2PO4/K2HPO4 buffer, pH 6.7, protease inhibitors) densities from bottom to top: 1.3 M sucrose (∼2.5 mL) →0.86 M sucrose (∼3 mL) →0.5 M PNS (∼4 to 5 mL) →0.25 M sucrose (2 to 3 mL). The gradients were centrifuged using a SW40 Ti rotor/swinging buckets (Beckman Coulter) at 100,000 × g for 1 h (slow acceleration/slow deceleration). The Golgi fraction at the 0.5 M/0.86 M sucrose interface was collected, and the molarity of the fraction was adjusted to 1.15 M using a 2.0-M sucrose buffer solution. This fraction was layered at the bottom of a second sucrose step gradient and overlaid with equal volumes of decreasing sucrose buffer densities from bottom to top: 1.15 M Golgi fraction →1.0 M sucrose→0.86 M sucrose →0.25 M sucrose. The gradients were centrifuged at 76,000 × g for 3 h. The enriched Golgi fraction was collected at the 0.25 M/0.86 M sucrose interface, diluted in HB, and pelleted for 1 h at 150,000 × g. The Golgi pellet was resuspended in lysis buffer (50 mM Tris, pH 7.5, 1 mM ethylenediaminetetraacetic acid [EDTA], 1 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid [EGTA], 0.27 M sucrose, 150 mM NaCl, 0.5% Nonidet P-40, protease inhibitors, phosphatase inhibitors; Roche), sonicated, and clarified by centrifugation to yield protein lysate.
TMT Quantitative Proteomics of Golgi-Enriched Proteins.
Cells were incubated with 25 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (MilliporeSigma) for 30 min at room temperature and alkylated with 15 mM chloroacetamide (MilliporeSigma) for 30 min at room temperature. The alkylation reaction was quenched with 10 mM DTT (MilliporeSigma) for 10 min at room temperature, and proteins were precipitated using chloroform/methanol. Protein pellets were resuspended in 200 mM 3-[4-(2-hydroxyethyl)piperazin-1-yl]propane-1-sulfonic acid (EPPS) (pH 8.5) and digested with Lys-C (Wako) for 2 h at 37 °C. Trypsin (Promega) was added to each sample and incubated for an additional 6 h at 37 °C. Lys-C and trypsin were used at a protease:substrate ratio of 1:100 (wt/wt). Digestion reactions were stopped by addition of 5% formic acid for 30 min. Peptides were dried via vacuum centrifugation and desalted by C18 stage tip (3M Empore). Peptides were resuspended in 200 mM EPPS (pH 8.5), quantified using micro BCA (Thermo Scientific), and labeled with TMT 6-plex reagents (Thermo Scientific) for 1 h at room temperature with samples from wild-type and wobbler cells in triplicate. Labeling was quenched by addition of 5% hydroxylamine to each reaction and incubating for 15 min at room temperature. As a ratio check for normalization, 5% of the labeled peptides was combined. Equal amounts of labeled peptides were combined, vacuum dried, and subjected to high pH reverse-phase fractionation (Thermo Fisher Scientific) according to manufacturer instructions. The six fractions were vacuum dried and desalted by C18 stage tip. Peptides were resuspended in 5% acetonitrile/5% formic acid and analyzed on an Orbitrap Fusion (Thermo Scientific) using synchronous precursor selection (SPS)-MS (53). Peptides were identified using SEQUEST and TMT reporter ion intensities used to determine relative peptide abundance as described (53).
Statistical Analyses.
Data were analyzed using Prism (GraphPad Software), and the tests are specified in the figure legends. Error bars represent the SD or SEM as indicated in the figure legend.
Data Availability.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD007981.
Supplementary Material
Acknowledgments
We thank the members of the J.W.H. and F&W (R.V.F. and T.C.W.) laboratories for comments on the manuscript; Dr. Alexander Bartelt for help with metabolic cage analyses; the Dana-Farber/Harvard Cancer Center in Boston, MA, for the use of the specialized Histopathology Core and the Rodent Histopathology Core, which provided H&E staining and GFAP staining of the spinal cords; and Gary Howard for editorial assistance. Dana-Farber/Harvard Cancer Center is supported in part by a National Cancer Institute Cancer Center Support Grant NIH 5 P30 CA06516. This work was supported by the Consortium for Frontotemporal Dementia Research (to C.S.P., T.C.W., and R.V.F.), the Mathers Foundation (to T.C.W.), NIH R37NS083524 (to J.W.H.), NIH R01NS110395 (to J.W.H.), the Harvard Brain Initiative ALS seed grant program (to J.W.H.), a generous gift from Ned Goodnow (to J.W.H.), the Canadian Institutes for Health Research (to S.S.), and the Howard Hughes Medical Institute (where T.C.W. is an investigator).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD007981.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913956117/-/DCSupplemental.
References
- 1.Noble W., Burns M. P., Challenges in neurodegeneration research. Front. Psychiatry 1, 7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization , Neurological Disorders: Public Health Challenges, (WHO Press, Geneva, Switzerland, 2006). [Google Scholar]
- 3.Small S. A., Petsko G. A., Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat. Rev. Neurosci. 16, 126–132 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Schreij A. M., Fon E. A., McPherson P. S., Endocytic membrane trafficking and neurodegenerative disease. Cell. Mol. Life Sci. 73, 1529–1545 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimprich A., et al. , A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 89, 168–175 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Small S. A., et al. , Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann. Neurol. 58, 909–919 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Gershlick D. C., et al. , A neurodevelopmental disorder caused by mutations in the VPS51 subunit of the GARP and EARP complexes. Hum. Mol. Genet. 28, 1548–1560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinstein M., et al. , VPS53 mutations cause progressive cerebello-cerebral atrophy type 2 (PCCA2). J. Med. Genet. 51, 303–308 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Falconer D. S., “Wobbler” in Mouse News Letter, (Oxford University Press, Oxford, 1956), Vol. 15, pp. 23–29. [Google Scholar]
- 10.Pérez-Victoria F. J., et al. , Structural basis for the wobbler mouse neurodegenerative disorder caused by mutation in the Vps54 subunit of the GARP complex. Proc. Natl. Acad. Sci. U.S.A. 107, 12860–12865 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt-John T., et al. , Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat. Genet. 37, 1213–1215 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Duchen L. W., Strich S. J., An hereditary motor neurone disease with progressive denervation of muscle in the mouse: The mutant ‘wobbler’. J. Neurol. Neurosurg. Psychiatry 31, 535–542 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt-John T., VPS54 and the wobbler mouse. Front. Neurosci. 9, 381 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fröhlich F., et al. , The GARP complex is required for cellular sphingolipid homeostasis. eLife 4, e08712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei J., et al. , The GARP complex is involved in intracellular cholesterol transport via targeting NPC2 to lysosomes. Cell Rep. 19, 2823–2835 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Spassieva S. D., et al. , Ectopic expression of ceramide synthase 2 in neurons suppresses neurodegeneration induced by ceramide synthase 1 deficiency. Proc. Natl. Acad. Sci. U.S.A. 113, 5928–5933 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanno T., Nishizaki T., Sphingosine induces apoptosis in hippocampal neurons and astrocytes by activating caspase-3/-9 via a mitochondrial pathway linked to SDK/14-3-3 protein/Bax/cytochrome c. J. Cell. Physiol. 226, 2329–2337 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Lin G., et al. , Phospholipase PLA2G6, a Parkinsonism-associated gene, affects Vps26 and Vps35, retromer function, and ceramide levels, similar to alpha-Synuclein gain. Cell Metab. 28, 605–618.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Victoria F. J., et al. , Ang2/fat-free is a conserved subunit of the Golgi-associated retrograde protein complex. Mol. Biol. Cell 21, 3386–3395 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez-Victoria F. J., Mardones G. A., Bonifacino J. S., Requirement of the human GARP complex for mannose 6-phosphate-receptor-dependent sorting of cathepsin D to lysosomes. Mol. Biol. Cell 19, 2350–2362 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyake Y., Kozutsumi Y., Nakamura S., Fujita T., Kawasaki T., Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Biophys. Res. Commun. 211, 396–403 (1995). [DOI] [PubMed] [Google Scholar]
- 22.Glaros E. N., et al. , Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J. Lipid Res. 49, 324–331 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Sillevis Smitt P. A., de Jong J. M., Animal models of amyotrophic lateral sclerosis and the spinal muscular atrophies. J. Neurol. Sci. 91, 231–258 (1989). [DOI] [PubMed] [Google Scholar]
- 24.Komen N., et al. , Colorectal anastomotic leakage: A new experimental model. J. Surg. Res. 155, 7–12 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Boillée S., Peschanski M., Junier M. P., The wobbler mouse: A neurodegeneration jigsaw puzzle. Mol. Neurobiol. 28, 65–106 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Phatnani H., Maniatis T., Astrocytes in neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 7, a020628 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laage S., Zobel G., Jockusch H., Astrocyte overgrowth in the brain stem and spinal cord of mice affected by spinal atrophy, wobbler. Dev. Neurosci. 10, 190–198 (1988). [DOI] [PubMed] [Google Scholar]
- 28.Moser J. M., Bigini P., Schmitt-John T., The wobbler mouse, an ALS animal model. Mol. Genet. Genomics 288, 207–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler R. G., Pedersen W. A., Camandola S., Rothstein J. D., Mattson M. P., Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann. Neurol. 52, 448–457 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Cutler R. G., et al. , Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 101, 2070–2075 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiménez-Rojo N., et al. , Membrane permeabilization induced by sphingosine: Effect of negatively charged lipids. Biophys. J. 106, 2577–2584 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu W. I., Lin Y. P., Wang E., Merrill A. H. Jr, Carman G. M., Regulation of phosphatidate phosphatase activity from the yeast Saccharomyces cerevisiae by sphingoid bases. J. Biol. Chem. 268, 13830–13837 (1993). [PubMed] [Google Scholar]
- 33.Pulli I., Asghar M. Y., Kemppainen K., Törnquist K., Sphingolipid-mediated calcium signaling and its pathological effects. Biochim. Biophys. Acta Mol. Cell Res. 1865, 1668–1677 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Pfizer Products , Inc, “Inhibitors Of Serine Palmitoyltransferases.” Patent WO 2008/084300 Al (2008).
- 35.Warner-Lambart Company LLC , “Use of a serine palmitoyltransferase (spt) inhibitor to treat atherosclerosis and dyslipidemia.” Patent WO2005/092325A1 (2005).
- 36.Dodge J. C., et al. , Glycosphingolipids are modulators of disease pathogenesis in amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U.S.A. 112, 8100–8105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandhoff K., My journey into the world of sphingolipids and sphingolipidoses. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 88, 554–582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lwin A., Orvisky E., Goker-Alpan O., LaMarca M. E., Sidransky E., Glucocerebrosidase mutations in subjects with parkinsonism. Mol. Genet. Metab. 81, 70–73 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Sidransky E., et al. , Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361, 1651–1661 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao L., et al. , A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation. PLoS Genet. 7, e1002063 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willnow T. E., Herz J., Genetic deficiency in low density lipoprotein receptor-related protein confers cellular resistance to Pseudomonas exotoxin A. Evidence that this protein is required for uptake and degradation of multiple ligands. J. Cell Sci. 107, 719–726 (1994). [PubMed] [Google Scholar]
- 42.Folch J., Lees M., Sloane Stanley G. H., A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957). [PubMed] [Google Scholar]
- 43.Bligh E. G., Dyer W. J., A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- 44.Tafesse F. G., et al. , Disruption of sphingolipid biosynthesis blocks phagocytosis of Candida albicans. PLoS Pathog. 11, e1005188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piccolis M., et al. , Probing the global cellular responses to lipotoxicity caused by saturated fatty acids. Mol. Cell 74, 32–44.e8, 10.1016/j.molcel.2019.01.036 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taguchi R., Ishikawa M., Precise and global identification of phospholipid molecular species by an Orbitrap mass spectrometer and automated search engine Lipid Search. J. Chromatogr. A 1217, 4229–4239 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Yamada T., et al. , Development of a lipid profiling system using reverse-phase liquid chromatography coupled to high-resolution mass spectrometry with rapid polarity switching and an automated lipid identification software. J. Chromatogr. A 1292, 211–218 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Lê S., Josse J., Husson F., FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 25, 1–18 (2008). [Google Scholar]
- 49.Gilles J. F., Dos Santos M., Boudier T., Bolte S., Heck N., DiAna, an ImageJ tool for object-based 3D co-localization and distance analysis. Methods 115, 55–64 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Carpenter A. E., et al. , CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rappsilber J., Ishihama Y., Mann M., Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Cox J., Mann M., MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 53.McAlister G. C., et al. , MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 86, 7150–7158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD007981.