Significance
Coformycin and pentostatin are potent nucleoside inhibitors of adenosine deaminase with anticancer activity due to their structurally unique 1, 3-diazepine nucleobases. Herein, the biosynthetic pathway of coformycin is reconstituted in vitro, demonstrating that it directly overlaps with the early stages of l-histidine biosynthesis. The key branch point between the coformycin and l-histidine pathways involves a seven-membered ring cyclization reaction that likely proceeds via Dieckmann cyclization and retro-aldol elimination. This reaction is catalyzed in an ATP-dependent manner, although phosphorylation of the substrate does not appear to be a part of the catalytic cycle. This suggests a regulatory role for ATP that may be important for modulating competition for shared resources between the metabolic processes of L-histidine and coformycin biosynthesis.
Keywords: coformycin, pentostatin, diazepine biosynthesis, histidine, SAICAR
Abstract
Coformycin and pentostatin are structurally related N-nucleoside inhibitors of adenosine deaminase characterized by an unusual 1,3-diazepine nucleobase. Herein, the cof gene cluster responsible for coformycin biosynthesis is identified. Reconstitution of the coformycin biosynthetic pathway in vitro demonstrates that it overlaps significantly with the early stages of l-histidine biosynthesis. Committed entry into the coformycin pathway takes place via conversion of a shared branch point intermediate to 8-ketocoformycin--monophosphate catalyzed by CofB, which is a homolog of succinylaminoimidazolecarboxamide ribotide (SAICAR) synthetase. This reaction appears to proceed via a Dieckmann cyclization and a retro-aldol elimination, releasing ammonia and D-erythronate-4-phosphate as coproducts. Completion of coformycin biosynthesis involves reduction and dephosphorylation of the CofB product, with the former reaction being catalyzed by the NADPH-dependent dehydrogenase CofA. CofB also shows activation by adenosine triphosphate (ATP) despite the reaction requiring neither a phosphorylated nor an adenylated intermediate. This may serve to help regulate metabolic partitioning between the l-histidine and coformycin pathways.
Coformycin (1) and pentostatin (2) (Fig. 1A) are N-nucleosides bearing an unusual -diazepine nucleobase that have been isolated from Streptomyces kaniharaensis SF-557 and Streptomyces antibioticus NRRL 3238, respectively (1, 2). The -diazepine moiety of both 1 and 2, and in particular the R configuration of their C-8 carbinol centers, is important for the strong binding ( nM) and inhibitory activity these nucleosides display toward adenosine deaminase (ADA) (3–6). Coformycin (1) and pentostatin (2) are thus respectively coproduced with the nucleoside antibiotics formycin A (3) (7) and vidarabine (4) (8, 9), which is an example of the frequently observed correlated production of ADA inhibitors and adenosine (5)-like nucleoside antibiotics that may help to prevent deactivation of the latter by ADA (8–10). Given these properties and interplay of activities, pentostatin has found clinical use as a chemotherapeutic agent in the treatment of graft-versus-host disease as well as proliferative diseases such as chronic lymphocytic leukemia and hairy cell leukemia (11–15).
Fig. 1.
(A) Structures of coformycin (1), pentostatin (2), formycin A (3), vidarabine (4), and adenosine (5). (B) Gene clusters of coformycin (cof) and pentostatin (pen) biosyntheis together with their annotated functions. Genes that are not necessary for biosynthesis are marked in gray (16).
The biosynthesis of coformycin and pentostatin is also of particular interest not only for the atypical -diazepine nucleobases they possess but also for the degree to which their respective biosynthetic pathways may overlap with that of l-histidine (6). The pentostatin (2) biosynthetic gene cluster (pen) in S. antibioticus has been previously described by Wu et al. (16) and includes the three genes penA, penB, and penC, which are all required for pentostatin production (Fig. 1B). PenA is annotated as an ATP phosphoribosyl transferase homologous to HisG, which catalyzes the coupling of phosphoribosyl diphosphate (PRPP, 7) with adenosine triphosphate (ATP, 8) to form 9 during the biosynthesis of l-histidine (Fig. 2). Moreover, feeding experiments by Hanvey et al. (17, 18) have shown that adenosine (5) is also a precursor to 2 and that the C-7 methylene carbon in the -diazepine ring derives from C-1 of d-ribose, which becomes the C- of l-histidine (19). PenB is a member of the short-chain dehydrogenase family of enzymes and has been shown to catalyze the interconversion of 2 and 19′ in vitro (16), implying that it is responsible for reduction of the 8-oxo group of the putative 1,3-diazepine intermediates 17′ and 19′. PenC is a homolog of succinylaminoimidazolecarboxamide ribotide (SAICAR) synthetase (16, 20–22); however, it has yet to be functionally characterized.
Fig. 2.
Proposed biosynthetic pathways of coformycin (COF, 1) and pentostatin (PTN, 2).
While the biosynthetic gene cluster for coformycin has not been definitively identified, the two genes cofA and cofB show high sequence homology to penB and penC (48% I/62% H and 56% I/68% H, respectively) and have been found in the vicinity of the formycin A biosynthetic gene cluster in S. kaniharaensis (23). These genes delineate the cof gene cluster (Fig. 1B) and suggest that cof is responsible for coformycin biosynthesis despite the absence of a HisG/PenA homolog. Based on these gene assignments, a pathway has been proposed for the biosynthesis of coformycin and pentostatin that overlaps significantly with that of l-histidine as shown in Fig. 2. In this pathway, HisG/PenA catalyzes formation of 9/9′ from 7 and 8/8′ (16, 24). The enzyme HisI from the l-histidine pathway is composed of a C-terminal pyrophosphorylase domain and an N-terminal cyclohydrolase domain capable of catalyzing the conversion of 9 to 11 (25) and may do the same for the -deoxy counterpart (i.e., 9′ 11′). An Amadori-like rearrangement (26) catalyzed by HisA then yields 12/12′ as the final common intermediate shared by the biosynthetic pathways for 1 and 2 versus l-histidine (6). Consequently, the as yet to be characterized SAICAR synthetase homologs PenC and CofB are hypothesized to be the key enzymes responsible for the respective conversion of 12 and 12′ to 17 and 17′ at this putative branch point.
Results and Discussion
To investigate these hypotheses, recombinant HisG, HisI, and HisA from Escherichia coli as well as CofB and CofA from S. kaniharaensis were overexpressed and purified as N-–tagged proteins. One-pot incubation of 1.5 M HisG, 1.2 M HisI, 1.2 M HisA, 3.5 M CofB, and 2.2 M CofA with 1 mM PRPP (7), 1 mM adenosine triphosphate (ATP) (8), 1 mM nicotinamide adenine dinucleotide phosphate (NADPH), and 5 mM MgCl2 in 100 mM TrisHCl buffer (pH 8.0) for 24 h resulted in the formation of a species having the same high-resolution mass spectrometry (HR-MS) profile and ultraviolet-visible (UV-Vis) spectrum as coformycin -monophosphate (18; Fig. 3A, trace 4, calculated for ; found 363.0710, nm) (27). Upon treatment with calf intestinal alkaline phosphatase (CIP), the isolated product was converted to coformycin (1) as determined by NMR spectroscopy (28, 29). In the absence of CofA, the most abundant product observed has the same exact mass as 8-ketocoformycin--monophosphate (17) (Fig. 3A, trace 3, calculated for ; found 361.0550), consistent with the hypothesis that the PenB homolog CofA catalyzes reduction of 17 to 18. When CofB was omitted from the incubation, only the l-histidine biosynthetic intermediates 11 and 12 were observed (Fig. 3A, trace 2). These observations are consistent with the hypothesis that CofB, and by extension PenC, is responsible for branching of the l-histidine biosynthetic pathway to form the unusual -diazepine nucleosides.
Fig. 3.
(A) HPLC analysis (UV absorbance monitored at 260 nm) of the in vitro one-pot, enzymatic reconstitution of coformycin biosynthesis. The assignment of each peak to a designated compound is based on HR-MS and UV-Vis spectroscopic results. The peak at ca. 7.2 min is a decomposition product of 12 (SI Appendix, Fig. S14). (B) LC-MS extracted ion chromatograms (EIC) of a d-erythronate standard and the in vitro CofB reaction following treatment with CIP. Traces were acquired at , which corresponds to the signal from d-erythronate.
To more carefully characterize the CofB-catalyzed reaction, a mixture containing 12 was prepared by incubating 1.5 M HisG, 1.2 M HisI, 1.2 M HisA with 1 mM ATP, and 1 mM PRPP for 3 h followed by removal of protein via filtration but without further purification (SI Appendix, section 2.B.3). Upon addition of 10 M CofB to the mixture containing 12, a product was formed within the first 15 min, having an absorption maximum at 352 nm (SI Appendix, Fig. S3A), which is consistent with previous spectroscopic characterizations of compound 17 and synthetic compound 19 (i.e., 17 without the -phosphate) (29). Upon prolonged incubation ( min), the product was observed by UV absorption to hydrolyze to 20 (Fig. 3A and SI Appendix, Fig. S3B). The structural assignment of these reaction products respectively as 17 and 20 was established by one-dimensional (1D) and two-dimensional (2D) NMR as well as HR-MS analysis following isolation and treatment with CIP (SI Appendix). CofB catalyzed conversion of 12 to 17 was also directly accompanied by the formation of equivalents of ammonium per turnover as detected and quantitated by coupled assay with l-glutamate dehydrogenase, which catalyzes the reductive amination of -ketoglutarate (-KG) to l-glutamate in the presence of ammonium and NADPH (30). Likewise, d-erythronate-4-phosphate (16) was also produced upon CofB-catalyzed conversion of 12 to 17 as determined by treatment with CIP and liquid chromatography (LC)-MS analysis against a potassium d-erythronate standard (Fig. 3B). These observations provide additional in vitro evidence indicating that CofB catalyzes the conversion of 12 to 17 via a mechanism that involves a retro-aldol–type elimination (15 17).
A possible mechanism for CofB-catalyzed formation of the -diazepine ring during the transformation of 12 to 17 is shown in Fig. 2. This mechanism involves a Dieckmann cyclization of 12 to yield the -diketone intermediate 14 and results in the elimination of ammonia. Consistent with this hypothesis, the resonances assigned to the -protons of substrate 12 were observed to vanish in buffered within 2 h (SI Appendix, Fig. S6). This suggests that the -carbon of 12 (or 13) is sufficiently acidic to serve as the nucleophile in the proposed Dieckmann cyclization (12 (13) 14). The cyclized intermediate 14 could subsequently undergo a retro-aldol–type elimination of d-erythronate-4-phosphate (16) to generate 8-ketocoformycin-5′-monophosphate (17). As elimination of the carboxylic acid 16 from 15 is expected to strongly favor product formation (31, 32), the CofB reaction is expected to be the first committed step in the formation of coformycin. Subsequent reduction catalyzed by CofA and dephosphorylation would complete the biosynthesis of coformycin (1). Formation of pentostatin (2) from 12′ may proceed in an analogous manner, being catalyzed by PenC and PenB.
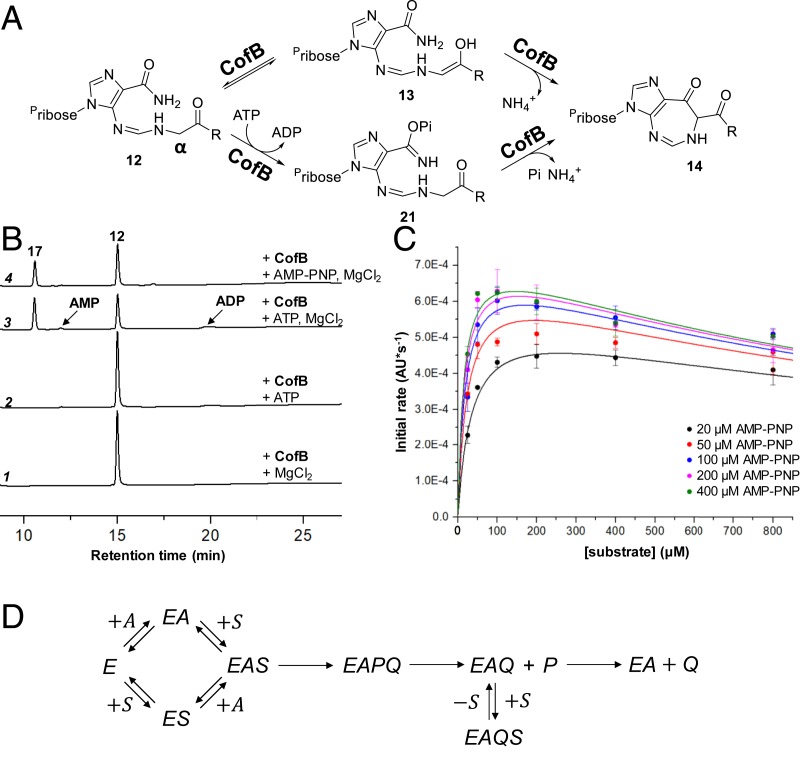
While PenC and CofB are annotated as homologs of SAICAR synthetase (PurC) in purine biosynthesis (16, 20, 21, 23), they exhibit only low to moderate sequence homology to PurC. Nevertheless, closer inspection of the CofB and SAICAR synthetase primary sequences revealed several conserved residues associated with substrate binding as well as a potential ATP binding site (SI Appendix, Fig. S9). However, the mechanism proposed in Fig. 2 does not involve substrate phosphorylation or ATPase activity of CofB. Since ATP was a potential contaminant of the unpurified preparation of 12 used to investigate the activity of CofB in vitro, the identification of a potential ATP binding site raised the possibility that the CofB-catalyzed cyclization reaction may alternatively involve an ATP-dependent phosphorylation step to facilitate the intramolecular cyclization as shown in Fig. 4A.
Fig. 4.
(A) Alternative pathways for the CofB-catalyzed cyclization of 12 with (Lower) and without (Upper) formation of a phosphorylated intermediate (21). (B) HPLC analysis (UV absorbance monitored at 260 nm) of 5 M CofB and 0.5 mM 12 after 1 h incubation with and without ATP, AMP-PNP (0.2 mM), and MgCl2 (1 mM) in TrisHCl buffer (50 mM, pH 8.0). (C) Dependence of the initial rate of the CofB-catalyzed reaction on the variable concentration of 12 with various constant concentrations of the activator AMP-PNP. Rates are reported as changes in absorbance at 352 nm per unit time, because the exact extinction coefficient of 17 at this wavelength in aqueous buffer is unknown. The turnover number (, see text) is thus computed using the extinction coefficient for the identical chromophore in 19′, which has been reported at 349 nm in methanol (33). Error bars represent one SD above and below the mean of two replicates. Curves represent cross-sections of the surface described by Eq. 1 at the indicated AMP-PNP concentrations given the fitted parameters reported in the text. Eq. 1 describes the initial rates associated with the kinetic model in D where equilibrium random binding of substrate and activator to enzyme is assumed.
To address the potential ATP dependence of CofB, the enzyme (10 M) was first incubated with 1 mM ATP, 10 mM MgCl2, and 100 mM TrisHCl buffer (pH 8.0) alone, whereupon significant hydrolysis of ATP to ADP and AMP was observed (SI Appendix, Fig. S7A). This indicated that CofB indeed possesses significant ATPase activity. Next, 12 was purified free of ATP from the enzymatic HisG/HisI/HisA reaction mixture described above by anion exchange chromatography and verified by 1D and 2D NMR spectroscopy. While incubation of purified 12 with either CofB/MgCl2 or CofB/ATP did not lead to the formation of 17 (Fig. 4B, traces 1 and 2), formation of this product was restored if CofB, MgCl2, and ATP were all present together (Fig. 4B, trace 3). However, conversion of 12 to 17 was also observed when ATP was replaced by the ATP analog adenylyl imidodiphosphate (AMP-PNP), which does not have a hydrolyzable -phosphate (Fig. 4B, trace 4). Only trace levels of AMP-PNP hydrolysis to AMP were observed under these conditions ( equivalents) or when substrate 12 was excluded from the reaction mixture (SI Appendix, Fig. S8). PenC was also found to have analogous behavior with ATP/AMP-PNP and MgCl2 when catalyzing the conversion of 12′ to 17′ (SI Appendix, Figs. S7B and S12B). These observations indicate that while ATP can be hydrolyzed by CofB and PenC in a magnesium-dependent manner and is necessary for the conversion of 12/12′ to 17/17′, neither an adenylated nor a phosphorylated reaction intermediate (e.g., 21) is likely to be a part of the catalytic cycle.
To further delineate the role of ATP in the CofB-catalyzed cyclization of 12, initial rates were measured in duplicate over a grid of concentration pairs of AMP-PNP (0 to 400 M) and 12 (25 to 800 M) in the presence of 5 M CofB, 3.75 mM MgCl2, 120 mM NaCl, and 37.5 mM TrisHCl buffer (pH 8.0) at 25 °C. Initial velocities were determined prior to conversion by monitoring changes in UV-Vis absorbance at the of 17 (i.e., 352 nm; SI Appendix, section 2.B.5). As shown in Fig. 4C, the initial rates show noticeable substrate inhibition with respect to the substrate 12 that becomes most pronounced upon saturation with the AMP-PNP activator.
This behavior can be explained by a kinetic model involving random binding of the substrate and activator (AMP-PNP) with at least two ordered product dissociation steps as shown in Fig. 4D. Under rapid-equilibrium binding of substrate and activator (34), the initial rates for such a model are given by
| [1] |
This bivariate function describes a surface of initial rates over the plane of AMP-PNP () and 12 () concentration pairs, where (5 M) is the total concentration of CofB in all assays. This surface was fitted to the complete set of initial rates versus all measured concentration pairs using least-squares nonlinear regression implemented in the OriginPro software package (35) to obtain the following parameters. In the absence of substrate inhibition, ( ) is the turnover number (Fig. 4 legend), ( M) is the dissociation constant for AMP-PNP from the free enzyme, and ( M) and ( M) are the Michaelis constants for 12 and AMP-PNP, respectively. The parameter ( mM) governs steady-state partitioning between formation of the dead-end complex and turnover. This result suggests that 12 can bind following the dissociation of the first product (i.e., 17 or alternatively d-erythronate-4-phosphate, 16), thereby locking the enzyme in a dead-end complex (e.g., in Fig. 4D) with the remaining product 16 or 17, respectively, corresponding to .
To complete the description of the coformycin biosynthetic pathway, the activity of CofA was also characterized in vitro with purified intermediate 17. When intermediate 17 was purified from the CofB reaction and incubated with CofA and NADPH, a single product was formed that coeluted with only one of the two products generated upon treatment of 17 with (Fig. 5A). Furthermore, dephosphorylation of the CofB/CofA product yielded coformycin as determined by NMR spectroscopy. These results indicate that CofA accepts 17 as a substrate and is stereospecific for hydride transfer to the si face of the C-8 carbonyl in 17 to generate 18. Moreover, coformycin (1) was also formed when 17 was first dephosphorylated to 19 before adding CofA (Fig. 5B), indicating that CofA is not specific for the -phosphate of 19. In this case, however, CofA could utilize either NADH or NADPH as the reductant apparently preferring the latter, while reduction of 17 appears to be specific for NADPH (Fig. 5). Thus, the final stages of coformycin biosynthesis may proceed in vivo with reduction either preceding or following dephosphorylation of 17.
Fig. 5.
(A) HPLC analysis (UV absorbance monitored at 260 nm) of the CofA-catalyzed reduction of isolated 17 (0.4 mM). Assays contained 2 M CofA and 0.4 mM NADPH (trace 2), 2 M CofA and 0.4 mM NADH (trace 5), or 4 mM (trace 3). (B) HPLC analysis (UV absorbance monitored at 260 nm) of the CofA (2 M)-catalyzed reduction of 0.4 mM 19 with 0.4 mM of either NADPH or NADH. Excess formation of NAD is observed due to background oxidation of NADH also seen in the absence of 19.
HisG is known to accept both ATP and -deoxy-ATP (dATP) as substrates with a possible slight preference for the former (24). To determine whether HisI/HisA/CofB/CofA can also accommodate -deoxy substrates, dATP (8′) and PRPP (7) were incubated with HisG/I/A and CofB for 24 h, upon which time the formation of 12′ and 17′ could be observed as shown in Fig. 6A (trace 3). Furthermore, 18′ was also formed in low levels when CofA was included in the incubation, suggesting that pentostatin can be synthesized in vitro via the CofB/CofA system (Fig. 6B). Endpoint competition assays involving HisG/HisI/HisA performed with equal starting concentrations (1 mM) of ATP, dATP, and PRPP demonstrated an approximately 6-fold greater formation of 12 versus 12′ by MS peak integration after a 4-h incubation at room temperature. Therefore, given the roughly 100-fold greater concentration of ATP versus dATP in vivo, the majority of the reaction flux in l-histidine biosynthesis is expected to favor ATP as the biosynthetic entry point (36). This is consistent with the observation that S. kaniharaensis produces only coformycin (2).
Fig. 6.
(A and B) HPLC analysis (A) (UV absorbance monitored at 260 nm) and LC-MS analysis (B) of one-pot in vitro reconstitution of pentostatin biosynthesis using CofA and CofB. Reaction conditions are the same as in Fig. 3A with ATP replaced by -deoxy-ATP. (C) HPLC analysis (UV absorbance monitored at 290 nm) of HisG and PenA activity in the presence of ATP versus dATP, 30 min incubation (SI Appendix, section 2.B.7). HisI (1.2 M) was present in all assays to prevent HisG/PenA product inhibition.
The requirement of PenA for pentostatin biosynthesis in S. antibioticus (16) may thus be a consequence of the need to augment flux through the minor pathway beginning with dATP. Consistent with this hypothesis, purified PenA was found to recognize dATP only as a substrate being otherwise inactive with respect to ATP as shown in Fig. 6C. These observations suggest that metabolic flux through the pentostatin biosynthetic pathway may be regulated primarily at the point of entry into the l-histidine (6) biosynthetic pathway as opposed to branching midway at intermediate 12′. However, PenC and PenB were also observed to accept 12 and 17 as substrates, respectively (SI Appendix, Figs. S12A and S13), suggesting that expression of the pen biosynthetic gene cluster can lead to shunting of l-histidine biosynthesis toward the production of coformycin. Indeed, S. antibioticus has been reported to coproduce low levels of coformycin relative to pentostatin (37).
Conclusions
In summary, the biosynthetic gene cluster for coformycin has been identified, and the biosynthetic pathway has been reconstituted in vitro. The pathway appears to show significant overlap with l-histidine biosynthesis essentially coopting the initial condensation of PRPP (7) with ATP (8) as well as the two ring-opening reactions to generate the branch-point intermediate 12. The first committed step of coformycin biosynthesis is thus the CofB-catalyzed cyclization of 12 to yield the phosphorylated oxo-derivative of coformycin (17). While the mechanism of this reaction awaits further study, the coproduction of ammonia and d-erythronate-4-phosphate is consistent with a Dieckmann cyclization followed by a retro-aldol elimination. Conversion of 17 to coformycin is then completed upon NADPH-dependent reduction catalyzed by CofA as well as dephosphorylation.
Coformycin biosynthesis thus appears to proceed as a fork of l-histidine biosynthesis that may result in direct competition between the two pathways for shared precursors. While the physiological implications and mechanism of the observed activation of CofB by ATP have yet to be fully explored, it may serve to modulate metabolic flux between pathways, leading to coformycin versus l-histidine in response to changing ATP levels. Furthermore, the ATPase activity of CofB may provide an “off switch” to prevent uncontrolled diversion of histidine biosynthetic intermediates into the coformycin pathway. In contrast, pentostatin biosynthesis is likely to be regulated primarily via the expression of penA, consistent with the typical paradigm of secondary metabolic control in Streptomyces (38). Furthermore, the ATP dependence of PenC may function to help minimize shunting of l-histidine intermediates into coformycin, which is likely to be a side effect of pen expression. While these hypothesized mechanisms of competition and control between the three metabolic pathways remain to be more rigorously tested, they offer at least one starting point for exploring the interface between primary and secondary metabolism as well as its regulation via gene expression versus enzyme activation.
Materials and Methods
All NMR spectra were acquired at the NMR Facility in the Department of Chemistry, University of Texas at Austin. All high-performance liquid chromatography (HPLC) data were acquired using an Agilent Technologies 1260 Infinity HPLC system with an inline diode array UV-visible absorbance detector. LC–electrospray ionization (ESI)–HR-MS data were acquired using an instrument of the same model equipped with an Agilent Technologies 6230 ToF ESI mass spectrometer. Further details regarding materials and instrumentation can be found in SI Appendix, section 1. Additional detail necessary to repeat all experimental procedures including cloning and expression of all proteins, preparation of compounds, and assays of enzyme activity are provided in SI Appendix, section 2. Finally, supporting NMR spectra, primer sequences, UV-absorbance spectra, and HPLC chromatograms are provided in SI Appendix, section 3.
Data Availability.
All data associated with these studies are included in the main text or SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (GM035906) and the Welch Foundation (F-1511). The Bruker AVANCE III 500 NMR at the University of Texas at Austin was supported by the NSF (1 S10 OD021508-01).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000111117/-/DCSupplemental.
References
- 1.Isono K., Nucleoside antibiotics: Structure, biological activity, and biosynthesis. J. Antibiot. 41, 1711–1739 (1988). [DOI] [PubMed] [Google Scholar]
- 2.Suhadolnik R. J., Nucleoside Antibiotics (Wiley-Interscience, New York, NY, 1970). [Google Scholar]
- 3.Sawa T., Fukagawa Y., Homma I., Takeuchi T., Umezawa H., Mode of inhibition of coformycin on adenosine deaminase. J. Antibiot. Ser. A 20, 227–231 (1967). [PubMed] [Google Scholar]
- 4.Agarwal R. P., Spector T., Parks R. E. Jr, Tight-binding inhibitors—IV. Inhibition of adenosine deaminases by various inhibitors. Biochem. Pharmacol. 26, 359–367 (1977). [DOI] [PubMed] [Google Scholar]
- 5.Cha S., Agarwal R. P., Parks R. E. Jr, Tight-binding inhibitors—II: Non-steady state nature of inhibition of milk xanthine oxidase by allopurinol and alloxanthine and of human erythrocytic adenosine deaminase by coformycin. Biochem. Pharmacol. 24, 2187–2197 (1975). [DOI] [PubMed] [Google Scholar]
- 6.Schramm V. L., Baker D. C., Spontaneous epimerization of ()-deoxycoformycin and interaction of ()-deoxycoformycin, ()-deoxycoformycin, and 8-ketodeoxycoformycin with adenosine deaminase. Biochemistry 24, 641–646 (1985). [DOI] [PubMed] [Google Scholar]
- 7.Hori M., et al. , A new antibiotic, formycin. J. Antibiot. Tokyo Ser. A 17, 96–99 (1964). [PubMed] [Google Scholar]
- 8.Dion H. W., Woo P. W. K., Ryder A., Isolation and properties of a vidarabine deaminase inhibitor, co-vidarabine. Ann. N. Y. Acad. Sci. 284, 21–29 (1977). [Google Scholar]
- 9.Woo P. W. K., Dion H. W., Lange S. M., Dahl L. F., Durham L. J., A novel adenosine and ara-A deaminase inhibitor, ()-3-(2-deoxy--d-erythro-pentofuranosyl)--tetrahydroimidazo[] []diazepin-8-ol. J. Heterocycl. Chem. 11, 641–643 (1974). [Google Scholar]
- 10.Takeuchi T., et al. , Antiviral effect of formycin derivatives. J. Antibiot. (Tokyo) 20, 297–298 (1967). [PubMed] [Google Scholar]
- 11.Brogden R. N., Sorkin E. M., Pentostatin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in lymphoproliferative disorders. Drugs 46, 652–677 (1993). [DOI] [PubMed] [Google Scholar]
- 12.Caron N., Lee S. H., Kimball A. P., Effects of -deoxycoformycin, 9--d-arabinofuranosyladenine -phosphate, and 1--d-arabinofuranosylcytosine triple combination therapy on intracerebral leukemia 1210. Cancer Res. 37, 3274–3279 (1997). [PubMed] [Google Scholar]
- 13.Ballet J. J., Insel R., Merler E., Rosen F. S., Inhibition of maturation of human precursor lymphocytes by coformycin, an inhibitor of the enzyme adenosine deaminase. J. Exp. Med. 143, 1271–1276 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grever M. R., Doan C. A., Kraut E. H., Pentostatin in the treatment of hairy-cell leukemia. Best Pract. Res. Clin. Haematol. 16, 91–99 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Goldberg J. D., et al. , Pentostatin for the treatment of chronic graft-versus-host disease in children. J. Pediatr. Hematol. Oncol. 25, 584–588 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Wu P., et al. , An unusual protector-protégé strategy for the biosynthesis of purine nucleoside antibiotics. Cell Chem. Biol 24, 171–181 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Hanvey J. C., Hardman J. K., Suhadolnik R. J., Baker D. C., Evidence for the conversion of adenosine to -deoxycoformycin by Streptomyces antibioticus. Biochemistry 23, 904–907 (1984). [DOI] [PubMed] [Google Scholar]
- 18.Hanvey J. C., et al. , Biosynthesis of -deoxycoformycin: Evidence for ring expansion of the adenine moiety of adenosine to a tetrahydroimidazo[-][]diazepine system. Biochemistry 26, 5636–5641 (1987). [DOI] [PubMed] [Google Scholar]
- 19.Winkler M. E., Ramos-Montañez S., Biosynthesis of histidine. EcoSal Plus, 10.1128/ecosalplus.3.6.1.9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levdikov V. M., et al. , The structure of SAICAR synthase: An enzyme in the de novo pathway of purine nucleotide biosynthesis. Structure 6, 363–376 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Lukens L. N., Buchanan J. M., Biosynthesis of the purines: XXIII. The enzymatic synthesis of N-(5-amino-1-ribosyl-4-imidazolylcarbonyl)-l-aspartic acid -phosphate. J. Biol. Chem. 234, 1791–1798 (1959). [PubMed] [Google Scholar]
- 22.Zhang Y., Morar M., Ealick S. E., Structural biology of the purine biosynthetic pathway. Cell. Mol. Life Sci. 65, 3699–3724 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S. A., et al. , Identification of the formycin A biosynthetic gene cluster from Streptomyces kaniharaensis illustrates the interplay between biological pyrazolopyrimidine formation and de novo purine biosynthesis. J. Am. Chem. Soc. 141, 6127–6131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ames B. N., Martin R. G., Garry B. J., The first step of histidine biosynthesis. J. Biol. Chem. 236, 2019–2026 (1961). [PubMed] [Google Scholar]
- 25.D’Ordine R. L., Klem T. J., Davisson V. J., N1-(-Phosphoribosyl)adenosine--monophosphate cyclohydrolase: Purification and characterization of a unique metalloenzyme. Biochemistry 38, 1537–1546 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Henn-Sax M., et al. , Two ()(8)-barrel enzymes of histidine and tryptophan biosynthesis have similar reaction mechanisms and common strategies for protecting their labile substrates. Biochemistry 41, 12032–12042 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Bzowska A., Lassota P., Shugar D., Phosphorylation of coformycin and -deoxycoformycin, and substrate and inhibitor properties of the nucleosides and nucleotides in several enzyme systems. Z. Naturforsch. 40, 710–714 (1985). [DOI] [PubMed] [Google Scholar]
- 28.Nakamura H., et al. , Structure of coformycin, an unusual nucleoside of microbial origin. J. Am. Chem. Soc. 96, 4327–4328 (1974). [DOI] [PubMed] [Google Scholar]
- 29.Hawkins L. D., Hanvey J. C., Boyd F. L. Jr, Baker D. C., Hollis Showalter H. D., Inhibitors of adenosine deaminase: Synthesis of coformycin and -deoxycoformycin. Nucleosides Nucleotides 2, 479–494 (1983). [Google Scholar]
- 30.Engel P. C., Dalziel K., The equilibrium constants of the glutamate dehydrogenase systems. Biochem. J. 105, 691–695 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grogan G., Emergent mechanistic diversity of enzyme-catalysed beta-diketone cleavage. Biochem. J. 388, 721–730 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grogan G., .-Diketone hydrolases. J. Mol. Catal. B Enzym. 19, 73–82 (2002). [Google Scholar]
- 33.Chan E., Putt S. R., Hollis Showalter H. D., Baker D. C., Total synthesis of ()-3-(2-deoxy--d-erythro-pentofuranosyl)--tetrahydroimidazo[-][]diazepin-8-ol (pentostatin), the potent inhibitor of adenosine deaminase. J. Org. Chem. 47, 3457–3464 (1982). [Google Scholar]
- 34.Cook P. F., Cleland W. W., Enzyme Kinetics and Mechanism (Garland Science, New York, NY, 2007). [Google Scholar]
- 35.OriginLab Corporation , Origin(Pro) (Version 9.0, OriginLab, Northhampton, MA, 2012).
- 36.Traut T. W., Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140, 1–22 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Hanvey J. C., Hawkins E. S., Baker D. C., Suhadolnik R. J., 8-Ketodeoxycoformycin and 8-ketocoformycin as intermediates in the biosynthesis of -deoxycoformycin and coformycin. Biochemistry 27, 5790–5795 (1988). [DOI] [PubMed] [Google Scholar]
- 38.Liu G., Chater K. F., Chandra G., Niu G., Tan H., Molecular regulation of antibiotic biosynthesis in Streptomyces. Microbiol. Mol. Biol. Rev. 77, 112–143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with these studies are included in the main text or SI Appendix.