Abstract
Purpose
This study examined differences in selected acoustic measures of speech and voice according to age and sex and across families.
Method
Participants included 169 individuals, 79 men and 90 women, from 18 families, ranging in age from 17 to 87 years. Participants reported no history of articulation disorders, stroke or active neurologic disease, or severe-to-profound hearing loss. They read aloud two passages to facilitate examination of the following speech and voice acoustic parameters: fricative spectral moments (center of gravity, standard deviation, skewness, and kurtosis), the proportion of time spent speaking, mean speaking fundamental frequency, semitone standard deviation (STSD), and cepstral peak prominence smoothed.
Results
The results indicated a significant age effect for fricative spectral center of gravity, spectral skewness, and speaking STSD. There was a significant sex effect for spectral center of gravity, spectral kurtosis, and mean fundamental frequency. Familial relationship was significant for spectral skewness, STSD, and cepstral peak prominence smoothed.
Conclusions
These findings revealed that certain speech and voice features change with age and some change differently for men and women. Additionally, speakers from the same family units may demonstrate similar patterns for prosody, voicing, and articulatory behavior. The results also demonstrated normal differences in speech and voice variation across age, sex, and family unit. Understanding patterns and differences across these demographic variables in healthy speakers is important to distinguishing more confidently between normal and disordered speech and voice patterns clinically.
It has been predicted that, by 2020, approximately 77 million people in the United States will be aged 65 years or older (U.S. Department of Health and Human Services, Administration for Community Living, 2017). Because communication disorders in adults become more prevalent with age, it is essential to understand patterns of typically aging speech in order to distinguish healthy speakers from those with disorders. This requires an understanding of how aging affects the systems that support communication. Research suggests that, after the third decade of life, there is a linear decline in the function of the cardiovascular, respiratory, skeletal, and muscular systems (Ramig et al., 2001). The typical aging process also involves neurological changes, both structural and functional. These include a diminished number of cerebellar cells and atrophy of the temporal lobes and precentral gyrus (Slawinski, 1994). Additionally, aged individuals are generally found to have decreased synaptic activity associated with a reduction in the release of neurotransmitters (Ramig et al., 2001). These neurological changes may cause a slowing of neural processes, which, in turn, may lead to decreased speed and accuracy of motor control as well as diminished cognitive–linguistic functioning (Torre & Barlow, 2009).
These age-related neuromuscular changes in the speech mechanism are often reflected in changes in the acoustic properties of speech. Many studies have examined the effects of aging on the voice, particularly changes in fundamental frequency (F0). Generally, men demonstrate a slight increase in F0, whereas women show a decrease with advancing age (Eichhorn et al., 2018; Nishio & Niimi, 2008). The aging voice is increasingly at risk for phonatory instability, which is often perceived as tremor, voice breaks, hoarseness, and/or breathiness (Gorham-Rowan & Laures-Gore, 2006). These perceptual qualities may be reflected in acoustic measures of perturbation, such as amplitude perturbation quotient and F0 standard deviation. Both of these metrics have been found to increase with age, indicating a reduction in amplitude and F0 stability, which listeners may rely on to estimate a speaker's age (Xue & Deliyski, 2001). The perceived hoarseness and/or breathiness in aging voices is also likely associated with noise in the voice signal, which can be measured as a harmonics-to-noise ratio (Spazzapan et al., 2018). Research indicates that this ratio is significantly lower in elderly speakers than in younger adults (Ferrand, 2002). This has been attributed to less periodic vocal fold vibration and/or incomplete vocal fold closure, which have been observed in older speakers (Gorham-Rowan & Laures-Gore, 2006).
Because harmonics-to-noise measures are only appropriate for sustained phonation, some researchers have used spectral and cepstral measures to describe voice quality in connected speech, which more closely reflects speakers' typical voice patterns. Measures of voice quality in connected speech are also preferred in the presence of voice disorders such as spasmodic dysphonia, in which phonation is less symptomatic in sustained phonation than in continuous speech (Awan et al., 2009). One such measure is cepstral peak prominence (CPP), which has been found to closely correlate with auditory–perceptual features of voice disorders (Awan et al., 2009). Additionally, CPP might distinguish younger and older speakers with no voice complaints (Garrett, 2013). Acoustic variability in F0, amplitude, and the presence of noise in the voice signal can also contribute to the perception of phonatory instability in older adults.
In addition to its impact on the larynx, the aging process also has an influence on several aspects of supraglottic vocal tract activity. Age-related neuromuscular changes affect the regulation of articulator movement amplitude, which results in generally decreased accuracy in speech production (Ramig et al., 2001; Torre & Barlow, 2009), although it has been reported that some older speakers may trade rate for accuracy and thus speak more slowly to articulate clearly (Benjamin, 1997). Therefore, not all older speakers would be anticipated to produce less precise sounds, and even those who do may remain fully intelligible. Articulatory precision has been measured through acoustic analysis of fricative spectral moments, which consider spectra as a distribution of frequencies and describe the center of gravity (centroid frequency), standard deviation (spread), skewness (spectral tilt), and kurtosis (peakedness) of that distribution (McFarland et al., 1996). Spectral moment analysis can reflect the configuration of the articulators during speech production. For example, the shorter, wider, and more posterior constriction for /ʃ/ is associated with relatively low spectral centroid frequencies compared with /s/, which is characterized by more anterior placement and a longer, deeper groove (Tjaden & Turner, 1997). Individuals with neurodegenerative diseases have been found to produce atypical fricative spectra associated with reduced articulatory precision (Tjaden & Turner, 1997). It can thus be anticipated that, as their neuromotor control declines, older speakers may produce fricative spectra that differ from those of younger adults.
In addition to changes in these characteristics of phoneme segments, older adults exhibit changes in prosodic features of speech production as well, affecting intonation, stress, and rhythm. Research suggests that, when reading aloud, older speakers produce larger intonational ranges and more frequent inflections than younger adults, perhaps to demarcate syntactic and semantic units in continuous speech in the presence of decreased articulatory precision (Barnes, 2013). Listeners also rely on pauses to understand syntactically complex sentences. In healthy adults, pausing patterns have been found to be closely linked with syntactic boundaries (Huber et al., 2012). However, these authors reported that older speakers exhibit different pausing structures than younger adults. Adults above the age of 45 years demonstrated more frequent breaths, fewer syllables per breath, increased frequency and duration of pauses, and greater intraspeaker variability in temporal parameters (Huber et al., 2012). A more recent study has reported that older adults pause more often than younger speakers at atypical linguistic locations (Lee et al., 2019).
Researchers have suggested several possible factors, which may contribute to these changes in speech motor control with age, including (a) declining neuromotor control, (b) impaired sensory feedback due to age-related hearing loss, (c) changes to respiratory physiology, and (d) slower cognitive processing (Huber et al., 2012; Torre & Barlow, 2009). Although these and likely other factors cause a general decline in voice quality and articulatory control among older adults, they are part of typical aging and must therefore be distinguished from pathological speech processes. An understanding of this distinction is crucial in carrying out clinical research and tracking treatment-related progress in older speakers.
Another important consideration for conducting clinical research is the need to evaluate the speech features of men and women separately. Research has clearly established the presence of biomechanical differences in the speech mechanism by sex. Perhaps the most apparent differences exist in F0. The average speaking F0 for men is typically in a range of 100–150 Hz, compared with 180–230 Hz for women. As noted earlier, F0 changes with age for speakers of both sexes. However, aging seems to affect male and female voices differently. For example, Eichhorn et al. (2018) found female voices to demonstrate a clear decrease in speaking F0 with advancing age, while male voices showed only a slight increase in F0 over the later adult life span.
Although research suggests that listeners rely primarily on F0 to determine speaker sex, there are other basic differences in the phonatory mechanisms of male and female speakers that require consideration. For example, male laryngeal structure differs from that of females in several key ways. Males have larynges whose cartilage is 20% larger than females' in the anterior–posterior dimension and vocal folds whose membranous portion is 60% longer than those of females (Titze, 1989). Also, male vocal folds are approximately 20%–30% thicker than female vocal folds, with a greater percentage of collagenous fibers (Titze, 1989). These structural laryngeal differences result in vocal fold vibration that is not only slower but also qualitatively different in males compared with females (Orlikoff et al., 2012; Yamauchi et al., 2014).
Male–female anatomical differences are also present in supraglottic features of the speech mechanism. On average, male vocal tracts—consisting of the structures between the lips and the vocal folds—are approximately 15% longer than female vocal tracts, with the result that men will generally resonate lower formant frequencies than women (Titze, 1989). One study reported that, while formant frequencies are generally higher in women, this is particularly true for F1 and F2 (Simpson, 2002). Although secondary to speaking F0, research indicates that vowel formant frequencies play an important role in speaker sex identification (Titze, 1989). Simpson (2002) suggested, however, that sex differences in acoustic metrics such as this are nonuniform because many anatomical features of the speech mechanism are not linearly proportional across sexes. For example, men and women present with different average ratios of oral-to-pharyngeal cavity length, which in turn produces nonuniform differences in resonance properties. More research is needed to understand the intricate ways in which male and female speech production differs and establish patterns of typical speech production for men and women.
The data set available for this study was collected as part of a much larger project that aimed to identify potential biomarkers in a number of biological systems in related individuals. This allowed us to test for potential familial influences on measure of speech and voice in addition to examining age- and sex-related differences. Several studies have examined data that suggest the presence of familial patterns in the speech production of related individuals. Untrained listeners have been found to reliably differentiate between related and unrelated speakers (Feiser & Kleber, 2012; Vanderydt, 1998). However, there is a paucity of research that adequately describes the speech features responsible for perceived similarity in the speech of family members. One study reported that accurate listener identification of related speakers was correlated with more pronounced dialectal features (Feiser & Kleber, 2012). Another study found that family members who were perceived as sounding related had similar F0, intonation patterns, and voice onset and offset timing (Vanderydt, 1998). Westrop (2000) also reported familial relationship to have a significant effect on segment durational differences, including stop gap durations and voice onset time. However, other studies have indicated that some acoustic similarities between pairs of unrelated speakers are often stronger than similarities between siblings, even those who are rated by listeners as sounding similar (Feiser, 2009). While the data suggest that acoustic trends in familial speech production exist, it remains unclear which speech features are affected by familial relationships and what the underlying mechanisms may be.
Research has established that acoustic measures reflect important changes in speech across the life span, differences between the sexes, and even similarities within family relationships across generations. This study was conducted to examine selected acoustic aspects of the speech of healthy individuals within families and across the adult life span. The availability of a data set with 169 individuals aged 17–87 years from 18 different families across three generations allowed for an acoustic examination of speech properties using measures reflective of laryngeal function, supraglottic articulation, and prosody. A portion of the data set examined in this study was used by Westrop (2000) to measure segment durational changes associated with age, sex, and familial relationship. After reporting differences by sex and age in measures including stop closure and vowel duration, as well as voice onset time, Westrop suggested several possible explanations for the findings. These included slower speech production with increasing age, differences in oral cavity shape and size between the sexes, and genetic patterning and/or environmental influences within families. In this study, it was hypothesized that these factors may also impact other acoustic characteristics of speech production.
The present investigation was conducted to examine the potential differences in segmental and suprasegmental speech features with aging, between sexes, and between families. Fricative spectral measures and spectral/cepstral analysis were selected as indices of articulatory precision and vocal quality, respectively. It was anticipated that both types of measure would indicate a change in speech performance with age and reflect physiological differences between the sexes and, potentially, unrelated individuals. In order to characterize prosodic differences among and between these participant groups, this study relied on F0 variability and a ratio of speaking/pausing time in connected speech. Taken together, these segmental and suprasegmental measures may describe normal variations in the speech production of persons of differing age, sex, and familial background. An understanding of normal speech differences in healthy individuals is important for distinguishing between typical and pathological speech patterns in a clinical setting.
Method
Participants
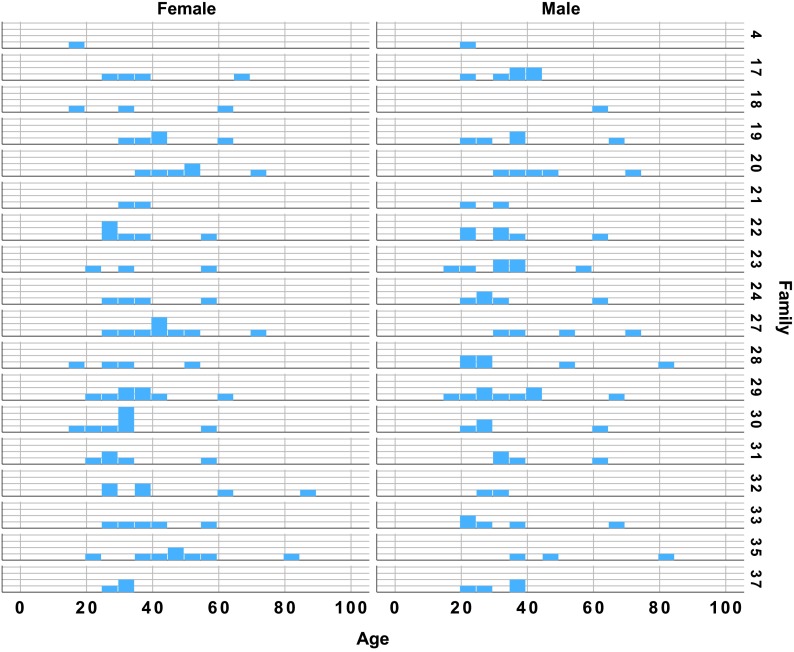
The data used in this analysis were collected as a part of the Utah Genetic Reference Project at the University of Utah, funded by the Keck Foundation. The broader goal of this project was to identify familial genetic factors that might be linked to identifiable biomarkers in a number of physiological systems, including speech and voice. This study included speech samples from 169 individuals in 18 families. Participants included 79 men and 90 women ranging in age from 17 to 87 years (see Table 1). Because the participants were recruited on the basis of family membership, rather than representing specific age brackets, the distribution of ages was not balanced across families (see Figure 1). There were more individuals in their 20s and 30s than middle age or older. All participants were native speakers of American English with no history of articulation disorders, stroke or active neurological disease, or severe-to-profound hearing loss. All participants were considered to have normal voices for their age and sex, as judged by two experienced speech-language pathologists and an otolaryngologist; they had not sought or received treatment for any voice complaints. Participants were not excluded for mild-to-moderate health problems typical of their age group, such as high blood pressure, thyroid problems, diabetes, or mild-to-moderate hearing loss. All speakers communicated easily with the experimenters, indicating functional hearing ability, although detailed audiometric data were not available.
Table 1.
Participant ages by sex.
| Sex | Age categories |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17–20 | 21–25 | 26–30 | 31–35 | 36–40 | 41–45 | 46–50 | 51–55 | 56–60 | 61–65 | 66–70 | 71–75 | 76–80 | 81–85 | 86–90 | Total | |
| Female | 5 | 6 | 18 | 17 | 13 | 6 | 4 | 6 | 7 | 3 | 1 | 2 | 0 | 1 | 1 | 90 |
| Male | 2 | 19 | 10 | 14 | 15 | 4 | 1 | 1 | 3 | 4 | 2 | 2 | 0 | 2 | 0 | 79 |
| Total | 7 | 25 | 28 | 31 | 28 | 10 | 5 | 7 | 10 | 7 | 3 | 4 | 0 | 3 | 1 | 169 |
Figure 1.
Participant ages by sex and family. The y-axis in each panel reflects the count of individuals in each family and age group.
Procedure
Participants were recorded in a sound booth with a digital audiotape (DAT) recorder and an AKG Acoustics C420 head-mounted condenser microphone with a constant 3-cm mic-to-mouth distance. Each sample was perceptually judged to be free of articulation deficits, with 100% agreement by two certified speech-language pathologists with at least 5 years of clinical experience. Recordings were transferred from DAT to 48-kHz audio files using a Dell computer with the Kay Elemetrics DAT interface system.
The data collection protocol for this study included two reading passages: (a) Goldilocks and the Three Bears and (b) the Rainbow Passage (see Appendix A for transcripts of these passages). Participants were given large print copies of each passage to read aloud. Prior to reading the Goldilocks passage, each speaker was given the following instruction: “Read this story as if you were reading to a small child, changing your voice for each bear and Goldilocks.” The rationale for including this task in the original data collection protocol was to evaluate pitch flexibility and prosodic range in the speakers. For the purposes of the present analysis, the recurrence of the word “said” (spoken as narration rather than in a character voice) allowed the averaging of fricative spectral measures across multiple tokens for each speaker (see below). For the Rainbow Passage, participants were instructed to read in their normal, conversational voice. This passage was included to allow a sampling of connected speech produced with typical suprasegmental features. These two passages were selected as connected speech tasks from which to extract measures that reflected both phonatory function and articulation. They included fricative spectral moments, mean F0, semitone standard deviation (STSD), speaking time ratio, and the CPP smoothed (CPPS).
Acoustic Analysis
For each participant, fricative spectral moments were computed from /s/ in each of five occurrences of the word “said” in the Goldilocks passage. This phonetic context was chosen to control for coarticulation effects and ensure consistency of fricative production. Using the Praat software program (Version 5.4; Boersma & Weenink, 2017), each /s/ was segmented and saved as a separate .wav file. The boundaries of each /s/ were identified using the combined audio waveform and spectrographic display in Praat. Next, the .wav files were read into a custom MATLAB application to further segment each fricative to save only the middle 50% of the total phoneme duration at the original 48-kHz sample rate. Praat's spectral slice function was then used with its default settings to compute the spectral center of gravity, standard deviation, skewness, and kurtosis in Hertz for each segmented fricative. The spectral moments for each participant were averaged for the five /s/ tokens. The spectral mean or center of gravity describes the average frequency distribution of spectral energy within a speech sound. The spectral variance describes the frequency variability over which the spectrum is distributed. The spectral skewness or tilt describes the asymmetry of the distribution of spectral energy frequencies. A positive spectral skewness signifies an asymmetrically long tail of the distribution extending into the higher frequencies. The spectral kurtosis describes the compactness of the spectral energy distribution. A higher kurtosis value is associated with a relatively peaked distribution, with the spectral energy more tightly clustered around the mean.
Measures of prosody, including intonational F0 variability (STSD) and the proportional amount of time participants spoke and paused, were computed from readings of the Rainbow Passage. Recordings of this passage were imported into Praat and trimmed to exclude experimenter speech, pauses at the beginning and end of each reading, and nonspeech behaviors (coughing, laughing, etc.) prior to analysis. F0 mean and standard deviation were measured from these trimmed recordings using Praat's voicing report, adjusting the F0 range as needed to avoid tracking errors. Because males and females usually have different F0 ranges, standard deviation values were converted into semitones using the following equation: STSD = 12/0.301 × log [(Hz mean + Hz SD / 2) / (Hz mean − Hz SD / 2)]. This allowed for a direct comparison of F0 variability for all speakers.
Trimmed recordings of the Rainbow Passage were also used to compute each speaker's speaking time ratio, expressed as a proportion of time spent speaking relative to the total passage duration. For example, a speaking time ratio of 1.0 would indicate all speaking and no pausing, while a ratio of 0.75 would indicate 75% speaking and 25% pausing. These ratios were computed using a custom MATLAB application, which defined pauses as lasting longer than 200 ms. The application generated a root-mean-square contour of the recording. A threshold was set at 10% of the file's maximum amplitude. Values above this threshold were operationally defined as speaking, with values below defined as pausing. This procedure was validated in a previous study by comparing the application's output with manually identified speech and pause segments (Dromey et al., 2008).
Finally, the Rainbow Passage files were also used to extract cepstral/spectral measures as an index of vocal quality in connected speech for each participant. Trimmed recordings of the Rainbow Passage were imported into Praat. Praat computed the CPPS, which is a measure reflecting the energy of the periodic signal above the linear regression line of other noise in the signal. Higher values indicate a less noisy signal with greater harmonic energy.
Statistical Analysis
The acoustic dependent variables were tested with Statistical Analysis System (Version 9.4) software by the fifth author with a mixed-model analysis of covariance (ANCOVA). The independent variables were age, sex, and family membership of the speakers. After establishing that there were differences in the mean values between families, the familial relationship was used as a blocking variable for the mixed-model analysis of the other independent variables. Dependent variables included fricative spectral moments (center of gravity, standard deviation, skewness, and kurtosis), F0 mean, STSD, speaking time ratio, and CPPS. To adjust for the multiple variables that were analyzed, a pseudo-Bonferroni correction was applied. Thus, statistical significance was reached with a p value of .01.
Results
The results reported below are for the individual variables representing articulatory precision, intonation, speech timing, and laryngeal function in connected speech. Appendix B presents descriptive statistics for each variable by family.
Fricative Spectral Moments
Age
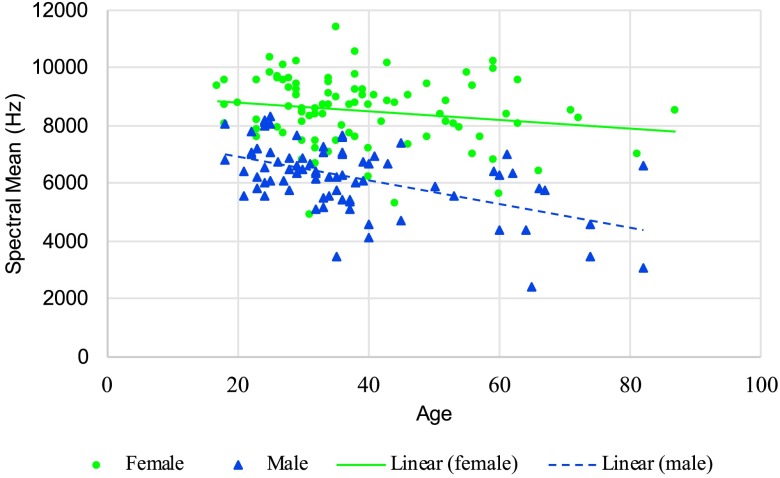
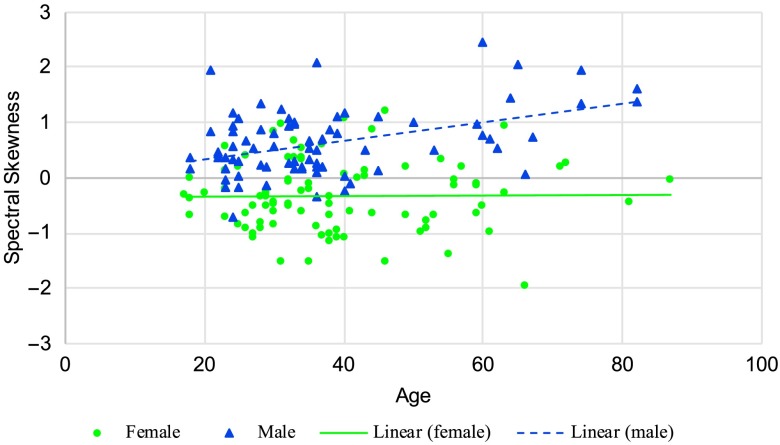
Speaker age was found to have a significant effect on spectral center of gravity for /s/, F(1, 131) = 24.19, p < .001. As shown in Figure 2, the decline for males was slightly greater than that for females, but the interaction was not significant at p < .01. Age had no significant effect on spectral standard deviation for /s/. There was, however, a significant age effect for spectral skewness, F(1, 131) = 9.78, p = .002. As seen in Figure 3, a significant Age × Sex interaction, F(1, 131) = 7.36, p = .008, revealed that while there was no age effect for females, male speakers' spectral skewness increased with age. There was no significant difference in spectral kurtosis for /s/ with age.
Figure 2.
Spectral mean for /s/ in Hertz for women and men as a function of age (lines represent a linear regression fit).
Figure 3.
Spectral skewness for /s/ for women and men as a function of age (lines represent a linear regression fit).
Sex
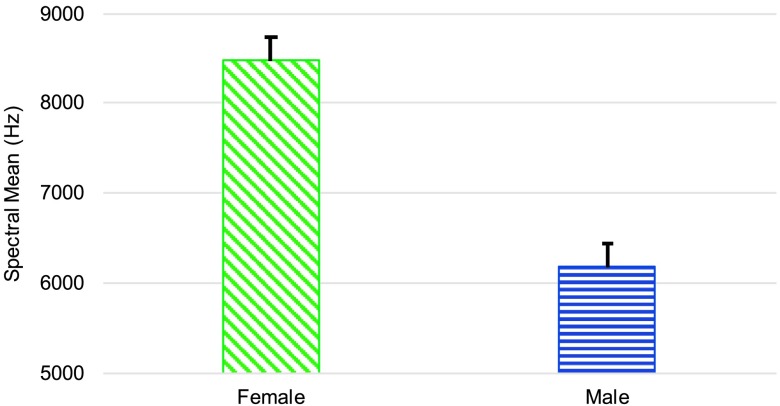
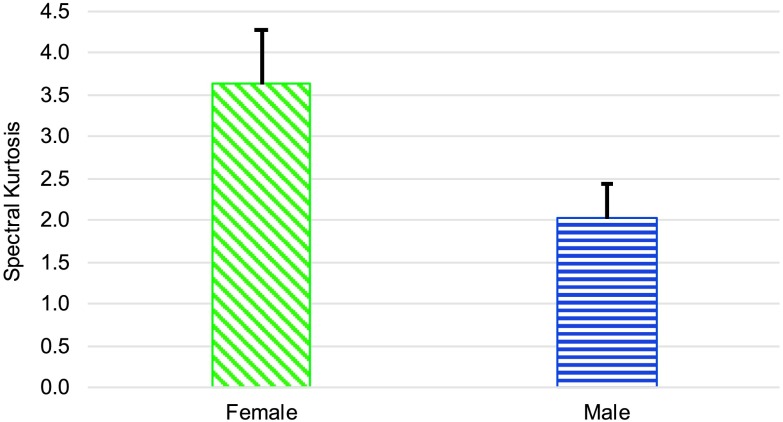
Testing revealed a significant difference in the spectral center of gravity for /s/ between male and female speakers, F(1, 17) = 8.74, p = .009, d = 1.253. As seen in Figure 4, females had a higher spectral center of gravity. There was no significant effect of sex on spectral standard deviation or spectral skewness for /s/. However, ANCOVA testing did reveal that females had a significantly higher spectral kurtosis measure for /s/ than males, F(1, 17) = 12.09, p = .003, d = 0.611, as shown in Figure 5.
Figure 4.
Mean (and 95% confidence interval) of the spectral mean for /s/ in Hertz for women and men.
Figure 5.
Mean (and 95% confidence interval) of the spectral kurtosis for /s/ for women and men.
Family
ANCOVA testing revealed that family relationship had no significant effect on spectral center of gravity, standard deviation, or kurtosis for /s/. However, there was significant variability among the families for spectral skewness, F(17, 147) = 2.47, p = .002.
F0 Measures
Age
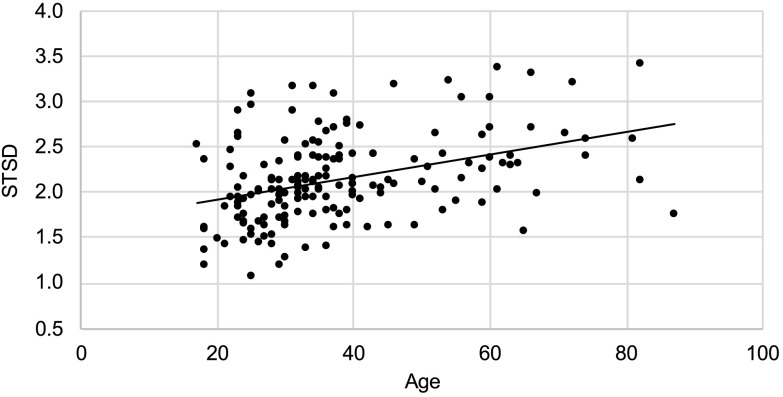
As shown in Figure 6, STSD was found to increase significantly with age, F(1, 132) = 29.09, p < .001.
Figure 6.
Semitone standard deviation (STSD) with age for all speakers (line represents a linear regression fit).
Sex
As would be expected, female speakers had a significantly higher F0 mean than male speakers, F(1, 17) = 195.82, p < .001, d = 5.934. Speaker sex had no significant effect on STSD.
Family
Family relationship had no significant effect on speaker F0 mean. However, there was a significant difference across the families for STSD, F(17, 147) = 2.10, p = .010.
Speaking Time Ratio
None of the factors of age, sex, or family relationship had a significant effect on speaking time ratio at p < .01.
CPP
Age
Speaker age had no significant effect on CPPS.
Sex
The effect of speaker sex on CPPS was not significant at p < .01.
Family
ANCOVA testing revealed significant differences across the families for CPPS, F(17, 147) = 3.51, p < .001.
Discussion
This study was conducted to examine the potential differences in segmental and suprasegmental speech features and vocal function with aging, across sexes, and between families. It was hypothesized that these measures would indicate a general decline in speech performance and vocal quality with age and reflect physiological differences between sexes and, potentially, unrelated individuals. The anticipated trends were only found for a few of the measures. Increasing age was associated with decreases in the /s/ spectral center of gravity and, for men, an increase in spectral skewness. Intonational F0 variability (STSD) increased with age. Women had a higher spectral mean and spectral kurtosis for /s/ along with a higher mean F0. Families differed on the measures of /s/ spectral skewness, F0 variability, and CPPS.
Age Effects
Research has established that typical aging is accompanied by atrophy of some neural structures and decreased synaptic activity (Ramig et al., 2001; Slawinski, 1994). These age-related neural changes are thought to affect the regulation of articulator movement amplitude, which may result in generally decreased accuracy in speech production (Ramig et al., 2001; Torre & Barlow, 2009). Tjaden and Turner (1997) reported that atypical fricative spectra were associated with reduced articulatory precision in individuals with neurodegenerative diseases. It was thus anticipated that, in the current study, typically aging speakers might demonstrate fricative spectra that differed from those of younger adults.
As hypothesized, we found that the spectral mean for /s/ decreased significantly with age, suggesting a possible change to the anterior resonating cavity by producing the fricative constriction in a more posterior location. However, it is important to note that changes in spectral mean with age may also be due to differences in other resonating cavities within the vocal tract. An earlier study of younger speakers (Dromey et al., 2018) found that perceptual ratings of /s/ precision declined along with the spectral center of gravity when kinematic sensors were placed on the tongue. If perceptual accuracy ratings had been part of the present investigation, it is possible that they may have declined with age along with the spectral center of gravity with age. This would be consistent with earlier reports of a reduction in articulatory precision with advancing age (Ramig et al., 2001). Future perceptual studies would be valuable in this connection. Although there was no age effect on spectral skewness for females, males' spectral skewness was found to increase significantly with age. A positive skewness is caused by a distribution of spectral energy that is asymmetrically less compact for frequencies above the mean, causing a right-tailed distribution with a left-leaning peak. While this finding may speak to age-related changes in production of /s/, the physiological mechanisms that underlie changes in spectral skewness are unclear (Nissen & Fox, 2009), and more research on the subject is needed. A clear association between spectral skewness and perceptual aspects of articulation has not yet been established in the literature.
Much research has been devoted to evaluating the effects of aging on F0. Several studies have described a decrease in F0 across the life span in females, whereas males experience a decrease in F0 during puberty and an increase after middle age (Cox & Selent, 2015; Debruyne & Decoster, 1999; Eichhorn et al., 2018; Hunter et al., 2011; Torre & Barlow, 2009). While the current study's results were consistent with this research, the sex differences were not significant. This may be due in part to the smaller number of older individuals in the data set compared to the younger speakers. The findings reported in the literature may be attributable to changes in the thickness and elastin content of the vocal folds with age, as described by Hammond et al. (1998). Had there been higher numbers of older speakers in our data set, it might have allowed these findings to be replicated here. This study found STSD to increase significantly with age, indicating greater intonational variability in F0 in older adults than in younger adults. This is consistent with previous findings that F0 standard deviation increases with age (Xue & Deliyski, 2001). Additionally, Barnes (2013) has suggested that older speakers produce larger intonational ranges and more frequent inflections than younger adults, perhaps to demarcate syntactic and semantic units in connected speech in the presence of reduced articulatory precision.
As an additional measure of prosody in this study, a speaking time ratio was computed for each participant, expressed as a proportion of time spent speaking relative to the total passage duration. Testing indicated that age had no significant effect on speaking time ratio. This is in contrast to a previous report that older adults tended to use more frequent breaths, fewer syllables per breath, increased frequency and duration of pauses, and greater intraspeaker variability in temporal parameters (Huber et al., 2012). It appears that methodological differences could partly account for this discrepancy. Huber et al. used a passage originally developed to elicit specific sounds that allowed the collection of aerodynamic data (Sapienza & Stathopoulos, 1995). As such, it contained a high proportion of bilabial stops in consonant–vowel–consonant–vowel contexts and would be less representative of typical speech than the Rainbow Passage. Also, the age distribution of the participants differed across the studies, making direct comparisons difficult.
Previous studies have used CPPS as an index of vocal quality, particularly in connected speech tasks. In one such study, Garrett (2013) found older speakers to have significantly lower CPP values in connected speech, indicating less periodicity and more prominent breathiness in the voice signal. This trend may be due to changes in laryngeal structure and function with age, which cause increased instability in elderly voices (Gorham-Rowan & Laures-Gore, 2006). It was thus hypothesized that CPPS would decrease with age in this study, indicating a decline in vocal quality. However, testing revealed no significant effect of speaker age on CPPS. Garrett reportedly used CPP versus CPPS, although the Analysis of Dysphonia in Speech and Voice program she used generally includes smoothing in the CPP calculation. Garrett also recorded speakers in a narrower age range than the present investigation, which may account for differences in the findings.
One factor that could have influenced each of the voice variables and how they change with age is the speaker's physiological as opposed to chronological age. Ramig and Ringel (1983) reported that men in better cardiovascular condition had less vocal perturbation than peers of the same age who were less fit. Indeed, older, healthier speakers tended to have voices that were similar to younger men in poorer cardiovascular condition. Because participants in this study were not stratified according to their level of physiological conditioning, it is likely that variability in the data could be attributable in part to differences in physiological aging.
Effects of Sex
The current study showed that females had higher spectral mean and kurtosis values for /s/ than males. It was hypothesized that spectral moment measures would differ between men and women due to sex differences in the size, shape, and proportions of the vocal tract (Nissen & Fox, 2009; Simpson, 2002; Titze, 1989). The physiological mechanisms that underlie differences in spectral moments are unclear. However, Tjaden and Turner (1997) reported that the anterior, longer, deeper tongue groove required for /s/ production is associated with higher spectral mean values relative to other phonemes with shorter, wider, and more posterior constriction. It may then be the case that females in this study produced /s/ with a more anterior and/or deeper tongue groove than males. It may also be that females had a smaller anterior resonating space within the oral cavity during /s/ production. Further investigation into the relationship between fricative spectral moment measures and physiological speech patterns is needed.
Previous research has reported that the average speaking F0 for males lies between 100 and 150 Hz, while the average for females is between 180 and 230 Hz (e.g., Gelfer & Mikos, 2005). The results of this study are consistent with this finding. The average F0 for males was 111.15 Hz, and the average for females was 198.16 Hz. These measures reflect sex differences in laryngeal structure. Male laryngeal cartilage is 20% larger than that of females in the anterior–posterior dimension. Also, male vocal folds are 60% longer and 20%–30% thicker than female vocal folds, with a greater percentage of collagenous fibers (Titze, 1989). It was hypothesized that STSD would also differ between sexes. However, no significant effect of sex on STSD was found. This indicates that males and females in the present data set spoke with similar intonational F0 variability.
Effects of Familial Relationship
Several studies have found that untrained listeners are able to reliably differentiate between related and unrelated speakers, indicating that acoustic trends in familial speech production exist (Feiser & Kleber, 2012; Vanderydt, 1998; Westrop, 2000). However, very little research exists, which adequately describes the speech features affected by familial relationship and what mechanisms might be responsible for such trends. Vanderydt found that related speakers who were perceived as sounding similar had similar mean F0 and intonation patterns. It was thus hypothesized that, in the current study, familial relationship would have an effect on mean F0, STSD, and possibly speaking time ratio.
As predicted, significant variability among the families was found for STSD, suggesting that related speakers demonstrate similarities in intonational F0 variability. However, average F0 and speaking time ratio were not significantly affected by familial relationship. One possible explanation for this finding is that, in Vanderydt's (1998) study, similarities in F0 and intonation patterns were only found in related speakers who were judged as sounding similar. In this study, we did not conduct perceptual evaluations to determine whether related individuals were perceived as similar in their intonation; this could be a useful line of inquiry in future work.
Testing revealed some additional, unanticipated results. There were significant differences across the families for spectral skewness for /s/. Although the physiological mechanisms responsible for changes in spectral skewness are unclear, this finding may suggest similarities in the articulation of /s/ for related speakers. Testing also revealed a significant effect of familial relationship on CPPS. This indicates that some families demonstrated a higher degree of periodicity in the voice signal than others that went beyond anticipated interspeaker differences. These findings could potentially be attributed to genetically based, anatomical similarities in the speech mechanism within families, learned articulation and voicing behaviors, or a combination of these factors. Further investigation into the speech and voice patterns affected by familial relationship is needed.
Limitations of This Study and Directions for Future Research
The relatively sparse representation of elderly speakers in the data set limits the extent to which inferences can confidently be extended to a speaker's later years. Future work that includes a higher proportion of older speakers would be valuable. Although spectral moment analysis has been used as an index of articulatory precision in previous studies (McFarland et al., 1996), research has yet to establish a clear relationship between fricative spectral moments and physiological speech patterns. It is therefore difficult to draw conclusions about trends in articulatory behavior from this study's findings that some fricative spectral moments vary with aging, between sexes, and across families. Future research could address this gap in the literature by pairing spectral moment analysis with kinematic measures to investigate possible links between spectral moments and changes in physiological speech patterns. Recent research has reported that the spectral and temporal aspects of an individual's speech may be linked to their hearing and speech perception abilities (Lowenstein & Nittrouer, 2019). Thus, a limitation of the current study is the lack of detailed data on the speakers' hearing acuity.
An additional constraint of this study is that all of the speech samples used were recordings of reading passages. While read speech still provides valuable information about speech patterns, it may not be entirely representative of natural, everyday speech, particularly for suprasegmental measures such as STSD and speaking time ratio, which were used in the current study. Future projects could collect these and similar measures during a spontaneous speech task to explore prosody patterns in a more naturalistic context.
Lastly, future studies could include a perceptual component to investigate what links, if any, exist between the acoustic trends we identified and perceived differences in speech and voicing behaviors. This could provide more information about the salience to listeners of differences in acoustic measures. The inclusion of a perceptual component may be especially valuable when investigating possible effects of familial relationship on acoustic metrics, since previous research suggests that these measures correlate more in related speakers who are judged as sounding similar by unfamiliar listeners (Vanderydt, 1998).
Conclusion
The availability of a large data set with a wide age range allowed for an investigation of acoustic changes across the life span. These data not only confirmed previous findings that certain speech and voicing features indicate a decline with age but also provided insight into how aging affects the speech of men and women differently. Additionally, few studies have considered these acoustic changes in the context of related individuals across multiple generations. Findings from the current study suggest that related speakers may demonstrate similar patterns for prosody, voicing, and articulation behavior, although the statistical testing did not allow us to draw specific inferences about such similarities.
These findings have potential clinical implications for speech treatment. The data from this study describe some normal variations in the speech production of persons who differ in age, sex, and familial background. This is particularly important for the treatment of older individuals, for whom it is normal to demonstrate some decline in speech and voicing performance compared to younger adults. An understanding of these normal speech differences in healthy individuals is important for differentiating between typical and pathological speech patterns in a clinical setting.
Acknowledgments
Data collection was funded by Public Health Service Grant RO1 DC04068 (awarded to Steven D. Gray) and through the cooperation and support of the Utah Genetic Reference Project, which was funded through the W. M. Keck Foundation. Funding for data analysis was provided by the David O. McKay School of Education at Brigham Young University. The authors express their appreciation to the families who took part in this research. This work was the first author's master's thesis.
Appendix A
Reading Passages
Goldilocks and the Three Bears
The three bears went into the living room. “Someone has been sitting in my chair,” said the father bear. “Someone has been sitting in my chair,” said the mother bear. “And someone has been sitting in my chair and has broken it all to pieces,” cried the baby bear. Then the three bears went upstairs. “Someone has been sleeping in my bed,” said the father. “Someone has been sleeping in my bed,” said the mother bear. “Someone has been sleeping in my bed,” cried the baby bear. Goldilocks opened her eyes and saw the three bears standing over her. “Oh dear,” Goldilocks said.
The Rainbow Passage
When the sunlight strikes rain drops in the air, they act like a prism and form a rainbow. The rainbow is a division of white light into many beautiful colors. These take the shape of a long, round arch, with its path high above and its two ends apparently beyond the horizon. There is, according to legend, a boiling pot of gold at one end. People look, but no one ever finds it. When a man looks for something beyond his reach, his friends say he is looking for the pot of gold at the end of the rainbow.
Appendix B
Descriptive Statistics by Family and Sex
Table B1.
Descriptive statistics for spectral mean and spectral standard deviation for /s/ in Hertz.
| Family | Spectral M
|
Spectral SD
|
||||||
|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||
| M | SD | M | SD | M | SD | M | SD | |
| 17 | 5244.50 | 1160.42 | 8307.68 | 1643.88 | 2910.47 | 497.82 | 2410.52 | 523.79 |
| 18 | N/A | N/A | 8080.40 | 797.98 | N/A | N/A | 3484.49 | 590.92 |
| 19 | 6761.62 | 1017.39 | 8458.04 | 2078.13 | 3227.29 | 580.67 | 2745.87 | 850.56 |
| 20 | 5612.52 | 1129.45 | 8296.53 | 618.05 | 3123.93 | 529.50 | 2886.99 | 687.35 |
| 21 | 6275.92 | 1042.06 | 7740.58 | 1336.01 | 3020.97 | 635.14 | 3615.92 | 80.73 |
| 22 | 6723.05 | 711.03 | 8474.94 | 965.25 | 3038.12 | 474.78 | 3061.20 | 1002.25 |
| 23 | 6754.48 | 999.17 | 8715.07 | 1081.23 | 2805.81 | 572.26 | 3288.65 | 1350.68 |
| 24 | 6460.15 | 454.79 | 7832.77 | 1081.60 | 3485.37 | 404.58 | 3148.78 | 863.34 |
| 27 | 5586.79 | 1562.06 | 8698.12 | 1030.10 | 3590.94 | 542.96 | 2661.05 | 548.95 |
| 28 | 6099.85 | 1660.35 | 8884.62 | 858.51 | 3106.64 | 411.35 | 2517.06 | 780.04 |
| 29 | 5977.93 | 884.56 | 8049.63 | 1327.85 | 3250.13 | 376.88 | 3001.14 | 469.39 |
| 30 | 6270.17 | 542.64 | 9005.98 | 586.83 | 2412.32 | 269.23 | 2953.12 | 1050.62 |
| 31 | 9281.61 | 1273.62 | 9038.94 | 1355.52 | 2488.33 | 248.66 | 2268.31 | 612.92 |
| 32 | 6698.32 | 2237.15 | 8355.89 | 1555.61 | 2340.71 | 161.22 | 2941.41 | 356.38 |
| 33 | 6088.02 | 2175.41 | 8731.69 | 2242.36 | 3197.13 | 428.20 | 3111.20 | 972.44 |
| 35 | 6668.06 | 667.65 | 8421.01 | 933.99 | 2826.16 | 176.97 | 2330.95 | 818.43 |
| 37 | 6402.08 | 1021.07 | 8600.23 | 1109.73 | 2923.47 | 414.71 | 2554.37 | 803.88 |
Note. N/A indicates a family with only one participant of the designated sex.
Table B2.
Descriptive statistics for spectral skewness and spectral kurtosis for /s/ in Hertz.
| Family | Spectral skewness |
Spectral kurtosis |
||||||
|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||
| M | SD | M | SD | M | SD | M | SD | |
| 17 | 0.03 | 0.42 | −1.04 | 0.63 | 0.81 | 1.19 | 4.09 | 2.30 |
| 18 | N/A | N/A | −0.35 | 0.55 | N/A | N/A | 0.92 | 1.33 |
| 19 | 0.29 | 0.43 | −0.24 | 0.84 | 2.46 | 2.51 | 3.70 | 1.87 |
| 20 | 0.67 | 0.85 | −0.35 | 0.57 | 2.59 | 2.60 | 3.38 | 1.96 |
| 21 | 0.29 | 0.18 | −0.76 | 0.41 | 2.08 | 2.04 | 1.50 | 0.34 |
| 22 | 0.39 | 0.52 | −0.39 | 0.52 | 1.34 | 1.22 | 2.01 | 1.28 |
| 23 | 0.92 | 0.59 | 0.22 | 0.40 | 2.46 | 1.53 | 2.88 | 3.58 |
| 24 | 0.49 | 0.48 | −0.25 | 0.53 | 0.48 | 0.52 | 2.97 | 2.04 |
| 27 | 0.93 | 0.43 | −0.04 | 0.72 | 1.83 | 1.52 | 4.64 | 3.62 |
| 28 | 0.45 | 0.65 | −0.83 | 0.51 | 1.91 | 1.95 | 5.73 | 3.66 |
| 29 | 0.49 | 0.35 | −0.02 | 0.73 | 1.48 | 1.05 | 2.45 | 1.47 |
| 30 | 0.67 | 0.48 | −0.43 | 0.44 | 3.82 | 1.03 | 3.34 | 2.23 |
| 31 | 1.04 | 1.05 | 0.00 | 0.34 | 2.68 | 3.88 | 3.69 | 2.24 |
| 32 | 0.48 | 0.63 | −0.38 | 0.64 | 3.20 | 0.75 | 2.42 | 1.89 |
| 33 | 1.21 | 0.76 | −0.29 | 0.95 | 2.96 | 2.28 | 3.25 | 2.97 |
| 35 | 1.11 | 0.25 | −0.78 | 0.59 | 2.30 | 0.63 | 6.96 | 4.84 |
| 37 | 0.75 | 0.37 | 0.43 | 0.09 | 2.08 | 0.76 | 5.79 | 5.53 |
Note. N/A indicates a family with only one participant of the designated sex.
Table B3.
Descriptive statistics for mean speaking F0 in Hertz and semitone standard deviation (STSD).
| Family | F0
|
STSD |
||||||
|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||
| M | SD | M | SD | M | SD | M | SD | |
| 17 | 105.71 | 8.57 | 212.20 | 25.86 | 2.02 | 0.41 | 2.40 | 0.67 |
| 18 | N/A | N/A | 177.58 | 7.77 | N/A | N/A | 2.17 | 0.17 |
| 19 | 105.09 | 15.66 | 183.42 | 3.90 | 2.47 | 0.39 | 2.35 | 0.30 |
| 20 | 111.07 | 8.31 | 202.77 | 9.07 | 2.03 | 0.35 | 2.08 | 0.61 |
| 21 | 104.12 | 4.48 | 180.96 | 0.71 | 2.30 | 0.23 | 2.18 | 0.26 |
| 22 | 111.69 | 6.87 | 187.99 | 15.09 | 2.20 | 0.26 | 2.11 | 0.28 |
| 23 | 95.37 | 8.43 | 206.06 | 11.92 | 1.63 | 0.37 | 2.07 | 0.21 |
| 24 | 106.19 | 12.88 | 220.40 | 13.63 | 2.17 | 0.58 | 2.14 | 0.03 |
| 27 | 120.34 | 21.29 | 200.38 | 9.31 | 2.31 | 0.20 | 2.25 | 0.36 |
| 28 | 106.12 | 9.03 | 192.70 | 10.72 | 2.27 | 0.37 | 2.27 | 0.72 |
| 29 | 113.57 | 15.48 | 215.23 | 13.59 | 1.90 | 0.40 | 2.00 | 0.28 |
| 30 | 115.41 | 8.35 | 195.92 | 8.82 | 2.22 | 0.80 | 2.28 | 0.69 |
| 31 | 115.57 | 15.54 | 190.92 | 8.75 | 2.28 | 0.82 | 2.16 | 0.33 |
| 32 | 121.98 | 9.33 | 199.99 | 22.24 | 1.73 | 0.92 | 1.77 | 0.51 |
| 33 | 115.65 | 11.95 | 190.53 | 34.79 | 1.93 | 0.51 | 2.20 | 0.25 |
| 35 | 123.27 | 26.99 | 191.77 | 15.23 | 2.53 | 0.76 | 2.62 | 0.44 |
| 37 | 114.98 | 17.53 | 205.35 | 7.63 | 2.12 | 0.74 | 1.94 | 0.48 |
Note. N/A indicates a family with only one participant of the designated sex.
Table B4.
Descriptive statistics for speaking/pausing ratio.
| Family | Male |
Female |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| 17 | 0.85 | 0.03 | 0.78 | 0.08 |
| 18 | N/A | N/A | 0.83 | 0.05 |
| 19 | 0.87 | 0.04 | 0.86 | 0.05 |
| 20 | 0.85 | 0.06 | 0.89 | 0.05 |
| 21 | 0.80 | 0.02 | 0.84 | 0.02 |
| 22 | 0.81 | 0.03 | 0.89 | 0.02 |
| 23 | 0.81 | 0.05 | 0.85 | 0.03 |
| 24 | 0.83 | 0.02 | 0.88 | 0.06 |
| 27 | 0.83 | 0.06 | 0.85 | 0.06 |
| 28 | 0.80 | 0.13 | 0.87 | 0.05 |
| 29 | 0.82 | 0.05 | 0.84 | 0.07 |
| 30 | 0.80 | 0.05 | 0.82 | 0.06 |
| 31 | 0.82 | 0.06 | 0.86 | 0.05 |
| 32 | 0.82 | 0.02 | 0.82 | 0.05 |
| 33 | 0.83 | 0.04 | 0.87 | 0.05 |
| 35 | 0.77 | 0.02 | 0.80 | 0.06 |
| 37 | 0.87 | 0.05 | 0.85 | 0.03 |
Note. N/A indicates a family with only one participant of the designated sex.
Table B5.
Descriptive statistics for cepstral peak prominence smoothed.
| Family | Male |
Female |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| 17 | 8.89 | 0.54 | 9.03 | 0.63 |
| 18 | N/A | N/A | 8.57 | 0.41 |
| 19 | 7.93 | 1.01 | 8.01 | 0.67 |
| 20 | 8.37 | 1.29 | 8.87 | 0.59 |
| 21 | 8.74 | 0.55 | 9.15 | 0.05 |
| 22 | 8.39 | 0.75 | 8.97 | 0.91 |
| 23 | 8.03 | 1.02 | 8.16 | 0.57 |
| 24 | 9.56 | 0.75 | 9.82 | 0.42 |
| 27 | 8.28 | 1.05 | 9.88 | 1.24 |
| 28 | 8.19 | 1.46 | 8.90 | 1.05 |
| 29 | 7.66 | 0.98 | 7.90 | 0.86 |
| 30 | 8.03 | 0.92 | 8.24 | 0.85 |
| 31 | 9.22 | 0.59 | 9.26 | 0.72 |
| 32 | 9.40 | 0.17 | 8.32 | 0.96 |
| 33 | 8.81 | 0.55 | 8.98 | 1.14 |
| 35 | 7.86 | 0.75 | 8.46 | 1.11 |
| 37 | 8.04 | 0.44 | 8.98 | 1.31 |
Note. N/A indicates a family with only one participant of the designated sex.
Funding Statement
Data collection was funded by Public Health Service Grant RO1 DC04068 (awarded to Steven D. Gray) and through the cooperation and support of the Utah Genetic Reference Project, which was funded through the W. M. Keck Foundation. Funding for data analysis was provided by the David O. McKay School of Education at Brigham Young University.
References
- Awan S. N., Roy N., & Dromey C. (2009). Estimating dysphonia severity in continuous speech: Application of a multi-parameter spectral/cepstral model. Clinical Linguistics & Phonetics, 23(11), 825–841. https://doi.org/10.3109/02699200903242988 [DOI] [PubMed] [Google Scholar]
- Barnes D. R. (2013). Age-related changes in the production of linguistic prosody [Master's thesis, Purdue University]. Purdue e-Pubs. https://docs.lib.purdue.edu/open_access_theses/17/ [Google Scholar]
- Benjamin B. J. (1997). Speech production of normally aging adults. Seminars in Speech and Language, 18(2), 135–141. https://doi.org/10.1055/s-2008-1064068 [DOI] [PubMed] [Google Scholar]
- Boersma P., & Weenink D. (2017). Praat: Doing phonetics by computer (Version 5.4) [Computer program]. http://www.praat.org
- Cox V. O., & Selent M. (2015). Acoustic and respiratory measures as a function of age in the male voice. Journal of Phonetics and Audiology, 1(1), 105 https://doi.org/10.4172/2471-9455.1000105 [Google Scholar]
- Debruyne F., & Decoster W. (1999). Acoustic differences between sustained vowels perceived as young or old. Logopedics Phoniatrics Vocology, 24(1), 1–5. https://doi.org/10.1080/140154399434490 [Google Scholar]
- Dromey C., Hunter E., & Nissen S. L. (2018). Speech adaptation to kinematic recording sensors: Perceptual and acoustic findings. Journal of Speech, Language, and Hearing Research, 61(3), 593–603. https://doi.org/10.1044/2017_JSLHR-S-17-0169 [DOI] [PubMed] [Google Scholar]
- Dromey C., Nissen S., Roy N., & Merrill R. M. (2008). Articulatory changes following treatment of muscle tension dysphonia: Preliminary acoustic evidence. Journal of Speech, Language, and Hearing Research, 51(1), 196–208. https://doi.org/10.1044/1092-4388(2008/015) [DOI] [PubMed] [Google Scholar]
- Eichhorn J. T., Kent R. D., Austin D., & Vorperian H. K. (2018). Effects of aging on vocal fundamental frequency and vowel formants in men and women. Journal of Voice, 32(5), 644.e1–644.e9. https://doi.org/10.1016/j.jvoice.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiser H. S. (2009). Acoustic similarities and differences in the voices of same-sex siblings. Paper presented at the 18th Annual Conference of the International Association for Forensic Phonetics and Acoustics (IAFPA), Cambridge, United Kingdom. [Google Scholar]
- Feiser H. S., & Kleber F. (2012). Voice similarity among brothers: Evidence from a perception experiment. Paper presented at the 21st Annual Conference of the International Association for Forensic Phonetics and Acoustics (IAFPA), Santander, Spain. [Google Scholar]
- Ferrand C. T. (2002). Harmonics-to-noise ratio: An index of vocal aging. Journal of Voice, 16(4), 480–487. https://doi.org/10.1016/S0892-1997(02)00123-6 [DOI] [PubMed] [Google Scholar]
- Garrett R. (2013). Cepstral- and spectral-based acoustic measures of normal voices [Master's thesis, University of Wisconsin, Milwaukee]. UWM Digital Commons. https://dc.uwm.edu/etd/217/ [Google Scholar]
- Gelfer M. P., & Mikos V. A. (2005). The relative contributions of speaking fundamental frequency and formant frequencies to gender identification based on isolated vowels. Journal of Voice, 19(4), 544–554. https://doi.org/10.1016/j.jvoice.2004.10.006 [DOI] [PubMed] [Google Scholar]
- Gorham-Rowan M. M., & Laures-Gore J. (2006). Acoustic–perceptual correlates of voice quality in elderly men and women. Journal of Communication Disorders, 39(3), 171–184. https://doi.org/10.1016/j.jcomdis.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Hammond T. H., Gray S. D., Butler J., Zhou R. X., & Hammond E. (1998). Age- and gender-related elastin distribution changes in human vocal folds. Otolaryngology—Head & Neck Surgery, 119(4), 314–322. https://doi.org/10.1016/S0194-5998(98)70071-3 [DOI] [PubMed] [Google Scholar]
- Huber J. E., Darling M., Francis E. J., & Zhang D. B. (2012). Impact of typical aging and Parkinson's disease on the relationship among breath pausing, syntax, and punctuation. American Journal of Speech-Language Pathology, 21(4), 368–379. https://doi.org/10.1044/1058-0360(2012/11-0059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E. J., Tanner K., & Smith M. E. (2011). Gender differences affecting vocal health of women in vocally demanding careers. Logopedics Phoniatrics Vocology, 36(3), 128–136. https://doi.org/10.3109/14015439.2011.587447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Huber J., Jenkins J., & Fredrick J. (2019). Language planning and pauses in story retell: Evidence from aging and Parkinson's disease. Journal of Communication Disorders, 79, 1–10. https://doi.org/10.1016/j.jcomdis.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Lowenstein J. H., & Nittrouer S. (2019). Perception–production links in children's speech. Journal of Speech, Language, and Hearing Research, 62(4), 853–857. https://doi.org/10.1044/2018_JSLHR-S-18-0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland D. H., Baum S. R., & Chabot C. (1996). Speech compensation to structural modifications of the oral cavity. The Journal of the Acoustical Society of America, 100(2), 1093–1104. https://doi.org/10.1121/1.416286 [DOI] [PubMed] [Google Scholar]
- Nishio M., & Niimi S. (2008). Changes in speaking fundamental frequency characteristics with aging. Folia Phoniatrica et Logopaedica, 60(3), 120–127. https://doi.org/10.1159/000118510 [DOI] [PubMed] [Google Scholar]
- Nissen S. L., & Fox R. A. (2009). Acoustic and spectral patterns in young children's stop consonant productions. The Journal of the Acoustical Society of America, 126(3), 1369–1378. https://doi.org/10.1121/1.3192350 [DOI] [PubMed] [Google Scholar]
- Orlikoff R. F., Golla M. E., & Deliyski D. D. (2012). Analysis of longitudinal phase differences in vocal-fold vibration using synchronous high-speed videoendoscopy and electroglottography. Journal of Voice, 26(6), 816.e13–816.e20. https://doi.org/10.1016/j.jvoice.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig L. A. O., & Ringel R. L. (1983). Effects of physiological aging on selected acoustic characteristics of voice. Journal of Speech and Hearing Research, 26(1), 22–30. https://doi.org/10.1044/jshr.2601.22 [DOI] [PubMed] [Google Scholar]
- Ramig L. A. O., Gray S., Baker K., Corbin-Lewis K., Buder E., Luschei E., Coon H., & Smith M. (2001). The aging voice: A review, treatment data and familial and genetic perspectives. Folia Phoniatrica et Logopaedica, 53(5), 252–265. https://doi.org/10.1159/000052680 [DOI] [PubMed] [Google Scholar]
- Sapienza C. M., & Stathopoulos E. T. (1995). Speech task effects on acoustic and aerodynamic measures of women with vocal nodules. Journal of Voice, 9(4), 413–418. https://doi.org/10.1016/S0892-1997(05)80203-6 [DOI] [PubMed] [Google Scholar]
- Simpson A. P. (2002). Gender-specific articulatory–acoustic relations in vowel sequences. Journal of Phonetics, 30(3), 417–435. https://doi.org/10.1006/jpho.2002.0171 [Google Scholar]
- Slawinski E. B. (1994). Acoustic correlates of [b] and [w] produced by normal young to elderly adults. The Journal of the Acoustical Society of America, 95(4), 2221–2230. https://doi.org/10.1121/1.408682 [DOI] [PubMed] [Google Scholar]
- Spazzapan E. A., Cardoso V. M., Fabron E. M. G., Berti L. C., Brasolotto A. G., & Marino V. C. C. (2018). Acoustic characteristics of healthy voices of adults: From young to middle age. CoDAS, 30(5), e20170225 https://doi.org/10.1590/2317-1782/20182017225 [DOI] [PubMed] [Google Scholar]
- Titze I. R. (1989). Physiologic and acoustic differences between male and female voices. The Journal of the Acoustical Society of America, 85(4), 1699–1707. https://doi.org/10.1121/1.397959 [DOI] [PubMed] [Google Scholar]
- Tjaden K., & Turner G. S. (1997). Spectral properties of fricatives in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 40(6), 1358–1372. https://doi.org/10.1044/jslhr.4006.1358 [DOI] [PubMed] [Google Scholar]
- Torre P., & Barlow J. A. (2009). Age-related changes in acoustic characteristics of adult speech. Journal of Communication Disorders, 42(5), 324–333. https://doi.org/10.1016/j.jcomdis.2009.03.001 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Administration for Community Living. (2017). Projected future growth of older population. Retrieved from https://www.acl.gov/node/579
- Vanderydt R. E. (1998). A study of voice similarities among family members. Massachusetts General Institute of Health Professions. [Google Scholar]
- Westrop R. M. (2000). Segment durational changes from young adults to elderly: A familial study. Utah State University. [Google Scholar]
- Xue S. A., & Deliyski D. (2001). Effects of aging on selected acoustic voice parameters: Preliminary normative data and educational implications. Educational Gerontology, 27(2), 159–168. https://doi.org/10.1080/03601270151075561 [Google Scholar]
- Yamauchi A., Yokonishi H., Imagawa H., Sakakibara K., Nito T., Tayama N., & Yamasoba T. (2014). Age- and gender-related difference of vocal fold vibration and glottal configuration in normal speakers: Analysis with glottal area waveform. Journal of Voice, 28(5), 525–531. https://doi.org/10.1016/j.jvoice.2014.01.016 [DOI] [PubMed] [Google Scholar]