Despite the availability of multiple immunosuppression methods, refractory immune checkpoint inhibitor-associated myocarditis remains a life-threatening toxicity associated with a high mortality of ∼40% as well as severe infectious complications.1 Here we report a case of a 57-year-old male receiving third-line treatment with a combination of checkpoint inhibitors (nivolumab and ipilimumab) for metastatic lung small-cell neuroendocrine carcinoma, stage IIIB. Before receiving the third treatment cycle, he presented a muscular weakness of the lower limbs with dyspnea of sudden onset associated with an oppressive retrosternal chest pain without syncope and a ptosis of the right eye with diplopia. He subsequently developed severe arrhythmias and third-grade atrioventricular block. Elevated concentrations of high-sensitivity troponins I and T, creatine kinase (CK), ferritin (Figure 1 ) and positron emission tomography/computed tomography with 68Ga-DOTATOC (gallium-68 DOTA-DPhe1, Tyr3-octreotate) confirmed the diagnosis of generalized myositis complicated by myocarditis and ocular myositis (see supplementary Figure S1, available at Annals of Oncology online). The left ventricular ejection fraction was preserved and coronary angiography showed normal arteries. A very broad infectious and myasthenia panel was negative. A myocarditis–myositis overlap syndrome was diagnosed and a pacemaker was placed. He received methylprednisolone sodium succinate pulse therapy at a dose of 1 g/day for 1 day followed by a dose of 200 mg/day for 5 days. Despite the repeated administrations of high intravenous methylprednisolone over a 1-week period, the patient's troponin I and T, CK and ferritin levels increased quickly (from 1291 to 18522 μg/l; Figure 1). The HScore was 211 points with a 93%–96% probability for associated reactive hemophagocytic syndrome. Intravenous tocilizumab (TCZ; at a dose of 8 mg/kg body weight weekly for two doses) was administered. The troponin T/I, CK and ferritin levels as well as inflammatory parameters rapidly decreased (Figure 1). The ejection fraction remained normal, and symptoms of myocarditis (arrhythmias) and myositis (muscular weakness and pain) progressively disappeared. Corticosteroids were progressively tapered and the patient did not experience any recurrence of cardiac or myositis adverse events. The immunotherapy was discontinued.
Figure 1.
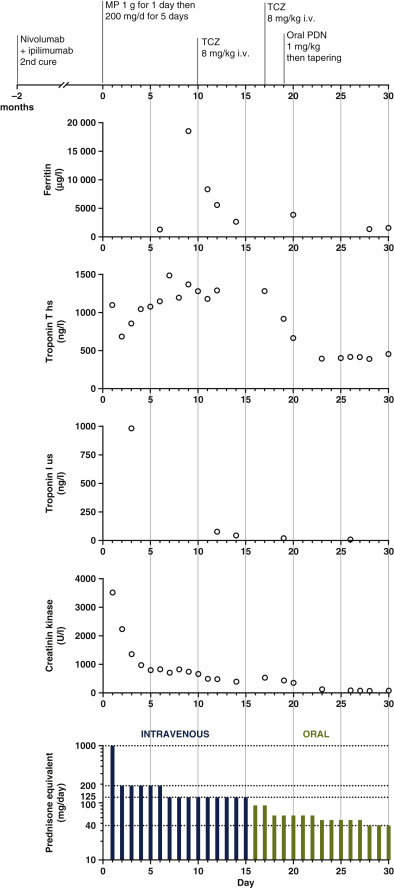
Kinetics of biochemical variables during treatment.
The patient began receiving methylprednisolone sodium succinate at a dose of 1 g/day for 1 day, followed by a dose of 200 mg/day with initial improvement of biochemical variables. Despite receiving high doses of methylprednisolone, the patient had an immune flare associated with a rapid increase in ferritin and troponin T levels. Tocilizumab (TCZ) at a dose of 8 mg/kg was administrated on days 7 and 14. This resulted in a rapid decrease of troponin T and I, creatine kinase (CK) and ferritin levels as well as inflammatory parameters and was associated with the resolution of the myocarditis and myositis, according to clinical and biochemical measures. The patient was then progressively weaned from corticosteroids and did not experience any recurrence of cardiac, myositis or hemophagocytic syndrome adverse events. ‡High-sensitivity troponin T is expressed by skeletal muscle, including regenerating skeletal muscle tissue, whereas high-sensitivity troponin I is specific to the myocardium.3,13 As reported previously,3 given that the patient had severe myositis related to immunotherapy, the high-sensitivity troponin T concentration reflected active skeletal muscle regeneration rather than active myocarditis in the context of normalization of the high-sensitivity troponin I concentration and CK level.3 hs, high-sensitivity; i.v., intravenous; MP, methylprednisolone sodium succinate pulse; PDN, prednisone; us, ultrasensitivity.
Severe and refractory immune checkpoint inhibitor-related myocarditis represents an important clinical challenge due to its high mortality, despite the use of immunosuppression escalation and the availability of multiple immunosuppressant (IS) drugs such as infliximab, rituximab, tacrolimus, antithymocyte globulin, mycophenolate mofetil or tacrolimus. The successful use of abatacept2 and alemtuzumab,3 two selective IS drugs, has been recently reported for this condition.
Interleukin (IL)-6 is a critical driver of acute and chronic inflammation. During inflammation, IL-6 signaling drives T-cell survival, expansion and proliferation.4 Moreover, IL-6 signaling promotes a protumorigenic immune-suppressive network.5 Compared with the other available selective IS drugs, the anti-IL-6R agent TCZ offers several strategic advantages without the risk of compromising immune checkpoint inhibitor efficacy.6 In addition, it carries complementary antitumor properties, because IL-6 blockade significantly improves the differentiation of CD4+ T cells into interferon-γ-producing effector T helper type 1 (Th1) cells.7 Furthermore, accumulating evidence suggests that the IL-6–Th17 pathway may have an important role in the pathogenesis of immune-related adverse events, especially in steroid-refractory cases.8 , 9 IL-17A-expressing CD4+ T cells (c-Kit− CD161+ MDR1+ Th17 cells) have been reported as key effectors of autoimmune inflammation refractory to glucocorticoids.8 The pathogenic effect of IL-6 is essential in the differentiation of proinflammatory Th17 cells from naïve CD4+ T cells, which might suggest a role for this Th17 subset in steroid-refractory immune-related adverse events.10 Another important report showed that among adults receiving chimeric antigen receptor T cells, early administration of TCZ to treat the cytokine release syndrome was associated with a lower rate of cardiovascular events.11 Recently, an approach of blocking the IL-6–IL-6 receptor (IL-6R) axis using TCZ suggested a promising immunomodulatory therapeutic strategy and could be beneficial to avoid rapid clinical deterioration from severe forms of coronavirus disease 2019 (COVID-19).12 Very interestingly, what we have noticed in our fragile patient is that he remained pauci-symptomatic despite having become COVID-19 positive during long immunosuppression. This observation could suggest a possible additional beneficial effect for TCZ on the clinical course of COVID-19. In our patient, the use of TCZ led to the rapid resolution of the refractory high-grade myocarditis, allowing a significant reduction in the total duration of immunosuppression.
Acknowledgments
Funding
MO received funding from the Leenaards Foundation (no grant number).
Disclosure
The authors have declared no conflicts of interest.
Supplementary Data
References
- 1.Wang D.Y., Salem J.E., Cohen J.V. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salem J.E., Allenbach Y., Vozy A. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380:2377–2379. doi: 10.1056/NEJMc1901677. [DOI] [PubMed] [Google Scholar]
- 3.Esfahani K., Buhlaiga N., Thebault P., Lapointe R., Johnson N.A., Miller W.H., Jr. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380:2375–2376. doi: 10.1056/NEJMc1903064. [DOI] [PubMed] [Google Scholar]
- 4.Li B., Jones L.L., Geiger T.L. IL-6 promotes T cell proliferation and expansion under inflammatory conditions in association with low-level RORgammat expression. J Immunol. 2018;201:2934–2946. doi: 10.4049/jimmunol.1800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukamoto H., Fujieda K., Senju S., Ikeda T., Oshiumi H., Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018;109:523–530. doi: 10.1111/cas.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mace T.A., Shakya R., Pitarresi J.R. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67:320–332. doi: 10.1136/gutjnl-2016-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohno Y., Toyoshima Y., Yurino H. Lack of interleukin-6 in the tumor microenvironment augments type-1 immunity and increases the efficacy of cancer immunotherapy. Cancer Sci. 2017;108:1959–1966. doi: 10.1111/cas.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramesh R., Kozhaya L., McKevitt K. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettelli E., Carrier Y., Gao W. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 10.Horisberger A., La Rosa S., Zurcher J.P. A severe case of refractory esophageal stenosis induced by nivolumab and responding to tocilizumab therapy. J Immunother Cancer. 2018;6:156. doi: 10.1186/s40425-018-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvi R.M., Frigault M.J., Fradley M.G. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T) J Am Coll Cardiol. 2019;74:3099–3108. doi: 10.1016/j.jacc.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.2005615117. (forthcoming) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes M., Lilleker J.B., Herrick A.L., Chinoy H. Cardiac troponin testing in idiopathic inflammatory myopathies and systemic sclerosis-spectrum disorders: biomarkers to distinguish between primary cardiac involvement and low-grade skeletal muscle disease activity. Ann Rheum Dis. 2015;74:795–798. doi: 10.1136/annrheumdis-2014-206812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


