Abstract
Background
Much of the focus regarding the global pandemic of coronavirus disease of 2019 (COVID-19) has been on the cardiovascular, pulmonary, and hematologic complications. However, neurologic complications have arisen as an increasingly recognized area of morbidity and mortality.
Objective
This brief report summarizes the neurologic complications associated with COVID-19 with an emphasis on the emergency medicine clinician.
Discussion
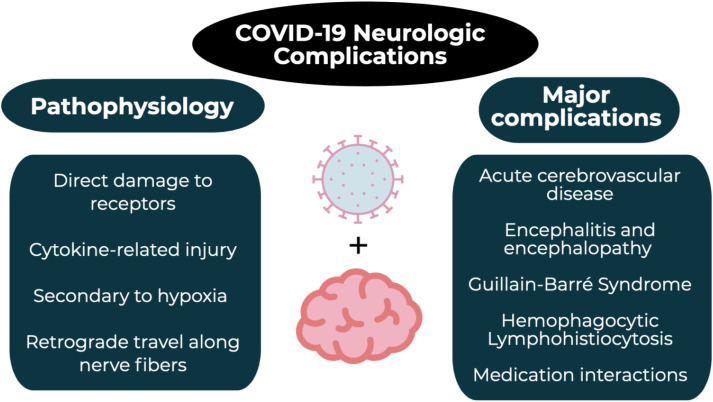
COVID-19 has infected over 3.5 million people and killed over 240,000 people worldwide. While pulmonary complications are profound, the neurologic system is also significantly impacted, with complications including acute cerebrovascular events, encephalitis, Guillain-Barré syndrome, acute necrotizing hemorrhagic encephalopathy, and hemophagocytic lymphohistiocytosis. Additionally, patients on immunosuppressive medications for pre-existing neurologic issues are at an increased risk for complications with COVID-19 infection, and many of the currently proposed COVID-19 therapies can interact with these medications.
Conclusions
When caring for COVID-19 patients, emergency medicine clinicians should be aware of the neurologic complications from COVID-19.
Keywords: COVID-19, Coronavirus, Infectious disease, Neurologic, HLH, Cerebrovascular disease, Encephalopathy
1. Introduction
First appearing in Wuhan, China, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease of 2019 (COVID-19), which the World Health Organization (WHO) declared a pandemic in March 2020 [1]. As of May 1, 2020, the COVID-19 pandemic has resulted in over 1 million cases and 62,406 deaths in the United States, and over 3.5 million cases and 240,000 deaths worldwide [[1], [2], [3]]. Despite the majority of the focus being placed on pulmonary and cardiovascular complications, emergency medicine clinicians must remain cognizant of the neurologic complications, which can present subtly and add substantially to the morbidity and mortality [[4], [5], [6], [7]]. The following article reviews the neurologic complications from COVID-19, with an emphasis on the emergency medicine clinician.
2. Methods
Authors searched PubMed and Google Scholar for articles using the keywords “COVID-19”, “SARS-CoV-2”, “neurologic”, “brain”, “cerebral”, “cerebrovascular accident”, “HLH”, “hemophagocytic lymphohistiocytosis”, “stroke”, “altered level of consciousness”, “decreased level of consciousness”, “encephalopathy”, “cerebrovascular disease”. Authors included case reports, retrospective studies, prospective studies, systematic reviews and meta-analyses, clinical guidelines, and narrative reviews focusing on COVID-19 and neurologic effects and complications. Preprinted articles were also included. The literature search was restricted to studies published in English. Emergency medicine physicians with experience in critical appraisal of the literature reviewed all of the articles and decided which studies to include for the review by consensus, with a focus on emergency medicine-relevant articles. A total of 60 articles were selected for inclusion.
3. Discussion
3.1. Pathophysiology and clinical features
A member of the beta-coronaviridae family, SARS-CoV-2 is an enveloped, non-segmented, single-stranded, positive-sense RNA virus [[8], [9], [10]]. The mechanisms by which SARS-CoV-2 causes neurologic damage are multifaceted, including direct damage to specific receptors, cytokine-related injury, secondary hypoxia, and retrograde travel along nerve fibers [[11], [12], [13], [14], [15]]. Much like its expression on lung epithelial cells, the expression of angiotensin converting enzyme 2 (ACE2) on endothelial cells of the blood-brain barrier can allow viral binding at this important site, facilitating viral entry into the central nervous system by attacking the vasculature [16,17]. The binding of SARS-CoV-2 at the pulmonary epithelial cells also generates a global systemic inflammatory response (SIRS), producing increased levels of interleukin (IL)-6, IL-12, IL-15, and tumor necrosis factor alpha (TNF-α); activating glial cells; and producing a massive pro-inflammatory central nervous system state [13]. In particular, IL-6 levels have been correlated with increased disease severity in COVID-19 [[11], [12], [13]]. These systemic effects combined with localized lung alveolar damage result in severe hypoxia, which can lead to cerebral vasodilation and may decompensate into cerebral edema and ischemia [13,14]. Finally, SARS-CoV-2 travels retrograde along the olfactory nerve and bulb, which provides an avenue between the nasal epithelium and the central nervous system and may also explain the common complaint of anosmia [15].
The prevalence of neurologic symptoms in COVID-19 patients has become more apparent, though pre-existing neurologic conditions have been linked to more severe COVID-19 infections [[4], [5], [6], [7],18]. In a recent review of patients diagnosed and hospitalized with COVID-19, 8% of the 4014 patients had pre-existing neurologic diseases, though this analysis mainly focused on those with prior strokes [6]. Interestingly, patients with prior neurologic conditions have less improvement of respiratory symptoms over their first 10 days of hospitalization and had a significantly increased risk of developing acute respiratory distress syndrome as compared to controls without neurologic disease [19,20]. In a separate cohort of 179 patients with SARS-CoV-2 pneumonia, pre-existing cerebrovascular disease was also found to be associated with increased mortality [21]. A similar mortality trend has been demonstrated in those with Parkinson's Disease [22]. A systematic review and meta-analysis also identified a 2.5-fold increased risk of severe infection among patients with a prior stroke [23].
While pre-existing neurologic conditions portend worse outcomes, the incidence of neurologic complications secondary to SARS-CoV-2 infection is also substantial. Among hospitalized COVID-19 patients, neurologic complications range from 6% to 36% [18,24]. Additionally, hypoxic ischemic encephalopathy was reported in 20% of patients in one series [25]. Focused efforts are also investigating the neurotropism of SARS-CoV-2 to account for the devastating brainstem-mediated complications in both the cardiovascular and pulmonary systems [16,26] (Fig. 1).
Fig. 1.
COVID-19 and the neurologic system.
3.2. Neurologic complications associated with COVID-19 infection
3.2.1. Acute cerebrovascular disease
Acute cerebrovascular disease remains one of the more common and serious neurologic complications seen in COVID-19 populations. However, this final common manifestation has a multifactorial etiology. SARS-CoV-2 causes a global inflammatory response and a hypercoagulable state evidenced by increased D-dimers, prolonged prothrombin time, and disseminated intravascular coagulation [20,27]. In an Italian cohort of COVID-19 patients admitted with confirmed infection, the rate of ischemic stroke was 2.5%, despite venous thromboembolism prophylaxis on admission [28]. In comparison, the rate of ischemic stroke in hospitalized COVID-19 patients in China was estimated to be as high as 5% [24]. Similarly, there was a 3.7% incidence of ischemic stroke in Dutch COVID-19 patients in the intensive care unit (ICU) despite venous thromboembolism prophylaxis [29]. Interestingly, COVID-19 has also led to younger patients presenting with ischemic stroke, including large vessel occlusions [30]. Additionally, COVID-19 patients can develop significant hypoxia leading to decreased cerebral oxygenation and infarcts, particularly in those with pre-existing cerebrovascular disease [13,14]. Infection, inflammation, and hypercoagulable states can further increase the risk of ischemic stroke, which can be even more pronounced in older patients [[31], [32], [33]].
When evaluating COVID-19 patients with stroke-like symptoms, it is important to protect the healthcare team while expediting this time-sensitive emergency. The American Heart Association has addressed this with guidelines for a protected code stroke, emphasizing screening guidelines, personal protective equipment, and crisis resource management [34]. However, once diagnosed with ischemic stroke, patients should still receive the standard of care based on their institution with consideration of intravenous thrombolytic medications and endovascular thrombectomy in the appropriate clinical scenarios, without any alteration to intervention criteria [35,36].
3.2.2. Encephalitis and encephalopathy
Encephalitis is characterized by acute onset of fever, vomiting, seizures, and decreased or changed consciousness [37]. While rare, SARS-CoV-2 encephalitis has been reported in several cases, though this was based on clinical and imaging findings as there has been no cerebral spinal fluid (CSF) evidence of SARS-CoV-2 to date [38,39]. The pathophysiology is unclear but may be related to edema secondary to inflammatory injury versus direct viral infection [39]. Similar to other cases of encephalitis, aggressive supportive care and treatment of increased intracranial pressure are paramount.
Acute necrotizing encephalopathy (ANE) is a rare neurologic complication caused by cytokine storm and damage to the blood-brain barrier [40]. Unlike other viral central nervous system infections, demyelination is not present in ANE [38]. Non-contrast head computed tomography (CT) can initially demonstrate symmetric, multifocal lesions, while magnetic resonance imaging (MRI) with T2-weighted-Fluid-Attenuated Inversion Recovery (FLAIR) may show hyperintense signal and internal hemorrhage [41]. The thalamus, brainstem, cerebellum, and cerebral white matter are the most common areas affected [41]. While this is more commonly associated with influenza or Zika infection, SARS-CoV-2 has also been associated with this condition [13]. The pathogenesis of ANE is poorly understood, but with the hyper-inflammatory state, treatment with intravenous immunoglobulin (IVIG) and steroids can be attempted [42,43].
3.2.3. Guillain-Barré Syndrome (GBS)
GBS is a symmetric, ascending flaccid paralysis, often preceded by respiratory or gastrointestinal infections from a virus or bacteria [44]. This progressive neuropathy has recently been linked to SARS-CoV-2 infection, with 5 cases reported in Italy and 2 additional cases from Wuhan, China [[45], [46], [47]]. All patients experienced a prodrome of an upper respiratory infection ranging from 5 to 14 days prior to the development of symmetric weakness, with 3 patients developing respiratory failure [[45], [46], [47]]. All patients had a positive nasopharyngeal polymerase chain reaction (PCR) test and chest imaging characteristic of SARS-CoV-2, but all cerebrospinal fluid (CSF) samples had a negative SARS-CoV-2 PCR [[45], [46], [47]]. While all of the patients received IVIG, those who developed respiratory failure had poor outcomes [45]. Interestingly, brain and spine MRI did not show abnormalities in half of the patients, highlighting the importance of consultation and additional testing, such as nerve conduction studies, when there is a high clinical suspicion even in the absence of radiographic findings [[45], [46], [47]].
3.2.4. Hemophagocytic Lymphohistiocytosis (HLH)
HLH is a severe dysregulation of T-lymphocyte, natural killer cell, and macrophage over-activation causing a massive cytokine storm and multiorgan injury [48]. This condition is often secondary to hematologic malignancy, immunosuppression, or critical infection, but has also been described in patients with SARS-CoV-2 [48]. HLH patients present with unremitting fevers, pancytopenia, coagulopathy, hepatic dysfunction, hypertriglyceridemia, and an elevated ferritin [[48], [49], [50]]. HLH is an underrecognized complication among COVID-19 patients as the innate immune system may result in uncontrolled cytokine storm, characterized by severely increased levels of IL-2, IL-6, IL-7, and TNFα. Up to one-third of COVID-19 patients with HLH develop neurologic abnormalities. Early recognition and scoring with the HScore allows for prompt consideration of immunosuppressive treatments (Table 1 ); an HScore of 200 predicts an 88% probability of HLH, while a score of 130 predicts a 9% probability of HLH [51]. Treatments include steroids and tocilizumab, both of which are currently under investigation for treatment in COVID-19 patients [51].
Table 1.
HScore.
| Category | Number of points |
|---|---|
| Temperature | |
| <38.4 ° | 0 |
| 38.4–39.4 °C | 33 |
| >39.4 °C | 49 |
| Organomegaly | |
| None | 0 |
| Hepatomegaly or splenomegaly | 23 |
| Hepatomegaly and splenomegaly | 38 |
| Number of cytopenias | |
| One lineage | 0 |
| Two lineages | 24 |
| Three lineages | 34 |
| Triglycerides | |
| <1.5 mmol/L | 0 |
| 1.5–4.0 mmol/L | 44 |
| >4.0 mmol/L | 64 |
| Fibrinogen | |
| >2.5 g/L | 0 |
| </=2.5 g/L | 30 |
| Ferritin | |
| <2000 ng/mL | 0 |
| 2000–6000 ng/mL | 35 |
| >6000 ng/mL | 50 |
| Serum aspartate aminotransferase | |
| <30 IU/L | 0 |
| ≥30 IU/L | 19 |
| Hemophagocytosis on bone marrow aspirate | |
| No | 0 |
| Yes | 35 |
| Known immunosuppression | |
| No | 0 |
| Yes | 18 |
3.3. Medication interactions
Many of the recently proposed medications have significant drugs interactions and side effects [4]. Azithromycin, corticosteroids, plasma exchange, biologic agents (tocilizumab), antivirals (e.g., remdesivir, ribavirin, lopinavir/ritonavir, favipiravir), and antimalarials (e.g., hydroxychloroquine, chloroquine) are all currently under investigation, with a recent announcement for an accelerated trial devoted to remdesivir [[52], [53], [54]]. Lopinavir/ritonavir and azithromycin interact with many common medications in patients with prior strokes including antihypertensives, antiplatelets, statins, and anticoagulants [[52], [53], [54], [55]]. These also carry an increased risk of neurocognitive impairment in longer courses [[55], [56], [57]]. Ribavirin and interferon alpha have both neuropathic and neuropsychiatric sequelae, while interferon caries a risk of retinopathy [58,59]. Similarly, antimalarials also carry the risk of neuropsychiatric side effects and less commonly ataxia, seizures, and limbic encephalitis [60]. A complete summary of mechanism of action, neurologic effects, and medication interactions can be found in Table 2 .
Table 2.
Medications and the nervous system.
| Medication | Mechanism | Neurologic effects and medication interactions |
|---|---|---|
| Remdesivir | Nucleotide-analog inhibitor of RNA polymerases |
|
| Ribavirin | RNA and DNA virus replication inhibitor |
|
| Lopinavir/Ritonavir | Lopinavir inhibits protease Ritonavir inhibits CYP3A metabolism |
|
| Favipiravir | Inhibits RNA-dependent RNA polymerases |
|
| Chloroquine and Hydroxychloroquine | Endosomal/organelle pH modifications |
|
| Azithromycin | Binds to 50s ribosome, inhibiting protein synthesis |
|
| Interferon | Activates immune system |
|
| Methylprednisolone | Inflammation reduction |
|
| Tocilizumab | IL-6 inhibitor |
|
RNA, ribonucleic acid; DNA, deoxyribonucleic acid; IL, interleukin.
4. Limitations
There are several limitations of this current literature review evaluating neurologic complications and effects connected to COVID-19. These include potential risk of bias, low patient numbers largely based on case reports, as well as heterogeneity in study design, outcomes, comparators, and patient populations. In this time of ultimate knowledge translation, a substantial portion of the literation is released as preprint without complete peer review; this highlights the need for further data to delineate these neurologic manifestations and complications secondary to SARS-CoV-2.
5. Conclusions
Significant neurologic complications are associated with COVID-19, such as impaired level of consciousness, cerebrovascular disease, encephalitis, encephalopathy, and GBS. Some of the medications utilized to treat COVID-19 also have potential neurologic effects and may interact with medications of pre-existing neurologic disease. Emergency medicine clinicians must be cognizant of these neurologic complications when treating COVID-19.
Conflicts of interest
None.
Acknowledgements
BL, RB, and MG conceived the idea for this manuscript and contributed substantially to the writing and editing of the review. This manuscript did not utilize any grants, and it has not been presented in abstract form. This clinical review has not been published, it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. This review does not reflect the views or opinions of the U.S. government, Department of Defense, U.S. Army, U.S. Air Force, or SAUSHEC EM Residency Program.
References
- 1.World Health Organization Coronavirus disease (COVID-19) situation report 102. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 2.CDC; Cases in the U.S. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 3.World Health Organization Coronavirus disease (COVID-19): situation report 105. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 4.Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3) doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sellner J., Taba P., Öztürk S., Helbok R. The need for neurologists in the care of COVID-19 patients. Eur J Neurol. 2020 doi: 10.1111/ene.14257. April. ene.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman C., Mayer K., Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009673. April. [DOI] [PubMed] [Google Scholar]
- 7.Nath A. Neurologic complications of coronavirus infections. Neurology. March 2020: 10.1212/WNL.0000000000009455. doi:. [DOI] [PubMed]
- 8.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA - J Am Med Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):1–22. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Y.H., Cai L., Cheng Z.S. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1) doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. February 2020:2020.02.10.20021832. doi: 10.1101/2020.02.10.20021832. [DOI]
- 13.Wu Y., Xu X., Chen Z. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y.R., Cao Q.D., Hong Z.S. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- a n update on the status. Mil Med Res. 2020;7(1) doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desforges M., Le Coupanec A., Dubeau P. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1) doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baig A.M. Updates on what ACS reported: emerging evidences of COVID-19 with nervous system involvement. ACS Chem Nerosci. 2020 doi: 10.1021/acschemsneuro.0c00181. April. [DOI] [PubMed] [Google Scholar]
- 17.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao L., Wang M., Chen S. February 2020. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: A retrospective case series study. medRxiv. 2020.02.22.20026500. [DOI] [Google Scholar]
- 19.Mo P., Xing Y., Xiao Y. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis March. 2020 doi: 10.1093/cid/ciaa270. [DOI] [Google Scholar]
- 20.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du R.H., Liang L.R., Yang C.Q. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020 doi: 10.1183/13993003.00524-2020. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng S.-Q., Peng H.-J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9(2):575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. April 2020:1747493020921664. doi: 10.1177/1747493020921664. [DOI] [PubMed]
- 24.Li Y., Wang M., Zhou Y. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electron J. March 2020:1–20. doi: 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González-Pinto T., Luna-Rodríguez A., Moreno-Estébanez A., Agirre-Beitia G., Rodríguez-Antigüedad A., Ruiz-Lopez M. Emergency room neurology in times of COVID-19: malignant ischemic stroke and SARS-COV2 infection. Eur J Neurol. 2020 doi: 10.1111/ene.14286. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oxley T.J., Mocco J., Majidi S. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020 doi: 10.1056/NEJMc2009787. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consoli D., Vidale S., Aguglia U. Previous infection and the risk of ischaemic stroke in Italy: the IN2 study. Eur J Neurol. 2015;22(3):514–519. doi: 10.1111/ene.12601. [DOI] [PubMed] [Google Scholar]
- 32.Siniscalchi A., Iannacchero R., Anticoli S., Romana Pezzella F., De Sarro G., Gallelli L. Anti-inflammatory strategies in stroke: a potential therapeutic target. Curr Vasc Pharmacol. 2015;14(1):98–105. doi: 10.2174/1570161113666150923111329. [DOI] [PubMed] [Google Scholar]
- 33.Siniscalchi A., Gallelli L., Malferrari G. Cerebral stroke injury: the role of cytokines and brain inflammation. J Basic Clin Physiol Pharmacol. 2014;25(2):131–137. doi: 10.1515/jbcpp-2013-0121. [DOI] [PubMed] [Google Scholar]
- 34.Khosravani H., Rajendram P., Notario L., Chapman M.G., Menon B.K. Protected code stroke: Hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke. 2020 doi: 10.1161/STROKEAHA.120.029838. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dafer R.M., Osteraas N.D., MD J.B. Acute stroke care in the Coronavirus Disease 2019 pandemic. J Stroke Cerebrovasc Dis. 2020:104881. doi: 10.1016/j.jstrokecerebrovasdis.2020.104881. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser J.F., Arthur A.S., Chen M. Society of NeuroInterventional surgery recommendations for the care of emergent neurointerventional patients in the setting of covid-19. J Neurointerv Surg. 2020 doi: 10.1136/neurintsurg-2020-016098. [DOI] [PubMed] [Google Scholar]
- 37.Ellul M., Solomon T. Acute encephalitis - diagnosis and management. Clin Med J R Coll Physicians London. 2018;18(2):155–159. doi: 10.7861/clinmedicine.18-2-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute Hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. March 2020:201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed]
- 39.Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.04.017. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi A. Imaging of acute disseminated encephalomyelitis. Neuroimaging Clin N Am. 2008;18(1):149–161. doi: 10.1016/j.nic.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Wong A., Simon E., Zimmerman R., Wang H., Toh C., Ng S. Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. Am J Neuroradiol. 2006;27(9):1919–1923. [PMC free article] [PubMed] [Google Scholar]
- 42.Alsolami A., Shiley K. Successful treatment of influenza-associated acute necrotizing encephalitis in an adult using high-dose oseltamivir and methylprednisolone: case report. Open Forum Infect Dis. 2017;4(3):145. doi: 10.1093/ofid/ofx145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das G., Mukherjee N., Ghosh S. Neurological insights of COVID-19 pandemic. ACS Chem Nerosci. 2020 doi: 10.1021/acschemneuro.0c00201. April. [DOI] [PubMed] [Google Scholar]
- 44.Sejvar J.J., Baughman A.L., Wise M., Morgan O.W. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36(2):123–133. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toscano G., Palmerini F., Ravaglia S. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 doi: 10.1056/nejmc2009191. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020 doi: 10.1016/j.jocn.2020.04.062. April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Samkari H., Berliner N. Hemophagocytic lymphohistiocytosis. Annu Rev Pathol Mech Dis. 2018;13(1):27–49. doi: 10.1146/annurev-pathol-020117-043625. [DOI] [PubMed] [Google Scholar]
- 49.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fardet L., Galicier L., Lambotte O. Development and validation of the hscore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;(0):0. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chavez S., Long B., Koyfman A., Liang S.Y. Coronavirus disease (COVID-19): a primer for emergency physicians. Am J Emerg Med. 2020;(0):0. doi: 10.1016/j.ajem.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.AbbVie Inc. KALETRA(R) Oral film coated tablets, Oral solution, Lopinavir ritonavir Oral film coated tablets, Oral solution. North Chicago, IL. 2013. www.fda.gov/medwatch
- 57.Pistell P.J., Gupta S., Knight A.G. Metabolic and neurologic consequences of chronic lopinavir/ritonavir administration to C57BL/6 mice. Antiviral Res. 2010;88(3):334–342. doi: 10.1016/j.antiviral.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sleijfer S., Bannink M., Van Gool A.R., Kruit W.H.J., Stoter G. Side effects of interferon-α therapy. Pharm World Sci. 2005;27(6):423–431. doi: 10.1007/s11096-005-1319-7. [DOI] [PubMed] [Google Scholar]
- 59.Russo M.W., Fried M.W. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124(6):1711–1719. doi: 10.1016/S0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 60.Maxwell N.M., Nevin R.L., Stahl S. Prolonged neuropsychiatric effects following management of chloroquine intoxication with psychotropic polypharmacy. Clin Case Reports. 2015;3(6):379–387. doi: 10.1002/ccr3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]