Abstract
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), represents the pandemic of the century, with approximately 3.5 million cases and 250,000 deaths worldwide as of May 2020. Although respiratory symptoms usually dominate the clinical presentation, COVID-19 is now known to also have potentially serious cardiovascular consequences, including myocardial injury, myocarditis, acute coronary syndromes, pulmonary embolism, stroke, arrhythmias, heart failure, and cardiogenic shock. The cardiac manifestations of COVID-19 might be related to the adrenergic drive, systemic inflammatory milieu and cytokine-release syndrome caused by SARS-CoV-2, direct viral infection of myocardial and endothelial cells, hypoxia due to respiratory failure, electrolytic imbalances, fluid overload, and side effects of certain COVID-19 medications. COVID-19 has profoundly reshaped usual care of both ambulatory and acute cardiac patients, by leading to the cancellation of elective procedures and by reducing the efficiency of existing pathways of urgent care, respectively. Decreased use of health care services for acute conditions by non-COVID-19 patients has also been reported and attributed to concerns about acquiring in-hospital infection. Innovative approaches that leverage modern technologies to tackle the COVID-19 pandemic have been introduced, which include telemedicine, dissemination of educational material over social media, smartphone apps for case tracking, and artificial intelligence for pandemic modelling, among others. This article provides a comprehensive overview of the pathophysiology and cardiovascular implications of COVID-19, its impact on existing pathways of care, the role of modern technologies to tackle the pandemic, and a proposal of novel management algorithms for the most common acute cardiac conditions.
Résumé
La maladie à coronavirus 2019 (COVID-19), causée par le SARS-CoV-2 (severe acute respiratory syndrome coronarivus-2 pour coronavirus du syndrome respiratoire aigu sévère 2), est la pandémie du siècle; en mai 2020, on dénombrait quelque 3,5 millions de cas et 250 000 décès dans le monde. Bien que les symptômes respiratoires dominent généralement le tableau clinique, on sait maintenant que la COVID-19 peut aussi avoir de graves conséquences sur le plan cardiovasculaire, par exemple des lésions myocardiques, des myocardites, des syndromes coronariens aigus, des embolies pulmonaires, des accidents vasculaires cérébraux, des arythmies, des insuffisances cardiaques et des chocs cardiogéniques. Les manifestations cardiaques de la COVID-19 pourraient être liées à la stimulation adrénergique, à l’inflammation généralisée et au syndrome de libération des cytokines causés par le SARS-CoV-2, à l’infection directe des cellules myocardiques et endothéliales par le virus, à l’hypoxie provoquée par l’insuffisance respiratoire, à un déséquilibre électrolytique, à une surcharge liquidienne et aux effets indésirables de certains médicaments utilisés pour traiter les symptômes de la COVID-19. En forçant l’annulation des interventions non urgentes et en réduisant l’efficacité des voies d’accès aux soins d’urgence, la COVID-19 a profondément transformé les soins usuels prodigués à tous les patients en cardiologie, qu’ils aient besoin de soins ambulatoires ou aigus. On a aussi observé une diminution de l’utilisation de services de soins de santé pour des problèmes aigus par les patients non atteints de COVID-19, une situation attribuée à la crainte de contracter le virus à l’hôpital. Des approches novatrices faisant appel aux technologies modernes ont été mises en œuvre pour pallier les restrictions imposées par la pandémie de COVID-19, entre autres : télémédecine, diffusion de matériel éducatif dans les médias sociaux, suivi des cas au moyen d’applications pour téléphone intelligent et modélisation de la pandémie grâce à l’intelligence artificielle. Les auteurs de cet article passent en revue les conséquences de la COVID-19 sur les plans physiopathologique et cardiovasculaire, ses répercussions sur les voies d’accès aux soins actuelles et le rôle des technologies modernes dans la lutte contre la pandémie, et proposent de nouveaux algorithmes de prise en charge des problèmes de santé cardiaque aigus les plus courants.
The coronavirus disease 2019 (COVID-19) is a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),1 which infected 3,524,429 patients and was linked to 247,838 deaths worldwide as of May 4, 2020.2 SARS-CoV-2 infection is triggered by binding to angiotensin-converting enzyme-2 (ACE2), which is highly expressed in the nasopharynx and lungs, as well as in the cardiovascular system and gastrointestinal and genitourinary tracts.3
Although respiratory symptoms usually dominate the clinical presentation of COVID-19, SARS-CoV-2 infection might also be responsible for a variety of potentially severe cardiovascular manifestations, particularly in patients with pre-existing cardiovascular conditions. 4, 5, 6 Indeed, subjects with cardiovascular diseases do suffer worse outcomes when infected with SARS-CoV-2.5 Moreover, COVID-19 could have an indirect impact on the delivery of cardiovascular care (both in patients with and without COVID-19) by reducing the efficiency of existing pathways (eg, primary percutaneous coronary intervention [PCI] networks and intensive care unit [ICU] bed availability)7 and through decreased use of health care services by patients because of concern about acquiring in-hospital infection.
This article provides a comprehensive overview of the pathophysiology and cardiovascular implications of COVID-19, its impact on existing pathways of care, the role of modern technologies to tackle the pandemic, and a proposal of novel management algorithms for the most common acute cardiac conditions.
Data Interpretation and Methodological Biases
We reviewed the published literature (including multiple search strategies in MEDLINE with PubMed interface) and critically assessed early reports on medRxiv (https://www.medrxiv.org/). An electronic search was executed employing the key words “cardiovascular” or “cardiac” or “heart” and “coronavirus 2019” or “COVID-19” or “SARS-CoV-2” between 2019, and May 4, 2020. No language restrictions were applied. The title, abstract, and full text of all articles captured with these search criteria were assessed. Social media (Twitter, LinkedIn, and Facebook) were also consulted.
In such times, methodologically sound research on COVID-19 and its cardiovascular manifestations is hampered by numerous challenges. These include SARS-CoV-2 test availability and accuracy, the decision of health care authorities not to screen for infection certain groups (which could lead to underestimation of the pandemic burden), heterogeneous reporting across countries, as well as the fact that estimates of the exact incidence of specific COVID-19 complications and prevalence of baseline comorbidities are often being carried out in a suboptimal fashion (eg, small single-centre cohorts and isolated reports on social media). For all these limitations, a high degree of caution and criticism should be adopted to identify selective reporting and biased data and when drawing conclusions based on small case series or anecdotal case reports.
Epidemiology and Pathophysiology of COVID-19 Infection
Epidemiology
SARS-CoV-2 is transmitted among people through respiratory droplets and fomites. The basic reproduction number (R0: the number of cases 1 infected individual can infect on average) of SARS-CoV-2 ranges from 2.2 to 3.2.8 However, to understand the full epidemic potential of SARS-CoV-2, it is necessary to take into consideration the high fraction (up to 86% of cases) of undocumented infections (asymptomatic or mild symptoms) that remains unrecognized and could expose a far greater portion of the population to the virus.9 The case fatality rate of COVID-19 widely varies across countries, ranging from 0.3% to 7.2%.10 , 11
Pathophysiology
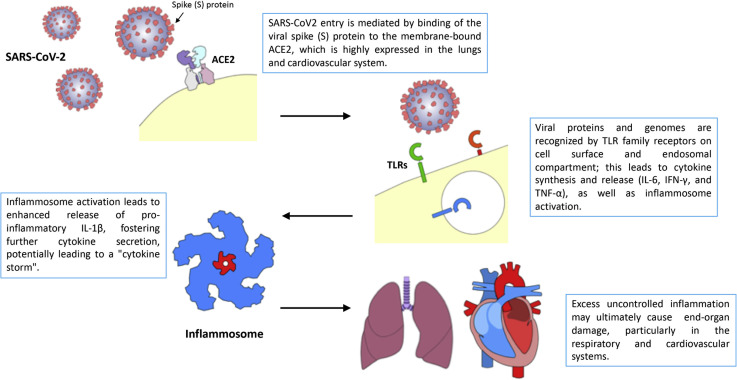
Figure 1 illustrates the pathophysiology of SARS-CoV-2 infection. Severe clinical presentations are generally associated with rapid viral replication, infiltration of inflammatory cells, and exaggerated release of cytokines (cytokine-release syndrome), leading to multiorgan damage, including an acute respiratory distress syndrome (ARDS).12 , 13 Patients requiring admission to ICUs have higher serum levels of granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, interferon-inducible protein-10, monocyte chemoattractant protein-1, macrophage inflammatory protein-1A, and tumor necrosis factor (TNF)-α, 6 further suggesting that the "intensity" of the “cytokine storm” modulates the severity of the disease. Interestingly, dramatically increased plasma interleukin (IL)-6 levels have been reported in SARS-CoV-2–infected patients with myocardial injury.6
Figure 1.
Pathophysiology of SARS-CoV-2 infection. Upon cell entry, mediated by binding of viral spike protein to angiotensin-converting enzyme-2 (ACE2), viral particles and genomes are recognized by pathogen-associated molecular pattern receptors of the Toll-like receptor (TLR) family. Upon TLR activation, cytokines and chemokines are secreted, and the inflammosome is subsequently activated, promoting an inflammatory drive. Excess inflammation is believed to underlie target organ damage in the lung and, eventually, in the cardiovascular system. IL-1β, interleukin-1β; IL-6, interleukin-6; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α.
Myocardial manifestations might be related to a stress cardiomyopathy or cytokine-related myocardial dysfunction that occurs in the setting of severe forms of COVID-19 and mimics the syndromes observed in secondary hemophagocytic lymphohistiocytosis and macrophage-activation syndrome, which are characterized by a fulminant and fatal cytokine release. 14 These unique features of COVID-19 pathophysiology might open a door for the treatment of severe forms of this infection by means of immune-response modulation therapy.
COVID-19 Clinical Presentations
The spectrum of COVID-19 infection presentations and severity widely vary among individuals6 , 15 , 16 (Table 1 ). A large study from the Chinese Center for Disease Control and Prevention revealed that among 72,314 COVID-19 patients, 81.4% had mild symptoms, whereas severe and critical presentations were only observed in 13.9% and 4.7% of cases, respectively.17 Mild presentations generally consist of symptoms common to other viral infections ("the flu-like syndrome").17 Anosmia and dysgeusia have also been reported, particularly in mild presentations.18 Severe COVID-19 may present as pneumonia, ARDS, with or without distributive and cardiogenic shock.17
Table 1.
Clinical presentation of COVID-19 infection
| Clinical symptoms |
| Common symptoms: Fever, dry cough, dyspnea, myalgias, fatigue, diarrhea, anosmia, dysgeusia. |
| Uncommon symptoms: Sputum production, headache, hemoptysis, rhinorrhea, sore throat, conjunctival injection. |
| Labs |
| Lymphopenia; prolonged prothrombin time; elevated D-dimer, alanine aminotransferase, total bilirubin and lactate dehydrogenase |
| Blood gas |
| PaO2/FiO2 < 200 if acute respiratory distress syndrome |
| Chest x-ray and computed tomography |
| Generally bilateral pneumonia with multiple infiltrates and ground-grass opacity |
| Viral confirmation |
| Real-time polymerase-chain reaction (RT-PCR) assay to detect viral RNA |
FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen.
Cardiovascular Disease and COVID-19: Vulnerability of Patients With Cardiovascular Disease
ACE2 represents a key counter regulatory enzyme of the renin-angiotensin-aldosterone system (RAAS) that degrades angiotensin II to angiotensin-(1-7), attenuating its effects on vasoconstriction, sodium retention, and fibrosis.3 After the initial engagement of ACE2 by the SARS-CoV-2 spike protein, there is subsequent downregulation of ACE2, leading to a reduction of angiotensin-(1-7),3 which triggers acute lung damage. Importantly, this injury can be attenuated in animal models by blocking the renin-angiotensin pathway with angiotensin converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs).19
Patients with underlying heart disease are among the highest-risk individuals for developing severe forms of the disease, including death.5 , 17 , 20 , 21 A meta-analysis including 1527 Chinese patients with COVID-19 reported the following prevalence of hypertension, diabetes, and cardiac and cerebrovascular disease: 17.1%, 9.7%, and 16.4%, respectively.21 Comorbidity burden has been associated with the severity of COVID-19 infection and the need for ICU admission.21 Although overall case fatality rate among 44,672 confirmed COVID-19 cases from Wuhan was 2.3%, higher rates were observed in patients with pre-existing cardiovascular disease (10.5%), diabetes (7.3%), and hypertension (6%).17 Data from Italy confirmed increased mortality rates in patients with comorbidities, particularly the older adults with pre-existent cardiovascular conditions.22
Patients with cardiovascular disease are generally older adults. Aging-related immunologic quiescence may predispose to higher rates of attack in older adults.23 Furthermore, diabetes and dyslipidemia—highly prevalent in this subset of patients—also represent markers of immunologic dysregulation having impact on COVID-19 infection susceptibility and outcomes.4 , 24 As previously reported with other viral infections—particularly influenza—COVID-19 might increase the occurrence of adverse cardiovascular events and induce acute exacerbation of chronic conditions such as ischemic heart disease or chronic heart failure.24
It has been speculated that some drugs could increase susceptibility to developing severe forms of COVID-19. Patients with pre-existing cardiovascular disease receiving ARBs or ACEIs have upregulation of ACE2, which would be therefore available in great amounts to offer a binding site for SARS-CoV-2.3 , 4 , 24 However, this concern, derived from in vitro and animal studies, has not found confirmation in clinical practice.24 Indeed, in a large multinational cohort of 8,910 COVID-19 patients, Mehra et al.25 did not observe an increase in the risk of in-hospital death associated with the use of ACEIs (2.1% vs 6.1%; odds ratio [OR], 0.33; 95% confidence interval [CI], 0.20-0.54) or ARBs (6.8% vs 5.7%; OR, 1.23; 95% CI, 0.87-1.74). Similarly, in a large population-based study from Northern Italy, the use of ACEIs and ARBs was more frequent among patients with COVID-19 than among controls because of their higher prevalence of cardiovascular disease. However, there was no evidence that such drugs affected the risk of COVID-19.26 Furthermore, Reynolds et al.27 found no substantial increase in the likelihood of a positive test for COVID-19 or in the risk of severe COVID-19 presentations in association with 5 common classes of antihypertensive drugs (ACEIs, ARBs, β-blockers, calcium-channel blockers, and thiazide diuretics). Therefore, given the balance of pros and cons of withholding such drugs, professional societies have warned against discontinuation of ACEIs and ARBs, as it could paradoxically lead to increased mortality related to the exacerbation of cardiovascular and renal conditions.4 , 24
Finally, older adults may also receive noncardiovascular medications, such as nonsteroid anti-inflammatory drugs, and may require insulin or secretagogues for diabetes mellitus. These drugs notoriously alter salt and water handling, particularly in the presence of impaired renal function, and may worsen respiratory complications, including pulmonary edema and consequent hypoxia.24
Cardiovascular Damage in COVID-19 Infection
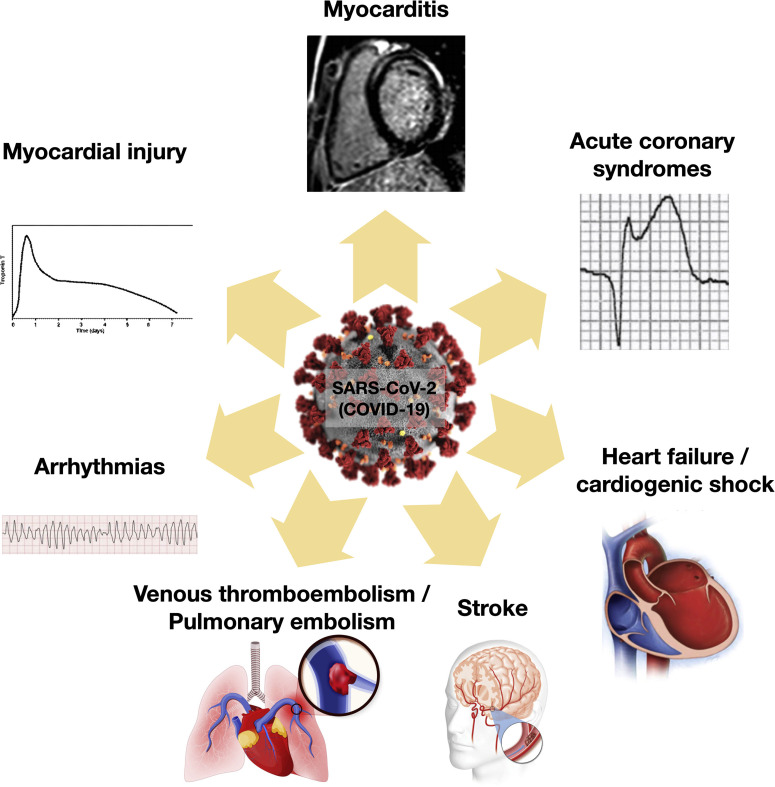
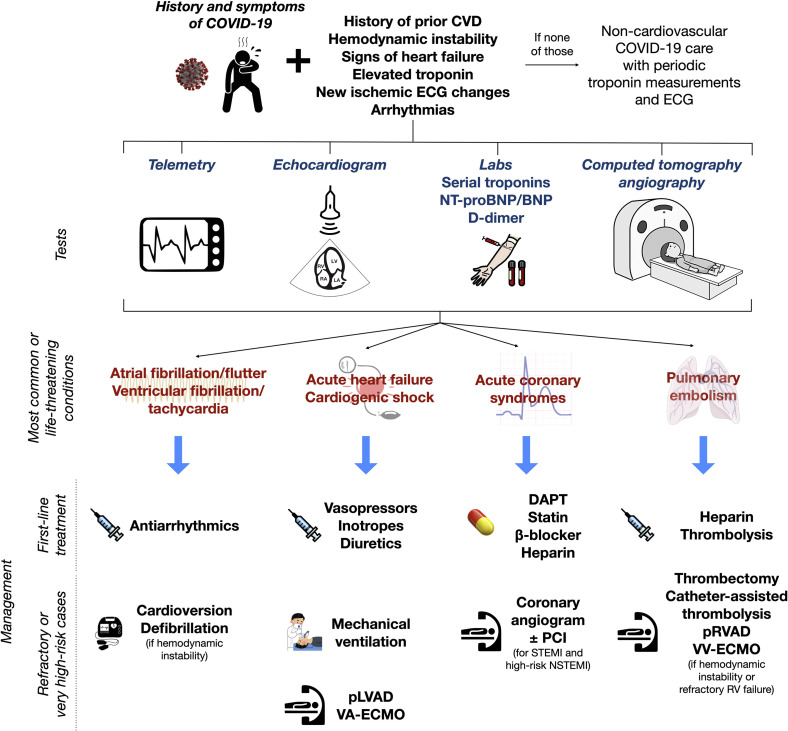
Figure 2 illustrates the different cardiovascular manifestations associated with COVID-19, and Figure 3 outlines the evaluation and management algorithms for the most common scenarios.
Figure 2.
Cardiovascular manifestations associated with COVID-19 infection.
Figure 3.
Proposed diagnostic workup and subsequent management of the most common or life-threatening cardiac manifestations of COVID-19. CVD, cardiovascular disease; DAPT, dual antiplatelet therapy; (N)STEMI, (non-)ST-segment elevation myocardial infarction; (NT-pro)BNP, (N-terminal pro-)B-type natriuretic peptide; PCI, percutaneous coronary intervention; pLVAD/pRVAD, percutaneous left-/right-ventricular assist device; VA-ECMO/VV-ECMO, venoarterial/venovenous extracorporeal membrane oxygenation.
Myocardial injury
Myocardial injury is detected by an increased troponin level and can be caused by myocardial ischemia or nonischemic myocardial injury, including myocarditis. Use of the Fourth Universal Definition of Myocardial Infarction is recommended for diagnosis.4
One possible mechanism of acute myocardial injury caused by SARS-CoV-2 infection could be its affinity to ACE2, which is widely expressed in the heart, and cause direct myocardial injury.5 , 17 Other proposed pathways include a cytokine storm triggered by an imbalanced response by type 1 and type 2 T-helper cells,5 sympathetic hyperactivity, anemia, and hypoxemic myocardial cells damage caused by respiratory dysfunction (type 2 myocardial infarction [MI]).
Chinese reports estimated that myocardial injury occurs in 7% to 20% of hospitalized COVID-19 patients.4 Myocardial injury associated with SARS-CoV-2 occurred in 5 of the first 41 COVID-19 patients (12.2%) in Wuhan, revealed by an increase in high-sensitivity cardiac troponin I levels (>28 pg/mL).6 In this study, 4 of 5 (80%) patients with myocardial injury were admitted to the ICU, which suggests the serious prognostic implications of myocardial injury in patients with COVID-19.6
In a small meta-analysis (4 studies, 341 patients), standardized mean differences of cardiac troponin I levels were significantly higher in those with severe COVID-19–related symptoms compared with those with nonsevere presentation.28 Myocardial injury, present in 19.7% of COVID-19 patients, was associated with higher levels of inflammatory biomarkers; more severe pulmonary involvement; higher need for noninvasive and invasive ventilation; and increased rates of ARDS, acute kidney injury, and coagulation disorders.29 Patients with myocardial injury were at higher risk of death.29
It is hence reasonable to perform an initial measurement of serum troponin upon admission for SARS-CoV-2 infection as well as longitudinal monitoring during hospital stay.30 This, in turn, could identify high-risk people who might be the target of advanced therapies (including immune-response modulation strategies).
Myocarditis and pericarditis
In a series reporting on 68 deaths in a cohort of 150 COVID-19 patients, 7% were attributed to myocarditis with hemodynamic collapse, whereas, in 33% of cases, myocarditis could have played a contributing role to patient death.31 Although the clinical picture is still referred to as "myocarditis" in many instances, myocardial infection by SARS-CoV-2 was not proven in most cases with COVID-19 myocardial involvement. To date, only isolated case reports provided data on the pathology of the myocardial tissue from COVID-19 patients, which preclude drawing definitive conclusions on this topic.32 , 33 Sala et al.32 reported on a 43-year-old woman with COVID-19 who presented with reverse Tako-Tsubo syndrome and mild left-ventricular systolic dysfunction. Cardiac magnetic resonance (CMR) revealed diffuse myocardial edema on the basal and mid–left-ventricular segments, with no detectable scar. Endomyocardial biopsy documented diffuse T-lymphocytic inflammatory infiltrates and huge interstitial edema without any other substantial damage. Molecular analysis showed absence of the SARS-CoV-2 genome within the myocardium.32 Tavazzi et al.33 described, for the first time, a biopsy-proven myocardial localization of viral particles in a COVID-19 patient presenting with cardiogenic shock. Although the clinical presentation suggested severe and necrotizing acute myocarditis, the pathology report demonstrated only low-grade myocardial inflammation and absence of myocyte necrosis.33 Importantly, SARS-CoV-2 was only demonstrated in interstitial cytopathic macrophages and their surroundings, whereas no viral particle was found in cardiac myocytes, which showed nonspecific damage (mainly focal myofibrillar lysis).33 Either transient viremia or infected macrophage migration from the lung might occur in COVID-19 patients with nonacute myocardial involvement. The available data seem to rule out a classic myocarditis presentation (ie, direct infection of myocardial cells by the virus), although they suggest that myocardial involvement in COVID-19 might rather be caused by the cytokine-release syndrome.
CMR availability issues, coupled with the potential for in-hospital spread of the virus related to the logistics of performing such tests, hampers the use of this valuable imaging modality. In this context, endomyocardial biopsy could provide key insights, whereas bedside echocardiography could give information on left-ventricular function.
No consensus exists on the appropriate treatment of myocarditis associated with COVID-19. In addition to mechanical circulatory support, antiviral therapy (lopinavir-ritonavir), immunoglobulin, and methylprednisolone were successfully used in the management of some cases of fulminant myocarditis.34 , 35
Pericardial effusion remains an uncommon finding in the literature, and few cases of tamponade in the setting of COVID-19 have been reported.36 , 37
Acute coronary syndromes
Kwong et al.38 previously demonstrated that patients with acute respiratory infections were at increased risk for subsequently developing acute MI after both influenza and noninfluenza viral illnesses, including other coronavirus species. COVID-19 might trigger an intense inflammatory response, which could also increase the risk of coronary plaque rupture.
The actual prevalence of acute coronary syndromes (ACS) in the setting of COVID-19 infection is unknown, given the gaps in testing witnessed in numerous countries in the early phases of the pandemic, particularly in the absence of typical symptoms suggesting COVID-19 infection.
In 28 Italian patients with ST-elevation myocardial infarction (STEMI) and COVID-19, Stefanini et al.39 reported that STEMI represented the first clinical manifestation of COVID-19 in the majority of cases (85.7%). Early mortality was 39.3%. Of note, angiography demonstrated the absence of obstructive coronary artery disease (CAD) in 39.3% of cases, a finding also reported in the United States by Bangalore et al.,40 who found nonobstructive disease in one-third of the patients who underwent coronary angiography. In this latter series, the prognosis of STEMI presentation was even worse than in the previous report, with a 72% rate of in-hospital mortality.
In addition to type 2 MI, “myocarditis” and stress cardiomyopathy, microvascular thrombosis has also been hypothesized as a mechanism underlying certain cases mimicking presentation of STEMI without obstructive CAD, given the endothelial dysfunction and hypercoagulable state associated with COVID-19.
Arrhythmias
In 138 hospitalized COVID-19 patients, cardiac arrhythmias represented the leading complication (19.6%) and were more common in patients requiring ICU admission (44.4% vs 6.9%).1 Guo et al.41 revealed that, in 187 COVID-19–positive patients stratified by the level of troponin, malignant ventricular arrhythmias was twice more frequent in the presence of elevated troponin levels (11.5% vs 5.2%). Ventricular arrhythmias might also represent the first clinical manifestation of SARS-CoV-2 infection.
In 136 COVID-19 patients who experienced in-hospital cardiac arrest, Shao et al.42 revealed that the most common initial rhythm was asystole in 89.7% of cases. Pulseless electrical activity was found in 4.4%, whereas a shockable rhythm was identified in only 5.9% of patients.
In the context of lockdown and overwhelmed health care systems unable to provide timely emergency responses, sudden cardiac death has been reported in patients with initially mild symptoms who were later found dead at home while in quarantine.16 In 4 Italian provinces, Baldi et al.43 reported an increase of 58% in out-of-hospital cardiac arrest during the 40 days of the COVID-19 outbreak in comparison with the same period in 2019. Moreover, the cumulative incidence of out-of-hospital cardiac arrest in 2020 was strongly associated with the cumulative incidence of COVID-19, and the increase in the number of cases over the number in 2019 followed the time course of the COVID-19 outbreak.43
In the setting of COVID-19, arrhythmias might be caused by the following mechanisms: (1) direct viral damage to myocardial cells and/or conduction system; (2) worsening of pre-existing myocardial conditions or conduction disturbances; (3) electrolytic derangements; (4) adrenergic stress leading to electrical instability; and (5) ACS with ongoing ischemia.
The high-grade systemic inflammatory state characteristic of COVID-19 represents another potential important proarrhythmic factor that should not be underestimated.44 Indeed, strong evidence points to inflammation as a novel risk factor for long QT-syndrome and torsades de pointes, primarily via direct electrophysiological effects of cytokines—particularly, IL-1, IL-6, and TNF-α—on the myocardium by modulating the expression and/or function of several cardiomyocyte ion (K+ and Ca++) channels.44
Finally, some therapies proposed for treatment of COVID-19 have been reported to cause arrhythmias45 (see following text and Supplemental Table S1).
Heart failure and cardiogenic shock
Concomitant heart failure was present in 23% to 49% of patients infected with COVID-19.46 , 47 Notably, it was associated with worse prognosis as it was almost 5 times more common in patients who did not survive the hospitalization (51.9% vs 11.7%).46 Similar to troponin, elevation of B-type natriuretic peptides (BNP/NT-proBNP) is associated with an unfavourable course among patients with ARDS.31
In the setting of COVID-19, heart failure could be attributable to either the exacerbation of underlying cardiovascular disease or the new onset of cardiomyopathy (particularly myocarditis or stress cardiomyopathy). Isolated right-heart failure can be observed in presence of pulmonary hypertension in the setting of severe ARDS or pulmonary embolism.
Moreover, older adults with cardiovascular disease often have left-ventricular hypertrophy and diastolic dysfunction. Thus, these patients may be prone to develop pulmonary edema when they are given copious amounts of intravenous fluids to maintain blood pressure or as a vehicle for parenteral drug infusion.24 However, pulmonary edema as observed in the setting of COVID-19 could also represent the manifestation of a pulmonary vascular injury, which might be direct or mediated by the excess of local angiotensin-2, inducing severe vasoconstriction and microvascular dysfunction and activating inflammation process.24
In the context of COVID-19, shock mechanisms could be either exclusively distributive (septic) or mixed (distributive and cardiogenic). Cardiogenic shock might be prominent in case of fulminant myocardial involvement. BNP and transthoracic echocardiogram are valuable to guide treatment.24 Right-heart catheterization can also be useful in case of discrepancy of other tests but at the expense of a higher risk of in-hospital spread of the virus.4
In patients not responding to conventional management, it is important to determine whether a concomitant cardiogenic component is present, particularly when considering mechanical respiratory and circulatory support with extracorporeal membrane oxygenation (ECMO), as this may have impact on selection of devices (venovenous vs venoarterial)48 (Fig. 3). Initial data in the setting of ARDS due to COVID-19 showed that the prognosis remained poor even with use of ECMO (mortality rate: 82.3%).49 In 12 critically ill COVID-19 patients requiring ECMO, Zeng et al.50 found that nearly half of them died of septic shock and multiorgan failure. Duration of ECMO support ranged from 3 to 28 days.50 A pooled analysis of early reports including 234 ARDS patients revealed that only 17 (7.2%) received ECMO.51 The mortality rate was 94.1% in patients who received ECMO and 70.9% in patients on conventional therapy.51 The pooled effect of ECMO vs conventional therapy on mortality was neutral (OR, 2.00, 95% CI, 0.49-8.16).51 The Extracorporeal Life Support Organization (ELSO) recommends use of ECMO only in expert centres for patients with severe ARDS (PaO2/FiO2 < 100) after collegial discussion on a case-by-case basis.52 ECMO can be considered futile, and the patient can be returned to conventional management, if no lung or cardiac recovery is observed after 21 days.52
Venous thromboembolism and pulmonary embolism
COVID-19 patients may be at increased risk of venous thromboembolism (VTE). In addition to prolonged immobilization, endothelial damage and vascular inflammation contribute to the development of a hypercoagulable state. In a multicentre Chinese study,53 elevated D-dimer levels (> 1 g/L) were independent predictors of in-hospital death. D-dimer and fibrin degradation products levels were significantly higher in nonsurvivors. Furthermore, disseminated intravascular coagulation was reported in 71.4% of patients who subsequently died.
In a study of 184 patients with severe COVID-19 from 3 centres in the Netherlands, 31% of patients developed VTE despite pharmacologic prophylaxis.54 Poissy et al.55 reported a cumulative incidence of pulmonary embolism (PE) of 20.4% (95% CI, 13.1%-28.7%) in a cohort of COVID-19 patients in critical conditions. In 90.1% of cases, PE occurred in patients already receiving prophylactic antithrombotic treatment. The incidence of PE in this cohort was significantly higher than that observed before the pandemic in patients with different conditions of similar severity or influenza infection. Therefore, in case of hemodynamic or respiratory deterioration in a patient with stable parenchymal pulmonary involvement, PE should be suspected.
The optimal thromboprophylactic regimen for patients hospitalized with COVID-19 remains unknown. Low-molecular-weight or unfractionated heparin, with or without mechanical compression stocking, can be considered according to the patient-risk profile.56
Several risk-stratification tools can be used for VTE risk assessment in this setting (eg, the Caprini, IMPROVE, and Padua models).56 Therapeutic anticoagulation, even in absence of obvious VTE complication, could be considered in severely ill patients with high D-dimer, although, at present, this recommendation is not substantiated by solid data.56 Systemic thrombolysis or catheter-based interventions might be considered in patients with massive PE and hemodynamic compromise. Percutaneous right-ventricular assist devices or venovenous ECMO might be contemplated in young patients with right-ventricular failure57 , 58 (Fig. 3).
Stroke
Ischemic stroke has been recognized as a complication of severe forms of COVID-19,59 which is thought to be associated with the highly prothrombotic state and major endothelial dysfunction caused by the infection. Beyrouti et al.60 reported a case series of 6 COVID-19 patients with stroke; large-vessel occlusion with markedly elevated D-dimer levels (≥1000 μg/L) were observed in all patients. Three patients had multiterritory infarcts; 2 had concurrent VTE; and, in 2, ischemic strokes occurred despite therapeutic anticoagulation.60 Similarly, Oxley et al.61 reported on 5 young COVID-19 patients (aged from 33 to 49 years) who presented with large-vessel ischemic stroke. Such an association between large-vessel stroke and COVID-19 in young patients requires further investigation. Therapeutic anticoagulation and thrombolysis could be beneficial to mitigate the consequences of stroke in this setting but must be balanced against the risk of hemorrhagic transformation, particularly considering the critical conditions of these patients and the delays in diagnosis because of sedation and other concomitant acute issues.
Redefining Priorities in Cardiovascular Care
The recent emergence of COVID-19 and the psychosocial aspects related to the pandemic have put health care systems under pressure, with an increasing number of infected patients, as well as shortage of personal protective equipment (PPE) among health care providers, ventilators, and certain drugs.
The following recommendations are proposals to inform clinical decision making among health care providers dealing with cardiovascular patients in the setting of the COVID-19 pandemic. These considerations are dynamic and might change in the coming weeks.
Emergency and elective procedures
The escalation of this health crisis has led to cancellation of elective cardiovascular procedures and outpatient visits owing to the concern of disease transmission among health care providers and other patients as well as to optimize resource allocation. Telemedicine is recommended for triage, management of nonemergent conditions, and renewal of prescriptions for patients with chronic stable disease.4 Any nonessential diagnostic investigation should be deferred. At the same time, it is important to continue guaranteeing care to cardiovascular emergencies and to perform such nondeferrable procedures after collegial discussion on a case-by-case basis.4 , 62 , 63
The optimal management strategy for patients with ACS has generated controversy during this pandemic period4 , 62 , 63 (Fig. 3). In patients with STEMI, Chinese providers have developed algorithms that emphasize rapid testing for COVID-19 infection and immediate fibrinolysis, as, in their health care system, the logistics of primary PCI have been severely disrupted by the critical need to protect health care providers from contagion.62 , 64 The Canadian Association of Interventional Cardiology instead recommends primary PCI for low COVID-19 probability in the absence of major logistic restrictions, whereas fibrinolysis could be considered in moderate to high probability or COVID-19–positive patients with STEMI, particularly in the presence of staff or resource limitations.65 PCI in catheterization laboratories with aerosol-level PPE should also be considered in case of fibrinolysis contraindication or failure or in cases of cardiogenic shock caused by ACS.65 Even in cases of successful initial fibrinolysis, planned PCI should be performed within 24 hours.65
For patients with non–ST-elevation ACS (NSTE-ACS), medical therapy should be pursued initially. Revascularization should be considered in case of refractory chest pain, malignant arrhythmia, and hemodynamic instability, with rapid discharge following revascularization.62 , 63 , 65
Efforts should be made to differentiate between type 1 and type 2 MI and other causes of troponin rise.62 , 63 , 65 Diagnostic and interventional procedures should be deferred in patients with type 2 MI, myocardial injury, and patients with suspected myocarditis without cardiogenic shock. Coronary computed tomography angiography—performed at the time of lung evaluation in cases with severe respiratory involvement—might be useful to rule out CAD.
Routine elective cardiac surgery procedures should be rescheduled, whereas patients at high risk for short-term adverse cardiac events should still be offered surgical treatment.4 , 66 Given ICU bed shortages and the risk for in-hospital contagion, medical management as a bridge to intervention after the pandemic should also be considered for selected patients. The Canadian Society of Cardiac Surgeons has issued detailed recommendations regarding elective and emergency procedures.66
As the initial peak of the COVID-19 pandemic starts to slowly abate in May 2020, professional cardiovascular societies indicated an ethical and logistic framework for the gradual reintroduction of invasive cardiovascular procedures and diagnostic tests.67 Patients with untreated cardiovascular disease are at increased risk of adverse outcomes, and efforts should be made to reestablish previous pathways of care while simultaneously minimizing the risk of COVID-19 resurgence.
How COVID-19 may affect delivery of cardiovascular care to noninfected patients
Little is known about the indirect impact of COVID-19 on cardiovascular outcomes of noninfected persons. US investigators reported a 38% decrease in cardiac catheterization laboratory STEMI activations.68 which is similar to reductions in PCI rates for ACS observed in Italy (32%) and Spain (40%).69 , 70 Emergency medical services from Hong Kong reported a mean increase of ∼4 hours in time from symptom onset to first medical contact for patients with STEMI,7 presumably because of patient hesitancy in seeking care because of concerns about possible in-hospital COVID-19 contagion. Such delays might have a detrimental impact on prognosis.71 Previously uncommon mechanical complications of MI are now being frequently observed, which could be again caused by delays in seeking care.71 Even more worrisome is the 58% increase in out-of-hospital cardiac arrest observed by Italian investigators during the pandemic, which could reflect the extreme consequences of such behavior of medical-care avoidance.43 Finally, further in-hospital delays can be expected among patients admitted to the hospital, which are related to COVID-19 infection testing and implementation of precautions for health care providers.7
Safety considerations for health care workers
Controlling exposures to occupational infections is fundamental to protect health care providers. Workers who enter the room of patients with known or suspected COVID-19 should adhere to standard precautions and use masks, face shields, gowns, gloves, and eye protection. When performing (or present for) an aerosol-generating procedure (AGP), the provider should wear an N95 mask and a face shield or a powered air-purifying respirator (PAPR). Additional control measures include limiting the number of health care providers to only those essential for patient care during the procedure and performing AGPs in an airborne infection isolation rooms (with negative air pressure). Considerations to prevent infection among cardiovascular providers are summarized in Table 2 . (See Supplementary Material for Ethical Issues and Challenges.)
Table 2.
Safety considerations for cardiovascular health care providers
| General precautions | COVID-19 testing
|
| Cardiopulmonary resuscitation |
|
| Catheterization laboratory environment |
|
| Specific consideration to subspecialty care teams |
|
PAPR, powered air-purifying respirator.
COVID-19 Treatment and Cardiovascular Implications
The COVID-19 pandemic has placed the scientific community under extraordinary pressure. The urgent need for a safe and effective treatment can also lead to less rigour and respect of essential research standards as well as relaxed generation and interpretation of data, which can have undesirable downstream impact.72
At least 30 clinical trials are currently being conducted to test the efficacy and safety of COVID-19 therapies. Broadly speaking, there are 3 therapeutic approaches (Supplemental Fig. S1): antiviral drugs, immune response modulators, and RAAS blockers.73 A list of the currently investigated drugs, their cardiovascular side effects, and potential interactions with cardiovascular medication are detailed in Supplemental Table S1. In this section, we focus exclusively on the cardiovascular side effects and drug interactions of commonly used anti-COVID-19 medications.
Antiviral drugs
Certain antivirals have shown an interaction with several cardiovascular medications. This is particularly true with lopinavir-ritonavir (protease inhibitors). Ritonavir is a potent inhibitor of cytochrome P450 (CYP3A). It can increase plasma concentration of class IA and III antiarrhythmics, potentially triggering severe ventricular arrhythmias through QTc prolongation.74 Similarly, clopidogrel active metabolite concentrations can be reduced, requiring platelet function monitoring to avoid excessive thrombotic risk or switching to prasugrel. Conversely, ticagrelor concentration increases with CYP3A inhibition. With regard to anticoagulants, most direct Xa and IIa inhibitors are also metabolized by CYP3A, and thus there is a potential increase in bleeding risk with ritonavir.75 , 76 Besides interactions, lopinavir-ritonavir association can directly cause hypotension and atrioventricular conduction disturbances.
Favipiravir is a novel antiviral drug that directly inhibits RNA-dependent-RNA-polymerase. It can prolong the QTc interval, increasing the risk for malignant ventricular arrhythmias.77
Immune response modulators
Chloroquine and hydroxychloroquine (CQ/HCQ) are well known to prolong the QTc interval, which is particularly concerning in COVID-19 patients.45 These patients are often hospitalized in critical conditions and, consequently, predisposed to electrolyte derangements (eg, hypokalemia), which can act synergistically with CQ/HCQ to prolong the QTc interval, thus triggering ventricular arrhythmias (torsade de pointes). Hypotension and atrioventricular conduction disturbances have also been reported with CQ/HCQ.
Azithromycin is an antibiotic of the macrolide family. Like CQ/HCQ, azithromycin prolongs the QTc interval. Hence, the frequent association of CQ/HCQ and azithromycin used in COVID-19 patients could increase the risk of torsade de pointes and therefore requires close electrocardiographic monitoring.45 Azithromycin can increase plasma concentration of edoxaban and, to a lesser extent, rivaroxaban.78 It may also increase international normalized ratio (INR) in patients on warfarin.79
Methylprednisolone and corticosteroids in general can increase blood pressure and increase fluid retention. They have also been associated with supratherapeutic INR values of patients on warfarin.80
RAAS blockers
Losartan is currently tested in RAAS blocker-naïve COVID-19 patients requiring or not requiring hospitalization (NCT04312009 and NCT04311177, respectively), with the rationale that ACE2 downregulation following pulmonary involvement results in angiotensin-2 upregulation (angiotensin-2 being a substrate of ACE2), which, in turn, is assumed to contribute to ARDS pathophysiology.3
Following the same rationale, another trial is testing the injection of recombinant soluble ACE2, functioning as a decoy receptor for SARS-CoV-2 virus in patients with evidence of COVID-19 viral pneumonia (NCT04287686). The cardiovascular effects of losartan are well known by cardiovascular clinicians, but the effects of recombinant ACE2 are unknown.
Innovative Approaches to Tackling Cardiovascular Disease During the COVID-19 Pandemic
Information technology has the potential to provide useful tools to help tackling the COVID-19 pandemic: specifically, to facilitate the early detection of cases (hence, optimizing patient workflow within health care systems), to disseminate key educational material to the general population and among health care providers, and to assist in the diagnosis of specific clinical features.81
Social media
Social media has recently become a vehicle for fast and massive dissemination of medical content among health care professionals. These platforms have been used to share protocols to optimize the care of COVID-19 patients and to disseminate information regarding optimal use of PPE to prevent in-hospital dissemination of the virus. Moreover, reports of novel manifestations of COVID-19 infection are actively being shared on social media; for example, the hashtag “#COVIDSTEMI” has recently been used on Twitter by many cardiologists to share cases of patients presenting with electrocardiographic and echocardiographic signs of MI, who were subsequently found to have no evidence of obstructive coronary artery disease. This initiative is facilitating the education and awareness of countless health care professionals on this new clinical entity and the organization of these data in peer-reviewed publications. Other initiatives include promoting the scientific debate on the role of RAAS blockers in COVID-19 patients and increasing the awareness of the inherent risk of QTc prolongation of CQ/HCQ and azithromycin early during the pandemic.81
Smartphone apps
The government of South Korea has been promoting COVID-19 case tracking and information sharing through smartphone apps early in the course of the pandemic, and this has been considered one of the reasons for the successful containment of the virus in such countries. An application under development would let people log their movements and compare them with those of known COVID-19 patients, using data supplied by health authorities to identify potential transmissions.82 Another team of researchers is working on a system, based on short-range Bluetooth signals, that allows automated tracing by public health authorities while preserving patient confidentiality.83
Artificial intelligence
The potential for artificial intelligence (AI) to assist in pandemic modeling and in the diagnosis of COVID-19 clinical manifestations is immense. Although epidemiologists and public health officials cannot be replaced, AI can serve to gather and systematically organize rapidly evolving information (eg, from social media and news media) to assist officials in decision-making.84 Moreover, AI is particularly suited for massive analysis of imaging data, which, at the moment, has been taken advantage of for the diagnosis of COVID-19 pneumonitis85 but is also potentially suitable for cardiac applications such as automated interpretation of electrocardiograms, imaging, and pathology specimens.
Conclusions
The COVID-19 pandemic represents the most important public health crisis of the century. The health, economic, and societal impacts will be felt for many years to come. Patients with cardiovascular disease are particularly vulnerable to COVID-19 and often develop severe forms of the infection. Several cardiovascular manifestations have been observed in COVID-19 patients, including myocardial injury, myocarditis, ACS, pulmonary embolism, stroke, arrhythmias, heart failure, and cardiogenic shock. COVID-19 has profoundly reshaped usual pathways of care of both elective and acute cardiac patients. Further research is needed to define the pathophysiology of COVID-19 precisely and to identify and properly evaluate drugs targeting the most relevant mechanisms that characterize severe manifestations of the disease. Collaborative initiatives should harness both conventional and novel tools to provide an effective and timely response to the COVID-19 pandemic on the global stage.
Acknowledgements
This article is dedicated to all health care professionals who have fallen fighting during the COVID-19 tragedy.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 1078 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjca.2020.05.018.
Supplementary Material
References
- 1.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University COVID-19 Resource Center. www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread Available at.
- 3.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tam C.C.F., Cheung K.-S., Lam S., et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13:e006631. doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Guan X., Wu P., et al. Early Transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R., Pei S., Chen B., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 11.Rajgor D.D., Lee M.H., Archuleta S., Bagdasarian N., Quek S.C. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020:S1473–S3099. doi: 10.1016/S1473-3099(20)30244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C., Zhou Y., Wang D.W. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020;45:230–232. doi: 10.1007/s00059-020-04909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region: case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z., McGoogan J.M. Characteristics of and important lessons rrom the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 18.Russell B., Moss C., Rigg A., Hopkins C., Papa S., Van Hemelrijck M. Anosmia and ageusia are emerging as symptoms in patients with COVID-19: What does the current evidence say? Ecancermedicalscience. 2020;14:ed98. doi: 10.3332/ecancer.2020.ed98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. JAMA. 2020;323:1499–1500. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 21.Li B., Yang J., Zhao F., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porcheddu R., Serra C., Kelvin D., Kelvin N., Rubino S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J Infect Dev Countries. 2020;14:125–128. doi: 10.3855/jidc.12600. [DOI] [PubMed] [Google Scholar]
- 23.Liu W.M., van der Zeijst B.A.M., Boog C.J.P., Soethout E.C. Aging and impaired immunity to influenza viruses: implications for vaccine development. Hum Vaccin. 2011;7:94–98. doi: 10.4161/hv.7.0.14568. [DOI] [PubMed] [Google Scholar]
- 24.Mehra M.R., Ruschitzka F. COVID-19 illness and heart failure. JACC Heart Fail. 2020;8:512–514. doi: 10.1016/j.jchf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds H.R., Adhikari S., Pulgarin C., et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis [epub ahead of print] Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China [epub ahead of print] JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman A.R., Bularga A., Mills N.L. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation. 2020;141:1733–1735. doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 31.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sala S., Peretto G., Gramegna M., et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavazzi G., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng J.H., Liu Y.X., Yuan J., et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights [epub ahead of print] Infection. 2020 doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) [epub ahead of print] JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua A., O’Gallagher K., Sado D., Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41:2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dabbagh M.F., Aurora L., D’Souza P., Weinmann A.J., Bhargava P., Basir M.B. Cardiac tamponade secondary to COVID-19 [epub ahead of print] JACC Case Reports. 2020 doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:2538–2541. doi: 10.1056/NEJMc1805679. [DOI] [PubMed] [Google Scholar]
- 39.Stefanini G.G., Montorfano M., Trabattoni D., et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19: a case series. N Engl J Med. 2020 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao F., Xu S., Ma X., et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldi E., Sechi G.M., Mare C., et al. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med. 2020 doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk and inflammation: mind the gap! [epub ahead of print] Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 45.Sapp J.L., Alqarawi W., MacIntyre C.J., et al. Guidance on minimizing risk of drug-induced ventricular arrhythmia during treatment of COVID-19: a statement from the Canadian Heart Rhythm Society. Can J Cardiol. 2020;36:948–951. doi: 10.1016/j.cjca.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;26 doi: 10.1136/bmj.m1091. 368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacLaren G., Fisher D., Brodie D. Preparing for the most critically ill patients with COVID-19. JAMA. 2020;323:1245. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 49.Ñamendys-Silva S.A. ECMO for ARDS due to COVID-19. Heart Lung. 2020;49:348–349. doi: 10.1016/j.hrtlng.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng Y., Cai Z., Xianyu Y., Yang B.X., Song T., Yan Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: a retrospective case series. Crit Care. 2020;24:148. doi: 10.1186/s13054-020-2840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henry B.M., Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–28. doi: 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartlett R.H., Ogino M.T., Brodie D., et al. Initial ELSO guidance document. ASAIO J. 2020;66:472–474. doi: 10.1097/MAT.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence [epub ahead of print] Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 56.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shokr M., Rashed A., Mostafa A., et al. Impella RP Support and catheter-directed thrombolysis to treat right ventricular failure caused by pulmonary embolism in 2 patients. Texas Heart Inst J. 2018;45:182–185. doi: 10.14503/THIJ-17-6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giraud R., Banfi C., Siegenthaler N., Bendjelid K. Massive pulmonary embolism leading to cardiac arrest: one pathology, two different ECMO modes to assist patients. J Clin Monit Comput. 2016;30:933–937. doi: 10.1007/s10877-015-9796-2. [DOI] [PubMed] [Google Scholar]
- 59.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beyrouti R., Adams M.E., Benjamin L., et al. Characteristics of ischaemic stroke associated with COVID-19 [epub ahead of print] J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welt F.G.P., Shah P.B., Aronow H.D., et al. Catheterization laboratory considerations during the coronavirus (Covid-19) pandemic: from ACC’s Interventional Council and SCAI. J Am Coll Cardiol. 2020;75:2372–2375. doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han Y., Zeng H., Jiang H., et al. CSC expert consensus on principles of clinical management of patients with severe emergent cardiovascular diseases during the COVID-19 epidemic. Circulation. 2020;141:e810–e816. doi: 10.1161/CIRCULATIONAHA.120.047011. [DOI] [PubMed] [Google Scholar]
- 64.Jing Z.C., Zhu H.D., Yan X.W., Chai W.Z., Zhang S. Recommendations from the Peking Union Medical College Hospital for the management of acute myocardial infarction during the COVID-19 outbreak. Eur Heart J. 2020;41:1791–1794. doi: 10.1093/eurheartj/ehaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wood D.A., Sathananthan J., Gin K., et al. Precautions and procedures for coronary and structural cardiac interventions during the COVID-19 pandemic: guidance from Canadian Association of Interventional Cardiology. Can J Cardiol. 2020;36:780–783. doi: 10.1016/j.cjca.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hassan A., Arora R.C., Adams C., et al. Cardiac surgery in Canada during the COVID-19 pandemic: a guidance statement from the Canadian Society of Cardiac Surgeons. Can J Cardiol. 2020;36:952–955. doi: 10.1016/j.cjca.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood D.A., Mahmud E., Thourani V.H., et al. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: from North American Society Leadership. Can J Cardiol. 2020;36:971–976. doi: 10.1016/j.cjca.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia S., Albaghdadi M.S., Meraj P.M., et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piccolo R., Bruzzese D., Mauro C., et al. Population trends in rates of percutaneous coronary revascularization for acute coronary syndromes associated with the COVID-19 outbreak. Circulation. 2020;141:2035–2037. doi: 10.1161/CIRCULATIONAHA.120.047457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodríguez-Leor O., Cid-Álvarez B., Ojeda S., et al. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en España. REC Interv Cardiol. 2020;2:82–89. [Google Scholar]
- 71.Moroni F., Gramegna M., Ajello S., et al. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic [Epub ahead of print] JACC Case Reports. 2020 doi: 10.1016/j.jaccas.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim A.H.J., Sparks J.A., Liew J.W., et al. A Rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med. 2020;172:819–821. doi: 10.7326/M20-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19) [Epub ahead of print] JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 74.AbbVie Full Prescribing Information for NORVIR tablets, oral solution and oral powder. https://www.rxabbvie.com/pdf/norpi2a.pdf Available at.
- 75.Pfizer Canada ULC B-MSCC PRODUCT MONOGRAPH ELIQUIS® apixaban tablets 2.5 mg and 5 mg. https://www.pfizer.ca/sites/default/files/201910/ELIQUIS_PM_229267_07Oct2019_Marketed_E.pdf Available at.
- 76.Williams D., Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41:343–370. doi: 10.2165/00003088-200241050-00003. [DOI] [PubMed] [Google Scholar]
- 77.Chinello P., Petrosillo N., Pittalis S., Biava G., Ippolito G., Nicastri E. QTc interval prolongation during favipiravir therapy in an ebolavirus-infected patient. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parasrampuria D.A., Mendell J., Shi M., Matsushima N., Zahir H., Truitt K. Edoxaban drug-drug interactions with ketoconazole, erythromycin, and cyclosporine. Br J Clin Pharmacol. 2016;82:1591–1600. doi: 10.1111/bcp.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hopkins S. Clinical toleration and safety of azithromycin. Am J Med. 1991;91:S40–S45. doi: 10.1016/0002-9343(91)90401-i. [DOI] [PubMed] [Google Scholar]
- 80.Hazlewood K.A., Fugate S.E., Harrison D.L. Effect of oral corticosteroids on chronic warfarin therapy. Ann Pharmacother. 2006;40:2101–2106. doi: 10.1345/aph.1H418. [DOI] [PubMed] [Google Scholar]
- 81.Vervoort D., Ma X., Luc J.G.Y., Zieroth S. Rapid scholarly dissemination and cardiovascular community engagement to combat the infodemic of the COVID-19 pandemic. Can J Cardiol. 2020;36:969.e1–969.e2. doi: 10.1016/j.cjca.2020.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knight W. Phones could track the spread of Covid-19. Is it a good idea? WIRED 2020. 2020 https://www.wired.com/story/phones-track-spread-covid19-good-idea/ [Google Scholar]
- 83.Foy K. MIT News; 2020. Bluetooth signals from your smartphone could automate Covid-19 contact tracing while preserving privacy.http://news.mit.edu/2020/bluetooth-covid-19-contact-tracing-0409 [Google Scholar]
- 84.Long J.B., Ehrenfeld J.M. The role of augmented intelligence (AI) in detecting and preventing the spread of novel coronavirus. J Med Syst. 2020;44:59. doi: 10.1007/s10916-020-1536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neri E., Miele V., Coppola F., Grassi R. Use of CT and artificial intelligence in suspected or COVID-19 positive patients: statement of the Italian Society of Medical and Interventional Radiology. Radiol Med. 2020;125:505–508. doi: 10.1007/s11547-020-01197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.