Significance
Ballistic tongue projection in lungless salamanders displays both extreme performance and thermal robustness, with the power of projection far exceeding the available muscle power even at low body temperatures. Our comparative analysis reveals that relatively minor changes in the musculoskeletal morphology of the tongue apparatus and in the timing of muscle activation have, through evolutionary time, transformed a muscle-powered system with modest performance and high thermal sensitivity into a spring-powered system with extreme performance and thermal robustness, in parallel in both major groups of this largest family of salamanders. High performance and thermal robustness evolve together, indicating they are both properties of the same elastic-recoil, “bow-and-arrow” mechanism. Similar evolutionary patterns may be found in other ectothermic animals with extreme performance.
Keywords: amphibian, biomechanics, evolution, feeding, temperature
Abstract
The evolution of ballistic tongue projection in plethodontid salamanders—a high-performance and thermally robust musculoskeletal system—is ideal for examining how the components required for extreme performance in animal movement are assembled in evolution. Our comparative data on whole-organism performance measured across a range of temperatures and the musculoskeletal morphology of the tongue apparatus were examined in a phylogenetic framework and combined with data on muscle contractile physiology and neural control. Our analysis reveals that relatively minor evolutionary changes in morphology and neural control have transformed a muscle-powered system with modest performance and high thermal sensitivity into a spring-powered system with extreme performance and functional robustness in the face of evolutionarily conserved muscle contractile physiology. Furthermore, these changes have occurred in parallel in both major clades of this largest family of salamanders. We also find that high-performance tongue projection that exceeds available muscle power and thermal robustness of performance coevolve, both being emergent properties of the same elastic-recoil mechanism. Among the taxa examined, we find muscle-powered and fully fledged elastic systems with enormous performance differences, but no intermediate forms, suggesting that incipient elastic mechanisms do not persist in evolutionary time. A growing body of data from other elastic systems suggests that similar coevolution of traits may be found in other ectothermic animals with high performance, particularly those for which thermoregulation is challenging or ecologically costly.
Elastic mechanisms that improve performance have evolved repeatedly in animals (1, 2), powering spectacularly athletic movements, such as the jumping of frogs and insects (3, 4), the striking of mantis shrimp and trap-jaw ants (5, 6), and the ballistic tongue projection of chameleons, frogs, and salamanders (7–10). These spring-powered actions are among the most dynamic of all animal movements, with accelerations reaching hundreds of Gs and power exceeding the capabilities of the muscles involved by orders of magnitude (2, 3, 7, 9, 11–13). Beyond their raw power, elastic mechanisms provide many benefits, including increased economy of movement, greater functional range of the involved muscles, protection against muscle damage, and increased muscle work (1, 14–19). In addition, elastic mechanisms allow animals to maintain high performance at low body temperatures (9, 10, 20, 21), which ectotherms often experience. This thermal robustness is in stark contrast to the thermal sensitivity of muscle-powered movements that suffer performance declines at low temperature (22–30). The ability of ectothermic animals to move rapidly to escape predators or capture food at a range of environmental conditions has clear ecological importance, yet the evolution of the derived mechanisms that enable such performance has been examined in only a few groups of animals (6, 31–33). Here we present a comparative evolutionary analysis of tongue projection in lungless salamanders (Plethodontidae) that reveals a pattern of repeated, parallel evolution of an elastic mechanism of extreme performance and thermal robustness. We show that the evolution of this elastic system occurred via relatively minor, coordinated changes in the morphology of the tongue apparatus coevolving with simple shifts in the timing of muscle activity. Given the relatively subtle changes required to produce dramatic performance benefits, we expect that similar evolutionary transitions in morphology and neural control can be found in other groups of ectothermic animals in which elastic-recoil mechanisms enhance performance and improve functional robustness.
Plethodontid salamanders have repeatedly evolved spring-powered tongue projection with extreme performance and thermal robustness (2, 34, 35) from an ancestral condition of muscle-powered tongue protrusion of modest performance (36). This parallel evolution provides multiple opportunities to explore the physiological mechanisms underlying extreme performance in the same system, and also yields insight into how high-performance systems are assembled through evolution. The family Plethodontidae is the largest among salamanders and its ∼470 species are diverse in many aspects of morphology, ecology, and life history. Plethodontids have long served as a model for the study of evolutionary processes and patterns, such as homoplasy (37), and the morphology of the tongue in particular has been a focus for phylogenetic analysis (38, 39). Plethodontid tongue function has also been a fruitful subject of study in biomechanics, motor control, physiology, and morphology, having been examined in many taxa (8, 21, 33, 36, 39–45) with both muscle-powered tongue protrusion and spring-powered tongue projection. Long-distance tongue projection is an evolutionarily significant innovation in plethodontids that may have facilitated their radiation in the neotropics; the ballistic-tongued clade Bolitoglossini includes over 260 species, or 36% of all salamander species and 55% of plethodontid species (https://amphibiaweb.org/).
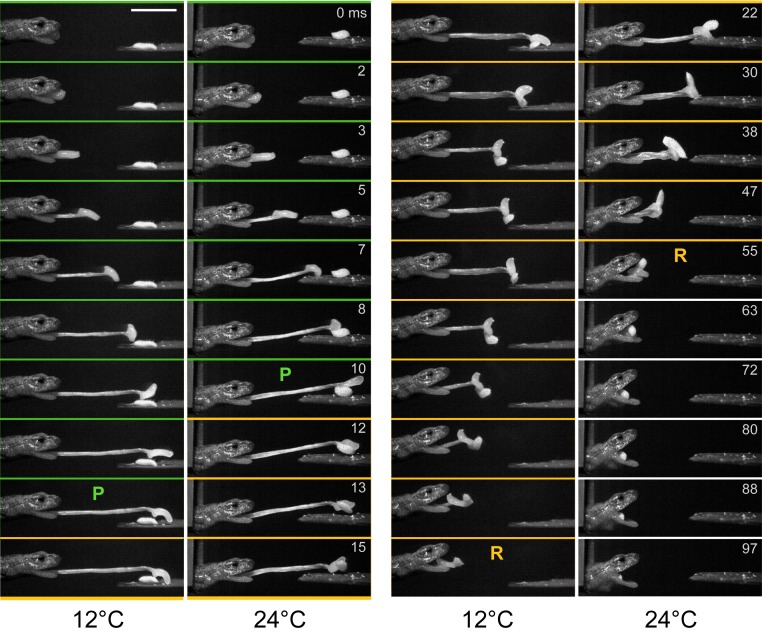
Members of the plethodontid lineages that have evolved long-distance ballistic tongue projection (2, 8, 36) provide compelling examples of extreme performance: Alpine Hydromantes can shoot its tongue up to 80% of body length in milliseconds and at a body temperature of 2 °C; tiny Thorius can accelerate its tongue at 600 G; and tropical Bolitoglossa can amplify the power of its tongue muscles 100 times (2, 8, 21, 46). Extremes include the highest acceleration and power output (i.e., rate of energy release) of any vertebrate movement (2), the longest-distance tongue projection among amphibians (8), and complete thermal independence of performance (21) (Fig. 1).
Fig. 1.
A salamander with ballistic tongue projection, Hydromantes platycephalus, capturing prey at 12 °C and 24 °C. The brief time to project the tongue using elastic recoil (green, P) is similar at two temperatures, while the time to retract the tongue (gold, R) using muscle shortening takes significantly longer at 12 °C than at 24 °C. Time in milliseconds relative to the start of tongue projection at time 0 is shown for each image pair. Note the time step between images is the same for 12 °C and 24 °C, and is greater during retraction (columns on right). (Scale bar, 1 cm.) Adapted with permission from ref. 21.
Systems with extreme performance, such as long-distance tongue projection, are ideal for understanding form–function relationships because they present these relationships more clearly than less extreme systems that do not approach physiological limits (2, 47). Extreme performance also often evolves hand in hand with a reduction in the number of functions performed, and as a result of this specialization, an extreme system is less likely to be compromised by the need to perform various functions. The tongue apparatus of plethodontid salamanders is used only for capturing prey (36). The hindlimbs of a frog, a contrasting example, are able to power ballistic jumping (4), but may embody trade-offs with other functions that they perform, such as swimming, climbing, grappling, digging, and wiping. Plethodontid tongues thus provide an opportunity to understand how a highly specialized system with extreme performance has evolved in the absence of apparent functional conflicts.
Results and Discussion
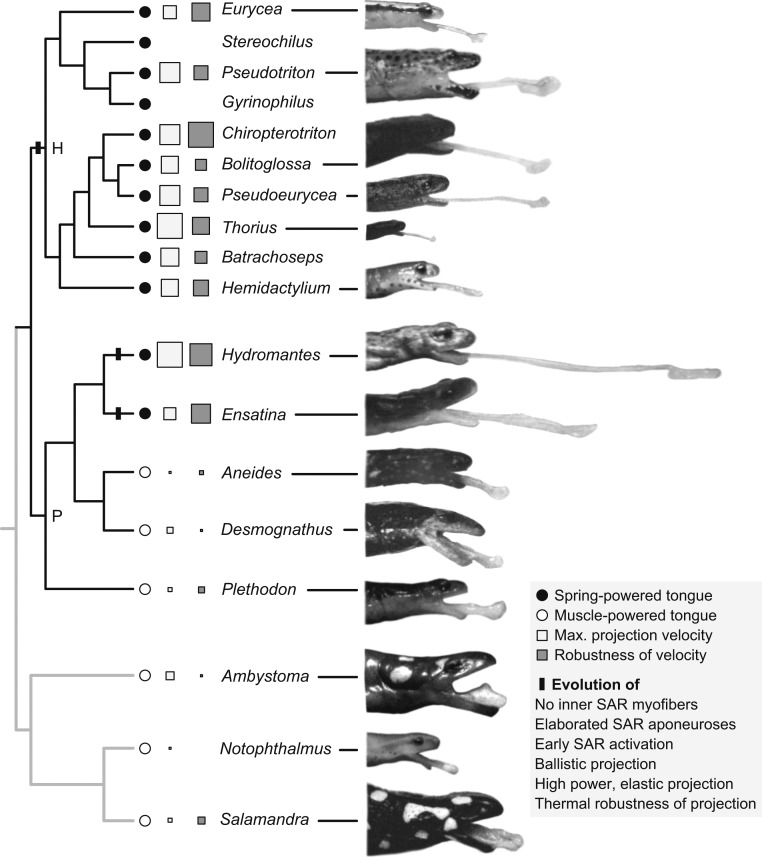
Our evolutionary analysis reveals that the most extreme case—truly ballistic projection—has evolved from nonballistic projection convergently in both major clades of plethodontids (Fig. 2). In each clade with ballistic projection (Plethodontinae and Hemidactylinae), the hyobranchial apparatus that makes up the tongue skeleton can be shot entirely from the body of the salamander (2, 8). The paired tongue projector muscles (the subarcualis rectus, SAR) surround and exert squeezing forces against the elongated, tapered posterior elements of tongue skeleton and launch it out rapidly, akin to shooting a melon seed from between the fingers. Collagen aponeuroses within the SAR muscle, arranged in similarly derived configurations in each clade (48) (Fig. 3), act as springs that store muscle energy and release it rapidly (2, 33). As a consequence of this elastic recoil of the aponeuroses, the tongue skeleton can be projected with sufficient velocity that it separates from the SAR muscles and carries the tongue pad to the prey under its own momentum. This truly ballistic tongue projection has evolved two or three times independently in the Plethodontidae, as revealed by our ancestral-state reconstruction using an all-rates-different evolutionary model and recent phylogenies (49–51) (Methods and Fig. 2).
Fig. 2.
Plethodontid salamander taxa (black branches) and outgroup taxa (gray branches) showing high-speed images of peak tongue projection. The suite of ballistic-projection characters (black bar) evolves in both major clades of plethodontids, Plethodontinae (P) and Hemidactyliinae (H). Symbols next to taxon names indicate tongue projection mechanism, peak projection velocity and thermal robustness of velocity, with higher performance and robustness indicated by larger symbols. Note the cooccurrence of velocity and its thermal robustness. Taxa without symbols have only morphological data. Branch lengths not indicated. See Methods for details of the phylogenetic analysis.
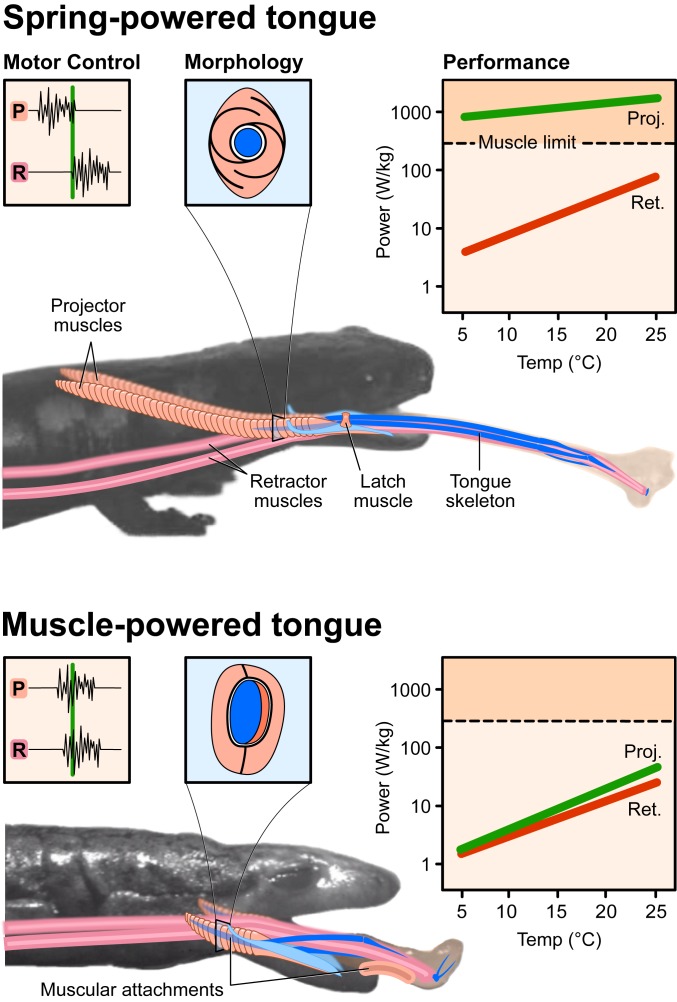
Fig. 3.
Summary of the major differences in tongue-projection mechanisms in plethodontid salamanders. The ballistic, spring-powered, high performance and thermally robust tongue (Upper), represented here by Bolitoglossa, shows a motor control pattern with early activation of the projector muscle (P) relative to tongue launch (vertical green line), a projector muscle (peach in anatomical drawing and in muscle cross-section) with spiral aponeuroses that act as springs surrounding but not attaching to a round skeletal element (dark blue) that can be launched completely from the muscle and the mouth. Peak projection power of this tongue type (green line in graph) is much higher, exceeding the muscle limit of ∼400 W/kg, and more thermally robust than muscle-powered tongue retraction (red line). The muscle-powered, thermally sensitive tongue (Lower), represented by Desmognathus, shows a motor control pattern with nearly simultaneous activation of projector and retractor muscles (pink) nearer to tongue launch, projector muscle without spiral aponeuroses and with inner myofibers (orange) inserting onto the elliptical tongue skeletal element, which cannot be launched entirely from the mouth. The tongue pad is attached to the lower jaw by a stout genioglossus muscle. Projection and retraction show similar, modest performance and high thermal sensitivity.
The nonballistic system from which ballistic tongue projection has evolved is retained in plethodontids as well as outgroup taxa (36, 52), indicating that it is ancestral for plethodontids (Fig. 2). This form of tongue protrusion uses direct muscle action and has lower performance and lower thermal robustness than ballistic projection (Fig. 2) (33, 35, 53). The sticky tongue pad is relatively massive and is protruded only a short distance from the mouth by movement of the tongue skeleton. This skeleton is pulled forward by the paired SAR muscles that insert directly on it and the skeleton does not separate from the SAR muscles as it does in ballistic projection (Figs. 3 and 4). The tongue skeleton is also shorter than in ballistic taxa and the tongue pad is linked to the lower jaw by a stout genioglossus muscle, limiting protrusion distance and performance (36, 41). Dynamic analysis of high-speed movies reveals that tongue projection in salamanders with this mode is powered by direct muscle action rather than elastically, including in plethodontid taxa Desmognathus and Plethodon (33–35), and in outgroups Salamandra and Notophthalmus (52), as well as Ambystoma (Table 1). Projection power is within the available power that has been measured for amphibian skeletal muscle [373 W/kg (19, 30)]; therefore, no elastic mechanism is necessary to produce the observed dynamics. Temperature manipulations also indicate that projection is muscle-powered in these taxa; projection velocity, acceleration, and power show relatively high thermal sensitivity (Q10 values of 1.6 to 1.9 for peak projection velocity across 10 to 20 °C and 2.8 to 6.9 for peak power) (Fig. 5 and Table 1), consistent with projection being limited by muscle contractile dynamics in the absence of an elastic mechanism.
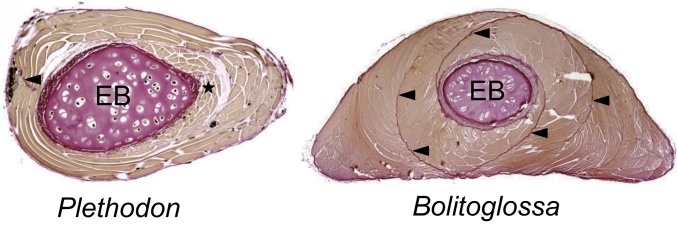
Fig. 4.
Histological cross-sections from the projector muscle and associated tongue skeleton from a muscle-powered (Plethodon, Left) and spring-powered (Bolitoglossa, Right) species. Myofibers (beige) surround the epibranchial cartilage of the tongue skeleton (EB, pink) and originate on collagen aponeuroses (arrowheads, pink). Inner myofibers (star) insert directly on the epibranchial in muscle-powered taxa, and are absent in spring-powered taxa. Anatomical orientations of sections and of inner and outer myofibers are indicated in Fig. 3.
Table 1.
Performance and thermal robustness mean values for each taxon
| Taxon | Tongue type | Peak power temperature (°C) | Peak velocity temperature (°C) | Peak projection velocity (m/s) | Peak projection power (W/kg) | Projection velocity Q10 | Projection power Q10 | Retraction power Q10 | Peak retraction power (W/kg) | n Individuals |
| Ambystoma | Muscle | 25 | 25 | 0.62 | 228 | 1.8 | 5.3 | 5.00 | 6 | |
| Aneides | Muscle | 20 | 25 | 0.20 | 10 | 1.8 | 6.9 | 3.28 | 6 | 4 |
| Batrachoseps | Spring | 25 | 20 | 2.56 | 4,200 | 1.2 | 1.8 | 2.63 | 10 | 8 |
| Bolitoglossa | Spring | 25 | 25 | 2.34 | 1,284 | 1.3 | 1.9 | 6.75 | 38 | 6 |
| Chiropterotriton | Spring | 10 | 10 | 2.83 | 5,864 | 0.9 | 0.7 | 3.05 | 48 | 6 |
| Desmognathus | Muscle | 20 | 25 | 0.47 | 82 | 1.9 | 4.6 | 4.54 | 60 | 6 |
| Ensatina | Spring | 25 | 25 | 1.73 | 2,134 | 1.0 | 1.0 | 4.04 | 30 | 7 |
| Eurycea | Spring | 25 | 25 | 1.87 | 2,231 | 1.0 | 1.3 | 4.67 | 21 | 14 |
| Hemidactylium | Spring | 15 | 20 | 2.34 | 4,336 | 1.1 | 1.6 | 3.99 | 30 | 5 |
| Hydromantes | Spring | 25 | 15 | 3.47 | 2,631 | 0.9 | 0.7 | 3.60 | 3 | |
| Notophthalmus | Muscle | — | — | 0.05 | 0.2 | — | — | — | 0.1 | 5 |
| Plethodon | Muscle | 25 | 25 | 0.26 | 18 | 1.7 | 4.7 | 7.56 | 9 | 6 |
| Pseudoeurycea | Spring | 25 | 25 | 2.77 | 3,033 | 1.1 | 1.6 | 3.89 | 39 | 12 |
| Pseudotriton | Spring | 15 | 25 | 2.76 | 3,310 | 1.2 | 1.4 | 2.88 | 65 | 8 |
| Salamandra | Muscle | 25 | 25 | 0.34 | 51 | 1.6 | 2.8 | 2.50 | 71 | 6 |
| Thorius | Spring | 15 | 15 | 3.45 | 6,765 | 1.1 | 1.2 | 1.05 | 52 | 4 |
See SI Appendix for additional data and sources.
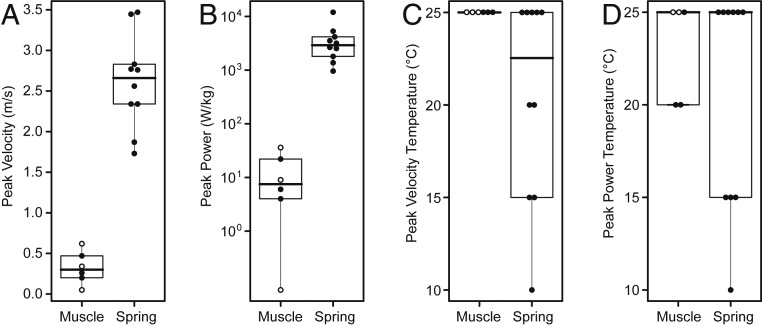
Fig. 5.
Comparison of salamander taxa showing higher peak velocity (A) and peak power (B) (on a log scale) of spring-powered tongue projection compared with muscle-powered projection, and the higher temperature at which peak velocity (C) and peak power (D) occur in muscle-powered taxa. Filled symbols are mean values from plethodontid taxa and open symbols are outgroup taxa, which are superimposed on box and whisker plots with the median indicated by the bold line.
We found that the peak instantaneous power of ballistic tongue projection is well in excess of available muscle power. This indicates that the energy of shortening of the projector muscles is stored in elastic structures and subsequently released more quickly. Power of projection reaches ∼18,000 W/kg in Bolitoglossa and ∼4,000 W/kg in Hydromantes (2, 33, 35, 46), compared with ∼300 to 400 W/kg of available muscle power.
We also found high-power, ballistic projection in taxa in both major clades that share several morphological traits: Elongate epibranchials, absence of a myofiber connection between the projector muscles and the tongue skeleton, and spiral aponeuroses within the projector muscles that can act as springs. We found other morphological correlates of high-power, ballistic projection including freedom of tongue pad from lower jaw through elongation or loss of the genioglossus muscle (Table 2), elongation of the retractor muscle and freedom from surrounding muscles, elongation and more complete folding of the tongue skeleton, greater extended tongue length, and a well-developed suprapeduncularis muscle in the floor of the mouth that can act as a latch that restrains the tongue skeleton prior to launch to permit loading of elastic elements (8, 33, 39–41, 48).
Table 2.
Morphology mean values for each taxon
| Taxon | Tongue type | Body mass (g) | SVL (mm) | Inner SAR myofibers | Genioglossus muscle | SAR/tongue mass | Aponeurosis spirality angle (°) | Epibranchial aspect ratio | n |
| Ambystoma | Muscle | 11.8 | 86 | Present | Stout | 0.16 | — | — | 3 |
| Aneides | Muscle | 1.4 | 40 | Present | Stout | 0.39 | 28 | 2.0 | 4 |
| Batrachoseps | Spring | 0.6 | 42 | Absent | Elongated | 0.75 | 130 | 1.2 | 15 |
| Bolitoglossa | Spring | 3.6 | 60 | Absent | Absent | 1.40 | 359 | 1.4 | 5 |
| Chiropterotriton | Spring | 0.4 | 32 | Absent | Absent | 0.91 | 383 | 1.3 | 4 |
| Desmognathus | Muscle | 11.2 | 84 | Present | Stout | 0.27 | 14 | 2.2 | 11 |
| Ensatina | Spring | 4.3 | 59 | Absent | Elongated | 0.47 | 212 | 1.3 | 10 |
| Eurycea | Spring | 1.7 | 53 | Absent | Absent | 0.61 | 296 | 1.3 | 6 |
| Gyrinophilus | Spring | 5.4 | 80 | Absent | Absent | 0.43 | 145 | 1.4 | 4 |
| Hemidactylium | Spring | 0.7 | 37 | Absent | Elongated | 0.81 | 116 | 1.2 | 8 |
| Hydromantes | Spring | 3.1 | 60 | Absent | Absent | 0.96 | 198 | 1.3 | 5 |
| Notophthalmus | Muscle | 2.0 | 44 | Present | Stout | 0.48 | 6 | 1.8 | 5 |
| Plethodon | Muscle | 2.4 | 56 | Present | Stout | 0.28 | 26 | 1.5 | 18 |
| Pseudoeurycea | Spring | 1.7 | 48 | Absent | Absent | 1.37 | 305 | 1.2 | 8 |
| Pseudotriton | Spring | 7.5 | 75 | Absent | Absent | 0.73 | 207 | 1.4 | 18 |
| Salamandra | Muscle | 24.2 | 97 | Present | Stout | 0.18 | 0 | 4.4 | 5 |
| Stereochilus | Spring | 1.1 | 48 | Absent | Elongated | 0.39 | 73 | 1.1 | 5 |
| Thorius | Spring | 0.1 | 21 | Absent | Absent | 1.77 | 226 | 1.3 | 8 |
See SI Appendix for additional data and sources.
Additionally, every high-power, ballistic species we examined has high thermal robustness of projection performance, significantly higher than that of muscle-powered tongue retraction (Fig. 3). The Q10 values of projection velocity are 0.9 to 1.3 across 10 to 20 °C for ballistic taxa. Muscle contractile experiments show that ballistic taxa have contractile dynamics of force production and thermal sensitivity typical of amphibian skeletal muscle (45, 53), excluding the possibility that unusual muscle properties confer the high performance and thermal robustness of projection.
An illuminating contrast to tongue projection is provided by tongue retraction. Tongue retraction is considerably slower than projection (by a factor of two to seven) and is accomplished in all plethodontids by the lengthy retractor muscles (the rectus cervicis profundus, RCP), which originate on the pelvis and run the length of the body and tongue to insert in the tongue pad. The low velocity and power of tongue retraction indicate that it is muscle-powered and does not involve appreciable elastic recoil, particularly when considered together with its high thermal sensitivity compared with projection (21, 34, 45). Even in ballistic-tongued taxa with high performance and high thermal robustness of projection, such as Hydromantes and Bolitoglossa, tongue retraction is relatively slow and thermally sensitive. This extends to other ballistic-tongued ectotherms, such as frogs and chameleons (9, 10, 20), providing a compelling illustration, within a single feeding event, of the benefits of elastic-recoil mechanisms.
For an elastic-recoil mechanism to operate, the muscles involved must be activated in advance of movement. Electromyographic recordings reveal that the projector muscles in ballistic plethodontids are activated up to 300 ms prior to tongue launch (34, 42, 44, 45), consistent with the myofibers stretching collagenous elastic structures (aponeuroses) within the muscles before movement begins. Early muscle activation by a similar interval has been observed in other spring-powered systems, such as suction feeding in pipefish and tongue projection in frogs and chameleons (9, 54–56). In contrast, muscle-activity onset in nonballistic plethodontids (Desmognathus and Plethodon) precedes tongue launch by a shorter interval (∼60 ms), although the duration of activity is similar. Unlike the activity of the SAR muscle in ballistic tongue projection, the activity of the RCP muscles continues throughout tongue retraction, consistent with the movement being driven directly by muscle shortening (34, 42, 44, 45). A motor control strategy of early activation is consistently present in all ballistic-tongued salamanders that have been examined, thus it likely evolved in concert with the morphological features that characterize ballistic taxa (Figs. 2 and 3).
Our evolutionary analysis shows that high-power projection, in excess of available muscle power, has evolved concordantly with ballistic projection in both major clades of plethodontids (Fig. 2). Ballistic projection, in turn, occurs only in species in which the projector muscle lacks myofibers that insert directly on the tongue skeleton (48); this lack of a myofiber connection allows the skeleton to separate from the muscle during projection and travel with higher acceleration and velocity. Ballistic projection is significantly correlated with both peak power and peak velocity of tongue projection as tested with phylogenetic analysis of variance (Methods, Fig. 2, and Table 3). Thus, the traits of high-power projection, spring-powered projection, and ballistic projection are perfectly correlated with one another, reflecting the same underlying mechanism.
Table 3.
Results of phylogenetic ANOVA that examine the effects of genioglossus muscle morphology (i.e., tongue attachment) and the loss of inner projector myofibers on performance and thermal robustness of performance
| F value | T value | P value | Adjusted P value | |
| Stout to elongated genioglossus | ||||
| Peak projection velocity | 95.568 | 9.875 | <0.001 | <0.001 |
| Peak projection power | 91.657 | 9.957 | <0.001 | <0.001 |
| Peak velocity Q10 | 26.939 | 5.351 | 0.002 | 0.004 |
| Peak power Q10 | 16.782 | 4.007 | 0.007 | 0.009 |
| Elongated to no genioglossus | ||||
| Peak projection velocity | 95.568 | 1.249 | 0.244 | 0.976 |
| Peak projection power | 91.657 | 0.810 | 0.433 | 0.866 |
| Peak velocity Q10 | 26.939 | 0.508 | 0.622 | 0.622 |
| Peak power Q10 | 16.782 | 0.707 | 0.492 | 0.656 |
| Stout to no genioglossus | ||||
| Peak projection velocity | 95.568 | 13.408 | <0.001 | <0.001 |
| Peak projection power | 91.657 | 13.024 | <0.001 | <0.001 |
| Peak velocity Q10 | 26.939 | 7.079 | <0.001 | <0.001 |
| Peak power Q10 | 16.782 | 5.661 | 0.002 | 0.004 |
| Loss of inner SAR myofibers | ||||
| Peak projection velocity | 95.568 | 1.249 | <0.001 | <0.001 |
| Peak projection power | 91.657 | 0.810 | <0.001 | <0.001 |
| Peak velocity Q10 | 26.939 | 0.508 | <0.001 | <0.001 |
| Peak power Q10 | 16.782 | 0.707 | <0.001 | <0.001 |
Adjusted P value is the Benjamini–Hochberg-adjusted P value for false discovery rate for each set of variables. Boldface indicates significance at P < 0.05.
Three morphological features that are central to the functioning of the derived elastic mechanism are significantly correlated with performance and its thermal robustness, as shown by our evolutionary analysis. The first such feature is the loss of the ancestral myofiber connection between the tongue skeleton and the projector muscle, which enables ballistic projection. The absence of these inner myofibers within the SAR muscle is correlated in our phylogenetic ANOVA with increases in peak velocity and peak power. Absence of inner myofibers is also correlated with greater thermal robustness of peak velocity and peak power, expressed as lower Q10 values (Table 3). The second feature that evolves concordantly with the loss of inner myofibers is a change in the shape of the element of the tongue skeleton on which the SAR muscle acts. This epibranchial cartilage is elliptical or irregular in cross-section in nonballistic taxa and assumes a rounder cross section in ballistic taxa (Fig. 4). The aspect ratio of the epibranchial is significantly correlated with tongue projection velocity and power, and as well as their thermal robustness as revealed by phylogenetic generalized least squares (PGLS) analysis (Tables 2 and 4). This shape change in the epibranchial is consistent with a change in the action of the SAR muscle from attaching to and pulling the tongue skeleton forward in the ancestral condition to squeezing the epibranchial radially to eject the tongue skeleton in the derived condition. The third morphological feature central to elastic projection is the derived configuration of the collagen structures within the SAR that store elastic energy. These structures are evident as spiral aponeuroses in the cross section of the SAR (Fig. 4) (described in ref. 48). Our PGLS analysis shows that greater elaboration of these aponeuroses (increased spirality) is significantly correlated with tongue projection velocity and power, as well as greater thermal robustness of velocity and power (Table 4).
Table 4.
Results of PGLS regression analyses that examine the relationships between morphology, performance and thermal robustness of performance
| Slope | T value | P value | Adjusted P value | |
| Morphology vs. performance | ||||
| Aponeurosis spirality vs. peak velocity | 0.134 | 2.971 | 0.012 | 0.018 |
| Aponeurosis spirality vs. peak power | 0.346 | 2.923 | 0.013 | 0.016 |
| Epibranchial aspect ratio vs. peak velocity | −2.234 | −2.981 | 0.011 | 0.022 |
| Epibranchial aspect ratio vs. peak power | −6.182 | −3.324 | 0.006 | 0.018 |
| SAR:tongue mass vs. peak velocity | 1.339 | 4.21 | 0.001 | 0.006 |
| SAR:tongue mass vs. peak power | 3.549 | 4.387 | 0.001 | 0.006 |
| Morphology vs. thermal robustness | ||||
| Aponeurosis spirality vs. velocity Q10 | −0.040 | −3.775 | 0.003 | 0.018 |
| Aponeurosis spirality vs. power Q10 | −0.119 | −3.543 | 0.004 | 0.012 |
| Epibranchial aspect ratio vs. velocity Q10 | 0.620 | 3.187 | 0.008 | 0.016 |
| Epibranchial aspect ratio vs. power Q10 | 1.576 | 2.405 | 0.033 | 0.040 |
| SAR:tongue mass vs. velocity Q10 | −0.273 | −2.523 | 0.027 | 0.041 |
| SAR:tongue mass vs. power Q10 | −0.782 | −2.333 | 0.038 | 0.038 |
| Performance vs. thermal robustness | ||||
| Peak velocity vs. velocity Q10 | −3.127 | −5.265 | <0.001 | <0.001 |
| Peak power vs. power Q10 | −2.817 | −6.164 | <0.001 | <0.001 |
Adjusted P value is the Benjamini–Hochberg-adjusted P value for false discovery rate for each set of variables. Boldface indicates significance at P < 0.05.
In addition to these changes in the propulsion mechanism, evolutionary changes to the size and morphology of the projectile also significantly affect performance. The SAR/tongue mass ratio is correlated with increased projection velocity and power as well as the thermal robustness of velocity and power, as shown in our PGLS analysis (Tables 2 and 4). A smaller tongue relative to the muscle places a lesser load on the muscle and thus enables the muscle to operate at a lower mass-specific work output. Reducing the load on a muscle has been shown in in vitro experiments on frog muscle to increase the thermal robustness of the work that the muscle can perform (19), so it can maintain higher work output at low temperatures. Reducing the mass of the projectile also directly translates to increased velocity, kinetic energy, and power of projection. Too great a reduction in projectile size could compromise the ability to deliver prey to the mouth, yet reducing the load on the projector muscle can also be accomplished by reducing drag on the projecting tongue. This occurs through elongation or elimination of the soft tissue connection between the tongue pad and the lower jaw, and this reduced load would be expected to improve projection performance. Indeed, our phylogenetic ANOVA reveals that evolutionary elongation of the genioglossus muscle that forms this connection from its ancestral condition as a stout connection results in significant increases in projection velocity and power and their thermal robustness, and loss of the genioglossus from the stout condition results in these same increases (Table 3).
Many morphological features that distinguish ballistic and nonballistic taxa are not only mechanistically linked to performance differences, but are also correlated with one another, as previously reported (48): Taxa with greater elaboration of the spiral aponeuroses in the SAR have greater SAR mass relative to tongue mass and lack inner myofibers (i.e., they are ballistic), and taxa that lack inner myofibers have significantly longer tongues and rounder epibranchials than (nonballistic) taxa that possess inner myofibers (Table 4). Ballistic and nonballistic taxa are separated in the principal-components space based on these characters (48).
Projection performance, measured as peak velocity and power, and the thermal robustness of performance are both significantly affected by the morphological characters of aponeurosis spirality, epibranchial aspect ratio, SAR/tongue mass, and loss of inner myofibers. It is therefore not surprising that each performance parameter is significantly correlated with its own thermal robustness in a PGLS analysis. For example, higher power projection correlates with higher thermal robustness of projection power, and likewise for projection velocity (Table 4).
Elastic-recoil mechanisms, such as those of plethodontid salamanders, are able to improve performance due to the inherent limits and thermal sensitivity of muscle rate properties, such as shortening velocity and power. The intrinsic maximum shortening velocity of muscle as well as its inherent force-length and force–velocity relations constrain the performance of muscle-powered movements (57, 58). Performance is further limited in ectothermic animals, such as salamanders, by the thermal sensitivity of muscle-shortening rates (22, 59, 60). Over a 10 °C drop in body temperature that ectotherms may experience, the speed and power of muscle shortening drop by at least half (i.e., temperature coefficient, Q10 > 2), with corresponding declines in whole-animal performance (22, 24, 26, 27, 59–65). This marked decline has been found in a diversity of muscles from an array of animals, including vertebrates and invertebrates, endotherms, and ectotherms (22–28, 30, 66, 67).
The evolutionary significance of elastic mechanisms in animals lies in their ability to circumvent constraints on rates imposed by muscle tissue itself. Muscle contractile physiology is evolutionarily conservative in its thermal dependence (59); therefore, adaptation at this level cannot be expected to buffer organisms against environmental challenges and we may expect to find similar evolutionary trajectories in other taxa. Muscle shows limited plasticity in its thermal-dependence values, both acutely and chronically (15, 24, 65, 67–69). Even clades with a long evolutionary history of functioning at low temperature, such as Antarctic fish, have not escaped the consequences of thermal effects on muscle contractile properties and organismal performance (70). Aspects of muscle contractile physiology are indeed conserved in plethodontid tongue projector and retractor muscles, with rate properties that are typical for amphibian muscle and, importantly, have similar values in ballistic and nonballistic taxa (45, 53).
Elastic-recoil mechanisms are able to circumvent these rate limitations by storing the mechanical energy that is produced by a shortening muscle temporarily in an elastic structure, such as a collagen tendon or aponeurosis in series with muscle fibers. The elastic structure subsequently recoils to propel an appendage or launch the animal’s whole body (1, 11). Because elastic tissue can typically shorten more quickly than the muscle can, but with the same force, performance is enhanced. Furthermore, the elastic modulus, or springiness, of elastic tissues in animals (collagen, resilin, and other animal protein rubbers) shows either very low thermal dependence or complete thermal independence (71, 72). Temperature, therefore, has only a minor effect on the rate of recoil of elastic structures and as a consequence it has little impact on the performance of movements powered by elastic recoil, such as the rapid tongue projection of chameleons, frogs, and salamanders (9, 10, 20, 21, 34, 45, 56).
High-performance, spring-powered movement coupled with thermal robustness has important ecological benefits for salamanders and other ectothermic animals. Decreased thermal dependence may be critical to ectothermic organisms that rely on rapid movement to escape predators or to obtain food. It can allow them to forage and otherwise function with high performance at low temperatures without the ecological costs of thermoregulatory behavior (73) or metabolic costs of regional endothermy. This may be particularly important to amphibians such as plethodontid salamanders, in which evaporative water loss restricts thermoregulatory opportunities to moist microhabitats (74). Amphibians that can feed over a wide a range of temperatures may reduce the costs of thermoregulation associated with high rates of water loss (75). Furthermore, spring-powered movements may be critical in organisms that exhibit slow, muscle-powered locomotion due to physiological constraints or the ecological requirements of crypsis (e.g., Hydromantes, some bolitoglossines, many toads, and chameleons) (20).
Evolution of elastic mechanisms in plethodontids and in other taxa indicate that these mechanisms likely evolved through morphological changes to nonelastic mechanisms, rather than by introduction of novel components or fundamental changes to muscle contractile physiology (2, 33, 34, 48, 53). This is seen in the few elastic systems in which evolution has been examined (6, 31–33), and also appears to hold for plethodontid salamander tongues (33, 35). In a clade that has evolved greater elastic storage, we can expect changes in morphological dimensions and other aspects of gross morphology (e.g., mechanical advantage of muscles, myofiber architecture, connective tissue elaboration) even in the face of possible changes at other levels that affect performance (e.g., titin isoforms, myofilament length) (76). We also expect that few incipient elastic mechanisms with intermediate performance persist in evolutionary time, because the advantages of speed are generally adaptive, and speed can be attained with little cost via elastic recoil. Systems in which selection for speed is countervailed by conflicting functions, such as force production or if the particular morphology of the system prevents the elaboration of connective tissue, might maintain relatively undeveloped elastic mechanisms. In this study, we expected to find intermediate forms in salamander tongues, but found only muscle-powered and fully fledged elastic systems with enormous performance differences.
Conclusions
Here we have examined the evolution of ballistic tongue projection in lungless salamanders, a spring-powered, high-performance, and thermally robust musculoskeletal system. Our studies of musculoskeletal morphology and whole-organism performance across a range of temperatures, combined with data on muscle contractile physiology and neural control, reveal that relatively minor evolutionary changes in morphology and neural control have produced a massive leap in performance and functional robustness in the face of conserved muscle physiology (2, 33–35, 51, 53). We conclude that such changes have likely evolved multiple times in parallel in salamanders to transform an ancestral, muscle-powered system of modest performance and high thermal sensitivity. Existing data from other elastic systems in animals [e.g., frogs and chameleons (9, 10, 20)] suggest that similar coevolution of morphological and motor-control traits has occurred in other ectotherms for which thermoregulation poses challenges or ecological costs.
Methods
Specimens.
Salamanders were purchased from commercial suppliers (Ambystoma maculatum, Notophthalmus viridescens, and Salamandra salamandra) or collected from natural populations in Mexico (Chiropterotriton chondrostega, Pseudoeurycea leprosa), California (Aneides flavipunctatus, Batrachoseps attenuatus), Georgia (Hemidactylium scutatum), and North Carolina (Pseudotriton ruber). Salamanders were housed individually in plastic containers with a substrate of moist paper towels at 14 to 19 °C and maintained on a diet of crickets or fruit flies. All procedures were approved by the Institutional Animal Care and Use Committee of the University of South Florida.
Feeding Experiments.
Feeding movements of salamanders were analyzed from a total of 1,952 feeding events from 106 individual salamanders: 1,055 feeding events of 55 individuals of 8 species collected for this study, combined with 897 feeding events of 51 individuals of 8 species from previous studies (21, 33, 35, 45, 46, 52) (Table 1 and SI Appendix, Table S1).
Prey capture was imaged in dorsal view at 6- to 15-kHz frame rate and 0.067- to 0.083-ms shutter speed with a Photron Fastcam 1024 PCI camera. Salamanders were placed on moist paper printed with a 5-mm grid for distance calibration, set on the surface of a ThermoElectric AHP-1200CPV temperature-controlled platform. Crickets or fruit flies were placed at varying distances in front of the salamanders, which were allowed to approach the prey prior to initiating prey capture. Salamanders, prey, and the substrate were illuminated by two Speco IR-200 near-infrared LED lights that provided cool light to avoid warming the salamanders. Feeding trials were conducted across a range of experimental temperatures (5, 10, 15, 20, and 25 °C) by varying the surface temperature of the feeding platform. Each salamander rested with its ventral surface against the moist paper on the temperature platform, acclimating to the experimental temperature for a period of at least 15 min prior to feeding trials, and its body temperature closely matched the temperature of the platform (±1 °C) (35). Body temperature was measured by directing a Sixth Sense LT300 thermal radiation thermometer at the dorsal surface of the head following each feeding event. The temperature sequence of feeding trials for each individual was randomized with one to three feedings recorded per experimental temperature before attempting a different, randomly selected temperature. Data from 10 and 20 °C trials was collected for the most species so it was used to calculate Q10 values from performance parameters.
Kinematic and Dynamic Analysis.
Digital image sequences from the Photron camera were used to quantify movements of the tongue during prey capture with respect to the upper jaw tip. The x, y coordinates of the tongue tip and the upper jaw tip were recorded from the image sequences using NIH ImageJ software running on an Apple iMac computer. Tongue-projection distance was computed as the straight-line distance between the upper jaw tip and tongue tip in each image of the feeding sequence. Coordinates were recorded beginning with the first appearance of the tongue beyond the upper jaw during tongue projection and ending with the withdrawal of the tongue pad into the mouth at the end of tongue retraction.
The dynamics of tongue movements were calculated using published methods (9, 45) by fitting a quintic spline to the distance–time data using the pspline package in R statistical software. First and second derivatives of the spline function were computed to yield instantaneous velocity and acceleration, respectively, at an interpolated rate of 10 to 15 kHz. The smoothing parameter of the spline was adjusted separately for tongue projection and tongue retraction of each feeding event to remove secondary oscillation artifacts from the velocity and acceleration traces. Instantaneous tongue mass-specific power was calculated as the product of the velocity at each point in time and its corresponding acceleration at the same point in time. Projector muscle mass-specific power was calculated by multiplying tongue mass-specific values by the average ratio of the mass of the tongue projectile to the mass of the projector (SAR) muscles for each species. Similarly, power achieved during tongue retraction was calculated by multiplying the power by the average ratio of the mass of the tongue projectile to the mass of the retractor (RCP) muscles.
Temperature effects on the maximum (i.e., peak) velocity and power of tongue projection and retraction from each feeding were examined in each salamander species separately. Biological rates are expected to have an exponential relationship with temperature; therefore, data were log10-transformed. Data were divided into three overlapping temperature intervals (5 to 15, 10 to 20, and 5 to 25 °C, each ±1 °C) based on the body temperature at which the data were gathered. An analysis of covariance (ANCOVA) was used separately on each subset of the data to examine the effects of temperature and individual salamander on peak velocity and power values. Individual was included in the model as a random effect to account for body size and other random individual differences. Measured body temperature as a continuous variable was included to examine how kinematic and dynamic variables responded to changes in body temperature across each temperature interval. Temperature coefficients indicating temperature sensitivity (i.e., Q10 values) were computed for each variable across each temperature interval as the base 10 antilogarithm of the partial regression coefficients of the temperature effect in the ANCOVAs multiplied by 10 (45, 46). In our discussion of temperature effects, “thermal robustness” is indicated by lower Q10 values, considerably lower than 2, and conversely “thermal sensitivity” is indicated by higher Q10 values; thermal independence is indicated by Q10 = 1.
Tongue Morphology.
We examined the relationships between the morphology of tongue apparatus with tongue projection performance and thermal robustness. Five morphological features were examined: 1) The spirality of the collagenous aponeurosis within the projector (SAR) muscles (i.e., the putative spring that propels the tongue in ballistic taxa) (Fig. 4); 2) the presence of inner SAR myofibers that insert directly on the epibranchial cartilage; 3) the aspect ratio of the cross section of the epibranchial cartilage; 4) the mass ratio of the SAR muscle to the tongue projectile; and 5) the extent of attachment of the tongue pad to the lower jaw (i.e., length of the genioglossus muscle). Data were compiled from previous studies (2, 21, 47, 48) with the exception of the salamandrid N. viridescens, for which new data were collected using the same methods (46, 48) (Table 2 and SI Appendix, Table S2).
Motor Control Data.
Electromyographic patterns of tongue muscle activation were examined from studies (2, 34, 42, 44, 45) that included both ballistic taxa (Bolitoglossa franklini, Ensatina eschscholtzii, Eurycea guttolineata, Hydromantes imperialis, Hydormantes supramontis, P. ruber) and nonballistic taxa (Desmognathus quadramaculatus, Plethodon metcalfi).
Evolutionary Analysis.
The phylogeny used for this study includes 18 species of salamanders, 15 ingroup species from Plethodontidae (Fig. 2 and Tables 1 and 2) and 3 outgroup species from Salamandridae (S. salamandra and N. viridescens) and Ambystomatidae (A. maculatum). Phylogenetic relationships of these species are based on the topology and branch lengths of the most recent phylogeny (51), as the backbone with additional taxa and branches inserted using published phylogenies (49, 50). A subset of taxa with the most complete set of dynamics and morphology data that included 13 plethodontids and Salamandra as the outgroup was used for analyses of quantitative performance traits. Values of morphological features, performance traits, and thermal robustness (i.e., Q10 values) for each taxon used in the evolutionary analysis were means calculated from all individuals of that taxon. In the case of peak velocity and peak power, the mean of the maximum value from each individual of the taxon was used. All morphological and performance data, except aponeurosis spirality and Q10 values, were log10-transformed prior to phylogenetic analyses.
PGLS regression was used to examine correlations between the continuous morphological and performance traits while taking phylogenetic relationships into account (77). PGLS was performed using a Brownian motion model of evolution with the nlme package in R. Phylogenetic ANOVA was conducted in R using the phylANOVA function in the phytools package (78) to examine the effect of discrete characters on performance and robustness variables, with 10,000 simulations and post hoc comparisons. A Benjamini–Hochberg adjustment for false-discovery rate was conducted and adjusted P values are reported, although this adjustment did not change the significance of any test at the P < 0.05 level.
Ancestral reconstruction was performed using 15 plethodontid taxa and two outgroup taxa for the evolution of ballistic tongue projection. Ballistic projection corresponds perfectly in the examined taxa with five other characters—1) No inner myofibers, 2) aponeurosis spirality angle of over 30°, 3) high-power projection exceeding muscle power, 4) early SAR activation relative to launch time (34, 42, 44, 45), and 5) an elastic-recoil mechanism—therefore, only one binary character representing this suite of features was reconstructed. Its evolution was estimated using the diversitree package in R (79), modeled under a constant-rate Markov model. The maximum-likelihood estimates for the transition rates of this model function were calculated using the find.mle function, and these parameters were used to estimate the marginal ancestral states at each node using the asr.marginal function (79). We used an all-rates-different model in which initial transition rates between the binary states were set at a range of values and final rates were permitted to differ. The state of the interior nodes, including the node at the base of the Plethodontidae, was left to vary as a range of likelihoods of ballistic projection present. The results of this analysis reconstructed the ancestral state for Plethodontidae as ballistic projection absent in most scenarios, followed by subsequent repeated gain of ballistic projection, depicted as bars in Fig. 2. The likelihoods at the interior nodes are nearly identical to our earlier phylogenetic analysis conducted for the presence of inner myofibers (48).
Data and Code Availability.
Data used in this study are provided in SI Appendix. R code and associated data files used in the phylogenetic analyses are available at DOI: 10.5281/zenodo.3688828.
Supplementary Material
Acknowledgments
We thank Chris Anderson, Ursula Dicke, Chris Evelyn, Maranda Holley, Gabriela Parra Olea, Jim O’Reilly, Bill Peterman, Gerhard Roth, Sean Rovito, Bill Ryerson, Nadja Schilling, and Kurt Schwenk for assistance obtaining salamanders; and members of the S.M.D. laboratory for help imaging salamander feeding behavior. This work was supported by NSF Grants IOS-0842626 and IOS-1350929 (to S.M.D.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The R code and associated data files used in the phylogenetic analyses are available at DOI: 10.5281/zenodo.3688828.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921807117/-/DCSupplemental.
References
- 1.Roberts T. J., Azizi E., Flexible mechanisms: The diverse roles of biological springs in vertebrate movement. J. Exp. Biol. 214, 353–361 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deban S. M., O’Reilly J. C., Dicke U., van Leeuwen J. L., Extremely high-power tongue projection in plethodontid salamanders. J. Exp. Biol. 210, 655–667 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Bennet-Clark H. C., “Energy storage in jumping animals” in Zoology, Davies P. S., Ed. (Pergamon, 1976), pp. 467–479. [Google Scholar]
- 4.Astley H. C., Roberts T. J., Evidence for a vertebrate catapult: Elastic energy storage in the plantaris tendon during frog jumping. Biol. Lett. 8, 386–389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gronenberg W., Brandao C. R. F., Dietz B. H., Just S., Trap-jaws revisited: The mandible mechanism of the ant Acanthognathus. Physiol. Entomol. 23, 227–240 (1998). [Google Scholar]
- 6.Claverie T., Chan E., Patek S. N., Modularity and scaling in fast movements: Power amplification in mantis shrimp. Evolution 65, 443–461 (2011). [DOI] [PubMed] [Google Scholar]
- 7.de Groot J. H., van Leeuwen J. L., Evidence for an elastic projection mechanism in the chameleon tongue. Proc. Biol. Sci. 271, 761–770 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deban S. M., Wake D. B., Roth G., Salamander with a ballistic tongue. Nature 389, 27–28 (1997).9288962 [Google Scholar]
- 9.Deban S. M., Lappin A. K., Thermal effects on the dynamics and motor control of ballistic prey capture in toads: Maintaining high performance at low temperature. J. Exp. Biol. 214, 1333–1346 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Sandusky P. E., Deban S. M., Temperature effects on the biomechanics of prey capture in the frog Rana pipiens. J Exp Zool A Ecol Genet Physiol 317, 595–607 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Aerts P., Vertical jumping in Galago senegalensis: The quest for an obligate mechanical power amplifier. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1607–1620 (1998). [Google Scholar]
- 12.Burrows M., Biomechanics: Froghopper insects leap to new heights. Nature 424, 509 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Patek S. N., Dudek D. M., Rosario M. V., From bouncy legs to poisoned arrows: Elastic movements in invertebrates. J. Exp. Biol. 214, 1973–1980 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Bennet-Clark H. C., The energetics of the jump of the locust Schistocerca gregaria. J. Exp. Biol. 63, 53–83 (1975). [DOI] [PubMed] [Google Scholar]
- 15.Biewener A., Baudinette R., In vivo muscle force and elastic energy storage during steady-speed hopping of tammar wallabies (Macropus eugenii). J. Exp. Biol. 198, 1829–1841 (1995). [DOI] [PubMed] [Google Scholar]
- 16.Roberts T. J., Azizi E., The series-elastic shock absorber: Tendons attenuate muscle power during eccentric actions. J. Appl. Physiol. 109, 396–404 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts T. J., Scales J. A., Mechanical power output during running accelerations in wild turkeys. J. Exp. Biol. 205, 1485–1494 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Alexander R. M., Tendon elasticity and muscle function. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133, 1001–1011 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Olberding J. P., Deban S. M., Effects of temperature and force requirements on muscle work and power output. J. Exp. Biol. 220, 2017–2025 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Anderson C. V., Deban S. M., Ballistic tongue projection in chameleons maintains high performance at low temperature. Proc. Natl. Acad. Sci. U.S.A. 107, 5495–5499 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deban S. M., Richardson J. C., Cold-blooded snipers: Thermal independence of ballistic tongue projection in the salamander Hydromantes platycephalus. J Exp Zool A Ecol Genet Physiol 315, 618–630 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Bennett A. F., Thermal dependence of muscle function. Am. J. Physiol. 247, R217–R229 (1984). [DOI] [PubMed] [Google Scholar]
- 23.Choi I. H., Cho Y., Oh Y. K., Jung N. P., Shin H. C., Behavior and muscle performance in heterothermic bats. Physiol. Zool. 71, 257–266 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Else P. L., Bennett A. F., The thermal dependence of locomotor performance and muscle contractile function in the salamander Ambystoma tigrinum nebulosum. J. Exp. Biol. 128, 219–233 (1987). [DOI] [PubMed] [Google Scholar]
- 25.Faulkner J. A., Zerba E., Brooks S. V., Muscle temperature of mammals: Cooling impairs most functional properties. Am. J. Physiol. 259, R259–R265 (1990). [DOI] [PubMed] [Google Scholar]
- 26.Herrel A., James R. S., Van Damme R., Fight versus flight: Physiological basis for temperature-dependent behavioral shifts in lizards. J. Exp. Biol. 210, 1762–1767 (2007). [DOI] [PubMed] [Google Scholar]
- 27.John-Alder H. B., Christopher Barnhart M., Bennett A. F., Thermal sensitivity of swimming performance and muscle contraction in northern and southern populations of tree frogs (Hyla crucifer). J. Exp. Biol. 142, 357–372 (1989). [Google Scholar]
- 28.Stevenson R. D., Josephson R. K., Effects of operating frequency and temperature on mechanical power output from moth flight muscle. J. Exp. Biol. 149, 61–78 (1990). [Google Scholar]
- 29.Rome L. C., Bennett A. F., Influence of temperature on muscle and locomotor performance. Am. J. Physiol. 259, R189–R190 (1990). [DOI] [PubMed] [Google Scholar]
- 30.Lutz G. J., Rome L. C., Muscle function during jumping in frogs. II. Mechanical properties of muscle: Implications for system design. Am. J. Physiol. 271, C571–C578 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Kaji T., Anker A., Wirkner C. S., Palmer A. R., Parallel saltational evolution of ultrafast movements in snapping shrimp claws. Curr. Biol. 28, 106–113.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Blanco M. M., Patek S. N., Muscle trade-offs in a power-amplified prey capture system. Evolution 68, 1399–1414 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Scales J. A., Stinson C. M., Deban S. M., Extreme performance and functional robustness of movement are linked to muscle architecture: Comparing elastic and nonelastic feeding movements in salamanders. J Exp Zool A Ecol Genet Physiol 325, 360–376 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Scales J. A., O’Donnell M. K., Deban S. M., Thermal sensitivity of motor control of muscle-powered versus elastically powered tongue projection in salamanders. J. Exp. Biol. 220, 938–951 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Deban S. M., Scales J. A., Dynamics and thermal sensitivity of ballistic and non-ballistic feeding in salamanders. J. Exp. Biol. 219, 431–444 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Wake D. B., Deban S. M., “Terrestrial feeding in salamanders” in Feeding: Form, Function and Evolution in Tetrapod Vertebrates, Schwenk K., Ed. (Academic Press, 2000), pp. 95–116. [Google Scholar]
- 37.Wake D. B., Wake M. H., Specht C. D., Homoplasy: From detecting pattern to determining process and mechanism of evolution. Science 331, 1032–1035 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Lombard R. E., Wake D. B., Tongue evolution in the lungless salamanders, Family Plethodontidae IV. Phylogeny of plethodontid salamanders and the evolution of feeding dynamics. Syst. Zool. 35, 532 (1986). [Google Scholar]
- 39.Wake D. B., Blackburn D. C., Lombard R. E., “Rampant homoplasy in complex characters: Repetitive convergent evolution of amphibian feeding structures” in Great Transformations in Vertebrate Evolution, Dial K. P., Shubin N., Brainerd E. L., Eds. (University of Chicago Press, 2015), pp. 395–405. [Google Scholar]
- 40.Lombard R. E., Wake D. B., Tongue evolution in the lungless salamanders, family plethodontidae. I. Introduction, theory and a general model of dynamics. J. Morphol. 148, 265–286 (1976). [DOI] [PubMed] [Google Scholar]
- 41.Lombard R. E., Wake D. B., Tongue evolution in the lungless salamanders, family Plethodontidae. II. Function and evolutionary diversity. J. Morphol. 153, 39–79 (1977). [DOI] [PubMed] [Google Scholar]
- 42.Deban S. M., Dicke U., Motor control of tongue movement during prey capture in plethodontid salamanders. J. Exp. Biol. 202, 3699–3714 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Deban S., Modulation of prey-capture behavior in the plethodontid salamander Ensatina eschscholtzii. J. Exp. Biol. 200, 1951–1964 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Deban S. M., Dicke U., Activation patterns of the tongue-projector muscle during feeding in the imperial cave salamander Hydromantes imperialis. J. Exp. Biol. 207, 2071–2081 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Anderson C. V., Larghi N. P., Deban S. M., Thermal effects on the performance, motor control and muscle dynamics of ballistic feeding in the salamander Eurycea guttolineata. J. Exp. Biol. 217, 3146–3158 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Deban S. M., Bloom S. V., Ballistic tongue projection in a miniaturized salamander. J. Exp. Zool. A Ecol. Integr. Physiol. 329, 62–71 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Dickinson M. H., et al. , How animals move: An integrative view. Science 288, 100–106 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Scales J., Bloom S. V., Deban S. M., Convergently evolved muscle architecture enables high-performance ballistic movement in salamanders. J. Morphol. 281, 196–212 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Adams D. C., Berns C. M., Kozak K. H., Wiens J. J., Are rates of species diversification correlated with rates of morphological evolution? Proc. Biol. Sci. 276, 2729–2738 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pyron R. A., Wiens J. J., A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 61, 543–583 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Shen X.-X., et al. , Enlarged multilocus data set provides surprisingly younger time of origin for the Plethodontidae, the largest family of salamanders. Syst. Biol. 65, 66–81 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Stinson C. M., Deban S. M., Functional morphology of terrestrial prey capture in salamandrid salamanders. J. Exp. Biol. 220, 3896–3907 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Olberding J. P., Scales J. A., Deban S. M., Movements of vastly different performance have similar underlying muscle physiology. J. Exp. Biol. 221, jeb166900 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Wainwright P. C., Bennett A. F., The mechanism of tongue projection in chameleons: I. Electromyographic tests of functional hypotheses. J. Exp. Biol. 168, 1–21 (1992). [Google Scholar]
- 55.Van Wassenbergh S., Strother J. A., Flammang B. E., Ferry-Graham L. A., Aerts P., Extremely fast prey capture in pipefish is powered by elastic recoil. J. R. Soc. Interface 5, 285–296 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson C. V., Deban S. M., Thermal effects on motor control and in vitro muscle dynamics of the ballistic tongue apparatus in chameleons. J. Exp. Biol. 215, 4345–4357 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Hill A. V., The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. B Biol. Sci. 126, 136–195 (1938). [DOI] [PubMed] [Google Scholar]
- 58.Katz B., The relation between force and speed in muscular contraction. J. Physiol. 96, 45–64 (1939). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett A. F., Temperature and muscle. J. Exp. Biol. 115, 333–344 (1985). [DOI] [PubMed] [Google Scholar]
- 60.James R. S., A review of the thermal sensitivity of the mechanics of vertebrate skeletal muscle. J. Comp. Physiol. B 183, 723–733 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Marsh R. L., Bennett A. F., Thermal dependence of isotonic contractile properties of skeletal muscle and sprint performance of the lizard Dipsosaurus dorsalis. J. Comp. Physiol. B 155, 541–551 (1985). [DOI] [PubMed] [Google Scholar]
- 62.Bauwens D., Garland T. Jr, Castilla A. M., Van Damme R., Evolution of sprint speed in lacertid lizards: Morphological, physiological, and behavioral covariation. Evolution 49, 848–863 (1995). [DOI] [PubMed] [Google Scholar]
- 63.Peplowski M. M., Marsh R. L., Work and power output in the hindlimb muscles of Cuban tree frogs Osteopilus septentrionalis during jumping. J. Exp. Biol. 200, 2861–2870 (1997). [DOI] [PubMed] [Google Scholar]
- 64.Navas C. A., James R. S., Wakeling J. M., Kemp K. M., Johnston I. A., An integrative study of the temperature dependence of whole animal and muscle performance during jumping and swimming in the frog Rana temporaria. J. Comp. Physiol. B 169, 588–596 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Marvin G. A., Effects of acute temperature and thermal acclimation on aquatic and terrestrial locomotor performance of the three-lined salamander, Eurycea guttolineata. J. Therm. Biol. 28, 251–259 (2003). [Google Scholar]
- 66.Barnes W. S., Ingalls C. P., Differential effects of temperature on contractile behavior in isolated frog skeletal muscle. Comp. Biochem. Physiol. A Comp. Physiol. 100, 575–580 (1991). [DOI] [PubMed] [Google Scholar]
- 67.Renaud J. M., Stevens E. D., The extent of short-term and long-term compensation to temperature shown by frog and toad sartorius muscle. J. Exp. Biol. 108, 57–75 (1984). [Google Scholar]
- 68.Wilson R. S., James R. S., Johnston I. A., Thermal acclimation of locomotor performance in tadpoles and adults of the aquatic frog Xenopus laevis. J. Comp. Physiol. B 170, 117–124 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Johnston I. A., Altringham J. D., Evolutionary adaptation of muscle power output to environmental temperature: Force-velocity characteristics of skinned fibres isolated from antarctic, temperate and tropical marine fish. Pflugers Arch. 405, 136–140 (1985). [DOI] [PubMed] [Google Scholar]
- 70.Wakeling J. M., Johnston I. A., Muscle power output limits fast-start performance in fish. J. Exp. Biol. 201, 1505–1526 (1998). [DOI] [PubMed] [Google Scholar]
- 71.Rigby B. J., Hirai N., Spikes J. D., Eyring H., The mechanical properties of rat tail tendon. J. Gen. Physiol. 43, 265–283 (1959). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denny M., Miller L., Jet propulsion in the cold: Mechanics of swimming in the Antarctic scallop Adamussium colbecki. J. Exp. Biol. 209, 4503–4514 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Huey R. B., Behavioral thermoregulation in lizards: Importance of associated costs. Science 184, 1001–1003 (1974). [DOI] [PubMed] [Google Scholar]
- 74.Feder M. E., Integrating the ecology and physiology of plethodontid salamanders. Herpetologica 39, 291–310 (1983). [Google Scholar]
- 75.Preest M. R., Pough F. H., Effects of body temperature and hydration state on organismal performance of toads, Bufo americanus. Physiol. Biochem. Zool. 76, 229–239 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Lindstedt S., Nishikawa K., Huxleys’ missing filament: Form and function of titin in vertebrate striated muscle. Annu. Rev. Physiol. 79, 145–166 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Symonds M. R. E., Blomberg S. P., “A primer on phylogenetic generalised least squares” in Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology: Concepts and Practice, Garamszegi L. Z., Ed. (Springer Berlin Heidelberg, 2014), pp. 105–130. [Google Scholar]
- 78.Revell L. J., phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012). [Google Scholar]
- 79.FitzJohn R. G., Diversitree: Comparative phylogenetic analyses of diversification in R. Methods Ecol. Evol. 3, 1084–1092 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study are provided in SI Appendix. R code and associated data files used in the phylogenetic analyses are available at DOI: 10.5281/zenodo.3688828.