Significance
Approaches to understanding change in plant function due to climate change often assume a fixed response: a given amount of climate forcing will cause a given amount of change in the measure of interest. Our study shows that this will not be the case for phenology. Effects of warming will be context dependent and vary with natural climate variability. Experimental warming in early, warm springs led to a greater advance of leafing, but more variation among species. In late, cool springs, there was a smaller effect of warming and species leafed out more simultaneously. Our results suggest that climate change could lead to more variable phenology, with consequences including greater risk of inappropriately early leafing and altered interactions among species.
Keywords: climate change, phenology, growing season length, boreal forest, temperate forest
Abstract
Changes in plant phenology associated with climate change have been observed globally. What is poorly known is whether and how phenological responses to climate warming will differ from year to year, season to season, habitat to habitat, or species to species. Here, we present 5 y of phenological responses to experimental warming for 10 subboreal tree species. Research took place in the open-air B4WarmED experiment in Minnesota. The design is a two habitat (understory and open) × three warming treatments (ambient, +1.7 °C, +3.4 °C) factorial at two sites. Phenology was measured twice weekly during the growing seasons of 2009 through 2013. We found significant interannual variation in the effect of warming and differences among species in response to warming that relate to geographic origin and plant functional group. Moreover, responses to experimental temperature variation were similar to responses to natural temperature variation. Warming advanced the date of budburst more in early compared to late springs, suggesting that to simulate interannual variability in climate sensitivity of phenology, models should employ process-based or continuous development approaches. Differences among species in timing of budburst were also greater in early compared to late springs. Our results suggest that climate change—which will make most springs relatively “early”—could lead to a future with more variable phenology among years and among species, with consequences including greater risk of inappropriately early leafing and altered interactions among species.
Changes in phenology have emerged as one of the most consistent signals of organismal response to climate change (1–3). Understanding the causes, consequences, and variability of changes in phenology is critical for predicting future pathways of ecological communities. Studies using long-term records and remote sensing have shown that in seasonally cold environments, climate change is causing earlier spring onset and delayed autumn senescence for many species (4–6) and altering the timing of reproduction (7–9). Studies of the response of spring phenology to experimental warming also show earlier leafing, emergence, and flowering (10–16) attributed to a variety of factors including earlier onset of temperatures favorable to growth and earlier snowmelt (8, 17). Autumn phenology has been less studied. There is evidence that late season flowering is less sensitive to warming (18, 19) and senescence is often delayed by warming (13, 15), but not always (7, 20). Although we have learned much, there remains considerable uncertainty.
In particular, it remains unclear whether and how climate forcing will interact with natural interannual temperature variability to affect phenology (21). For example, will warming alter phenology to the same degree in a warm, early spring compared to a cool, late spring? Moreover, questions remain about the factors that can explain interspecific differences (17, 22) in climate sensitivity such as species' geographic origin, leaf habit, physiology, microclimate, or interactions with nonclimate cues (e.g., photoperiod). Finally, while experiments are often upheld as a way to disentangle the multiple factors that could affect phenology, there have been critiques of the value of inferences gained from warming experiments compared to other methods (23, 24).
It is likely that climate warming will interact with natural temperature variability to alter phenology. There is evidence from remote sensing that vegetation green-up is slower in early, warm springs (25) and that there is more differentiation among species and individuals in such years (26). This differentiation could be due to a number of factors such as similar degree day requirements spread over longer or shorter times (26), the alteration of key windows of developmental sensitivity (14, 22), differences in requirements for phenological events to occur, or a combination of these. Thus, responses to a generally warming future climate might have different impacts in years with early vs. late springs (relative to decadal scale variation). For example, one might expect that warming may not have equal effects during early versus late springs if increased chilling results in faster rates of budburst under forcing temperatures (27–29) or photoperiod constrains response (26, 30). To date, using experimental warming to explore the interaction of interannual temperature variability with climate forcing has been limited due to the short time span of most warming studies (21).
Warming may affect species and individuals differently due to the proximate mechanisms that cause phenological responses. These mechanisms can be environmental (e.g., temperature, photoperiod, resource availability) or organismal (e.g., sensitivity to conditions, speed of response to conditions, physiology; ref. 31). Although leafing phenology is cued by a combination of temperature and daylength in most tree species in seasonally cold forests (32–37), there is growing evidence that species differ in the relative importance of each (27–29, 38). Such differences affect the ability to respond to earlier onset of warm temperatures in spring and the extension of warm temperatures into the fall. For budburst, some species are most sensitive to spring warming (i.e., forcing temperatures). Such species may be most responsive to earlier onset of spring. For other species, rates of budburst also depend strongly on winter chilling (27–29) or are constrained by photoperiod (30); such species might be less responsive to earlier onset of spring. For senescence, much less is known. A large meta-analysis of trees from seasonally cold forests suggests that October temperatures were the strongest predictors of leaf senescence followed by cooling degree days and photoperiod. However, the strength of the relationships vary with latitude and geographic region (39).
There is growing evidence that suggests interannual spring temperature variability (STV) influences regional strategies in phenology (40). Species from areas with high STV have higher chilling requirements to ward against inappropriate leafing and subsequent frost damage (40). In addition, research suggests that regions with warmer, earlier springs house species with greater extent of photoperiodic control (29). Both high chilling requirements and photoperiodic control will constrain phenological shifts in response to climate change. Species with such constraints might be less able to take advantage of a longer active season but would likely also be less vulnerable to frost damage. Strong effects of geographic–climatic history mean that the effects of climate change on phenology of mixed species communities will be more difficult to predict.
Overall, a number of knowledge gaps exist because of limitations of prior experimental work. These include (a) short time frame (most 2 to 3 y) limiting inferences regarding whether response to warming depends on weather in any particular year (e.g., warm versus cold spring); (b) paucity of research on needle-leaved conifers that dominate northern latitude forests (but see ref. 15); (c) strong focus on budburst, leaving autumn phenology poorly characterized; (d) warming of either soil/roots or foliage, but not both (but see ref. 13); and (e) results that are inconsistent with long-term observational data (23, 24) raising questions about the realism of experiments for understanding climate change effects on phenology.
To address several of these knowledge gaps, we tested the extent to which experimental warming changed spring and autumn leafing phenology over 5 y in 10 co-occurring tree species grown in two habitat conditions at two sites in the temperate-boreal ecotone. The data from this experiment represents an order of magnitude richer data set (200 habitat–species–year combinations, each at 3 temperature levels) than any prior phenology-warming experiment. We hypothesized that species would differ in the degree that warming advanced budburst in spring or delayed senescence in autumn. We expected to see a stronger response in boreal species due to lower sensitivity to chilling and greater sensitivity to forcing temperatures (29, 37, 41). We had competing hypotheses about interannual variability on the effect size of warming on budburst: if spring forcing temperatures were the dominant cue, then there would be no difference among years in effect size. Alternately, if winter chilling or photoperiod played a role, then effect size would vary among years.
Our study is part of a larger project (Boreal Forest Warming at an Ecotone in Danger; B4WarmED). B4WarmED explores the potential for projected climate warming to alter tree function and species composition at the boreal-temperate forest ecotone through effects on juvenile physiology, growth, and survival (42, 43). Variation in phenological response to warming among co-occurring tree species could play an important role in determining climate change impacts on forested communities. This is because phenology impacts tree growth and productivity (44–48), frost risk, reproductive fitness (49–51), success of invasive species (52, 53), and abundance and health of herbivores (54). Our work is unique in its use of local genotypes, length, and large number of functionally diverse species, including broad- and needle-leaved species and those tolerant and intolerant of shade. In addition, we use active warming of above and belowground plant parts and include both spring and autumn phenophases. Finally, due to the considerable interannual variability in spring and autumn temperature regimes (SI Appendix, Fig. S1), multiple years of data increase our ability to make inferences across a range of seasonal weather conditions (e.g., early versus late springs). Multiple years of data also allow us to integrate responses to natural interannual variation in temperature with response to experimental warming (16, 55).
Results
Budburst Phenology.
The average day of year of budburst differed among years, canopy cover, warming treatments, and species (SI Appendix, Table S1). Among species, Betula papyrifera generally broke bud the earliest and Abies balsamea the latest. Experimental warming advanced budburst of all species (F2,75 = 150; P < 0.0001). Days advanced by species averaged across years ranged from 2.4 to 7.2 d for the +3.4 °C treatment and 1.8 to 4.6 d for +1.7 °C treatment. However, averaging across years masks interannual variability in budburst, which varied considerably from year to year (F3,3603 = 4551; P < 0.0001); using the ambient treatment as a reference, average day of year of budburst varied by 31 d among years. Moreover, the effect of warming on budburst differed among years (year × warming interaction; F6,3748 = 11.2; P < 0.0001) and species differed in the extent of such interannual variation sensitivity (year × warming × species interaction; F54,5272 = 2.676; P < 0.0001; SI Appendix, Fig. S2). Budburst advanced most in spring 2012 with seedlings of Populus tremuloides breaking bud 13 and 15 d earlier in +1.7 °C and +3.4 °C, respectively. In contrast, in 2013, several species (Picea glauca, Acer rubrum, and Pinus strobus) did not advance budburst in either warming treatment. Finally, canopy cover was related to budburst for Betula papyrifera, Pinus banksiana, Pinus strobus, and Quercus macrocarpa. Generally, budburst was delayed in closed relative to open sites. There was a single interaction involving warming and canopy cover (year × habitat × warming; F54,5201 = 2.218, P < 0.0001; SI Appendix, Table S1) that stemmed from a single year (2010) showing slightly stronger response to warming in the open compared to closed.
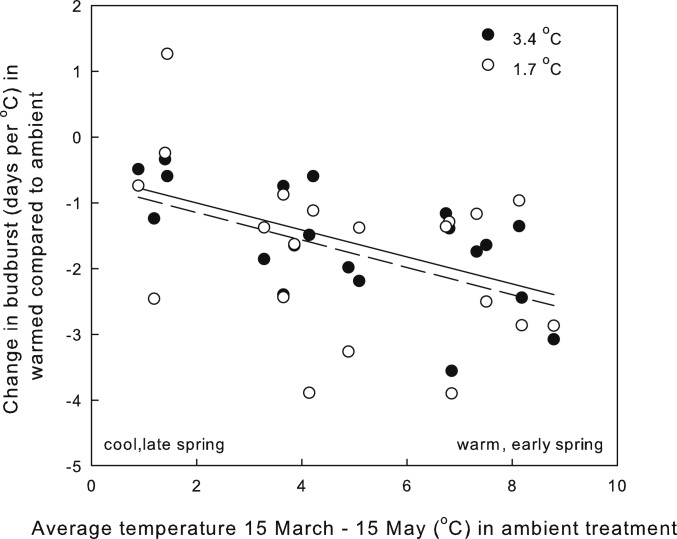
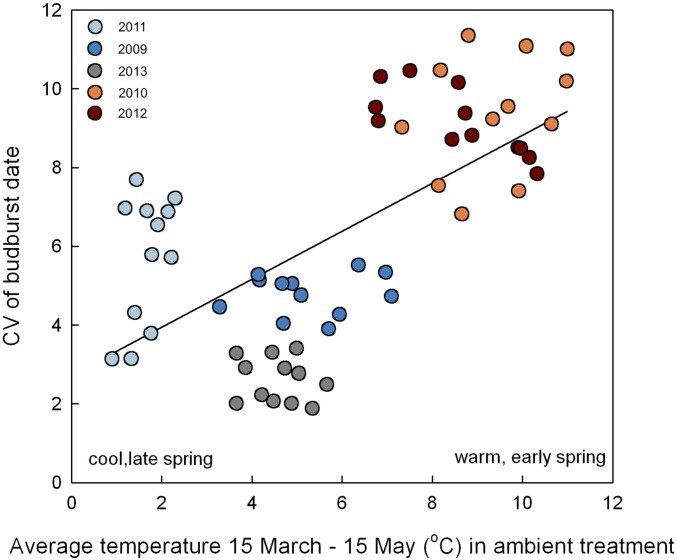
The differences among years in response of budburst to warming were related to the timing of spring warm-up. Plotting the effect size of warming versus the average spring temperature in ambient plots (March 15 to May 15 [TMAM] as an indicator of warmth and timing of spring) shows a negative relationship, indicating that budburst advances more with warming in early, warm springs compared to late, cold springs (Fig. 1; R2 = 0.21, P < 0.0001, no difference between treatments in the relationship). We interpret this evidence as showing, with >99.9% confidence, that warmer temperatures will have a larger effect (size) in warmer, earlier springs, but that a lot of unexplained variation remains, some of which may be related to other mechanisms we did not examine and some which may simply be experimental "noise." In addition, differences among species in budburst timing were more pronounced in warm versus cold springs. For example, in 2011, a late, cold spring, time from average budburst of the first species to the last was ∼14 d. In contrast, in 2012, an early, warm spring, the difference between the first and last species was 32 d. There was a positive relationship between TMAM and the coefficient of variation of budburst among species [Fig. 2; R2 = 0.41; CV of budburst = 2.74 + 0.609 TMAM, CV calculated among species means (n = 10) for each year (5) * site (2) * habitat (2) * treatment (3) combination, n = 60].
Fig. 1.
Average change in budburst of per degree warming (day per degree Celsius) as a function of average March 15 through May 15 temperature (TMAM) in the ambient treatment. Each point is the average change in budburst of 10 species for each year, site, canopy condition, and warming treatment (y axis; n = 10 per point) and corresponding average TMAM in the ambient treatment for each year, site, and canopy condition (x axis; n = 93 per point). Filled dots and solid line are from +3.4 °C treatment. Open dots and dashed line are from +1.7 °C. R2 = 0.21, P < 0.0001.
Fig. 2.
Coefficient of variation (CV) among 10 species in budburst date as a function of average March 15 to May 15 temperature (TMAM) in the ambient treatment. Each point represents CV of species means (n = 10) for one site × year × habitat × treatment combination (e.g., CFC, 2009, Closed canopy, +3.4 °C, n = 60). Colors indicate year.
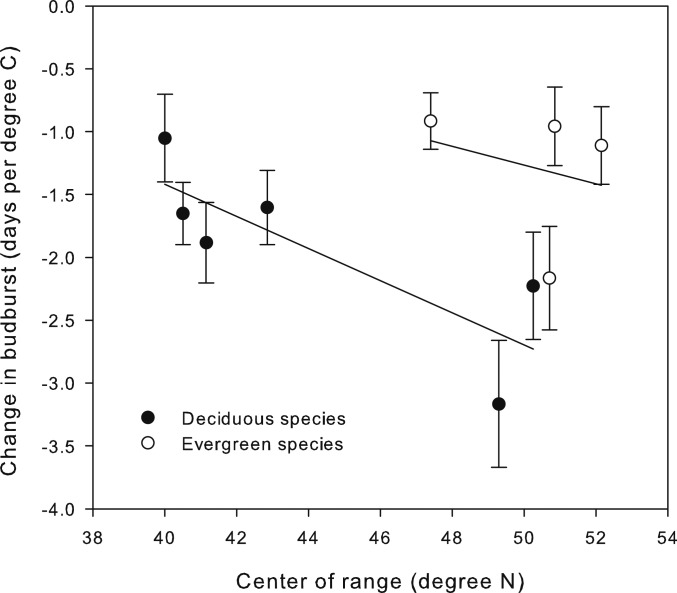
We also found differences in effect size of warming among plant functional types and in relation to the species range (using center of geographic range in midcontinent as a proxy). Evergreen species had a lower effect size than deciduous species (Fig. 3; F1,1143 = 20.4, P < 0.0001). Within functional groups, species from more northern, colder climates advanced budburst more in response to warming (Fig. 3; F1,1143 = 10.4, P < 0.0013); low sample sizes within functional groups suggest this latter result should be considered cautiously.
Fig. 3.
Average change in budburst of per degree warming (days) as a function of an index of the center of a species latitudinal geographic range (in midcontinent). Each point represents 1 of our 10 study species. Average ± SEM were calculated across sites, canopy conditions, and years (2 sites × 2 canopy conditions × 5 y = 20). There were significant differences between evergreen and deciduous species (F1,1143 = 20.4, P < 0.0001), a significant negative relationship between geographic range and effect size (F1,1143 = 10.4, P < 0.0013) and no interaction.
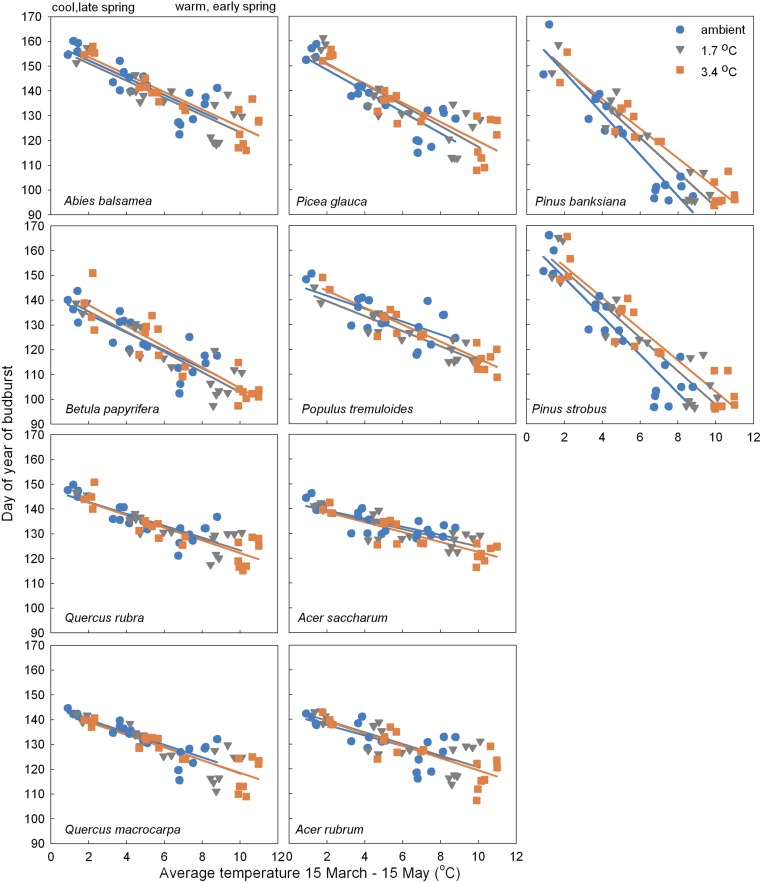
Finally, the advancement of budburst as a result of experimental warming was consistent with the advancement of budburst induced by natural interannual changes in spring temperatures. For all but the two pine species, experimental warming and natural interannual changes in climate moves budburst along a common line (Fig. 4). Overall, we found a strong relationship between day of year of budburst and TMAM measured in each treatment (F1,573 = 1,062; P < 0.0001), a small effect of warming treatment (F1,573 = 3.294, P = 0.04), and no interaction of warming and TMAM measured in each treatment (e.g., no difference in slope; F1,573 = 0.1654, P = 0.85). This indicates that phenological responses were similar to warming temperatures whether they were from experimental warming or ambient variability.
Fig. 4.
Relationship between of day of year of budburst versus average temperature March 15 through May 15 temperature (TMAM) in ambient, +1.7 °C, and +3.4 °C across 5 y (2009 to 2013) for 10 tree species. Each panel represents a species. Each point is average of individuals within sites and canopy condition and year (y axis; n = 12 per point, 2 individuals * 3 plots) and corresponding average TMAM in each treatment for each year, site, and canopy condition (x axis; n = 93 per point).
Growing Degree Days at Time of Budburst.
Spring phenology is often modeled using a measure of thermal time (e.g., growing degree days, GDD). In this framework, there is often an expectation that the thermal time at budburst will be the same across treatments and years if spring warming is the main driver of budburst. In contrast, we found that growing degree days at time of budburst differed among years, warming treatments, and species (SI Appendix, Table S2). Similar to our analyses of date of budburst, we found significant year × warming (F6,3956 = 8.478, P < 0.0001) and year × warming × species (F54,5201 = 2.218, P < 0.0001) interactions suggesting interannual variation in the effect size of warming on GDDs accumulated on the date of budburst. Generally, individuals in warming treatments required more GDDs to break bud compared to those in ambient. Species differed in GDD at time of budburst, with Betula papyrifera and Pinus banksiana requiring least and Abies balsamea the most among years.
Senescence Phenology.
Like budburst, timing of senescence differed among years, canopy cover, warming treatments, and species (SI Appendix, Table S3). In general, there was lower variation among years in the timing of senescence compared to budburst, and species tended to be the strongest effect. Warming delayed senescence for deciduous species in all years, and either had no effect or advanced senescence in needle-leaved, evergreen conifers depending on year (SI Appendix, Fig. S3). Average delay of senescence by deciduous species averaged across years ranged from 1.7 to 11.6 d for the +3.4 °C treatment and 1.7 to 7.8 d for the +1.7 °C treatment. Maximum delays occurred in autumn 2009: seedlings of Acer saccharum delayed senescence by 17 d in the +3.4 °C treatment and Acer rubrum delayed senescence by 13 d in the +1.7 °C treatment. Minimum delays in senescence for deciduous species occurred in autumn 2012 when Populus tremuloides began senescence 5 d earlier in the +3.4 °C treatment compared to ambient and Betula papyrifera began to senesce 3 d earlier in the +1.7 °C treatment compared to ambient. Using the ambient treatment as a reference, start of senescence varied across years by 14 d; 2013 was the earliest year (average start of senescence was September 16) and 2009 was the latest (average start of senescence was September 30).
We found species differences in the degree of delay of senescence and in whether responses were linear or nonlinear (species × warming interaction; F18,4543 = 10; P < 0.001). Acer saccharum was the most responsive to warming, delaying senescence from 7 to 17 d depending on year. Finally, we found canopy differences in senescence for Acer saccharum, Acer rubrum, and Quercus rubra (species × habitat interaction; SI Appendix, Table S3). Generally, senescence was delayed in closed relative to open for these species. This was especially pronounced in Acer spp., which senesced on average 9 d later in closed canopy treatments. There were also several three-way interactions that included canopy cover and warming (SI Appendix, Table S3). These interactions were related to the overall lack of response of evergreen species to any treatments, whereas all deciduous species delayed senescence more with warming, some delayed senescence more in closed canopy treatments, and for some the magnitude of delay differed among years. For the latter, there did not appear to be any consistent order among years across species.
Growing Season Length.
For the deciduous species, growing season length differed among years, warming treatments, and species (SI Appendix, Table S4). Year was the strongest effect (F4,180 = 366; P < 0.0001). Using the ambient treatment as a reference, growing season length varied by >30 d among years; 2013 was the shortest (115 d) and 2010 the longest (148 d) growing season. Given that evergreen species maintain foliage year-round, estimating growing season length based on leafing phenology may not be the most appropriate measure. Thus, we only examined change in growing season length for seasonally deciduous species.
Warming extended the growing season in all deciduous species (SI Appendix, Fig. S4). Among years, average extension ranged from 10 to 17 d for the +3.4 °C treatment and 6 to 11 d for +1.7 °C treatment. Maximum extension occurred was in 2010: seedlings of Acer saccharum extended the growing season by 22 d in +3.4 °C and Acer rubrum by 16 d in +1.7 °C. Minimum extension occurred in 2013 when Acer rubrum showed a 0- and 3-d change in growing season length in +1.7 and +3.4 °C, respectively.
In both treatments with elevated temperatures, contribution to growing season length of early leafing compared to later senescence varied among years (SI Appendix, Table S5; +1.7 °C, F4,29 = 4.37, P = 0.0082; +3.4 °C, F4,29 = 17.05, P < 0.0001), but not among species (SI Appendix, Table S5; +1.7 °C, F5,29 = 0.874, P = 0.513; +3.4 °C, F5,29 = 0.7344, P = 0.605). In 2012, early leaf out contributed most to growing season length (64% and 81% in 1.7 and 3.4 °C), whereas in 2013 late leaf fall in autumn contributed the most (77% in both warming treatments), with other years showing more equal contributions of the two (Tukey’s honestly significant difference, α = 0.05).
Discussion
Phenology has emerged as one of the most coherent signals of organismal response to a changing climate (2, 56). Both historical records and experimental studies show alteration of phenology linked to altered temperature regimes, especially in seasonally cold climates (13, 15, 39). Our research extends and advances prior research by demonstrating strong interannual variation in the effect of experimental warming on phenology (Figs. 1 and 2). We found significant differences in the magnitude and nature of phenological responses to warming among a suite of co-occurring species. Responses to warming differed as a function of leaf habit and geographical range with deciduous species and boreal species more responsive to warming (Fig. 3). Moreover, we found a larger spread between when the first and last species break bud in early springs. We hypothesize that these differences are related to differences among species in the relative importance of environmental cues for phenology, the relative sensitivity of species to cues across the growing season (14), and in the speed of warming in early versus late springs (25, 26). Finally, integrating natural with experimental changes in temperature revealed consistent budburst responses to warming (Fig. 4). This suggests that our experiments were realistic manipulations of natural climate variation, but also supports using natural variation as proxies of future warming at least within the range of temperature examined. Our results are consistent with other studies that warmed only aboveground suggesting that studies that only warmed aboveground were not confounded by incomplete (e.g., lack of belowground warming) warming treatments. Overall, our findings suggest that phenology could be among the suite of characteristics that affect how co-occurring species interact under climate change. Changing phenology can affect plant growth and productivity (44–48), frost risk, reproductive fitness (49–51), success of invasive species (52, 53), and abundance and health of herbivores (54). In general, changing species interactions associated with changing phenology would have consequences for community composition and function.
Effect of Experimental Warming Stronger in Early Springs.
One of our most compelling results showed strong interannual variation in the strength of treatment effects related to variation in weather in spring (Fig. 1). In the 5 y reported here, the start of phenological spring and autumn varied by as much as 30 and 14 d, respectively. To date, little attention has been paid to whether the effects of climate warming are contingent on whether conditions in spring are average, cooler than average, or warmer than average. Our data suggest that in the future, early, warm springs (relative to other years) will show a larger effect size and greater variance associated with additional warming. These patterns may result from the changing relative importance of temperature and photoperiod as spring progresses. For example, if forcing temperatures dominate in early spring (e.g., late March) we might expect stronger responses to warming especially for species that lack photoperiodic sensitivity and have low chill requirements. In late spring, chilling requirements and photoperiod thresholds have likely been fulfilled for all species and the response of all species to forcing is strong and rapid (21, 28). Additional warming in a late spring may not further stimulate budburst. This supposition is supported in part by evidence that long photoperiods can substitute for forcing in some species (38).
Longer Forestwide Green-Up Period in Early Springs.
In addition to interannual variation in the average effect size of warming, differences among species in timing of budburst were also greater in early compared to late springs. This suggests that climate change (by advancing spring on average) could increase asynchrony of leafing in forested communities. For example, in 2012, an early, warm spring, the number of days between the first species breaking bud and the last was 32, while in 2011, a cold, late spring, it was 14. Again, these differences may be related to species differences in relative importance of cues during spring development. In early springs, the earliest leafing species may be those for whom forcing temperatures dominate cues for budburst. Early springs often also involve less chilling hours and shorter photoperiods when forcing temperatures begin. Species whose rate of response to forcing temperature increases with longer chill exposures (27, 28, 57, 58) or require a particular length of photoperiod to respond to forcing (30) will be slower to respond, resulting in later leafing.
Another potential explanation for greater phenological differentiation is related to the within-season warming speed (59). There is some evidence that in late springs, individuals reach their forcing sums closer in time to one another, compressing spring phenology in ordinal time, reducing differentiation among individuals and species (26), and inducing more rapid green-up (25). Our results using experimental warming further extend these ideas by showing greater differentiation among species and warming treatments in early, warm springs compared to late, cool springs. Examining the mechanisms that may underlie these results such as similar degree day requirements spread over longer or shorter times; alteration of key windows of developmental sensitivity; interactions of chilling, photoperiod, and warming; or some combination can be tested using a modeling framework, which represents a next step for this work. Such modeling would employ several approaches. First, process-based models (60) and a model selection approach (25) allow flexibility in defining the window for degree day accumulation, base temperature, and role of chilling and photoperiod. Second, continuous developmental models (14, 61) can more fully examine the potential for changing developmental sensitivity during the growing season and under experimental warming.
Regardless of the underlying mechanisms that create greater differentiation among species in early springs, such patterns would extend the period in which a mixed species forest leafs out in early springs. This could affect species' interactions among plants and between plants and animals. First, species that leaf out earlier might gain a greater amount of carbon due to higher irradiance (less neighbor shading), which could be especially important for seedlings, saplings, and young trees in understory conditions where that extra light can help drive a considerable increase in total annual carbon flux (62). Alternately, it could increase vulnerability to invasive species due to extended periods of incomplete canopy closure (48, 51). Second, generalist insect herbivores that feed on juvenile foliage of multiple species may have more food resources if young foliage is available longer. Since finding and capturing prey is easier and less energy intensive early in the season when foliage is small (63), a longer period between when the budburst of the first and last species may also increase food accessibility for insect-feeding birds (64). For mammalian herbivores, extended spring could have positive consequences by extending the period of high quality forage (54).
Autumn Leaf Phenology Extended Only in Deciduous Species.
Autumn is a time in which temperatures are falling and sun angles become lower. Although we found approximately equal contribution of delayed senescence in autumn and earlier budbreak in spring to extended growth season in deciduous species (SI Appendix, Table S5), the broader consequences of extended autumn are perhaps less than extended spring. For deciduous species photosynthetic capacity at this time is generally reduced (65), thus the positive consequences may be lower. Moreover, autumn extension could have overall negative impacts. In particular, while warming has been shown to increase assimilation and ecosystem respiration (i.e., aggregate leaf, stem, root, and soil microbe) in both spring and autumn, in autumn respiration exceeds assimilation (47). Thus, if autumns warm faster than springs the net impact on carbon balance might be negative (47). In addition, autumn extension could increase the potential for frost to affect leaf drop prior to full translocation of nutrients from leaves.
Autumn leaf senescence was not sensitive to warming in evergreen, needle-leaved species. For these, senescence represents loss of the oldest and least functional foliage cohorts. In addition, given the high cost of construction of needle leaves, a resource-conservative strategy means that retranslocation of nutrients may be prioritized over extension of the life of the needle, constraining any delay in senescence.
Geographical Range Predicts Response to Warming.
Across geographic gradients, some studies of leafing phenology have focused on ecotypic variation within species (37, 66–69), while others compared species with different climatic ranges (29, 40, 70, 71). Results of the role of geography provide conflicting or incomplete stories. For example, there are few comparisons of whether populations or species from cold versus warm climates differ in sensitivity to spring warming. Some suggest that there is little difference in sensitivity (70), while others find that those from colder regions are more sensitive to spring warming (37). Recent work suggests that species from geographic regions with short winters rely on photoperiodism (29). Studies of chilling requirements suggest strong clinal variation but the direction of response can differ among species. Some studies found that northern populations have longer chill requirements than southern (32, 67), whereas others show the opposite (37, 72). Differences among studies may be related to local climate conditions. For example, several studies have suggested that maritime populations have longer chill requirements to guard against improper leafing during the warm–cold fluctuations common near oceans (37, 41). Recently, Zohner and colleagues showed that species from geographic regions with higher spring temperature variability have higher chill requirements (40). Our results also point to geographic–climate history as a key factor in leaf-out strategies. We saw stronger response to spring warming in more boreal species. For species from northern latitudes, the selective pressure for chilling and photoperiod may be absent or lessened given the predictability of onset of forcing temperatures. In this case, forcing dominates the cues for budburst making these species more responsive to early spring forcing. This means that these species have enhanced ability to take advantage of early springs that are predicted under climate change but also are more at risk for tissue damage due to a late season frost (15, 73). There is evidence that while onset of spring temperatures is advancing, the date of last frost is not or less so (1, 74).
Experimental Warming Matches that Induced by Natural Variation.
Natural interannual variation in spring temperatures across our study years allowed us to integrate natural changes in spring temperatures with those induced by our experiment. In the quest to understand ecosystem responses to climate change, field observations across climate gradients or through time and experimental manipulations of climate offer complementary approaches, both with strengths and weaknesses (16, 55). Few studies integrate both approaches (16). In our study, integrating natural with experimental changes in temperature revealed consistent budburst responses to warming; i.e., responses were similar to experimental and natural temperature variation. This suggests that our experiments were realistic manipulations of natural climate forcing and contrast with recent critiques of the value of inferences gained from warming experiments compared to long-term records (23, 24). A meta-analysis suggested that experiments showed a systematically lower effect size of warming compared to long-term records (23). Our results do not support these conclusions. The bivariate relationship of day of year of budburst versus spring temperature experienced to that point in time results in a common line along which phenological responses shift among years and with experimental warming (Fig. 4). The fact that the slope of the relationship between spring temperature and date of budburst is constant regardless of warming treatment provides strong support for our supposition that our experiments do not systematically underestimate the effect of warming on phenology. Our average effect sizes were lower than the 5 d/degree warming predicted for many long-term datasets (23). However, averages of effect size in short-term experiments may not be the best metric to judge the efficacy of experiments as they mask patterns of response: we found that in years with early, warm springs, effect sizes were similar to historical records.
In summary, projections of future climate change indicate that weather will be more variable with greater extremes, springs will occur earlier and autumns later, last spring frost dates will not change as rapidly as spring warming, and in northern regions winter will warm more than other seasons (1, 74). Coupling these projections with our experimental data suggests a future with more variable phenology among years and among species, greater spread among species especially in early springs and autumns, greater risk of inappropriate leafing (e.g., in January through March) or frost damage of those species most sensitive to forcing temperatures and lower response to climate change in chill sensitive species. Differences among species in phenological responses could be one mechanism for shifts in the species interactions (e.g., competitive hierarchies, trophic interactions) that structure current communities. Our research suggests changing community patterns of growing season length, carbon gain, and risk. These patterns could affect species interactions, such as plant–herbivore interactions, and ecosystem processes, such as carbon and nutrient cycling. Finally, the fact that effect size of warming differs among years suggests that models that employ fixed parameters (e.g., base temperature), fail to account for interacting factors (e.g., chilling) or ignore the potential for changing windows of developmental sensitivity for phenology may not adequately represent interannual patterns of phenology.
Materials and Methods
We established open-air warming systems at two sites, ∼150 km apart, in forests that span the transition from temperate to boreal biomes in northern Minnesota. One site was located close to the boreal-temperate ecotone boundary at the Cloquet Forestry Center (CFC), Cloquet, Minn. (46°40′46″ N, 92°31′12 W, 382 m a.s.l., 4.5 °C mean annual temperature [MAT], 807 mm mean annual precipitation [MAP]) and a higher latitude site was at the Hubachek Wilderness Research Center (HWRC) near Ely, Minn., (47°56′ 46″ N, 91°45′29″ W, 415 m a.s.l., 3.0 °C MAT, 722 mm MAP). Both sites had coarse-textured upland soils and research plots were located in 40 to 60 y old mixed aspen–birch–fir stands scattered with pine, spruce, and other species. Plots occur in both closed (∼5% to 10% of full sunlight) and relatively open (∼40% to 60% of full sunlight) overstory conditions. We included both closed and open plots, because regeneration in both habitat types is important in determining boreal forest canopy composition, given the spatial and temporal patterns of natural and anthropogenic disturbances (75–77). Each site had six experimental blocks. Blocks designated as open canopy sites were harvested and stumps cut to less than 30 cm in winter 2006 and 2007 at CFC and early spring 2008 at HWRC. Blocks were brush cut to remove existing saplings and shrubs. Plots were established by selecting patches of level ground at least 1 m from canopy trees or remaining stumps and at least 2 m from one another.
Experimental Design.
The experimental design was a 2 (site) × 2 (habitat) × 3 (warming treatment) factorial, with 6 replicates (2 per block) for a total of 72, 7.1-m2 circular plots. Treatments included two target levels of simultaneous plant and soil warming (+ 1.7 °C, +3.4 °C) and an ambient control. Treatment targets were chosen to bracket the anticipated warming ∼75 to 100 y from now (78) and to enable assessment of nonlinear responses to warming.
Belowground warming was accomplished through buried resistance-type heating cable systems. Resistance-heating cables (146 m, 240V VAC, GX, Devi A/B, Denmark) were installed by hand at a depth of 10 and 20 cm apart. For ambient plots, a PVC tube matching the resistance cable sleeve was buried to emulate the soil disturbance of the warmed treatments. Aboveground heating was achieved in open air (i.e., without chambers) following established methods with some adaptations to the forested ecosystem and treatment levels. Ceramic heating elements (Model FTE-1000, 240V, 245 mm × 60 mm; Mor Electric Heating Assoc.) warm aboveground structures (see ref. 79 for details of experimental design and performance).
Planting of Seedlings.
We planted juveniles of 10 common tree species in the boreal-temperate ecotone into each experimental plot. Seedlings planted were obtained from the Minnesota Department of Natural Resources nursery program. All seeds came from Minnesota sources <80 km from the Cloquet site. Species included five boreal and five temperate species that represented a mix of ecological tolerances (SI Appendix, Table S6). Approximately, 10 2-y-old bare root individuals of each species were planted into each plot in May 2008 into the existing vegetation at each plot for a total of 8,712 juveniles planted. In 2012, we initiated a second planting as we harvested individuals that had grown too large for the treatment plots. In the open canopy plots, we harvested all but two individuals per species per plot. In the closed canopy habitat we harvested ∼50% of the individuals of each species in each plot.
Phenology Measurements.
Phenology measurements were made on the 2008 cohort in all years (2009 to 2013). In 2012 and 2013, we supplemented our sampling with 2012 cohort trees as necessary to obtain a sufficient sample size (two individuals/species/plot). In 2012, for both habitats, we measured autumn phenology on both 2008 and 2012 cohorts using ∼50% of individuals of each. In 2013, in the closed canopy habitat, we sampled spring and autumn phenology on ∼50% trees from the 2008 cohort and 50% from 2012 cohort trees, whereas in open canopy we sampled only 2012 cohort trees. As there were some differences in cohort behavior we included cohort as a random effect in our statistical models.
We measured phenology every 3 to 4 d (twice weekly) in spring and autumn. From mid June to mid August plants were censused weekly. We censused two individuals per plot and scored phenological state as a binary variable (yes, no). We used two phenophases to define the growing season: budbreak and start of leaf senescence. We defined budbreak when bud scales were parted revealing underlying leaf tissue. Start of leaf senescence marked the beginning of breakdown of chlorophyll that revealed underlying pigments. Plants were scored "yes" in this category when ∼1/3 of leaves were in this state (of those that would senescence for conifers).
Observers were trained in phenology monitoring using a 35-page protocol that included pictures and detailed descriptions of the characteristics of each phase for each species. New observers worked with staff until they consistently scored phenophases the same as long-term staff and each other. Observers regularly compared observations for consistency including sending real-time images using smartphones to each other and to long-term staff.
Statistical Analysis.
In our analysis, we focus on two phenophases, budbreak and start of leaf senescence, as key indicators of the start and end of the growing season for the majority of our species. Using our event-based binary data, we determined the first day that each individual was in a particular phenophase (budbreak or start of leaf senescence) for each year. We used date of budbreak (BB) and date of start of leaf senescence (SLS) to calculate length of the growing season (GSL) as SLS minus BB. We only examined change in growing season length for the seasonally deciduous species. The start and end of the growing season for conifers corresponds to up- and down-regulation of photosynthesis in evergreen needles. The timing of this may not correspond to leafing phenology. We used a mixed-effect model to test the individual effects of year, warming treatment, canopy and species, and their two- and three-way interactions. Site, block, plot, and seedling cohort were random effects. Block was nested in site and plot was nested in block. While we included conifers in analyses of budburst and senescence phenology, we did not include evergreen conifers in our analysis of growing season length as their active growing season is decoupled from the timing of the appearance of new leaves and loss of leaves from the oldest cohort.
If forcing temperatures are the only cue for budburst, then growing degree days accumulated at time of budburst should not vary across years or warming treatments. To test this, we calculated the growing degree days at time of budburst as follows:
where T stands for temperature. We assumed Tbase to be 4 °C and used the minimum and maximum temperature settings from sensors located within research plots. Our results do not change if a slightly higher or lower Tbase is used. We summed GDD from January 1 until budburst. We then ran the same mixed effects models as described above with GDD as the response variable.
To examine factors contributing to variation in the effect size of warming, we first calculated the effect size per degree of warming. For each species in each warming treatment in each block, we calculated the average date of budburst (10 species × 3 warming treatments × 12 blocks = 360). For each species, we calculated the difference in budburst between seedlings grown in ambient versus elevated conditions (deltaBB) by site, canopy, and block (10 species × 2 sites × 2 canopy × 3 blocks = 120). For each block, we calculated the difference in air temperature between each warming treatment and the ambient treatment for each day from the date the treatment was turned on to the average date of budburst for each species (deltaT; 10 species × 12 blocks [2 sites × 2 canopy × 3 blocks] = 120). We used these data to calculate the change in budburst per degree Celsius warmed by dividing deltaBB by deltaT for each species × site × canopy combination. We compared the effect size of warming to a measure of spring weather calculated as the average temperature between March 15 and May 15 temperature (TMAM) in ambient treatments for each year, site, canopy, and block (n = 12). In addition, we compared effect size of warming to an index of the center of the latitudinal range of each species in central North America (42). The center of the latitudinal range distribution (west of 86 degrees longitude and east of 98 degrees longitude) was defined as the midpoint between the northern and southern range limits and obtained for all species from range maps in the US Forest Service publications, the Silvics of North America, Volumes 1 and 2 (80). We used a mixed-effect model to test the individual effects of warming treatment, canopy, and index of latitudinal range and their two- and three-way interactions. Year, site, and block were random effects. Block was nested in site. Graphical examination of these data suggested that broad-leaved, deciduous trees had a different response compared to needle-leaved evergreen. We thus ran the prior model but included plant functional group (evergreen or deciduous) as another predictor variable.
To examine whether our treatments led to changes in phenology similar to that induced by natural variation, we calculated the average temperature March 15 to May 15 temperature (TMAM) for each year, site, canopy condition, and warming treatment. We calculated the average day of year of budburst for each year, site, canopy condition, warming treatment, and species. We then used TMAM and warming treatment as explanatory variables to predict day of year of budburst. An overall model included species as a random effect. We also ran species-specific models. The interaction between TMAM and warming treatment provided a test of whether our treatment responses matched response to natural variation in spring weather.
Data Availability.
Data are available at the Environmental Data Initiative, https://environmentaldatainitiative.org/ (81).
Supplementary Material
Acknowledgments
We thank the numerous summer interns who contributed to collection of phenological data and the staff at HWRC and CFC for ongoing support of the research. We thank three anonymous reviewers whose comments improved the manuscript. This work was funded by the US Department of Energy, Grant DE-FG02-07ER64456; the Wilderness Research Foundation; and Minnesota Agricultural Experiment Station, project MIN-42-060 (R.A.M.) and MIN-42-050 (P.B.R.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Data used for all analyses are available at the Data Repository for University of Minnesota (DRUM), https://conservancy.umn.edu/handle/11299/212268. This includes datasets of budburst, senescence, growing season length, and growing degree days at the time of budburst.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1917508117/-/DCSupplemental.
References
- 1.Schwartz M. D., Ahas R., Aasa A., Onset of spring starting earlier across the Northern Hemisphere. Glob. Change Biol. 12, 343–351 (2006). [Google Scholar]
- 2.Parmesan C., Yohe G., A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Root T. L., et al. , Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Menzel A., Fabian P., Growing season extended in Europe. Nature 397, 659 (1999). [Google Scholar]
- 5.Ahl D. E., et al. , Monitoring spring canopy phenology of a deciduous broadleaf forest using MODIS. Remote Sens. Environ. 104, 88–95 (2006). [Google Scholar]
- 6.Zhu W., et al. , Extension of the growing season due to delayed autumn over mid and high latitudes in North America during 1982-2006. Glob. Ecol. Biogeogr. 21, 260–271 (2012). [Google Scholar]
- 7.Prevéy J. S., et al. , Warming shortens flowering seasons of tundra plant communities. Nat. Ecol. Evol. 3, 45–52 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Inouye D. W., Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89, 353–362 (2008). [DOI] [PubMed] [Google Scholar]
- 9.CaraDonna P. J., Iler A. M., Inouye D. W., Shifts in flowering phenology reshape a subalpine plant community. Proc. Natl. Acad. Sci. U.S.A. 111, 4916–4921 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherry R. A., et al. , Divergence of reproductive phenology under climate warming. Proc. Natl. Acad. Sci. U.S.A. 104, 198–202 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norby R. J., Hartz-Rubin J. S., Verbrugge M. J., Phenological responses in maple to experimental atmospheric warming and CO2 enrichment. Glob. Change Biol. 9, 1792–1801 (2003). [Google Scholar]
- 12.Bronson D. R., Gower S. T., Tanner M., Van Herk I., Effect of ecosystem warming on boreal black spruce bud burst and shoot growth. Glob. Change Biol. 15, 1534–1543 (2009). [Google Scholar]
- 13.Gunderson C. A., et al. , Forest phenology and a warmer climate–Growing season extension in relation to climatic provenance. Glob. Change Biol. 18, 2008–2025 (2012). [Google Scholar]
- 14.Clark J. S., Melillo J., Mohan J., Salk C., The seasonal timing of warming that controls onset of the growing season. Glob. Change Biol. 20, 1136–1145 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Richardson A. D., et al. , Ecosystem warming extends vegetation activity but heightens vulnerability to cold temperatures. Nature 560, 368–371 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Dunne J. A., Saleska S. R., Fischer M. L., Harte J., Integrating experimental and gradient methods in ecological climate change research. Ecology 85, 904–916 (2004). [Google Scholar]
- 17.Wadgymar S. M., Ogilvie J. E., Inouye D. W., Weis A. E., Anderson J. T., Phenological responses to multiple environmental drivers under climate change: Insights from a long-term observational study and a manipulative field experiment. New Phytol. 218, 517–529 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Dunne J. A., Harte J., Taylor K. J., Subalpine meadow flowering phenology responses to climate change: Integrating experimental and gradient methods. Ecol. Monogr. 73, 69–86 (2003). [Google Scholar]
- 19.Price M. V., Waser N. M., Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 79, 1261–1271 (1998). [Google Scholar]
- 20.Fu Y. S. H., et al. , Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc. Natl. Acad. Sci. U.S.A. 111, 7355–7360 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchin R. M., Salk C. F., Hoffmann W. A., Dunn R. R., Temperature alone does not explain phenological variation of diverse temperate plants under experimental warming. Glob. Change Biol. 21, 3138–3151 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Thackeray S. J., et al. , Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Wolkovich E. M., et al. , Warming experiments underpredict plant phenological responses to climate change. Nature 485, 494–497 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Polgar C. A., et al. , Tree leaf out response to temperature: Comparing field observations, remote sensing, and a warming experiment. Int. J. Biometeorol. 58, 1251–1257 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Klosterman S., Hufkens K., Richardson A. D., Later springs green-up faster: The relation between onset and completion of green-up in deciduous forests of North America. Int. J. Biometeorol. 62, 1645–1655 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Zohner C. M., Mo L., Renner S. S., Global warming reduces leaf-out and flowering synchrony among individuals. eLife 7, e40214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laube J., et al. , Chilling outweighs photoperiod in preventing precocious spring development. Glob. Change Biol. 20, 170–182 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Nanninga C., Buyarski C. R., Pretorius A. M., Montgomery R. A., Increased exposure to chilling advances the time to budburst in North American tree species. Tree Physiol. 37, 1727–1738 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Zohner C. M., Benito B. M., Svenning J.-C., Renner S. S., Day length unlikely to constrain climate-driven shifts in leaf-out times of northern woody plants. Nat. Clim. Chang. 6, 1120–1123 (2016). [Google Scholar]
- 30.Basler D., Körner C., Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agric. Meteorol. 165, 73–81 (2012). [Google Scholar]
- 31.Chmura H. E., et al. , The mechanisms of phenology: The patterns and processes of phenological shifts. Ecol. Monogr. 89, e01337 (2019). [Google Scholar]
- 32.Perry T. O., Wu W. C., Genetic variation in the winter chilling requirement for date of dormancy break for Acer rubrum. Ecology 41, 790–794 (1960). [Google Scholar]
- 33.Cannell M. G. R., Smith R. I., Climatic warming, spring budburst and frost damage on trees. J. Appl. Ecol. 23, 177–191 (1986). [Google Scholar]
- 34.Hunter A. F., Lechowicz M. J., Predicting the timing of budburst in temperate trees. J. Appl. Ecol. 29, 597–604 (1992). [Google Scholar]
- 35.Morin X., et al. , Leaf phenology in 22 North American tree species during the 21st century. Glob. Change Biol. 15, 961–975 (2009). [Google Scholar]
- 36.Partanen J., Koski V., Hänninen H., Effects of photoperiod and temperature on the timing of bud burst in Norway spruce (Picea abies). Tree Physiol. 18, 811–816 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Myking T., Heide O. M., Dormancy release and chilling requirement of buds of latitudinal ecotypes of Betula pendula and B. pubescens. Tree Physiol. 15, 697–704 (1995). [DOI] [PubMed] [Google Scholar]
- 38.Caffarra A., Donnelly A., Chuine I., Jones M. B., Modelling the timing of Betula pubescens budburst. I. Temperature and photoperiod: A conceptual model. Clim. Res. 46, 147–157 (2011). [Google Scholar]
- 39.Gill A. L., et al. , Changes in autumn senescence in northern hemisphere deciduous trees: A meta-analysis of autumn phenology studies. Ann. Bot. 116, 875–888 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zohner C. M., Benito B. M., Fridley J. D., Svenning J.-C., Renner S. S., Spring predictability explains different leaf-out strategies in the woody floras of North America, Europe and East Asia. Ecol. Lett. 20, 452–460 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Campbell R. K., Sugano A. I., Phenology of bud burst in douglas-fir related to provenance, photoperiod, chilling, and flushing temperature. Bot. Gaz. 136, 290–298 (1975). [Google Scholar]
- 42.Reich P. B., et al. , Geographic range predicts photosynthetic and growth response to warming in co-occurring tree species. Nat. Clim. Chang. 5, 148–152 (2015). [Google Scholar]
- 43.Rice K. E., Montgomery R. A., Stefanski A., Rich R. L., Reich P. B., Experimental warming advances phenology of groundlayer plants at the boreal-temperate forest ecotone. Am. J. Bot. 105, 851–861 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Richardson A. D., et al. , Influence of spring phenology on seasonal and annual carbon balance in two contrasting New England forests. Tree Physiol. 29, 321–331 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Richardson A. D., et al. , Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philos. Trans. R. Soc. B 365, 3227–3246 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dragoni D., et al. , Evidence of increased net ecosystem productivity associated with a longer vegetated season in a deciduous forest in south-central Indiana, USA. Glob. Chang. Biol. 17, 886–897 (2011). [Google Scholar]
- 47.Piao S., et al. , Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451, 49–52 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Oleksyn J., Reich P. B., Tjoelker M. G., Chalupka W., Biogeographic differences in shoot elongation pattern among European Scots pine populations. For. Ecol. Manage. 148, 207–220 (2001). [Google Scholar]
- 49.Chuine I., Beaubien E. G., Phenology is a major determinant of tree species range. Ecol. Lett. 4, 500–510 (2001). [Google Scholar]
- 50.Morin X., Augspurger C., Chuine I., Process-based modeling of species’ distributions: What limits temperate tree species’ range boundaries? Ecology 88, 2280–2291 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Chuine I., Why does phenology drive species distribution? Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 3149–3160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolkovich E. M., Cleland E. E., The phenology of plant invasions: A community ecology perspective. Front. Ecol. Environ. 9, 287–294 (2011). [Google Scholar]
- 53.Wolkovich E. M., Cleland E. E., Phenological niches and the future of invaded ecosystems with climate change. AoB Plants 6, plu013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Searle K. R., Rice M. B., Anderson C. R., Bishop C., Hobbs N. T., Asynchronous vegetation phenology enhances winter body condition of a large mobile herbivore. Oecologia 179, 377–391 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Harte J., Kueppers L. M., Insight from integration. Nature 485, 449 (2012). [Google Scholar]
- 56.Cohen J. M., Lajeunesse M. J., Rohr J. R., A global synthesis of animal phenological responses to climate change. Nat. Clim. Chang. 8, 224–228 (2018). [Google Scholar]
- 57.Cannell M. G. R., Smith R. I., Thermal time, chill days and prediction of budburst in Picea sitchensis. J. Appl. Ecol. 20, 951–963 (1983). [Google Scholar]
- 58.Harrington C. A., Gould P. J., St.Clair J. B., Modeling the effects of winter environment on dormancy release of Douglas-fir. For. Ecol. Manage. 259, 798–808 (2010). [Google Scholar]
- 59.Wang C., Tang Y., Chen J., Plant phenological synchrony increases under rapid within-spring warming. Sci. Rep. 6, 25460 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chuine I., Régnière J., Process-based models of phenology for plants and animals. Annu. Rev. Ecol. Evol. Syst. 48, 159–182 (2017). [Google Scholar]
- 61.Clark J. S., Salk C., Melillo J., Mohan J., Tree phenology responses to winter chilling, spring warming, at north and south range limits. Funct. Ecol. 28, 1344–1355 (2014). [Google Scholar]
- 62.Harrington R. A., Brown B. J., Reich P. B., Fownes J. H., Ecophysiology of exotic and native shrubs in southern Wisconsin: II. Annual growth and carbon gain. Oecologia 80, 368–373 (1989). [DOI] [PubMed] [Google Scholar]
- 63.Wood E. M., Pidgeon A. M., Liu F., Mladenoff D. J., Birds see the trees inside the forest: The potential impacts of changes in forest composition on songbirds during spring migration. For. Ecol. Manage. 280, 176–186 (2012). [Google Scholar]
- 64.Wood E. M., Pidgeon A. M., Peters D. P. C., Extreme variations in spring temperature affect ecosystem regulating services provided by birds during migration. Ecosphere 6, art216 (2015). [Google Scholar]
- 65.Bauerle W. L., et al. , Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proc. Natl. Acad. Sci. U.S.A. 109, 8612–8617 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kreibel H. B., Wang C.-W., The interaction between provenance and degree of chilling on bud-break of sugar maple. Silvae Genet. 11, 125–130 (1962). [Google Scholar]
- 67.Nienstaedt H., Chilling requirements in seven Picea species. Silvae Genet. 16, 65–68 (1967). [Google Scholar]
- 68.Oleksyn J., Tjoelker M. G., Reich P. B., Growth and biomass partitioning of populations of European Pinus sylvestris L under simulated 50-degrees and 60-degrees-N daylengths–Evidence for photoperiodic ecotypes. New Phytol. 120, 561–574 (1992). [Google Scholar]
- 69.Oleksyn J., et al. , Growth and physiology of Picea abies populations from elevational transects: Common garden evidence for altitudinal ecotypes and cold adaptation. Funct. Ecol. 12, 573–590 (1998). [Google Scholar]
- 70.Vitasse Y., et al. , Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 149, 735–744 (2009). [Google Scholar]
- 71.Zohner C. M., Renner S. S., Common garden comparison of the leaf-out phenology of woody species from different native climates, combined with herbarium records, forecasts long-term change. Ecol. Lett. 17, 1016–1025 (2014). [DOI] [PubMed] [Google Scholar]
- 72.Sharik T. L., Barnes B. V., Phenology of shoot growth among diverse populations of yellow birch (Betula alleghaniensis) and sweet birch (B. lenta). Can. J. Bot. 54, 2122–2129 (1976). [Google Scholar]
- 73.Augspurger C. K., Spring 2007 warmth and frost: Phenology, damage and refoliation in a temperate deciduous forest. Funct. Ecol. 23, 1031–1039 (2009). [Google Scholar]
- 74.Liu Q., et al. , Extension of the growing season increases vegetation exposure to frost. Nat. Commun. 9, 426 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frelich L. E., Reich P. B., Spatial patterns and succession in a Minnesota southern-boreal forest. Ecol. Monogr. 65, 325–346 (1995). [Google Scholar]
- 76.Grigal D. F., Ohmann L. F., Classification, description, and dynamics of upland plant communities within a Minnesota wilderness area. Ecol. Monogr. 45, 389–407 (1975). [Google Scholar]
- 77.Heinselman M. L., Fire in the virgin forest of the Boundary Waters Canoe Area, Minnesota. J Quat Res 3, 329–382 (1973). [Google Scholar]
- 78.IPCC “Climate change 2014: Synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change” (IPCC, Geneva, Switzerland, 2014). [Google Scholar]
- 79.Rich R. L., et al. , Design and performance of combined infrared canopy and belowground warming in the B4WarmED (Boreal Forest Warming at an Ecotone in Danger) experiment. Glob. Change Biol. 21, 2334–2348 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Burns R. M., Honkala B. H., “Silvics of North America” in Agriculture Handbook 654 (U.S. Department of Agriculture, Forest Service, Washington, DC, 1990), Vol. 2. [Google Scholar]
- 81.Montgomery R., et al. , Phenological data (2009-2013) for ten tree species grown under experimental warming in northern Minnesota, USA. Data Repository for the University of Minnesota. https://conservancy.umn.edu/handle/11299/212268. Deposited 25 March 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at the Environmental Data Initiative, https://environmentaldatainitiative.org/ (81).