Abstract
Purpose
The purpose of this study was to compare Words-in-Noise (WIN) data between young adults with perinatal HIV (PHIV) infection and those with PHIV exposure but uninfected (PHEU) and to evaluate associations between antiretroviral therapy (ART) exposures and WIN data.
Method
The WIN test and cognitive function were assessed in participants of the Pediatric HIV/AIDS Cohort Study Adolescent Master Protocol Up. Impaired WIN (IWIN) performance was defined as a signal-to-babble ratio of > +10 dB. Cognitive function was determined based on fluid cognition composite scores (FCCSs) and crystallized cognition composite scores, and < 70 was considered a fluid or crystallized cognitive impairment. Log binomial models were used to calculate the relative risks of IWIN between PHIV and PHEU.
Results
PHIV (n = 334) and PHEU (n = 52) participants had similar WIN thresholds and IWIN percentages. For young adults with FCCS ≥ 70, participants with PHIV were less likely to have IWIN for the better ear and worse ear as compared to participants with PHEU. For young adults with FCCS < 70, there was no association between HIV status and risk of IWIN for the better ear or worse ear. For those adults with crystallized cognition composite score of ≥ 70, young adults with PHIV were less likely to have IWIN for the better ear than young adults with PHEU; there was no association between HIV status and IWIN for the worse ear. For young adults with PHIV without a Centers for Disease Control and Prevention Class C diagnosis, a longer combination ART duration was associated with a higher risk of IWIN for the better ear.
Conclusions
For those without cognitive impairment, young adults with PHEU had poorer WIN thresholds than those young adults with PHIV. In young adults with PHIV who had no prior Centers for Disease Control and Prevention Class C diagnosis, a longer combination ART duration was associated with IWIN only in the better ear.
Human immunodeficiency virus (HIV) is a virus transmitted via bodily fluids or acquired perinatally at or around the time of birth (PHIV). HIV attacks the body's immune system by destroying CD4 cells, a type of white blood cell (or T-cell), which fight off infections. As the disease progresses, the HIV RNA viral load (amount of HIV virus in the blood) increases, destroying more CD4 cells, leading to the body's inability to fight off other diseases or infections. HIV disease severity is categorized by the Centers for Disease Control and Prevention (CDC) based on various measures of HIV RNA viral load and CD4 count and has been demonstrated to be a useful predictor of outcomes for people living with HIV.
Children living with PHIV are at a greater risk for morbidities during childhood (Slogrove et al., 2012), including episodes of otitis media (Principi et al., 1991; Weber et al., 2006), and have a higher risk of hearing loss compared to HIV-uninfected children (Chao et al., 2012; Taipale et al., 2011; Torre, Cook, et al., 2015). Furthermore, data from the Pediatric HIV/AIDS Cohort Study (PHACS) Adolescent Master Protocol (AMP) indicated that children with PHIV have a higher risk of hearing loss than children who are PHIV-exposed but uninfected (PHEU; Torre et al., 2012). Among PHACS AMP children with PHIV, those with a prior AIDS diagnosis (CDC Class C diagnosis, the most severe HIV disease status) had almost three times the odds of hearing loss compared to those without a prior AIDS diagnosis, and those with a nadir CD4% of < 20 had more than double the odds of hearing loss compared to those with a nadir CD4% of ≥ 20. Nadir CD4% refers to an individual's lowest recorded CD4%, a ratio of CD4 T-cells to total lymphocytes—an indicator of lowest immune functioning. Not included in the PHACS AMP analyses was an evaluation of speech communication abilities since no measures of speech audiometry were obtained as part of the hearing protocol.
The ability of an individual to recognize and repeat back speech stimuli is critical for successful spoken language processing and, ultimately, a person's ability to effectively participate in academic, social, and workplace environments. However, it is a complicated process that is dependent on the effective functioning of the whole information-processing system working together. Initially, successful word recognition requires precise encoding of auditory information in the speech signal, and then neurocognitive and language processes are employed for word recognition and response. There are a number of components of neurocognition and executive function that are key for successful processing and responding that include auditory attention, inhibition, and short-term memory (Roman et al., 2017). Additionally, vocabulary and language comprehension are core factors in a person's ability to provide verbal responses. Impairments in any one of these processes can affect a person's ability to process language, especially in complex listening environments.
Children and adolescents with PHIV are at a greater risk for poorer neurocognitive outcomes (e.g., working memory, processing speed, and perceptual reasoning) compared to children with PHEU (Smith et al., 2012) and HIV-unexposed uninfected children (Bisiacchi et al., 2000). Children with PHIV and early and severe disease progression are at a particular risk for poor neurocognitive and executive functioning outcomes compared to either youth with PHEU or youth with PHIV but slower disease progression. These findings applied even with recovery from high viral load, low CD4 T-cell counts, and a previous AIDS-defining illness (Nichols et al., 2016; Smith et al., 2012). Both groups of youth (PHIV and PHEU) in PHACS AMP had significantly poorer cognition and academic achievement scores compared to population norms (Garvie et al., 2014).
Although there is a complex interplay of factors, hearing and neurocognitive abilities are important contributors to a young adult's ability to accomplish educational goals, obtain and maintain employment, and make responsible health behavior decisions (Blair, 1985; Perkins-Dock et al., 2015; Qi & Mitchell, 2012). Given that children and adolescents with PHIV are at a higher risk for cognitive impairment and hearing loss, early identification is critical to facilitate a positive transition into adulthood as they work to attain age-appropriate milestones, including entering the workforce or continuing their education.
The presence of background noise, including multitalker babble, is an important factor in a person's ability to hear and comprehend speech and can impact their ability to successfully navigate through their day at school or a workplace (Wilson, 2003). Background noise can exacerbate speech misunderstanding for anyone, but particularly for those with hearing loss whose signal (speech) recognition is distorted, and require an increase in signal-to-distortion ratio for effective hearing. Assessing speech recognition in noise can be an important factor in understanding an individual's educational or workplace needs and necessary accommodations. However, routine audiometric procedures do not assess this, nor is there an accepted standardized procedure.
One clinically useful assessment tool, the Words-in-Noise (WIN) test (Wilson, 2003), examines a listener's ability to correctly repeat words in the presence of multitalker babble. It was developed to evaluate communication abilities in military veterans with high-frequency sloping sensorineural hearing loss (Wilson et al., 2003). Later, Wilson et al. (2010) established normative data for the WIN test in children and young adults. However, WIN data have not been collected in young adults with hearing loss or HIV.
Although hearing data exist for both children with HIV (Chao et al., 2012; Maro et al., 2016; Taipale et al., 2011; Torre et al., 2012) and adults living with HIV (ALHIV) (Luque et al., 2014; Maro et al., 2014; Torre, Hoffman, et al., 2015; van der Westhuizen et al., 2013), there are no hearing data or speech communication data in young adults with HIV aged 18–24 years. The overall purpose of this study was to evaluate hearing performance in young adults with PHIV and PHEU who have enrolled in the PHACS AMP Up study. The objectives of this study were (a) to compare WIN test performance between young adults with PHIV and those with PHEU, while accounting for cognitive ability, and (b) to evaluate associations between HIV treatment exposures, known as antiretroviral therapy (ART), and WIN test results adjusting for HIV disease severity measures among PHACS AMP Up young adults with PHIV.
Method
Study Participants
PHACS AMP is a prospective cohort study conducted at 15 sites within the United States and Puerto Rico that enrolled 451 PHIV and 227 PHEU children and adolescents between 2007 and 2009. PHACS AMP Up is a prospective cohort study of young adults who are 18 years and older, enrolling previous PHACS AMP participants with PHIV and with PHEU as well as additional young adults with PHIV. The current data included PHACS AMP Up young adults, most of whom were involved in the original PHACS AMP study and have available lifetime health history. The main goal of PHACS AMP Up is to examine the specific health and psychosocial issues that young adults with PHIV and PHEU face as they transition into adulthood. Participation in the study includes three in-person study visits at entry, 3 years, and 6 years of study enrollment, during which cognitive and WIN hearing assessments are administered; completion of yearly online surveys; and yearly lab testing for sexually transmitted infections. PHACS AMP Up enrollment began in early 2014 and is ongoing with a participation goal of 600 PHIV and 250 PHEU young adults. Due to funding limitations and the extensive PHACS AMP Up research protocol that consisted of an entry visit of 3–5 hr, pure-tone thresholds could not be obtained. As a result, the WIN test as part of the NIH Toolbox Version 2.0 (Gershon et al., 2013) was chosen. The NIH Toolbox is a comprehensive assessment tool for 3- to 85-year-olds that provides tests in cognition, sensation, motor, and emotion. The current study sample data were extracted on July 1, 2018, and included PHACS AMP Up participants with PHIV or PHEU who completed the WIN test at their entry visit. Participants who reported exposure to very loud noise within the 24 hr before completing the exam were excluded.
Outcome Measures
WIN Hearing Test
The WIN hearing test has been shown to be an efficient screener for hearing loss in adults (Wilson et al., 2007), and the test can be administered by someone other than a certified audiologist. The WIN test was set up, earphones were calibrated, and personnel were trained based on the NIH Toolbox Administration Manual (see http://www.NIHToolbox.org for further details). Briefly, each set of headphones (Sennheiser HDA200) was calibrated for each specific soundcard loaded onto a laptop computer or iPad, and this was completed before WIN testing could be performed. Trained PHACS AMP Up staff followed explicit instructions from the NIH Toolbox Administration Manual on how to complete the WIN test, and all testing was completed in a quiet room. Both ears were evaluated in one session. The NIH Toolbox WIN test uses 35 monosyllabic words that vary in presentation level in the presence of a fixed level (70 dB SPL) of multitalker babble. Five words are presented at each of seven signal-to-babble ratios (SBRs) in a descending approach from +24 dB SBR (i.e., signal of 24 dB above the babble) to 0 dB in 4-dB steps. The outcome measure of the WIN test is the interpolated SBR, in dB, where the Spearman–Kärber equation is used to determine the ratio at which the person undergoing testing repeats 50% of the words correctly. Wilson et al. (2003) established that an SBR of +6 dB on the WIN test could be used as the cutoff point to differentiate normal hearing from hearing loss in adults, but other dB SBRs can be used. All the reported WIN tests in this study were conducted in English.
Cognitive Testing
Cognitive ability was also assessed using the NIH Toolbox. Within the cognitive domain measures, there are subdomains including executive function, attention, episodic memory, language, processing speed, and working memory. Measures of cognitive function were obtained from the fluid and crystallized composite scores. The fluid cognition composite score (FCCS) represents primarily nonverbal function, derived from subtests in the executive functioning, attention, episodic memory, processing speed, and working memory subdomains. The crystallized cognition composite score (CCCS) represents primarily verbal function, derived from subtests in the language subdomain.
Exposures
For Objective 1, HIV infection status (i.e., PHIV and PHEU) was the exposure of interest, and for Objective 2, ART was the exposure of interest, with the main focus on duration, in years, of combination ART (cART).
Possible Confounders
Possible confounding variables considered in the analyses included demographic characteristics (age, sex, race, and ethnicity), ever having a pressure equalization tube, substance use (alcohol, marijuana, cigarettes, or other illicit drugs, evaluated individually), period between PHACS AMP hearing examination and PHACS AMP Up WIN test, and years of school completed. For each cognition composite score, an age-adjusted score of 100 (SD = 15) is considered average cognitive ability, a score of < 85 is considered below average cognitive ability, and a score of < 70 is considered a significant cognitive impairment; a cutoff of < 70 was considered an FCCS or CCCS cognitive impairment for the purposes of this analysis. As a result, all analyses were stratified by levels of the age-adjusted FCCS or, separately, the CCCS from the NIH Toolbox.
Possible confounding variables for the analyses restricted to participants with PHIV included historical measures of HIV disease severity. The specific severity variables were the percentage of lifetime HIV RNA viral load measurements of > 400 copies/ml and CD4 nadir (cells per cubic millimeters). Furthermore, current HIV RNA viral load (copies per milliliter), CD4 count (cells per cubic millimeters), CD4% at the time of WIN test, and most severe CDC class were included. Current HIV RNA, CD4 count, and CD4% were all evaluated as both continuous variables and also as dichotomous variables.
Statistical Analyses
WIN performance was defined for a better and worse ear and examined by HIV status in two ways. First, summary statistics of the continuous WIN dB SBR thresholds were presented, and the dB SBR thresholds for the PHIV and PHEU cohorts were compared by the Wilcoxon rank sum test. Second, the proportion of PHIV and PHEU with impaired WIN (IWIN) performance, defined as an SBR > +10 dB (equivalent to < 20 words correct of 35 words presented, categorized as moderate or worse hearing loss in the NIH Toolbox), was calculated as the estimate for the prevalence of IWIN and compared between PHIV and PHEU participants by Fisher exact test for worse ear and better ear separately. Additionally, the prevalence of IWIN was estimated and compared between the cohorts stratified by FCCS (≥ 70, < 70) and by CCCS (≥ 70, < 70).
For better and worse ear WIN data, a log binomial model was used to calculate the unadjusted relative risk (RR) of IWIN based on HIV infection status. Generalized estimating equations were used in all models to account for the correlation in the outcome of participants at the same PHACS clinical site, with an assumed compound symmetry correlation structure. These analyses were further stratified by higher versus lower FCCS and by CCCS, separately. Log binomial models were then fit to determine the adjusted association between HIV status and risk of IWIN including each potential confounder. The percent change in the RR for IWIN with inclusion of each confounder as compared to the unadjusted models was calculated, and only covariates that changed the RR by ≥ 10% were considered confounders and included in the final adjusted model. If more than one variable met the criteria to be considered a confounder, a final multivariable model was constructed that adjusted for all confounders.
The association between duration of cART and the risk of IWIN was assessed in PHACS AMP Up participants with PHIV who had previously been enrolled in PHACS AMP (n = 174) due to consideration of the completeness of the HIV-related measures. These analyses were stratified by CDC class (CDC Class C vs. not CDC Class C) and conducted for the better and worse ear. A log binomial model was used to determine the RR of IWIN per 1-year increase in the duration of cART exposure adjusted for historical HIV disease severity. Additional confounding variables were identified, and adjusted log binomial models were constructed using the same methodology described above by including only those covariates that resulted in changes to the RR for IWIN of at least 10%.
Results
Overall, 561 WIN assessments were completed in PHACS AMP Up as of July 1, 2018, 513 (91%) of which were completed in English. Of these, 402 (78%) had data that met quality control checks for both ears. Data were considered as failing quality control checks if any of the following occurred: no data, no correct word ever recorded, a nonzero dB SBR but no additional data at the next lower dB SBR, a drop-off from five words correct to none from 1 dB SBR to the next, and an increase of more than two words correct from 24 to 20 dB SBR or from 20 to 16 dB SBR. Of the 402 participants, 16 (4%) were exposed to very loud noise within 24 hr before completing the WIN assessment and were excluded from the analysis sample. As a result, there were a total of 386 PHACS AMP Up participants used in this study: 334 had PHIV, and 52 had PHEU.
Those participants with valid WIN data (n = 386) and those participants without WIN data (n = 216) in PHACS AMP Up did not differ significantly by HIV infection status, age at enrollment, or sex. However, the participants with WIN data were more likely to be Black (71% vs. 55%) and less likely to be Hispanic (22% vs. 46%).
Demographic characteristics of the analysis sample by HIV infection status are displayed in Table 1. At the time of WIN assessment, the young adults with PHIV were almost 3 years older than the PHEU group (median age = 21.4 vs. 18.5 years). There was no difference between the two cohorts in sex, race, or ethnicity distributions. The percentage reporting substance use (including history of drinking alcohol, using marijuana, smoking cigarettes/e-cigarettes, or other illicit drugs) was similar by HIV status. More of the youth with PHIV had a history of pressure equalization tubes (10% compared to 2% in PHEU). In addition, more young adults with PHIV (11%) left high school without a degree than young adults with PHEU (4%).
Table 1.
Demographic characteristics of the Pediatric HIV/AIDS Cohort Study Adolescent Master Protocol Up participants with valid Words-in-Noise data by HIV status.
| Characteristic | PHIV (n = 334) | PHEU (n = 52) | Total (N = 386) | p * | |
|---|---|---|---|---|---|
| Age (years) | Median (Q1, Q3) | 21.35 (19.10, 25.00) | 18.50 (18.15, 19.65) | 20.80 (18.80, 24.50) | < .01 |
| Sex | Male | 124 (37%) | 24 (46%) | 148 (38%) | .22 |
| Race | Black | 241 (72%) | 34 (65%) | 275 (71%) | .33 |
| Ethnicity | Hispanic | 70 (21%) | 13 (25%) | 83 (22%) | .59 |
Note. Values shown are median and interquartile range (Q1, Q3) or n (%). PHIV = perinatal HIV; PHEU = perinatal HIV exposure but uninfected.
P value by Wilcoxon rank sum test for continuous covariates and by Fisher exact test for binary covariates.
Medians and interquartile ranges (Q1, Q3) for FCCS and CCCS along with percentages of participants defined as being cognitively impaired based on the NIH Toolbox Cognition Domain are presented in Table 2. Median age-corrected FCCS and CCCS were similar in young adults with PHIV and those with PHEU. Additionally, when participants were categorized by cognitive impairment, 33% and 6% were defined as being cognitively impaired based on FCCS and CCCS, respectively, for both cohorts of young adults with PHIV and PHEU. In general, however, more cognition data were missing from the PHIV group compared to the PHEU group.
Table 2.
Cognition data from the NIH Toolbox, specifically fluid and crystallized cognition age-corrected composite scores, by HIV status.
| Cognition data | PHIV (n = 334) | PHEU (n = 52) | Total (N = 386) | p * |
|---|---|---|---|---|
| Fluid composite cognition score (FCCS): age corrected, median (Q1, Q3) | 77.3 (65.6, 92.0) a | 78.1 (67.0, 88.0) b | 77.4 (65.8, 91.0) | .90* |
| FCCS: age corrected, < 70 | ||||
| Yes | 95 (33%) | 16 (33%) | 111 (33%) | 1.00 |
| No | 194 (67%) | 33 (67%) | 227 (67%) | |
| Unknown | 45 | 3 | 48 | |
| Crystallized composite cognition score (CCCS): age corrected, median (Q1, Q3) | 93.0 (81.1, 104.0) c | 93.0 (83.5, 106.0) b | 93.0 (82.0, 104.0) | .65 |
| CCCS: age corrected, < 70 | ||||
| Yes | 17 (6%) | 3 (6%) | 20 (6%) | 1.00 |
| No | 269 (94%) | 46 (94%) | 315 (94%) | |
| Unknown | 48 | 3 | 51 |
Note. Values shown are median and interquartile range (Q1, Q3) or n (%). “Yes” indicates those who had a fluid or crystallized cognitive impairment. “No” indicates those who did not have a fluid or crystallized cognitive impairment. PHIV = perinatal HIV; PHEU = perinatal HIV exposure but uninfected.
Forty-five missing.
Three missing.
Forty-eight missing.
P value by Wilcoxon rank sum test for continuous covariates and by Fisher exact test for binary covariates.
WIN Performance and HIV Infection Status
WIN thresholds and percentages of participants with IWIN for the better and worse ear are displayed in Table 3, by HIV infection status. Without considering cognitive test results, PHIV and PHEU participants had similar WIN thresholds, but young adults with PHIV had slightly lower percentages of IWIN for the better and worse ear. For example, 19% of young adults with PHIV had IWIN for the worse ear, while 23% of young adults with PHEU had IWIN for the worse ear.
Table 3.
Better ear and worse ear Words-in-Noise (WIN) scores by HIV status.
| WIN outcome | PHIV (n = 334) | PHEU (n = 52) | Total (N = 386) | p * |
|---|---|---|---|---|
| Better ear: WIN score, median (Q1, Q3) | 4.8 (3.6, 6.8) | 5.2 (2.8, 7.6) | 5.2 (3.6, 6.8) | .50 |
| Better ear: IWIN (SBR > +10 dB) | 15 (4%) | 4 (8%) | 19 (5%) | .30 |
| Worse ear: WIN score, median (Q1, Q3) | 7.6 (5.2, 9.2) | 7.2 (5.2, 10.0) | 7.6 (5.2, 9.2) | .75 |
| Worse ear: IWIN (SBR > +10 dB) | 63 (19%) | 12 (23%) | 75 (19%) | .46 |
Note. Values shown are median and interquartile range (Q1, Q3) or n (%). PHIV = perinatal HIV; PHEU = perinatal HIV exposure but uninfected.
P value by Wilcoxon rank sum test for continuous outcomes and by Fisher exact test for binary outcomes.
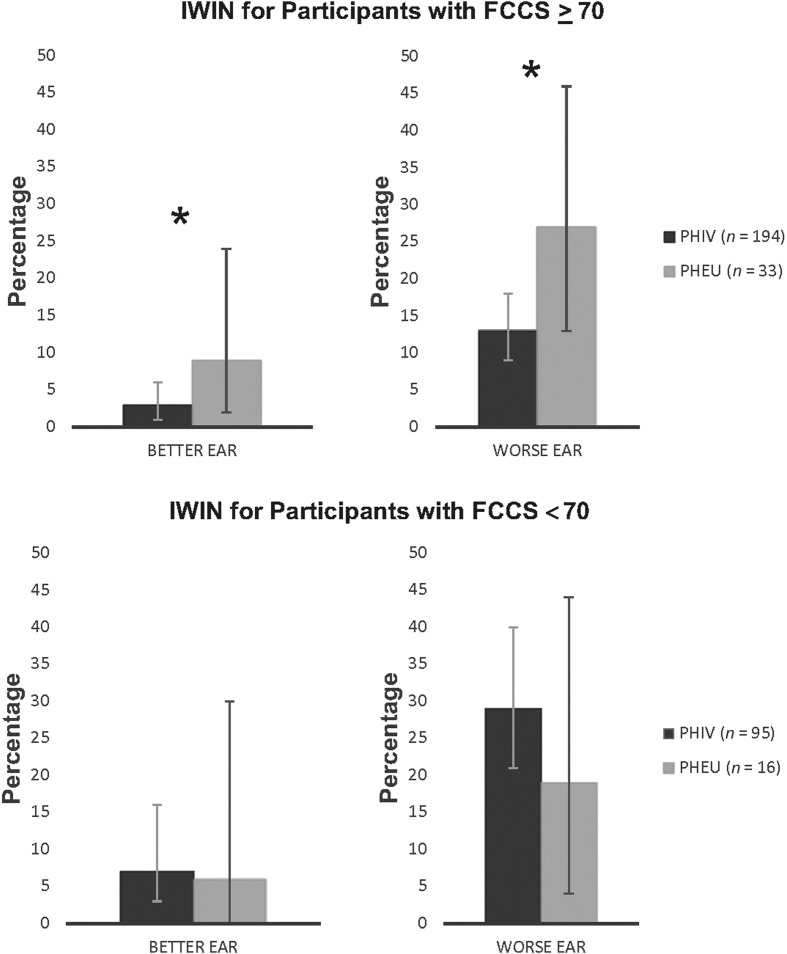
IWIN percentages are shown for better and worse ear for young adults with PHIV and PHEU, stratified by FCCS (see Figure 1) and CCCS (see Figure 2). In Figure 1, for young adults with FCCS ≥ 70, participants with PHIV were significantly less likely to have IWIN for the better ear as compared to PHEU participants (RR = 0.28, 95% confidence interval [CI] [0.12, 0.65], p = .003). Similarly, young adults with PHIV and FCCS ≥ 70 were significantly less likely to have IWIN for the worse ear than PHEU young adults (RR = 0.52, 95% CI [0.30, 0.90], p = .02). No variables met the criteria to be considered confounders of this association for both the better ear and the worse ear. For young adults with FCCS < 70, after adjusting for alcohol use, there was no association between HIV status and IWIN for the better ear (RR = 0.87, 95% CI [0.35, 2.16]), and after adjusting for age and alcohol use, there was again no association between HIV status and IWIN for worse ear data (RR = 1.21, 95% CI [0.51, 2.85]).
Figure 1.
The percentages for IWIN for the better ear and worse ear are shown for PHIV and PHEU young adults stratified by those with FCCS ≥ 70 (top panel) and those with FCCS < 70 (bottom panel). Error bars represent 95% confidence intervals. Asterisk (*) indicates statistically significant differences with p < .05 by Fisher exact test. IWIN = impaired Words-in-Noise; PHIV = perinatal HIV infection; PHEU = perinatal HIV exposure but uninfected; FCCS = fluid cognition composite score.
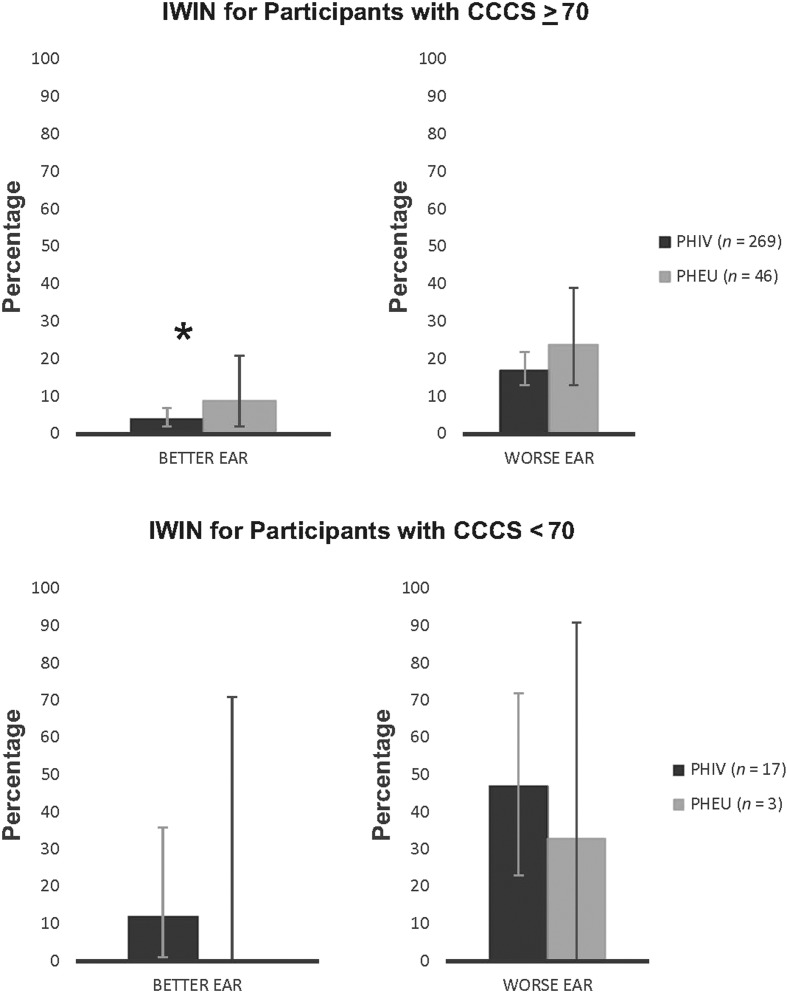
Figure 2.
The percentages for IWIN for the better ear and worse ear are shown for PHIV and PHEU young adults stratified by those with CCCS ≥ 70 (top panel) and those with CCCS < 70 (bottom panel). Error bars represent 95% confidence intervals. Note that no PHEU young adult had IWIN for the better ear. Asterisk (*) indicates statistically significant differences with p < .05 by Fisher exact test. IWIN = impaired Words-in-Noise; PHIV = perinatal HIV infection; PHEU = perinatal HIV exposure but uninfected; CCCS = crystallized cognition composite score.
In Figure 2, for those participants with CCCS ≥ 70, after adjusting for cigarette smoking, young adults with PHIV were less likely to have IWIN for the better ear than PHEU young adults (RR = 0.46, 95% CI [0.25, 0.82], p = .009). However, for worse ear data, after adjusting for age, this association between HIV status and IWIN was weaker (RR = 0.69, 95% CI [0.44, 1.10]). There were only 20 participants (17 PHIV and three PHEU) with CCCS < 70, and as a result, there were too few participants for subsequent statistical analyses for this group.
Because young adults with PHEU had poorer WIN thresholds compared to young adults with PHIV, contrary to published research, the histories of ever being diagnosed with (a) attention-deficit/hyperactivity disorder, (b) learning disorder, or (c) speech/language/communication disorder were evaluated. Young adults with FCCS or CCCS < 70 had higher percentages of these diagnoses. Stratified by FCCS or CCCS, there was a higher percentage of young adults with PHIV who had one or more of these three diagnoses than that of young adults with PHEU. WIN thresholds, however, were similar for those young adults with and without these diagnoses across the cognitive function categories.
Of the 386 PHACS AMP Up participants with WIN data, only 199 (51.6%), 150 young adults with PHIV and 49 young adults with PHEU, had complete left and right ear pure-tone data from PHACS AMP hearing testing. The median and interquartile range (Q1, Q3) for length of time between hearing testing in PHACS AMP and WIN testing in PHACS AMP Up were 4.9 years (3.5, 6.6 years). Pure-tone averages (PTAs) of 0.5, 1, 2, and 4 kHz were calculated for each ear, and a better and worse ear PTA was determined. Because at the time of PHACS AMP hearing testing, participants were children and adolescents, the definition of hearing loss used was a PTA of ≥ 20 dB. The median and interquartile range (Q1, Q3) for worse ear PTA in the young adults with PHIV were 10.0 dB (6.25, 13.75), slightly higher than that of young adults with PHEU (7.5 dB [5.0, 10.0]). Furthermore, 16 (11%) young adults with PHIV had a worse ear PTA of ≥ 20 dB, and this group did have a slightly higher median WIN threshold (9.2 dB SBR [5.6, 11.2]) compared to the median WIN threshold of those with a worse ear PTA of < 20 dB (7.6 dB SBR [5.2, 10.0]). Only one (2%) young adult with PHEU had a worse ear PTA of ≥ 20 dB. Just over half of the PHACS AMP Up participants had PTA data, and most of them had hearing within normal limits, even with a more strict definition of hearing loss. Although more young adults with PHIV had hearing loss, these adults still had a lower percentage of IWIN compared to young adults with PHEU.
WIN Performance and ART Exposure
A descriptive summary of HIV disease-related characteristics is provided in Table 4. Briefly, over half of young adults with PHIV received their first cART before 5 years of age and have been on cART, on average, over 15 years. Lifetime measures of HIV RNA and CD4 are also shown in Table 4, and the median percentage for lifetime unsuppressed HIV viral load was 45.1%. In other words, slightly less than half of recorded lifetime viral load measurements were unsuppressed. In terms of past disease severity, the median for CD4 nadir count was 274.5 cells/mm3, and 23% (n = 41) had a prior CDC Class C diagnosis. Almost 90% of young adults with PHIV were receiving some cART regimen at the time of WIN testing. Among these cART regimens, 63 (36%) contained an integrase inhibitor, 74 (43%) contained a protease inhibitor, and 58 (33%) contained a nonnucleoside reverse transcriptase inhibitor (non-NRTI).
Table 4.
HIV-related variables for the Pediatric HIV/AIDS Cohort Study (PHACS) Adolescent Master Protocol (AMP) Up young adults with perinatal HIV who were previously enrolled in PHACS AMP (N = 174).
| Age of first cART (years) | 3.3 (1.4, 6.1) |
| cART duration (years) | 15.6 (12.1, 17.0) |
| Percentage of lifetime HIV RNA measurements > 400 copies/ml | 45.1 (22.1, 68.1) |
| CD4 nadir (cells/mm3) | 274.5 (114, 404) |
| Ever CDC Class C | 41 (23%) |
| Currenta HIV RNA (copies/ml; N = 165) | 40 (20, 1,567) |
| Current HIV RNA > 400 copies/ml (N = 165) | 55 (33%) |
| Current CD4 count (cells/mm3; N = 164) | 580 (402, 770) |
| Current CD4 count (cells/mm3) < 350 (N = 164) | 31 (19%) |
| Current CD4% (N = 163) | 33 (23, 39) |
| Current CD4% < 25 (N = 163) | 49 (30%) |
Note. Values shown are median and interquartile range (Q1, Q3) or n (%). cART = combination antiretroviral therapy; CDC = Centers for Disease Control and Prevention.
“Current” was defined as 3 months prior to 3 months after Words-in-Noise testing.
When stratified by CDC class, the median WIN threshold for the better ear was 4.4 and 5.2 dB SBR for non–Class C young adults compared to Class C young adults, respectively. Also, for the worse ear, the median was 6.8 and 7.8 dB SBR for non–Class C and Class C young adults, respectively. For young adults with PHIV without a CDC Class C diagnosis, cART duration was associated with IWIN for the better ear (RR = 1.13 per year, 95% CI [1.01, 1.25], p = .03) after adjusting for HIV disease severity history, and no other variables met the criteria to be considered confounders. In other words, each additional year of increase in cART duration was associated with a 13% higher risk of IWIN for the better ear. For these participants, however, cART duration was not associated with IWIN for the worse ear (RR = 0.96, 95% CI [0.90, 1.03]). Additionally, for those young adults with a CDC Class C diagnosis, cART duration was not associated with either IWIN for the better ear (RR = 0.94, 95% CI [0.76, 1.15]) or IWIN for the worse ear (RR = 1.00, 95% CI [0.90, 1.11]).
Discussion
The goals of this study were to compare WIN test performance between young adults with PHIV and those with PHEU and to evaluate the association between ART exposure and WIN test results. In PHACS participants without cognitive impairment, young adults with PHIV actually performed better on the WIN test compared to young adults with PHEU. Additionally, findings from this study suggest that, among young adults with PHIV, without a CDC Class C diagnosis, longer cART duration was associated with a higher risk of IWIN for the better ear, but not for the worse ear. There were no significant associations between duration on cART and IWIN in either ear for young adults with CDC Class C.
Despite some methodological differences, Wilson et al. (2010) provide limited reference data from WIN testing of 24 healthy young adults aged 18–27 years. The current study suggests that the PHACS young adults with PHIV as well as those with PHEU both demonstrated worse scores on WIN testing than the Wilson et al. cohort. For young adults in Wilson et al.'s study, the monaural mean was 4.5 dB SBR, compared to the median values of 7.2 and 5.2 dB SBR for worse and better ear, respectively, in PHACS young adults with PHEU and 7.6 and 4.8 dB SBR for worse and better ear, respectively, in young adults with PHIV. It is not known why worse ear WIN thresholds are poorer (the better ear WIN thresholds are similar) in PHACS AMP Up participants compared to the Wilson et al. data, particularly when not all PHACS AMP Up participants completed previous pure-tone testing. However, differences in important characteristics, such as cognitive functioning, or in the implementation of the WIN measure itself cannot be ruled out as contributing factors to discrepancies between study findings. It may also suggest a need for continued monitoring of risk from in utero HIV and/or ART exposures, as well as psychosocial factors concomitant with families affected by HIV.
To date, there are no large-scale research studies that included speech audiometry measures in children or young adults with HIV. There is, however, a growing literature on speech audiometry in ALHIV. Zhan et al. (2018) found that ALHIV, and with normal hearing (thresholds ≤ 20 dB HL at 500, 1000, 2000, and 4000 Hz), experienced difficulty with speech-in-noise tasks. Since these researchers used the Hearing-In-Noise Test (Nilsson et al., 1994) in only ALHIV, without a control group that included adults without HIV, the interpretation of their results is limited. In another study that did include a control group, Torre et al. (2016) collected speech audiometry data in 262 men (117 ALHIV) and 134 women (105 ALHIV). Word recognition scores in quiet (presented at 40-dB sensation level in reference to the speech recognition threshold) were collected in all participants. There were similar percentages of ALHIV and adults without HIV with < 90% word recognition scores, but there was a higher percentage of adults without HIV with word recognition scores of 100%. Among ALHIV, HIV-related variables, such as CD4 count and HIV viral load, were not significantly associated with word recognition scores. Using presentation levels at higher dB sensation levels, such as those used by Torre et al. (2016), most likely will overcome the effects of hearing loss and therefore generate ceiling effects. Word recognition testing in quiet is a routine part of the standard audiological test battery and has been used with ALHIV (Luque et al., 2014; Torre et al., 2016), although this is not typically representative of real-life communication.
Given the early and lifelong exposures to HIV and ART, some of which may be ototoxic, as well as research showing that children and adolescents with PHIV have poorer hearing sensitivity (Chao et al., 2012; Torre et al., 2012), increased episodes of otitis media (Prinicipi et al., 1991; Weber et al., 2006), and more otologic complications (Taipale et al., 2011), it was expected that young adults with PHIV would have poorer WIN thresholds. Therefore, the finding that, within young adults without cognitive impairment, those with PHEU had worse WIN thresholds than those with PHIV was surprising and not easily explained. There is the possibility that young adults with PHIV may have received closer monitoring of their health and social exposures from both the medical community and caregivers than young adults with PHEU. This may confer more protection from exposures that affect this audiometric outcome, including earlier identification and treatment of ear infections and other morbidities and, in later years, hazardous noise exposure, for example.
The role of cognition was also an important finding. Despite the fact that both groups experienced equal percentages of cognitive impairment, when the comparison of risk for IWIN was stratified by those with and without cognitive impairment, for those with cognitive impairment, young adults with PHIV had higher IWIN percentages than young adults with PHEU, particularly for the worse ear. The relationship between poor cognition and increased IWIN in the worse ear is not as clear in young adults with PHEU, complicated by the low prevalence of those young adults with cognitive impairment. Given that attention-deficit/hyperactivity disorder as well as learning or speech/language disorders were not the underlying cause of WIN threshold differences, it may be that, in young adults with PHIV and cognitive impairment, there is residual impairment in executive functions or other neurocognitive abilities from early and chronic viral or treatment exposure. This residual impairment could negatively affect the language processing portion of the WIN task.
For those young adults with PHIV, those with more severe disease history (as defined as prior CDC Class C diagnosis) had higher dB SBR, and among those with less disease severity, duration of cART was associated with an increased risk of IWIN, but only in the better ear. There are limited studies on the effects of HIV medications on hearing, and those studies are confounded with other risk factors for hearing loss (i.e., age and noise exposure). Some have suggested drug-induced hearing loss in ALHIV regardless of HIV disease severity (Bankaitis & Schountz, 1998), while others have reported ototoxic effects of NRTIs (Simdon et al., 2001). Conversely, Torre, Hoffman, et al. (2015) found that total years on NRTIs, total years on non-NRTIs, and total years on protease inhibitors were not associated with hearing loss in ALHIV after adjusting for age, sex, race, and noise exposure. Similarly, in children with HIV, there was no association between cART at the time of hearing testing and hearing loss (Chao et al., 2012; Torre et al., 2012), nor was there an association between duration of cART or NRTIs with hearing loss (Torre et al., 2012). The results of the current study and those from previous results are mixed regarding ART ototoxicity and hearing, and further study is needed on the association between ART and speech audiometry.
The current study is the first to show young adults with PHIV and PHEU to have poorer WIN thresholds compared to young adults without exposure to HIV. For those without cognitive impairment, young adults with PHEU had poorer WIN thresholds than young adults with PHIV. Previously, Torre et al. (2012) showed the prevalence of hearing loss in the poorer ear to be 20.0% in children with PHIV compared to 10.5% in children with PHEU. The participants from Torre et al. (2012) were recruited for this current study. The proportion of IWIN in the worse ear of the current study was slightly higher, where 19% of young adults with PHIV and 23% of young adults with PHEU had IWIN across the cognitive function categories. Pure-tone data were collected as part of PHACS AMP, but only 199 participants (51.5%) in the current study have complete PTA data from that earlier study. From those data, young adults with PHIV had a higher percentage of hearing loss compared to young adults with PHEU. This is contrary to current results where young adults with PHEU had a higher percentage of IWIN. As a result, hearing loss may not explain the IWIN, although caution must be used regarding the contribution of hearing loss to the present results since, as mentioned, only just over half of the PHACS AMP Up had complete PTA data.
There are limitations to this study. First, because of funding restrictions and the length of the PHACS AMP Up protocol, extensive audiometric testing was not feasible. As a result, no pure-tone data were collected as part of the current study, but there were some historical pure-tone threshold data from PHACS AMP. From the sample of PHACS AMP Up with PTA data, most had normal hearing, but hearing sensitivity profiles for the entire cohort were not obtained, and thus, the number of PHACS AMP Up participants with normal hearing could not be confirmed. Second, the entry visit of the PHACS AMP Up protocol is very long, lasting between 3 and 5 hr. The variability in results may have been a result of inconsistencies in when the WIN test was administered, participant fatigue, and/or lack of attention. Another limitation was that trained PHACS AMP UP staff administered the WIN test rather than audiologists, and it was completed in a non–sound-treated room. This introduces the possibility of visual distraction and background noise during testing. The trained technicians may not be as precise with earphone placement, possibly allowing additional extraneous background noise during testing, resulting in erroneous WIN thresholds that would be higher than expected. This staff, however, adhered to the thorough NIH Toolbox Manual for WIN test setup, calibration, and administration.
In conclusion, both groups of young adults (PHIV and PHEU) had poorer WIN thresholds than what has been reported in unexposed, uninfected young adults. However, in the current participants without cognitive impairment, young adults with PHEU had poorer WIN thresholds than those with PHIV, which is contrary to most hearing research. In young adults with PHIV who had no prior CDC Class C diagnosis, longer cART duration was associated with an increased risk of IWIN in the better ear. More research is needed in this area given these young adults are likely in either academic or employment settings or both; even mild hearing loss, early in life, could adversely affect communication and academic performance and limit vocational options (Matkin & Wilcox, 1999). Hearing loss is associated with social isolation, mental health problems, and stigmatization (Tambs, 2004), and in a population of young adults who may already be experiencing multiple layers of stigmatization (PHIV or PHEU status, lower socioeconomic status), speech communication problems can add further burden to the existing struggle. Understanding hearing problems, specifically word recognition in background noise, may help to target critical interventions to mitigate this communication problem and improve the quality of life for both young adults with PHIV and PHEU.
Acknowledgments
We thank the children and families for their participation in Pediatric HIV/AIDS Cohort Study (PHACS) and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with cofunding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Cancer Institute, the National Institute on Alcohol Abuse and Alcoholism, and the National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T. H. Chan School of Public Health (HD052102; principal investigator [PI]: George R. Seage III; Program Director: Liz Salomon) and the Tulane University School of Medicine (HD052104; PI: Russell Van Dyke; co-PI: Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc. (PI: Julie Davidson).
The following institutions, clinical site investigators, and staff participated in conducting PHACS Adolescent Master Protocol and Adolescent Master Protocol Up in 2018, in alphabetical order: Ann & Robert H. Lurie Children's Hospital of Chicago: Ellen Chadwick, Margaret Ann Sanders, Kathleen Malee, and Yoonsun Pyun; Baylor College of Medicine: William Shearer, Mary Paul, Chivon McMullen-Jackson, Mandi Speer, and Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Mirza Baig, and Alma Villegas; Children's Diagnostic & Treatment Center: Lisa Gaye-Robinson, Sandra Navarro, and Patricia Garvie; Boston Children's Hospital: Sandra K. Burchett, Michelle E. Anderson, and Adam R. Cassidy; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Ray Shaw, and Raphaelle Auguste; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson, and Karen Surowiec; St. Christopher's Hospital for Children: Janet S. Chen, Maria Garcia Bulkley, Taesha White, and Mitzie Grant; St. Jude Children's Research Hospital: Katherine Knapp, Jill Utech, Megan Wilkins, and Jamie Russell-Bell; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, and Vivian Olivera; Tulane University School of Medicine: Margarita Silio, Medea Gabriel, and Patricia Sirois; University of California, San Diego: Stephen A. Spector, Megan Loughran, Veronica Figueroa, and Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Carrie Chambers, Emily Barr, and Mary Glidden; University of Miami: Gwendolyn Scott, Grace Alvarez, Juan Caffroni, and Anai Cuadra.
Funding Statement
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with cofunding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Cancer Institute, the National Institute on Alcohol Abuse and Alcoholism, and the National Heart, Lung, and Blood Institute through cooperative agreements with the Harvard T. H. Chan School of Public Health (HD052102; principal investigator [PI]: George R. Seage III; Program Director: Liz Salomon) and the Tulane University School of Medicine (HD052104; PI: Russell Van Dyke; co-PI: Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc. (PI: Julie Davidson).
References
- Bankaitis A. E., & Schountz T. (1998). HIV-related ototoxicity. Seminars in Hearing, 19(2), 155–163. [Google Scholar]
- Bisiacchi P. S., Suppiej A., & Laverda A. (2000). Neuropsychological evaluation of neurologically asymptomatic HIV-infected children. Brain and Cognition, 43(1–3), 49–52. [PubMed] [Google Scholar]
- Blair J. C. (1985). The effects of mild sensorineural hearing loss on academic performance of young school-age children. The Volta Review, 87, 87–93. [Google Scholar]
- Chao C. K., Czechowicz J. A., Messner A. H., Alarcón J., Roca L. K., Larragán Rodriguez M. M., Villafuerte C. G., Montano S. M., & Zunt J. R. (2012). High prevalence of hearing impairment in HIV-infected Peruvian children. Otolaryngology—Head & Neck Surgery, 146(2), 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvie P. A., Zeldow B., Malee K., Nichols S. L., Smith R. A., Wilkins M. L., Williams P. L., &. Pediatric HIV/AIDS Cohort Study (PHACS). (2014). Discordance of cognitive and academic achievement outcomes in youth with perinatal HIV exposure. The Pediatric Infectious Disease Journal, 33(9), e232–e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. C., Wagster M. V., Hendrie H. C., Fox N. A., Cook K. F., & Nowinski C. J. (2013). NIH toolbox for assessment of neurological and behavioral function. Neurology, 80(11, Suppl. 3), S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque A. E., Orlando M. S., Leong U. C., Allen P. D., Guido J. J., Yang H., & Hulin W. (2014). Hearing function in patients living with HIV/AIDS. Ear and Hearing, 35(6), e282–e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro I. I., Fellows A. M., Clavier O. H., Gui J., Rieke C. C., Wilbur J. C., Chambers R. D., Jastrzembski B. G., Mascari J. E., Bakari M., Matee M., Musiek F. E., Waddell R. D., von Reyn C. F., Palumbo P. E., Moshi N., & Buckey J. C. (2016). Auditory impairments in HIV-infected children. Ear and Hearing, 37(4), 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro I. I., Moshi N., Clavier O., MacKenzie T. A., Kline-Schoder R. J., Wilbur J. C., Chamber R. D., Fellows A. M., Jastrzembski B. G., Mascari J. E., Bakari M., Matee M., Musiek F. E., Waddell R. D., von Reyn C. F., & Buckey J. C. (2014). Auditory impairments in HIV-infected individuals in Tanzania. Ear and Hearing, 35(3), 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkin N. D., & Wilcox A. M. (1999). Considerations in the education of children with hearing loss. Pediatric Clinics of North America, 46(1), 143–152. [DOI] [PubMed] [Google Scholar]
- Nichols S. L., Chernoff M. C., Malee K. M., Sirois P. A., Woods S. P., Williams P. L., Yildrim C., Delis D., Kammerer B., & Memory and Executive Functioning Study of the Pediatric HIV/AIDS Cohort Study. (2016). Executive functioning in children and adolescents with perinatal HIV infection and perinatal HIV exposure. Journal of the Pediatric Infectious Diseases Society, 5(Suppl. 1), S15–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M., Soli S. D., & Sullivan J. A. (1994). Development of the Hearing-in-Noise Test for the measurement of speech reception thresholds in quiet and in noise. The Journal of the Acoustical Society of America, 95(2), 1085–1099. [DOI] [PubMed] [Google Scholar]
- Perkins-Dock R. E., Battle T. R., Edgerton J. M., & McNeill J. N. (2015). A survey of barriers to employment for individuals who are deaf. Journal of the American Deafness & Rehabilitation Association, 49(2), 66–85. [Google Scholar]
- Principi N., Marchisio P., Tornaghi R., Onorato J., Massironi E., & Picco P. (1991). Acute otitis media in human immunodeficiency virus-infected children. Pediatrics, 88(3), 566–571. [PubMed] [Google Scholar]
- Qi S., & Mitchell R. E. (2012). Large-scale academic achievement testing of deaf and hard-of-hearing students: Past, present, and future. Journal of Deaf Studies and Deaf Education, 17(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Roman A. S., Pisoni D. B., Kronenberger W. G., & Faulkner K. F. (2017). Some neurocognitive correlates of noise-vocoded speech perception in children with normal hearing: A replication and extension of Eisenberg et al. (2002). Ear and Hearing, 38(3), 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simdon J., Watters D., Bartlett S., & Connick E. (2001). Ototoxicity associated with use of nucleoside analog reverse transcriptase inhibitors: A report of 3 possible cases and review of the literature. Clinical Infectious Diseases, 32(11), 1623–1627. [DOI] [PubMed] [Google Scholar]
- Slogrove A., Reikie B., Naidoo S., De Beer C., Ho K., Cotton M., Bettinger J., Speert D., Esser M., & Kollmann T. (2012). HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. Journal of Tropical Pediatrics, 58(6), 505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Chernoff M., Williams P. L., Malee K. M., Sirois P. A., Kammerer B., Wilkins M., Nichols S., Mellins C., Usitalo A., Garvie P., Rutstein R., & Pediatric HIV/AIDS Cohort Study (PHACS) Team. (2012). Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. The Pediatric Infectious Disease Journal, 31(6), 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale A., Pelkonen T., Taipale M., Roine I., Bernardino L., Peltola H., & Pitkäranta A. (2011). Otorhinolaryngological findings and hearing in HIV-positive and HIV-negative children in a developing country. European Archives of Oto-Rhino-Laryngology, 268(10), 1527–1532. [DOI] [PubMed] [Google Scholar]
- Tambs K. (2004). Moderate effects of hearing loss on mental health and subjective well-being: Results from the Nord-Trøndelag hearing loss study. Psychosomatic Medicine, 66(5), 776–782. [DOI] [PubMed] [Google Scholar]
- Torre P. III, Cook A., Elliott H., Dawood G., & Laughton B. (2015). Hearing assessment data in HIV-infected and uninfected children of Cape Town, South Africa. AIDS Care, 27(8), 1037–1041. [DOI] [PubMed] [Google Scholar]
- Torre P. III., Hoffman H. J., Springer G., Cox C., Young M., Margolick J. B., & Plankey M. (2015). Hearing loss among HIV-seropositive and HIV-seronegative men and women. JAMA Otolaryngology—Head & Neck Surgery, 141(3), 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre P. III., Hoffman H. J., Springer G., Cox C., Young M. A., Margolick J. B., & Plankey M. (2016). Speech audiometry findings from HIV+ and HIV− adults in the MACS and WIHS longitudinal cohort studies. Journal of Communication Disorders, 64, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre P. III., Zeldow B., Hoffman H. J., Buchanan A., Siberry G. K., Rice M., Sirois P. A., Williams P. L., & Pediatric HIVAIDS Cohort Study. (2012). Hearing loss in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents. The Pediatric Infectious Disease Journal, 31(8), 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Westhuizen Y., Swanepoel D., Heinze B., & Hofmeyr L. M. (2013). Auditory and otological manifestations in adults with HIV/AIDS. International Journal of Audiology, 52(1), 37–43. [DOI] [PubMed] [Google Scholar]
- Weber R., Neto C. D. P., Miziara I. D., & Filho B. C. A. (2006). Haart impact on prevalence of chronic otitis media in Brazilian HIV-infected children. Brazilian Journal of Otorhinolaryngology, 72(4), 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. H. (2003). Development of a speech-in-multitalker-babble paradigm to assess word-recognition performance. Journal of the American Academy of Audiology, 14(9), 453–470. [PubMed] [Google Scholar]
- Wilson R. H., Abrams H. B., & Pillion A. L. (2003). A word-recognition task in multitalker babble using a descending presentation mode from 24 dB to 0 dB signal to babble. Journal of Rehabilitation Research and Development, 40(4), 321–327. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., Carnell C. S., & Cleghorn A. L. (2007). The Words-in-Noise (WIN) test with multitalker babble and speech-spectrum noise maskers. Journal of the American Academy of Audiology, 18(6), 522–529. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., Farmer N. M., Gandhi A., Shelburne E., & Weaver J. (2010). Normative data for the Words-in-Noise test for 6- to 12-year-old children. Journal of Speech, Language, and Hearing Research, 53(5), 1111–1121. https://doi.org/10.1044/1092-4388(2010/09-0270) [DOI] [PubMed] [Google Scholar]
- Zhan Y., Fellows A. M., Qi T., Clavier O. H., Soli S. D., Shi X., Gui J., Shi Y., & Buckey J. C. (2018). Speech in noise perception as a marker of cognitive impairment in HIV infection. Ear and Hearing, 39(3), 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]