Abstract
Purpose
The goal of this work was to evaluate the low-frequency hearing preservation of long electrode array cochlear implant (CI) recipients.
Method
Twenty-five participants presented with an unaided hearing threshold of ≤ 80 dB HL at 125 Hz pre-operatively in the ear to be implanted. Participants were implanted with a long (31.5-mm) electrode array. The unaided hearing threshold at 125 Hz was compared between the preoperative and postoperative intervals (i.e., initial CI activation, and 1, 3, 6, 9, and 12 months after activation).
Results
Eight participants maintained an unaided hearing threshold of ≤ 80 dB HL at 125 Hz postoperatively. The majority (n = 5) demonstrated aidable low-frequency hearing at initial activation, whereas 3 other participants experienced an improvement in unaided low-frequency hearing thresholds at subsequent intervals.
Conclusions
CI recipients can retain residual hearing sensitivity with fully inserted long electrode arrays, and low-frequency hearing thresholds may improve during the postoperative period. Therefore, unaided hearing thresholds obtained within the initial weeks after surgery may not reflect later hearing sensitivity. Routine measurement of postoperative unaided hearing thresholds—even for patients who did not demonstrate aidable hearing thresholds initially after cochlear implantation—will identify CI recipients who may benefit from electric–acoustic stimulation.
Supplemental Material
Appropriate audiologic assessment is important for determining candidacy for cochlear implantation and in the postoperative management of cochlear implant (CI) recipients. The preoperative test battery is relatively consistent across CI teams, including unaided pure-tone detection and aided speech recognition. In contrast, the postoperative test battery is less consistent. Postoperative evaluations are used to determine the optimal external device configuration (i.e., CI-alone or electric–acoustic stimulation [EAS] 1 ), individualize device settings, and assess associated outcomes. Specifically, the assessment of unaided hearing detection may not be included in the management of CI recipients—especially those who presented with moderate-to-profound low-frequency hearing thresholds preoperatively and were implanted with electrode arrays not designed for hearing preservation—due to the presumption of postoperative hearing loss. However, there is new evidence of long electrode array CI recipients with postoperative low-frequency hearing preservation that challenges this belief (Helbig et al., 2018). Inclusion of postoperative measurement of unaided hearing detection in all CI recipients with aidable (e.g., ≤ 80 dB HL) thresholds preoperatively may improve the quality of audiologic care by identifying patients who could be fit with EAS as opposed to a CI alone.
Provided that there is sufficient postoperative acoustic hearing, speech recognition with EAS is better than that observed with the CI alone (Dillon, Buss, Adunka, Buchman, & Pillsbury, 2015; Dunn, Perreau, Gantz, & Tyler, 2010; Gantz & Turner, 2003; Gifford, Dorman, Sheffield, Teece, & Olund, 2014; Helbig et al., 2011; Pillsbury et al., 2018). Benefits of EAS may be observed even when acoustic amplification is limited to 125 Hz (Zhang, Dorman, & Spahr, 2010). Candidates for EAS are presumed to be those who presented preoperatively with normal-to-moderate low-frequency thresholds and received a short electrode array. However, some patients with preoperative low-frequency acoustic hearing may receive long electrode arrays. CI teams face a paradox when selecting the electrode array for a patient with low-frequency acoustic hearing. Short lateral wall electrode arrays are associated with a high incidence of low-frequency hearing preservation (Suhling et al., 2016; Wanna et al., 2018). Unfortunately, patients with short lateral wall electrode arrays who cannot take advantage of acoustic input postoperatively typically demonstrate poorer speech recognition than those with longer lateral wall electrode arrays (Buchman et al., 2014; Büchner, Illg, Majdani, & Lenarz, 2017; O'Connell et al., 2016; Zhou, Li, Galvin, Fu, & Yuan, 2017). As a consequence, long lateral wall electrode arrays are recommended for CI candidates with moderate-to-profound low-frequency hearing loss. Since low-frequency hearing preservation is possible with long electrode arrays (Helbig et al., 2018), there is a need to document the prevalence of low-frequency hearing preservation in long electrode array recipients, particularly if the preserved acoustic hearing is within the EAS fitting range.
The present report evaluates the prevalence of low-frequency hearing preservation with long electrode arrays, and demonstrates when in the first year after CI activation that preserved hearing is evident. The low-frequency hearing thresholds were evaluated for a cohort of prospectively enrolled participants who received a full insertion of the same 31.5-mm electrode array and completed unaided hearing threshold measurement at specific intervals during the first year of device use. The aim was to identify if and when long electrode array recipients should be considered for the fitting of an EAS external device.
Method
Participant data were obtained from a cohort of adults with unilateral hearing loss or asymmetric hearing loss who underwent cochlear implantation as part of a clinical trial investigating outcomes of CI use in cases of substantial hearing in the contralateral ear (Buss et al., 2018; Dillon, Buss, Anderson, et al., 2017; Dillon, Buss, Rooth, et al., 2017). The U.S. Food and Drug Administration approved an investigational device exemption for the clinical trial, and the procedures were approved by the study site's institutional review board. Participants provided informed consent prior to cochlear implantation. Inclusion in the clinical trial required a pure-tone average (PTA; 500, 1000, and 2000 Hz) of ≥ 70 dB HL in the ear to be implanted. The present report evaluates the subset of participants with preoperative unaided hearing thresholds of ≤ 80 dB HL at 125 Hz in the ear to be implanted.
Twenty-five participants (15 female, 12 unilateral hearing loss) had a preoperative unaided hearing threshold at 125 Hz of ≤ 80 dB HL in the ear to be implanted and were evaluated with respect to hearing preservation in the present report. The reported etiologies included Ménière's disease (n = 3), viral infection (n = 1), and noise-induced hearing loss (n = 1), but the cause of hearing loss was unknown in the majority of participants (n = 20). The mean preoperative low-frequency PTA (125, 250, and 500 Hz) was 69 dB HL (SD = 12 dB), and the mean PTA was 80 dB HL (SD = 10 dB). Unaided hearing thresholds at 125 Hz ranged from 20 to 80 dB HL, with a mean of 62 dB HL (SD = 16 dB). The age at implantation ranged from 29 to 79 years (M = 61 years, SD = 13 years). Participants received the standard electrode array (MED-EL Corporation) inserted via a round window approach. The standard electrode array is 31.5 mm in length and was the only full-length electrode array available in the United States at the time of the clinical trial.
Unaided hearing thresholds were measured pre-operatively; at initial CI activation (2–4 weeks postoperatively); and at 1, 3, 6, 9, and 12 months postactivation. The present report focuses on the unaided hearing thresholds at 125 Hz. A value of 100 dB HL was recorded when there was no response to the stimulus; the maximum output of the audiometer at 125 Hz was 95 dB HL.
Unaided hearing thresholds at 125 Hz were first compared between the preoperative and initial activation intervals to assess the change in low-frequency hearing as a result of cochlear implantation using a paired-samples t test (SPSS, Version 23). Second, a generalized linear mixed-effects model (GLME; MATLAB 2019a) assessed whether the unaided hearing thresholds at 125 Hz changed after device activation (activation to 12 months). There were missing data at the 9-month (n = 2) and 12-month (n = 1) intervals; GLME models accommodate missing data better than comparable models (Oleson, Brown, & McCreery, 2019).
Results
At initial activation, there was a significant elevation in the unaided hearing thresholds at 125 Hz as compared to the preoperative thresholds, t(24) = −9.25, p < .001, with the majority of participants (n = 16) providing no response to the stimulus.
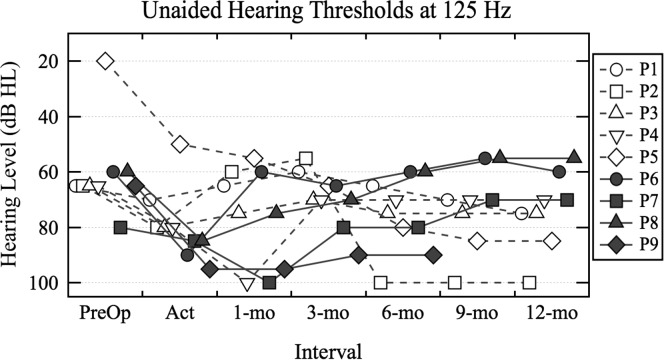
Nine participants maintained an unaided hearing threshold at 125 Hz of ≤ 95 dB HL at initial activation. Figure 1 plots their unaided hearing thresholds at 125 Hz at each interval to review individual results over the study period. Participant P9 withdrew from the study prior to the 12-month interval. A GLME model assessed a main effect of interval (initial activation and 1, 3, 6, 9, and 12 months postactivation) on the unaided hearing thresholds at 125 Hz for the nine participants with hearing preservation. There was no significant main effect of interval on the unaided hearing thresholds, t(50) = −0.73, p = .47. Review of Figure 1 reveals that, although the majority experienced relatively stable thresholds postactivation, some participants experienced a decrement in thresholds (e.g., P5), whereas others experienced an improvement in thresholds (e.g., P8).
Figure 1.
Unaided hearing thresholds (dB HL) at 125 Hz measured over the study period for participants who presented with hearing preservation at initial activation (labeled Act). Open symbols and dashed lines indicate the results for the participants who met the electric–acoustic stimulation fitting criterion of ≤ 80 dB HL at initial activation. Closed symbols and solid lines indicate the results for participants whose unaided hearing thresholds exceeded the electric–acoustic stimulation fitting criterion at initial activation.
It is of interest whether the participants with postoperative hearing preservation should be fit with EAS. Clinically, EAS fitting is attempted when a patient presents with an unaided hearing threshold at 125 Hz of ≤ 80 dB HL. At initial activation, five participants (labeled P1–P5) presented with an unaided hearing threshold that met this criterion (open symbols and dashed lines indicate individual results in Figure 1). P2 and P5 experienced a change in their unaided hearing thresholds after the 6-month interval, such that they no longer fell within the fitting range for EAS. The unaided hearing thresholds of four participants (labeled P6–P9) exceeded the EAS fitting criterion at initial activation (closed symbols and solid lines indicate individual results in Figure 1). Interestingly, three of these four participants (P6, P7, and P8) demonstrated improvement in unaided hearing thresholds such that they met the EAS fitting criterion at a subsequent interval. The fitting of EAS was indicated at the 1-month interval for P6 and P8, and at the 3-month interval for P7. By the 12-month interval, the fitting of EAS was indicated for six participants; three of these participants would not have been identified for EAS fitting if unaided hearing thresholds were measured at initial activation only. Two of the participants with no 125-Hz audibility at CI activation (not shown in Figure 1) produced thresholds of 90 dB HL at the 12-month interval (see Supplemental Material S1). There was also some evidence of threshold improvement in the postactivation period at 250 and 500 Hz, although thresholds remained above the 80–dB HL criterion in all but two cases (P7 and P8 at 250 Hz; see Supplemental Material S1). Changes of 5–10 dB, however, may be measurement error. These data demonstrate that low-frequency thresholds at activation do not necessarily indicate residual hearing at subsequent intervals; as a result, it may be advisable to assess an individual's unaided hearing at multiple postoperative intervals during the first year of device listening experience.
Discussion
The current report provides additional evidence of low-frequency hearing preservation after implantation of a long electrode array. Low-frequency hearing preservation was not the primary aim of cochlear implantation in the evaluated cohort; however, 32% of participants with pre-operative hearing at 125 Hz presented with aidable (≤ 80 dB HL) acoustic hearing at 125 Hz at one or more postoperative test intervals.
In general, lateral wall electrode arrays are currently preferred for hearing preservation cases, as they increase success rates and decrease the incidence of intracochlear damage when compared to precurved electrode arrays (Wanna et al., 2014, 2018). However, CI teams are often faced with the dilemma of whether to implant a short lateral wall electrode array to increase likelihood of hearing preservation, or a longer lateral wall electrode array to support speech recognition in the event that acoustic hearing is subsequently lost. This scenario is most relevant to CI candidates with moderate to moderately severe low-frequency thresholds; in this group, even a relatively modest (15–30 dB HL) shift in low-frequency PTA can preclude EAS fitting. The data in the present report demonstrate hearing preservation with a long lateral wall electrode array in roughly one third of participants receiving a 31.5-mm electrode array at one or more postoperative test intervals. These results are preliminary but have implications for clinical practice in that the risk of full insertion of a long electrode array on hearing preservation may be less than currently believed. Additionally, these results were obtained with the standard electrode array; the currently available FlexSOFT electrode array is the same length (31.5 mm) but is thinner and has more flexible mechanical properties, potentially supporting better hearing preservation. Initial investigations of the FlexSOFT electrode array have demonstrated aidable unaided hearing thresholds postoperatively for some recipients (Mick et al., 2014; Usami et al., 2014).
When the cohort of patients considered in the present report was implanted, it was not anticipated that they would meet the indications for EAS fitting, considering the inclusion criterion of a PTA of ≥ 70 dB HL in the ear to be implanted and use of a long electrode array. As such, clinical trial participants were fitted with a CI-alone device. Ongoing work is investigating the effectiveness of EAS fitting in CI recipients of long electrode arrays and low-frequency acoustic hearing, in addition to modified mapping procedures incorporating the angular insertion depth of individual electrode contacts (Dillon, O'Connell, Canfarotta, Rooth, & Buss, 2019).
The criteria for when to fit EAS as opposed to a CI-alone device vary across devices and clinical practice. Although the benefits of EAS over CI-alone has been demonstrated for patients with residual hearing, the criterion for determining residual hearing varies from a maximum unaided threshold of 60–80 dB HL (Helbig et al., 2011; James et al., 2005; Pillsbury et al., 2018). Additionally, it is unclear whether aidable acoustic hearing above 125 Hz is needed to observe a benefit with EAS over CI alone. For instance, Gantz et al. (2016) define functional hearing with EAS as a PTA (125–1000 Hz) of better than 85–90 dB HL. However, CI recipients with limited acoustic hearing in the implanted ear may also experience a benefit from EAS. Speech recognition in quiet and noise is significantly improved for CI recipients when 125-Hz low-pass filtered acoustic information is added to the contralateral ear (Zhang et al., 2010). Ongoing work is assessing the effectiveness of EAS on masked speech recognition in spatially separated noise when acoustic input in the implanted ear is limited to 125 Hz.
Perhaps the most compelling finding herein is that some participants demonstrated improvement in low-frequency unaided hearing thresholds after CI activation. Although unaided hearing thresholds at activation would not have supported fitting EAS for some CI recipients, subsequent testing as part of the clinical trial protocol revealed improvement and EAS candidacy. Considering the evidence of postoperative hearing preservation in the present report, the clinical postoperative test battery at our center now consists of unaided hearing threshold assessment, aided sound-field thresholds, and aided speech recognition using the Minimum Speech Test Battery (2011). Recommended follow-up intervals include 1, 3, 6, 9, and 12 months postactivation and then annually thereafter. We think measuring unaided hearing detection for CI recipients with preoperative acoustic hearing of ≤ 80 dB HL, independent of the implanted electrode array, will improve the quality of our audiologic care by identifying patients who may benefit from EAS. For patients with hearing preservation, unaided hearing thresholds are measured at each interval and used in the fitting and verification of EAS. For patients with no response to 125-Hz pure tone during unaided hearing threshold assessment at device activation, our audiology team repeats unaided hearing threshold assessment at 6 and 12 months postactivation to evaluate whether thresholds have improved to an EAS fitting range.
There are a number of factors that have been proposed to account for changes in unaided hearing thresholds after implantation. The elevation of unaided hearing detection thresholds or complete loss of residual hearing initially after cochlear implantation is thought to be due to surgical trauma (Roland & Wright, 2006). Mechanisms that may contribute to loss of hearing sensitivity later in the postoperative period include inflammation (Seyyedi & Nadol, 2014) and fibrosis (Quesnel et al., 2016). It does not appear that electric stimulation is associated with elevation in unaided hearing thresholds (Dillon, Bucker, et al., 2015), and in fact, it may have a neurotrophic effect (Leake, Stakhovskaya, Hradek, & Hetherington, 2008). Improvements in acoustic hearing thresholds over the first year of device use may be due to resolution of a conductive hearing loss (Chole, Hullar, & Potts, 2014); therefore, some patients may not meet the EAS fitting criterion until a few months after cochlear implantation.
Incorporating the measurement of unaided acoustic hearing into the postoperative test battery may improve the quality of the audiologic management of CI recipients by understanding the incidence of hearing preservation with specific electrode arrays, identifying when the fitting of EAS is warranted, and individualizing the fitting of devices.
Supplementary Material
Acknowledgments
The clinical trial was supported by a research grant from MED-EL Corporation. Margaret T. Dillon and Meredith A. Rooth are supported by a research grant from MED-EL Corporation provided to their university. The authors acknowledge the regulatory assistance of the North Carolina Translational and Clinical Sciences Institute, which is supported by the National Center for Advancing Translational Sciences, through Grant Award UL1TR002489.
Funding Statement
The clinical trial was supported by a research grant from MED-EL Corporation. Margaret T. Dillon and Meredith A. Rooth are supported by a research grant from MED-EL Corporation provided to their university. The authors acknowledge the regulatory assistance of the North Carolina Translational and Clinical Sciences Institute, which is supported by the National Center for Advancing Translational Sciences, through Grant Award UL1TR002489.
Footnote
CI alone refers to electric stimulation of the frequency range (e.g., 70–8500 Hz). EAS refers to presenting low-frequency information acoustically and presenting mid- to high-frequency information electrically. EAS devices are indicated for CI recipients with aidable low-frequency residual hearing.
References
- Buchman C. A., Dillon M. T., King E. R., Adunka M. C., Adunka O. F., & Pillsbury H. C. (2014). Influence of cochlear implant insertion depth on performance: A prospective randomized trial. Otology & Neurotology, 35(10), 1773–1779. https://doi.org/10.1097/MAO.0000000000000541 [DOI] [PubMed] [Google Scholar]
- Büchner A., Illg A., Majdani O., & Lenarz T. (2017). Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLOS ONE, 12(5), e0174900 https://doi.org/10.1371/journal.pone.0174900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E., Dillon M. T., Rooth M. A., King E. R., Deres E. J., Buchman C. A., … Brown K. D. (2018). Effects of cochlear implantation on binaural hearing in adults with unilateral hearing loss. Trends in Hearing, 22 https://doi.org/10.1177/2331216518771173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chole R. A., Hullar T. E., & Potts L. G. (2014). Conductive component after cochlear implantation in patients with residual hearing conservation. American Journal of Audiology, 23(4), 359–364. https://doi.org/10.1044/2014_AJA-14-0018 [DOI] [PubMed] [Google Scholar]
- Dillon M. T., Bucker A. L., Adunka M. C., King E. R., Adunka O. F., Buchman C. A., & Pillsbury H. C. (2015). Impact of electric stimulation on residual hearing. Journal of the American Academy of Audiology, 26(8), 732–740. https://doi.org/10.3766/jaaa.15013 [DOI] [PubMed] [Google Scholar]
- Dillon M. T., Buss E., Adunka O. F., Buchman C. A., & Pillsbury H. C. (2015). Influence of test condition on speech perception with electric-acoustic stimulation. American Journal of Audiology, 24(4), 520–528. https://doi.org/10.1044/2015_AJA-15-0022 [DOI] [PubMed] [Google Scholar]
- Dillon M. T., Buss E., Anderson M. L., King E. R., Deres E. J., Buchman C. A., … Pillsbury H. C. (2017). Cochlear implantation in cases of unilateral hearing loss: Initial localization abilities. Ear and Hearing, 38(5), 611–619. https://doi.org/10.1097/AUD.0000000000000430 [DOI] [PubMed] [Google Scholar]
- Dillon M. T., Buss E., Rooth M. A., King E. R., Deres E. J., Buchman C. A., … Brown K. D. (2017). Effect of cochlear implantation on quality of life in adults with unilateral hearing loss. Audiology and Neuro-Otology, 22(4–5), 259–271. https://doi.org/10.1159/000484079 [DOI] [PubMed] [Google Scholar]
- Dillon M., O'Connell B., Canfarotta M., Rooth M., & Buss E. (2019). Incorporating electrode angular insertion depth in electric-acoustic stimulation programming. Podium presentation at the 46th Annual Scientific and Technology Meeting of the American Auditory Society, Scottsdale, AZ. [Google Scholar]
- Dunn C. C., Perreau A., Gantz B., & Tyler R. S. (2010). Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. Journal of the American Academy of Audiology, 21(1), 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz B. J., Dunn C., Oleson J., Hansen M., Parkinson A., & Turner C. (2016). Multicenter clinical trial of the Nucleus Hybrid S8 cochlear implant: Final outcomes. The Laryngoscope, 126(4), 962–973. https://doi.org/10.1002/lary.25572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz B. J., & Turner C. W. (2003). Combining acoustic and electrical hearing. The Laryngoscope, 113(10), 1726–1730. [DOI] [PubMed] [Google Scholar]
- Gifford R. H., Dorman M. F., Sheffield S. W., Teece K., & Olund A. P. (2014). Availability of binaural cues for bilateral implant recipients and bimodal listeners with and without preserved hearing in the implanted ear. Audiology and Neuro-Otology, 19(1), 57–71. https://doi.org/10.1159/000355700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig S., Adel Y., Leinung M., Stöver T., Baumann U., & Weissgerber T. (2018). Hearing preservation outcomes after cochlear implantation depending on the angle of insertion: Indication for electric or electric-acoustic stimulation. Otology & Neurotology, 39(7), 834–841. https://doi.org/10.1097/MAO.0000000000001862 [DOI] [PubMed] [Google Scholar]
- Helbig S., Van de Heyning P., Kiefer J., Baumann U., Kleine-Punte A., Brockmeier H., … Gstoettner W. (2011). Combined electric acoustic stimulation with the PULSARCI100 implant system using the FLEXEAS electrode array. Acta Otolaryngology, 131(6), 585–595. https://doi.org/10.3109/00016489.2010.544327 [DOI] [PubMed] [Google Scholar]
- James C., Albegger K., Battmer R., Burdo S., Deggouj N., Deguine O., … Fraysse B. (2005). Preservation of residual hearing with cochlear implantation: How and why. Acta Oto-Laryngologica, 125(5), 481–491. https://doi.org/10.1080/00016480510026197 [DOI] [PubMed] [Google Scholar]
- Leake P. A., Stakhovskaya O., Hradek G. T., & Hetherington A. M. (2008). Factors influencing neurotrophic effects of electrical stimulation in the deafened developing auditory system. Hearing Research, 242(1–2), 86–99. https://doi.org/10.1016/j.heares.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick P., Arnoodi H., Shipp D., Friesen L., Symons S., Lin V., … Chen J. (2014). Hearing preservation with full insertion of the FLEXsoft electrode. Otology & Neurotology, 35(1), e40–e44. https://doi.org/10.1097/MAO.0b013e318291c66d [DOI] [PubMed] [Google Scholar]
- Minimum Speech Test Battery. (2011). Minimum Speech Test Battery (MSTB) for Adult Cochlear Implant Users 2011 (Version 1.0). Goodyear, AZ: Auditory Potential LLC; Retrieved from http://www.auditorypotential.com/MSTBfiles/MSTBManual2011-06-20%20.pdf [Google Scholar]
- O'Connell B. P., Cakir A., Hunter J. B., Francis D. O., Noble J. H., Labadie R. F., … Wanna G. B. (2016). Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otology & Neurotology, 37(8), 1016–1023. https://doi.org/10.1097/MAO.0000000000001125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson J. J., Brown G. D., & McCreery R. (2019). The evolution of statistical methods in speech, language, and hearing sciences. Journal of Speech, Language, and Hearing Research, 62(3), 498–506. https://doi.org/10.1044/2018_JSLHR-H-ASTM-18-0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillsbury H. C., Dillon M. T., Buchman C. A., Staecker H., Prentiss S. M., Ruckenstein M. J., … Adunka O. F. (2018). Multicenter US clinical trial with an electric-acoustic stimulation (EAS) system in adults: Final outcomes. Otology & Neurotology, 39(3), 299–305. https://doi.org/10.1097/MAO.0000000000001691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel A. M., Nakajima H. H., Rosowski J. J., Hansen M. R., Gantz B. J., & Nadol J. B. Jr. (2016). Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hearing Research, 333, 225–234. https://doi.org/10.1016/j.heares.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland P. S., & Wright C. G. (2006). Surgical aspects of cochlear implantation: Mechanisms of insertional trauma. Advances in Oto-Rhino-Laryngology, 64, 11–30. https://doi.org/10.1159/000094642 [DOI] [PubMed] [Google Scholar]
- Seyyedi M., & Nadol J. B. Jr. (2014). Intracochlear inflammatory response to cochlear implant electrodes in humans. Otology & Neurotology, 35(9), 1545–1551. https://doi.org/10.1097/MAO.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhling M. C., Majdani O., Salcher R., Leifholz M., Büchner A., Lesinski-Schiedat A., & Lenarz T. (2016). The impact of electrode array length on hearing preservation in cochlear implantation. Otology & Neurotology, 37(8), 1006–1015. https://doi.org/10.1097/MAO.0000000000001110 [DOI] [PubMed] [Google Scholar]
- Usami S., Moteki H., Tsukada K., Miyagawa M., Nishio S. Y., Takumi Y., … Tono T. (2014). Hearing preservation and clinical outcome of 32 consecutive electric acoustic stimulation (EAS) surgeries. Acta Otolaryngology, 134(7), 717–727. https://doi.org/10.3109/00016489.2014.894254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanna G. B., Noble J. H., Carlson M. L., Gifford R. H., Dietrich M. S., Haynes D. S., … Labadie R. F. (2014). Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. The Laryngoscope, 124(Suppl. 6), S1–S7. https://doi.org/10.1002/lary.24728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanna G. B., O'Connell B. P., Francis D. O., Gifford R. H., Hunter J. B., Holder J. T., … Haynes D. S. (2018). Predictive factors for short- and long-term hearing preservation in cochlear implantation with conventional-length electrodes. The Laryngoscope, 128(2), 482–489. https://doi.org/10.1002/lary.26714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Dorman M. F., & Spahr A. J. (2010). Information from the voice fundamental frequency (F0) region accounts for the majority of the benefit when acoustic stimulation is added to electric stimulation. Ear and Hearing, 31(1), 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Li H., Galvin J. J. III., Fu Q. J., & Yuan W. (2017). Effects of insertion depth on spatial speech perception in noise for simulations of cochlear implants and single-sided deafness. International Journal of Audiology, 56(Suppl. 2), S41–S48. https://doi.org/10.1080/14992027.2016.1197426 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a