Abstract
Purpose
The aim of this study was to find the most useful marker of endometriosis-related infertility and evaluate predictive and diagnostic values of systemic inflammatory response markers (preoperative white blood–cell subtypes, neutrophil:lymphocyte ratio [NLR], platelet:lymphocyte ratio [PLR], and monocyte:lymphocyte ratio [MLR]) and CA125 levels in endometriosis patients.
Methods
This study comprised 662 women who had undergone laparoscopic surgery and been pathologically confirmed as having endometriosis and 83 patients pathologically confirmed with benign ovarian tumors. Related inflammatory factors in endometriosis complicated by infertility were analyzed via logistic regression analysis. Diagnostic values of the inflammatory response markers were obtained by receiver operating–characteristic analysis.
Results
We firstly identified that lower NLR level was an independent risk factor of infertility. Serum lymphocytes were significantly higher in endometriosis patients, while serum CA125, NLR, MLR, and PLR were elevated. For differentiating endometriosis from other benign ovarian tumors, the combination of NLR and CA125 achieved greater sensitivity than CA125 alone. In addition, both CA125 and NLR were positively correlated with stage, oviduct adhesion, and diameter of ovarian ectopic cysts.
Conclusion
NLR may be used as a simple and easily obtained predictive marker for endometriosis with infertility. Moreover, NLR can be a neoadjuvant biomarker for serum CA125 to diagnose endometriosis.
Keywords: endometriosis, endometrioma, uterine leiomyoma, infertility, NLR, PLR, CA125
Introduction
Endometriosis is a common chronic disease in women, and is characterized byectopia of endometrial cells and stroma outside the uterine cavity. Endometriosis causes chronic pelvic pain and infertility, the major clinical symptoms of endometriosis, in 5%–10% of women during reproductive years.1 Moreover, although endometriosis is a benign disease, it shows characteristics of malignant tumors, such as neovascularization, local infiltration, distant metastasis, and recurrence.2 The diagnosis of endometriosis requires invasive procedures, such as laparoscopic surgery and histological examination of lesions.3 CA125 has been studied extensively as a noninvasive serological biomarker of endometriosis. Elevated CA125 levels have been observed in sera, menstrual effluent, and peritoneal fluid of women with endometriosis, but its specificity is not enough.4
Correlations between systemic inflammatory response markers and prognosis of cancers has been widely investigated. A high neutrophil:lymphocyte ratio (NLR) or platelet:lymphocyte ratio (PLR) is associated with adverse overall survival in many solid tumors.5,6 Numerous evidence suggests that endometriosis may be a localized inflammatory disease with subclinical systemic manifestations.7,8 The systemic inflammatory response involves changes in relative levels of circulating white blood cells (WBCs), and neutrophilia is accompanied by relative lymphocytopenia.9 However, to date, little research has been done on the diagnostic and predictive values of SIS markers in endometriosis. Recently, there have been several studies evaluating the diagnostic value of the combination of SIS markers and CA125 in endometriosis, with contradictory conclusions.3,10,11 In order to screen neoadjuvant biomarkers for serum CA125 in the diagnosis of endometriosis, we evaluated the sensitivity and specificity of preoperative WBC counts, NLR, PLR, monocyte:lymphocyte ratio (MLR), and CA125 and their combination.
The major complication of endometriosis is infertility: 30%–50% of women with endometriosis are infertile.12 Distorted pelvic anatomy, such as pelvic adhesions, could result in reduced fecundity in endometriosis patients. These disruptions impair oocyte release or pickup, alter sperm motility, cause disordered myometrial contractions, and impair fertilization and embryo transport.13 In addition to the mechanical factors associated with infertility, growing evidence supports chronic inflammation being an important cause of endometriosis-associated infertility.14 Therefore, we wished to figure out the association between SIS markers and clinicopathological features of endometriosis, thus identifying risk factors of endometriosis-related infertility.
Methods
A retrospective study was performed at the Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, China from January 1, 2006 to December 30, 2015. A total of 662 women who had undergone laparoscopic surgery were diagnosed with endometriosis according to pathological confirmation: 83 had pathologically confirmed benign ovarian tumors, including 20 cases of teratoma, 33 cases of serous cystadenoma, 27 cases of mucinous cystadenoma, and three cases of other benign conditions. Exclusion criteria were patients with hematopoietic system diseases, liver diseases, tuberculosis, or immune-system disease. Patients with incomplete or unavailable data were excluded. The healthy control group comprised 45 women who had had premenopausal health checks at the Physical Examination Center of Qilu Hospital who had no previous history of gynecological disease or abnormalities on laboratory examination. This retrospective study was approved by the ethics committee of Qilu Hospital. We also obtained informed consent from each patient.
Symptoms of pelvic pain, dysmenorrhea, reproductive status, menstrual cycle, and complications, pathological reports, surgical data, and differential WBC counts and CA125 levels in serum estimated 1 week before surgery were recorded. SIS markers, such as NLR, MLR, and PLR, were defined as absolute neutrophil, monocyte, or platelet count divided byabsolute lymphocyte count. Combined values were obtained by multiplying CA125 levels by NLR, MLR, or PLR.
SPSS 12.0 was used for statistical analysis, and p<0.05 considered statistically significant. The Shapiro–Wilk test was used to ascertain whether continuous variables had normal distribution. Continuous and normally distributed variables are presented as means ± SD, and intragroup differences were investigated using Student's t-test or one-way ANOVA. Continuous variables with abnormal distribution are expressed as medians, and differences between variables were analyzed using the Mann–Whitney U test. Pearsons chi-square test or Fisher exact chi-square test was used to analyze the percentage of patients with abnormally elevated values. Sensitivity and specificity for each marker were assessed with ROC curves. Area under the ROC curve (AUC) was used to calculate the ability of each potential marker to discriminate between endometriosis cases and controls (benign-tumor group and healthy control group). Correlations between groups were evaluated by Pearson’s correlation coefficient or Spearman's rank-correlation coefficient. To evaluate independent prognostic factors related to infertility, univariate and multivariate logistic regression analyses were conducted after adjustment for confounding factors.
Results
NLR and Serum CA125 Levels Independent Risk Factors of Endometriosis with Infertility
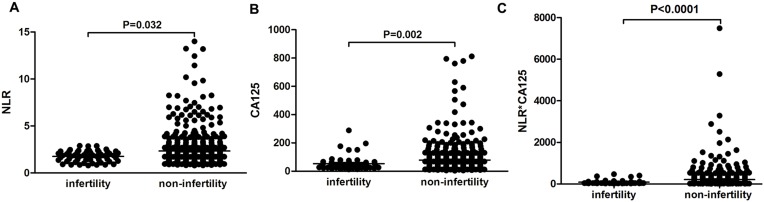
All the endometriosis patients were divided into infertile and fertile (with reproductive history) groups. NLR was significantly lower in the infertile group than the fertile group (p=0.032, Figure 1A). Interestingly, CA125 levels and the combination of NLR and CA125 were both significantly lower in infertile patients than fertile ones (p=0.002, p<0.0001, respectively; Figure 1, B and C). Infertility is one of the major complications of endometriosis. We performed logistic regression analysis to examine risk factors related to infertility. Firstly, univariate logistic analysis indicated that age, diameter of ovarian endometriomas, oviduct adhesions, pelvic adhesion, clinical stage, NLR, and CA125 levels were statistically significant (Table 1). Then, these factors were included in multivariate logistic analysis (Table 2), which suggested that diameter of cysts, bilateral ovarian endometriosis cysts, pelvic adhesion, clinical stage, and lower NLR levels were independent risk factors associated with infertility.
Figure 1.
Comparison of NLR and serum CA125 levels in infertie and fertile groups. Mean levels of NLR (A), CA125 (B), and combination of CA125 and NLR (C) in fertile and infertile endometriosis patients.
Table 1.
Univariate Logistic Analysis of Risk Factors of Endometriosis-Associated Infertility
| OR | 95% CI | p-value | |
|---|---|---|---|
| Age (years) | 2.682 | 1.467–4.903 | 0.001 |
| Unilateral or bilateral cyst | 4.32 | 1.821–10.250 | 0.001 |
| Diameter of cyst | 6.733 | 3.582–12.653 | <0.001 |
| Pain | 1.368 | 0.763–2.451 | 0.293 |
| Endometrioma | 1.572 | 0.368–6.727 | 0.542 |
| Uterine leiomyoma | 0.59 | 0.315–1.106 | 0.1 |
| Endometrial polyp | 0.738 | 0.320–1.705 | 0.477 |
| Oviduct adhesion | 4.038 | 1.435–11.362 | 0.008 |
| Pelvic adhesion | 4.792 | 2.675–8.585 | <0.001 |
| Clinical stage | 24.843 | 13.007–47.448 | <0.001 |
| NLR | 0.527 | 0.342–0.814 | 0.004 |
| CA125 (U/mL) | 0.99 | 0.982–0.997 | 0.009 |
Note: p<0.05 displayed in bold.
Table 2.
Multivariate Logistic Analysis of Risk Factors of Endometriosis-Associated Infertility
| OR | 95% CI | p-value | |
|---|---|---|---|
| Age (years) | 1.925 | 0.904–4.101 | 0.09 |
| Unilateral or bilateral | 2.967 | 1.108–7.944 | 0.03 |
| Diameter of cyst | 2.656 | 1.185–5.955 | 0.018 |
| Oviduct adhesion | 1.644 | 0.486–5.554 | 0.424 |
| Pelvic adhesion | 3.648 | 1.773–7.506 | <0.001 |
| Clinical stage | 8.477 | 3.632–19.785 | <0.001 |
| NLR | 0.632 | 0.401–0.995 | 0.047 |
| CA125 (U/mL) | 0.992 | 0.983–1.001 | 0.084 |
Note: p<0.05 displayed in bold.
Lower Mean Lymphocyte Levels in Endometriosis Patients; Serum CA125, NLR, PLR, and Combined Markers (NLR–CA125, MLR–CA125, PLR–CA125) Elevated
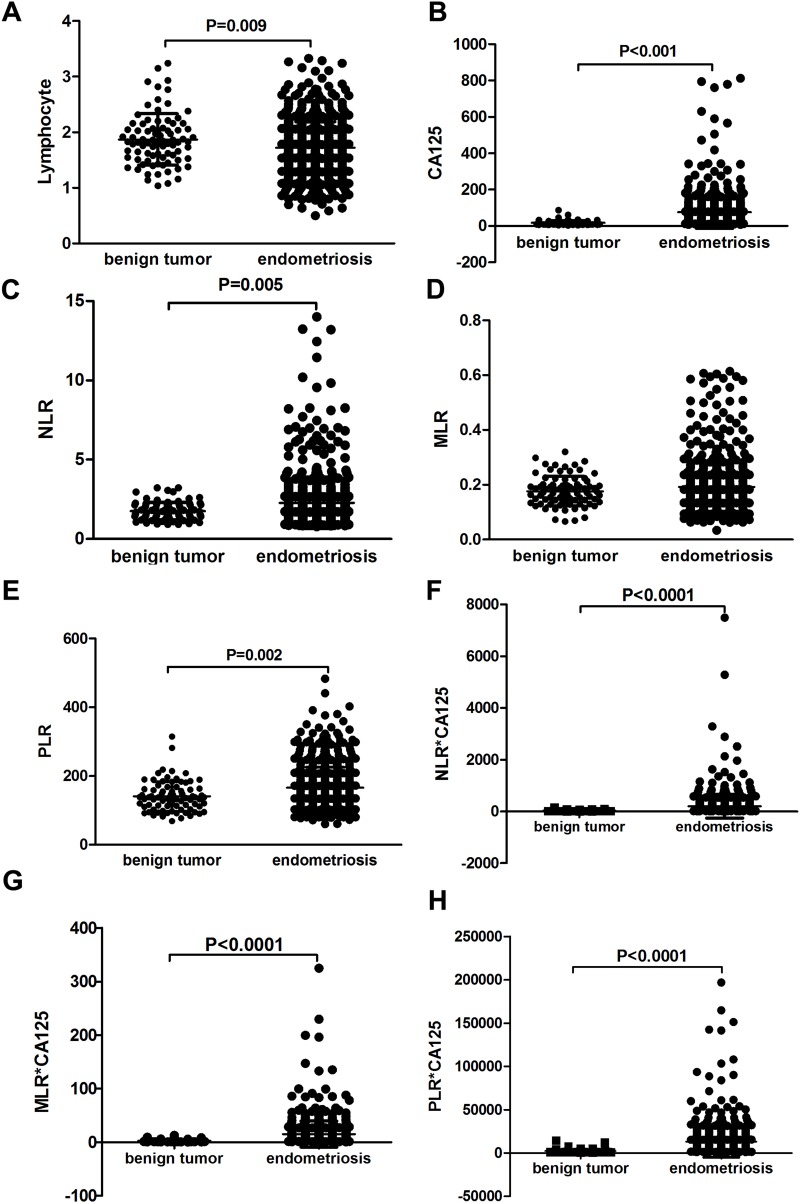
The mean age of all participants was 34.94±9.05 years, and the mean age of the endometriosis group and benign ovarian tumor group was 34.08±7.59 and 33.29±12.59 years, respectively. There was no significant difference in regard to age between the two groups (p=0.288). Mean values of NLR, MLR, PLR, and serum CA125 were higher in the endometriosis group, whereas lymphocyte counts were significantly lower in these patients (Figure 2). For other WBC subtypes, such as neutrophil, monocyte, and platelet counts, no significant difference was noted between the two groups. As shown in Figure 2A, the mean of lymphocyte counts were significantly higher in the endometriosis group than the benign-tumor group (p<0.001). On the contrary, mean serum CA125 was significantly higher in the endometriosis group than the benign-tumor group (p<0.001, Figure 2B). NLR was significantly higher in the endometriosis group than the benign-tumor group (p=0.005, Figure 2C). For MLR, no significant difference was noted between the the endometriosis group and the benign-tumor group (p>0.05, Figure 2D). PLR was significantly higher in the endometriosis group than the benign-tumor group (p<0.001, Figure 2E). Mean values of the combination markers (NLR–CA125, MLR–CA125, PLR–CA125) were higher in the endometriosis group than the benign-tumor group (Figure 2F–H).
Figure 2.
Mean values of NLR, PLR, serum CA125 levels, and combined markers in the two groups. Mean lymphocyte counts (A); serum CA125 (B); NLR (C); MLR (D); PLR (E); combination of NLR and CA125 (F); combination of MLR and CA125 (G); combination of PLR and CA125 (H) in endometriosis group and benign ovarian tumor group.
Diagnostic Value of NLR, MLR, PLR, and Serum CA125 Levels and Combined Markers in Endometriosis Patients
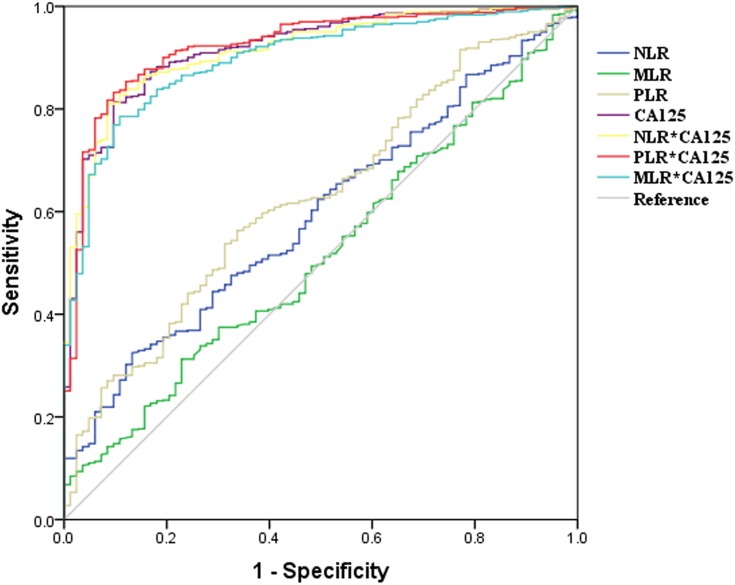
To determine the diagnostic value of NLR, MLR, PLR, and CA125 levels and combined markers in identifying endometriosis patients, we derived ROC curves to differentiate endometriosis from other benign ovarian tumors or healthy controls. Only markers with AUC >.5 were evaluated further for sensitivity, specificity, and cutoff value. For differentiating endometriosis from other benign ovarian tumors, the AUC for NLR was 0.56, with sensitivity of 32.9% and specificity of 80.2% (p=0.047). The AUC for PLR was 0.59, with sensitivity of 56.0% and specificity of 62.5% (p=0.002), while the AUC for MLR was 0.51, with sensitivity of 7.1% and specificity of 97.9% (p=0.73). As expected, diagnostic sensitivity and specificity of CA125 were 80.6% and 85.4%, respectively. The cutoff value for CA125 was 27.03. Combined biomarkers were calculated by multiplying CA125 level by NLR or PLR. The AUC for the combination of CA125 and NLR was 0.85, with sensitivity of 81.3% and specificity of 84.4%. The combination of CA125 and PLR, with the highest AUC of 0.86, showed the highest sensitivity of 85.1%, but also demonstrated lower specificity — 82.3% (Figure 3, Table 3).
Figure 3.
Receiver operating–characteristic curves of NLR, MLR, PLR, CA125, and combined markers. Discrepancy in diagnoses between endometriosis patients and benign-tumor patients assessed using ROC curves. Discrepancy in diagnoses between endometriosis patients and healthy controls assessed using ROC curves.
Table 3.
Diagnostic Sensitivity and Specificity of NLR, MLR, PLR, and CA125 and Combined Markers in Differentiating Endometriosis from Other Benign Ovarian Tumors
| AUC (95% CI) | Sensitivity, % | Specificity, % | Cutoff Value | p-value | |
|---|---|---|---|---|---|
| NLR | 0.56 (0.50–0.62) | 32.90 | 80.20 | 2.26 | 0.047 |
| MLR | 0.51 (0.45–0.57) | 7.10 | 97.90 | 0.35 | 0.729 |
| PLR | 0.59 (0.54–0.66) | 56.00 | 62.50 | 146.15 | 0.002 |
| CA125 (U/mL) | 0.86 (0.81–0.91) | 80.60 | 85.40 | 27.03 | 0 |
| NLR–CA125 | 0.85 (0.80–0.90) | 81.30 | 84.40 | 46.42 | 0 |
| PLR–CA125 | 0.86 (0.81–0.91) | 85.10 | 82.30 | 3,477.90 | 0 |
Note: p<0.05 displayed in bold.
Platelets and PLR Elevated in Endometriosis Patients with Endometrioma, While PLR Upregulated and Lymphocytes Lower in Endometriosis Patients with Uterine Leiomyoma
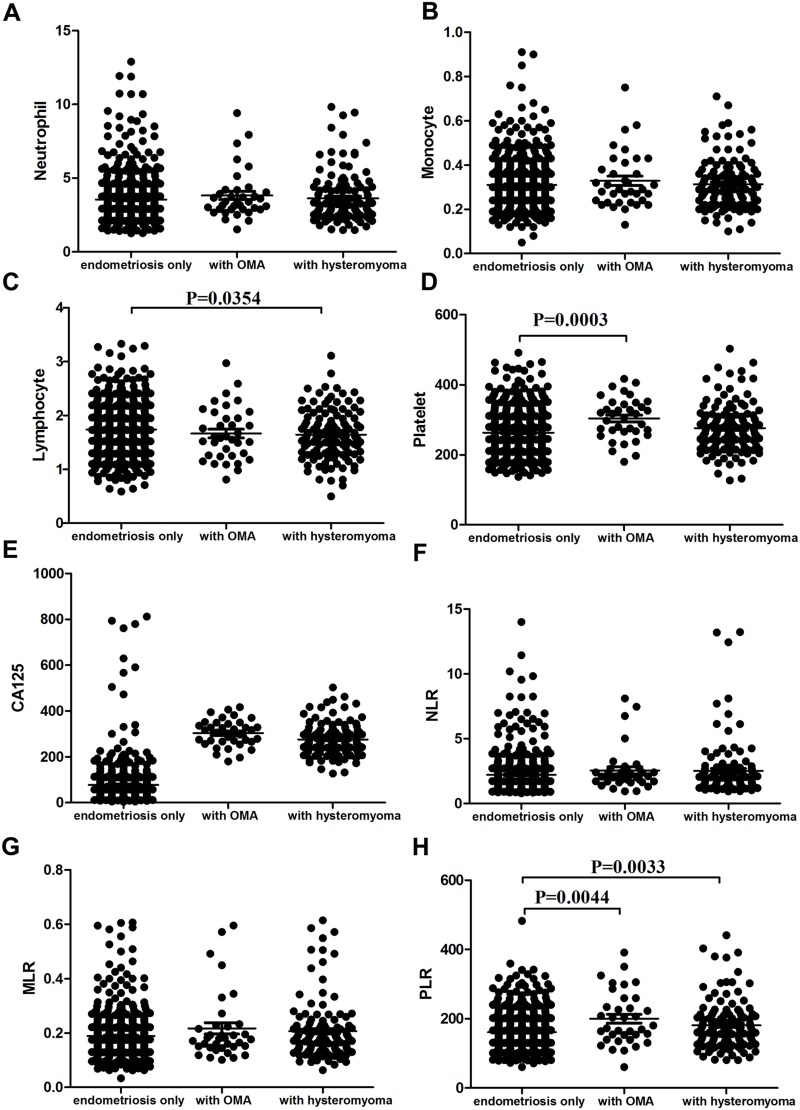
There was no significant difference among the endometriosis-only, ovarianendometrioma (OMA) or uterine leiomyoma groups in regard to neutrophil counts, monocyte counts, NLR, MLR, or serum CA125 levels (Figure 4, A, B, E–G). Lymphocyte counts were significantly lower in endometriosis patients with uterine leiomyoma than those with nonuterine leiomyoma (p=0.0354, Figure 4C). Platelet counts were significantly higher in endometriosis patients with OMA than those with non-OMA (p=0.0003, Figure 4D). PLR was significantly higher in patients with OMA and uterine leiomyoma than controls (p=0.0044 and p=0.0033, respectively; Figure 4H).
Figure 4.
Comparison of endometriosis patients with ovarian endometrioma (OMA), patients with uterine leiomyoma, and controls in terms of WBC subtypes, NLR, MLR, PLR, and CA125. Mean of neutrophil counts (A), monocyte counts (B), lymphocyte counts (C), platelet counts (D), serum CA125 (E), NLR (F), MLR (G), and PLR (H) in endometriosis group, endometriosis patients with uterine leiomyoma, and endometriosis patients with OMA.
Correlation Analysis Between Systemic Inflammatory Response Markers or Serum CA125 Levels and Clinicopathological Features of Endometriosis
All the endometriosis patients were divided into minimal-to-moderate disease (stages I–II) and moderate-to-severe disease (stages III–IV) according to the revised American Society of Reproductive Medicine classification of endometriosis. A total of 61 patients were classified as stages I–II and 601 patients as stages III–IV, while 67.4% of endometriosis patients had unilateral ovarian endometriosis cysts, 32.6% bilateral cysts, 71.6% symptoms of pelvic pain, 8.7%were infertile, with 5.5% having endometrioma, 18.9% uterine leiomyoma, 10.5%endometrial polyps, and 68.5% of endometriosis pelvic adhesions.
A comparison of lymphocyte, NLR, MLR, PLR, and serum CA125 levels based on different clinicopathological parameters is shown in Table 4. There was no significant difference in age with lymphocyte, NLR, MLR, PLR, or serum CA125 levels. NLR was significantly higher in patients with endometriotic cysts of >5 cm diameter or oviduct adhesions (p=0.008, p=0.048, respectively). Serum CA125 levels was related to endometriotic cysts, chronic pelvic pain, unilateral endometrioma, bilateral ovarian endometriosis cysts, and oviduct adhesions (p<0.001, p<0.001, p=0.007, p=0.019, respectively). The endometriosis group was also evaluated according to severity of disease and divided into stages I–II and III–IV according to the revised American Society of Reproductive Medicine classification of endometriosis. NLR and CA125 levels were significantly higher in patients at stages III–IV (p=0.044, p=0, respectively).
Table 4.
Correlations between lymphocyte, NLR, MLR, PLR, and serum CA125 levels and clinicopathological parameters of endometriosis
| n | Lymphocytes (109/L) | p-value | NLR | p | MLR | p-value | PLR | p-value | CA125 (U/mL) | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | |||||||||||
| <34 | 328 | 1.73±0.48 | 0.495 | 2.39±1.75 | 0.309 | 0.19±0.09 | 0.708 | 274.02±66.08 | 0.169 | 78.25±87.38 | 0.120 |
| ≥34 | 334 | 1.71±0.46 | 2.18±1.46 | 0.19±0.08 | 259.78±61.71 | 74.83±91.89 | |||||
| Cyst | |||||||||||
| Unilateral | 446 | 1.72±0.48 | 0.890 | 2.31±1.69 | 0.566 | 0.19±0.09 | 0.370 | 265.11±63.52 | 0.563 | 72.16±90.28 | <0.001 |
| Bilateral | 216 | 1.72±0.45 | 2.25±1.43 | 0.19±0.09 | 270.39±65.78 | 85.54±87.79 | |||||
| Cyst diameter | |||||||||||
| <5 cm | 225 | 1.74±0.49 | 0.430 | 2.09±1.28 | 0.008 | 0.19±0.08 | 0.205 | 266.45±63.31 | 0.664 | 71.40±91.06 | 0.007 |
| ≥5 cm | 437 | 1.71±0.46 | 2.38±1.75 | 0.19±0.09 | 267.03±64.82 | 79.16±88.87 | |||||
| Pain | |||||||||||
| No | 188 | 1.72±0.44 | 0.814 | 2.14±1.11 | 0.851 | 0.19±0.08 | 0.626 | 269.94±66.34 | 0.577 | 57.20±59.37 | <0.001 |
| Yes | 474 | 1.72±0.48 | 2.34±1.77 | 0.20±0.09 | 265.60±63.45 | 84.19±98.11 | |||||
| Endometrial polyp | |||||||||||
| No | 596 | 1.73±0.47 | 0.475 | 2.30±1.64 | 0.410 | 0.19±0.09 | 0.728 | 267.61±64.83 | 0.884 | 78.33±92.57 | 0.069 |
| Yes | 66 | 1.67±0.45 | 2.13±1.27 | 0.19±0.07 | 259.83±58.90 | 60.22±54.29 | |||||
| Oviduct adhesion | |||||||||||
| No | 512 | 1.72±0.46 | 0.936 | 2.23±1.57 | 0.048 | 0.19±0.08 | 0.384 | 265.07±64.35 | 0.323 | 73.70±89.57 | 0.019 |
| Yes | 150 | 1.73±0.51 | 2.47±1.72 | 0.20±0.11 | 272.87±63.82 | 86.16±89.48 | |||||
| Ovary adhesion | |||||||||||
| No | 208 | 1.69±0.45 | 0.254 | 2.18±1.43 | 0.471 | 0.19±0.08 | 0.130 | 260.21±67.25 | 0.325 | 72.47±95.59 | 0.088 |
| Yes | 454 | 1.73±0.48 | 2.33±1.68 | 0.19±0.09 | 269.87±62.69 | 78.38±86.81 | |||||
| Disease severity | |||||||||||
| Stages I–II | 61 | 1.80±0.49 | 0.752 | 1.95±1.12 | 0.044 | 0.18±0.07 | 0.166 | 161.13±65.28 | 0.260 | 17.28±5.02 | 0 |
| Stages III–V | 601 | 1.72±0.47 | 2.37±1.69 | 0.19±0.09 | 166.11±59.04 | 82.71±92.06 |
Notes: Student's t-test used. p<0.05 are displayed in bold.
In addition, we analyzed the diagnostic sensitivity and specificity of NLR, MLR, PLR, CA125, and combined markers in differentiating endometriosis from other benign ovarian tumors and in differentiating endometriosis from healthy controls. The results are shown in Tables S1 and S2. Then, we analyzed the diagnostic sensitivity and specificity of lymphocytes, platelets, and PLR in endometriosis patients complicated with OMA andlymphocytes, platelets, and platelet receptors in the diagnosis of endometriosis with hysteromyoma. Results are shown in Tables S3 and S4.
Discussion
Endometriosis is defined as the implantation and growth of endometrial cells outside the uterine cavity,15 which may be viewed as a local disease with systemic inflammation.16 The peritoneal microenvironment in women with endometriosis includes increased WBCs, especially macrophages, and an abundance of inflammatory cytokines and prostaglandins, which activate/alter the peritoneal microenvironment, creating the conditions for differentiation, adhesion, proliferation, and survival of ectopic endometrial cells.17 It has been reported that inflammation is present in ectopic endometria of women with endometriosis.7,8 Furthermore,abnormal coagulation has been observed in moderate and severe ovarian endometriosis patients.18,19 It has been reported that endometriosis is associated with systemic inflammation, which constitutes shifts in differential WBC counts with an increase in neutrophils and a decrease in lymphocytes.20 Similarly, our results support a significant association of lymphopenia with endometriosis.
The mechanism of infertility in endometriosis is still unclear. Women who are infertile are more likely to have advanced stages of endometriosis.21 It is accepted that inflammation plays a central role in the development and progression of endometriosis. What is more, the growth and adhesion of endometrial cells in the peritoneal cavity due to reactive oxygen species (ROS) and free radicals leads to disease onset, with ensuing symptoms of pain and infertility.22 As reported in our study, clinical stage, diameter of cysts, and pelvic adhesion are independent risk factors associated with infertility, which is in accordance with previous reports. Substantial evidence supports chronic inflammation being an important cause of endometriosis-associated infertility. Endometriosis is an inflammatory process associated with a change in immune-cell functions. Neutrophils in the abdominal cavity can secrete VEGF, an effective proangiogenic factor. Activated macrophages release cytokines or growth factors that promote the growth of endometriosis. Infertile patients have elevated serum IL2, IL4, IL6, IL21, TNFα, and IFNγ levels compared to fertile individuals. Inflammatory factors reduce the motility of sperm. Secondly, implantation of fertilized eggs is impaired in endometria with inflammation, or the endometrial antibody results in implantation dysfunction, showing embryo toxicity.14 Our data showed that NLR in infertile endometriosis patients was significantly lower than fertile ones and lower NLR was an independent factor associated with infertility, which has not been reported previously.
One of the diagnostic markers already well established is CA125 in endometriosis patients. Preoperative CA125 levels in patients were positively correlated with endometriosis, which is in accordance with a previous study.23 Sensitivity and specificity of CA125 in this study were high (80.6% and 85.4%, respectively), with a cutoff level of 27.03 IU/mL. Recently, there have been several studies evaluating the diagnostic value of the combination of SIS markers and CA125 in endometriosis patients, with contradictory conclusions.3,10,11 In a previous study, the AUC of serum CA125 was higher than the combination of CA125 and PLR in detecting moderate or severe endometriosis.24 Our data show that the combination of PLR and CA125 had higher sensitivity (85.1%) than CA125 alone in differentiating endometriosis from benign ovarian tumors. It has also been reported that the combination of NLR and CA125 achieved greater sensitivity (69.3%) when compared with CA125 alone (55.8%), but yielded lower specificity (83.9%) than CA125 alone (92.8%) in diagnosing endometriosis.11 Tokmak et al reported that sensitivity (80%) and specificity (82%) of NLR combined with CA125 in diagnosing endometriosis was higher than CA125 alone.9 We found that NLR–CA125 sensitivityincreased and specificity decreased compared with CA125 alone in differentiating endometriosis from benign ovarian tumors or healthy controls.
In this study, 18.9% of endometriosis patients had uterine leiomyoma, while 5.5% of endometriosis patients had OMA. Our data showed that lymphocytes and PLR were correlated with uterine leiomyoma. Furthermore, platelets and PLR were positively correlated with OMA. Therefore, it may be helpful to analyze complications and clinical decisions in endometriosis patients. Consistently with a previous study, we foundthat CA125 was positively associated with the stage of endometriosis. Furthermore, serum CA125 levels in patients with pelvic pain, pelvic adhesion, and of ovarian ectopic cyst diameter>5 cm were significantly lower.25 The elevation of CA125 in women with endometriosis may be due to inflammatory reactions that alter endothelial permeability, leading the marker to reach the circulation.26 It has also been reported that menstruation and adhesions appear to be the main factors affecting pretreatment serum CA125 level in patients with endometriosis.27
In addition, our data showed that stage, pelvic adhesion, and the diameter of ovarian ectopic cyst endometriomas had positive correlations with NLR. This is again consistent with previous studies, indicating that inflammatory markers are significantly elevated in patients with advanced stages of endometriosis.1,9 In our study, no significant correlation was observed between PLR and stage of endometriosis. Similarly, a previous study that assessed the diagnostic use of PLR in endometriosis also failed to show a significant relationship between PLR and advanced-stage endometriosis.24 Recently, PLR and NLR have been considered a more useful indicator of excessive inflammatory status, especially in patients with malignancy. Many studies have reported that higher neutrophil or lower lymphocyte counts predict poorer survival in ovarian cancer.28 Peritoneal neutrophils and macrophages secrete proinflammatory cytokines, such as VEGF, IL8, IL12, and CXCL10, which may promote endometriotic cell growth, invasion, and angiogenesis. Lymphocytes in endometriosis have limited capability of eliminating endometrial cells in the peritoneal cavity. Furthermore, the interaction between endothelial cells and platelets causes the release of inflammatory substances, which induces adhesion and migration of leukocytes.19 The novelty of our study is that NLR can be a neoadjuvant biomarker for serum CA125 to diagnose endometriosis. NLR may be used as a simple and easily obtained predictive marker for endometriosis with infertility. There were some limitations in our research. For example, healthy subjects were not included.
We conclude that serum CA125, NLR, and PLR values and combined biomarkers are significantly elevated in patients with endometriosis. The combination of serum CA125 and NLR showed higher diagnostic value than serum CA125 alone in diagnosing endometriosis, indicating NLR may be a neoadjuvant biomarker for serum CA125 in the diagnosis of endometriosis. Nevertheless, serum CA125 still plays an important role in diagnosing endometriosis. Also, platelets and PLR could be used for prediction of endometriosis with OMA or uterine leiomyoma. In addition, our findings demonstrate that lower NLR may represent a potential risk factor for infertility in endometriosis.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31470885, 81772879, 31300752, 81572070), National Science Foundation for Young Scientists of China (31500741), and Promotive Research Fund for Young and Middle-Aged Scientists of Shandong Province (BS2014SW022).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zondervan KT, Becker CM, Koga K, et al. Endometriosis. Nat Rev Dis Primers. 2018;4:9. doi: 10.1038/s41572-018-0008-5 [DOI] [PubMed] [Google Scholar]
- 2.Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366–373. doi: 10.1016/j.fertnstert.2011.05.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SK, Park JY, Jee BC, Suh CS, Kim SH. Association of the neutrophil-to-lymphocyte ratio and CA 125 with the endometriosis score. Clin Exp Reprod Med. 2014;41:151–157. doi: 10.5653/cerm.2014.41.4.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May KE, Conduit-Hulbert SA, Villar J, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16:651–674. doi: 10.1093/humupd/dmq009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 6.Li B, Zhou P, Liu Y, et al. Platelet-to-lymphocyte ratio in advanced cancer: review and meta-analysis. Clin Chim Acta. 2018;483:48–56. doi: 10.1016/j.cca.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Osuga Y, Koga K, Hirota Y, et al. Lymphocytes in endometriosis. Am J Reprod Immunol. 2011;65:1–10. doi: 10.1111/j.1600-0897.2010.00887.x [DOI] [PubMed] [Google Scholar]
- 8.Berbic M, Fraser IS.Regulatory T cells and other leukocytes in the pathogenesis of endometriosis. J Reprod Immunol. 2011;88:149–155. doi: 10.1016/j.jri.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 9.Tokmak A, Yildirim G, Öztaş E, et al. Use of neutrophil-to-lymphocyte ratio combined with CA125 to distinguish endometriomas from other benign ovarian cysts. Reprod Sci. 2016;23:795–802. doi: 10.1177/1933719115620494 [DOI] [PubMed] [Google Scholar]
- 10.Yang HD, Lang J-H, Zhu L, et al. Diagnostic value of the neutrophil-to-lymphocyte ratio and the combination of serum CA125 for stages III and IV endometriosis. Chin Med J(Engl). 2013;126:2011–2014. [PubMed] [Google Scholar]
- 11.Cho S, Cho H, Nam A, et al. Neutrophil-to-lymphocyte ratio as an adjunct to CA125 for the diagnosis of endometriosis. Fertil Steril. 2008;90:2073–2079. doi: 10.1016/j.fertnstert.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 12.Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39:535–549. doi: 10.1016/j.ogc.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holoch KJ, Lessey BA. Endometriosis and infertility. Clin Obstet Gynecol. 2010;53:429–438. doi: 10.1097/GRF.0b013e3181db7d71 [DOI] [PubMed] [Google Scholar]
- 14.Tola EN. The association between in vitro fertilization outcome and the inflammatory markers of complete blood count among nonobese unexplained infertile couples. Taiwan J Obstet Gynecol. 2018;57:289–294. doi: 10.1016/j.tjog.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Chmaj-Wierzchowska K, Kampioni M, Wilczak M, Opala T. Do inflammatory factors play a significant role in etiopathogenesis of endometrial cysts? Part 1. Ann Agric Environ Med. 2013;20:854–858. [PubMed] [Google Scholar]
- 16.Agic A, Xu H, Finas D, et al. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62:139–147. doi: 10.1159/000093121 [DOI] [PubMed] [Google Scholar]
- 17.Laganà AS, Vitale SG, Salmeri FM, et al. Unus pro omnibus, omnes pro uno: a novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses. 2017;103:10–20. doi: 10.1016/j.mehy.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 18.Viganò P, Ottolina J, Sarais V, et al. Coagulation status in women with endometriosis. Reprod Sci. 2018;25:559–565. doi: 10.1177/1933719117718273 [DOI] [PubMed] [Google Scholar]
- 19.Yavuzcan A, Caglar M, Ustun Y, et al. Evaluation of mean platelet volume, neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in advanced stage endometriosis with endometrioma. J Turk Ger Gynecol Assoc. 2013;14:210–215. doi: 10.5152/jtgga.2013.55452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MA, Han GH, Kwon JY, Kim YH. Clinical significance of platelet-to-lymphocyte ratio in women with preeclampsia. Am J Reprod Immunol. 2018;80:e12973. doi: 10.1111/aji.12973 [DOI] [PubMed] [Google Scholar]
- 21.D’Hooghe TM, Debrock S, Hill JA, Meuleman C. Endometriosis and subfertility: is the relationship resolved? Semin Reprod Med. 2003;21:243–254. doi: 10.1055/s-2003-41330 [DOI] [PubMed] [Google Scholar]
- 22.Vitale SG, Capriglione S, Peterlunger I. The role of oxidative stress and membrane transport systems during endometriosis: a fresh look at a busy corner. Oxid Med Cell Longev. 2018;21:7924021. doi: 10.1155/2018/7924021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karimi-Zarchi M, Dehshiri-Zadeh N, Sekhavat L, Nosouhi F. Correlation of CA-125 serum level and clinico-pathological characteristic of patients with endometriosis. Int J Reprod Biomed (Yazd). 2016;14:713–718. doi: 10.29252/ijrm.14.11.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H, Zhu L, Wang S, Lang J, Xu T. Noninvasive diagnosis of moderate to severe endometriosis: the platelet-lymphocyte ratio cannot be a neoadjuvant biomarker for serum cancer antigen 125. J Minim Invasive Gynecol. 2015;22:373–377. doi: 10.1016/j.jmig.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 25.Amaral VF, Ferriani RA, Sá MFSD, et al. Positive correlation between serum and peritoneal fluid CA125 levels in women with pelvic endometriosis. Sao Paulo Med. 2006;J124:223–227. doi: 10.1590/S1516-31802006000400010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng YM, Wang ST, Chou CY. Serum CA125 in preoperative patients at high risk for endometriosis. Obstet Gynecol. 2002;99:375–380. doi: 10.1016/s0029-7844(01)01731-8 [DOI] [PubMed] [Google Scholar]
- 27.Ramos I, Podgaec S, Abrão M, Oliveira R, Baracat E. Evaluation of CA-125 and soluble CD-23 in patients with pelvic endometriosis: a case-control study. Rev Assoc Med Bras(1992). 2012;58:26–32. doi: 10.1016/S0104-4230(12)70151-3 [DOI] [PubMed] [Google Scholar]
- 28.Williams KA, Labidi-Galy SI, Terry KL, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014;132:542–550. doi: 10.1016/j.ygyno.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]