Abstract
Congenital hyperinsulinism (HI) is the most frequent cause of persistent hypoglycemia in infants and children. Delays in diagnosis and initiation of appropriate treatment contribute to a high risk of neurocognitive impairment. HI represents a heterogeneous group of disorders characterized by dysregulated insulin secretion by the pancreatic beta cells, which in utero, may result in somatic overgrowth. There are at least nine known monogenic forms of HI as well as several syndromic forms. Molecular diagnosis allows for prediction of responsiveness to medical treatment and likelihood of surgically-curable focal hyperinsulinism. Timely genetic mutation analysis has thus become standard of care. However, despite significant advances in our understanding of the molecular basis of this disorder, the number of patients without an identified genetic diagnosis remains high, suggesting that there are likely additional genetic loci that have yet to be discovered.
Keywords: Hypoglycemia, insulin, beta-cell, KATP channel, pancreas
Introduction
Congenital hyperinsulinism (HI) is the most common cause of persistent hypoglycemia in infants and children. To date, at least nine monogenic forms of HI have been identified in addition to several syndromic forms. Prompt identification of congenital HI is crucial to minimize the risk of permanent neurological damage that results from untreated hypoglycemia. Despite significant advances which have expanded our understanding of the pathophysiology of this disorder, the cause of HI remains unknown in up to 50% of patients suggesting the role of additional genetic loci that have yet to be discovered (Kapoor et al., 2013; Martinez et al., 2016; Snider et al., 2013). In this article, we review the known monogenic and syndromic forms of HI as well as the approach to genetic testing.
Clinical Diagnosis
Infants with congenital hyperinsulinism typically present with persistent hypoglycemia shortly after birth, but they can also present later in infancy. Clinical clues to the diagnosis of HI include increased glucose utilization requiring high glucose infusion rates to maintain euglycemia and large for gestational age birth weight. The latter results because fetal insulin secretion induces cellular hyperplasia via insulin-like growth factor 1 receptor (IGF1R) mediated signaling and by promoting anabolic metabolism (Hill, 1982) and is thus a major determinant of in utero growth.
The diagnosis of HI is made based upon the critical sample, the blood specimen obtained at the time of spontaneous or provoked hypoglycemia, and the glycemic response to glucagon. The threshold plasma glucose for obtaining the critical sample is < 50mg/dL to permit investigation of the biochemical counter-regulatory response to hypoglycemia and to limit the likelihood of false positive results. Biochemical findings consistent with inappropriate insulin action (Table 1) include inappropriately low plasma beta-hydroxybutyrate and free fatty acid concentrations, and a glycemic response to glucagon of 30 mg/dL or more at the time of hypoglycemia (Finegold, Stanley, & Baker, 1980). While a detectable insulin level at the time of hypoglycemia is inappropriate and consistent with insulin excess, the absence of measurably elevated insulin does not exclude a diagnosis of HI. In many cases, increased insulin levels are not observed because the serum insulin concentration is below the detection threshold of the insulin assay utilized (Palladino, Bennett, & Stanley, 2008). Hemolysis of the blood sample may also result in undetectable insulin concentration (D. D. De Leon & Stanley, 2013). Conversely, due to improvements in assay sensitivity and differences in laboratory reporting practices, serum insulin may be reported as detectable in the absence of hyperinsulinism. Thus, accurate diagnosis requires a comprehensive interpretation of biochemical markers of insulin action, and does not rely solely upon an insulin level.
Table 1.
Sensitivity and Specificity of Critical Sample Laboratory Values for the Diagnosis of Hyperinsulinism (C. Ferrara, Patel, Becker, Stanley, & Kelly, 2016)
| Sensitivity, % | Specificity, % | |
|---|---|---|
| DETECTABLE INSULIN | 82.2 | 100 |
| ELEVATED C-PEPTIDE (≥0.5 ng/mL) | 88.5 | 100 |
| SUPPRESSED IGFBP1 (≤ 110 ng/mL) | 85 | 97 |
| SUPPRESSED β-HYDROXYBUTYRATE (<1.8 mM) | 100 | 100 |
| SUPPRESSED FREE FATTY ACIDS (<1.7 mM) | 86.9 | 100 |
| POSITIVE GLYCEMIC RESPONSE TO 1MG GLUCAGON INJECTION (Δ plasma glucose ≥30mg/dL) | 88.9 | 100 |
IGFBP1: insulin-like growth factor binding protein 1
Therapeutic Approach
The primary goal for hyperinsulinism treatment is to maintain plasma glucose > 70 mg/dL, above the threshold for activation of neuroendocrine responses to hypoglycemia. This target is supported by physiology and reflects current consensus opinion recognizing an absence of outcomes data comparing different therapeutic thresholds (Thornton et al., 2015). Diazoxide, which acts to open pancreatic β-cell ATP-sensitive potassium (KATP) channels and decrease insulin secretion, is the first-line therapeutic agent. However, diazoxide is ineffective in treating hyperinsulinism caused by inactivating mutations in the genes encoding the KATP channel. Diazoxide-responsiveness is typically the starting point for distinguishing congenital hyperinsulinism phenotypes and it is defined operationally as the demonstration that the cardinal features of hyperinsulinemic hypoglycemia (i.e. fasting hypoketotic hypoglycemia) are corrected while on treatment with diazoxide at doses ≤ 15mg/kg/day. Practically, this is assessed by demonstrating: 1) appropriate beta-hydroxybutyrate elevation (>2 mmol/L) prior to the decline of plasma glucose concentration below 50–60 mg/dL during a provocative fasting test, and 2) correction of protein-induced hypoglycemia, when present.
Treatment options for diazoxide-unresponsive cases are limited. Second-line medical therapy for hyperinsulinism includes octreotide and the longer-acting somatostatin analogs, which act downstream of the KATP channel to inhibit insulin secretion (Hosokawa et al., 2017; Modan-Moses, Koren, Mazor-Aronovitch, Pinhas-Hamiel, & Landau, 2011). As explained later, the treatment of choice for focal hyperinsulinism is localized surgical excision. For non-focal cases unresponsive to medical therapy, subtotal pancreatectomy may be required. That the optimal management of hyperinsulinism is dependent upon the underlying cause accentuates the importance of early ascertainment of a genetic diagnosis.
Monogenic forms of congenital HI
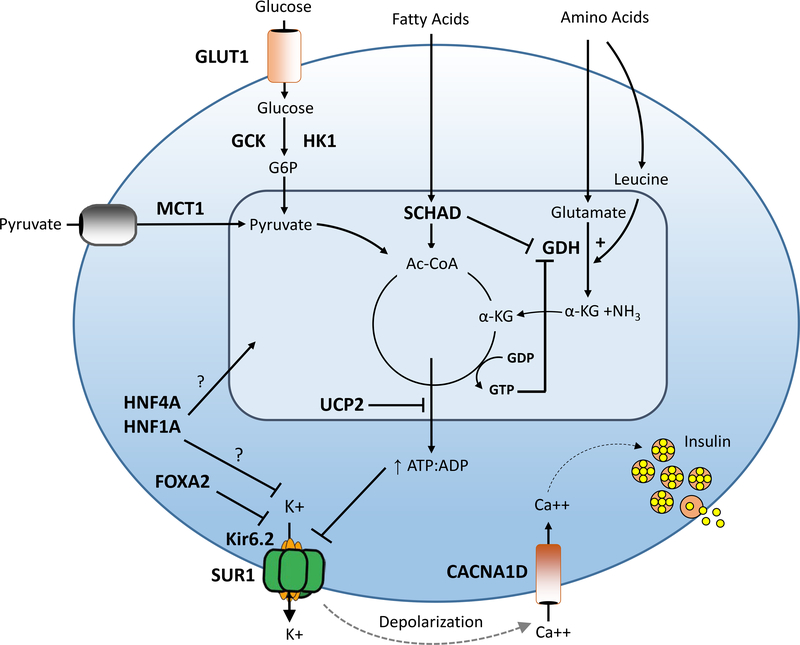
The incidence of congenital HI is estimated at 1 in 40000 live births in the general population and as high as 1 in 2500 in populations with high rates of consanguinity (Mathew et al., 1988; Otonkoski et al., 1999). Defects in key genes involved in regulating pancreatic β-cell insulin secretion cause this heterogeneous clinical condition characterized by recurrent hyperinsulinemic hypoglycemia (Figure 1). Notably, several of the genes associated with monogenic congenital HI are also involved in the pathogenesis of monogenic diabetes.
Figure 1. Genetic causes of congenital HI.
Pancreatic β-cell insulin secretion is predominately controlled by oxidation of glucose and amino acids. Glucose is transported into the β-cell by an insulin-independent glucose transporter (GLUT), predominantly GLUT1, and is phosphorylated by glucokinase (GCK). Glucose metabolism leads to an elevated intracellular ATP/ADP ratio resulting in sequential closure of plasma membrane ATP-sensitive KATP channels (composed of SUR1 and Kir6.2 subunits), membrane depolarization, activation of voltage-gated calcium channels, elevation of cytosolic calcium, and release of insulin from storage granules into the circulation. Amino acids stimulate insulin secretion via a variety of mechanisms. Leucine stimulates insulin secretion by allosterically activating glutamate dehydrogenase (GDH), increasing the oxidation of glutamate to alpha-ketoglutarate which increases the ATP/ADP ratio and triggers the insulin secretion cascade. GDH is allosterically inhibited by GTP and SCHAD. Diazoxide activates KATP channels thereby inhibiting insulin secretion. Defects in the triggering pathway for insulin secretion cause monogenic HI. Genes associated with congenital HI are highlighted in bold and include: SUR1 (sulfonylurea receptor), Kir6.2 (inwardly rectifying potassium channel), GCK (glucokinase), HK1 (hexokinase 1), GDH (glutamate dehydrogenase), SCHAD (short-chain 3-OH acyl-CoA dehydrogenase), HNF4a (hepatocyte nuclear transcription factor 4alpha), and HNF1a (hepatocyte nuclear transcription factor 1alpha), MCT1 (monocarboxylate transporter 1), UCP2 (uncoupling protein 2).
KATP-HI
The most common and severe form of monogenic HI is caused by inactivating mutations in ABCC8 and KCNJ11 located on chromosome 11p15.1, which respectively encode the two subunits, SUR-1 (sulfonylurea receptor) and Kir6.2 (potassium pore), of the hetero-octameric β-cell plasma membrane ATP-sensitive potassium channel (P. Thomas, Ye, & Lightner, 1996; P. M. Thomas et al., 1995). KATP-HI mutations are inherited in either a recessive or dominant manner. Biallelic inheritance of recessive KATP channel mutations result in complete absence of plasma membrane KATP channels and therefore diazoxide-unresponsiveness (Cartier, Conti, Vandenberg, & Shyng, 2001). Monoallelic paternally inherited recessive KATP channel mutations in combination with a somatic loss of maternal 11p15 result in absence of plasma membrane KATP channels only in a localized area of the pancreas (see later description of focal hyperinsulinism) (Verkarre et al., 1998). Monoallelic dominant KATP channel mutations allow for normal protein trafficking but result in the incorporation of mutant subunits into the KATP channel complex and impaired channel function. Mutations that severely diminish channel activity cause diazoxide-unresponsive HI. In contrast, mutations that permit residual KATP activity result in a diazoxide-responsive form (Macmullen et al., 2011; Pinney et al., 2008). Biallelic recessive and dominant diazoxide-unresponsive KATP-HI are clinically indistinguishable from each other at presentation. Affected neonates are born large for gestational age and present with severe hypoglycemia in the first few days of life (D. D. De Leon, Stanley, C.A., 2012). Both fasting and protein-induced hypoglycemia are observed; the latter likely results from glutamine-mediated amplification of glucagon-like peptide 1 (GLP-1) receptor signaling (D. D. De Leon et al., 2008). Near-total pancreatectomy is frequently required to control hypoglycemia. Dominant diazoxide-responsive forms typically manifest with milder hypoglycemia due to retained partial KATP function. Presentation may be delayed beyond infancy, and in some cases, hypoglycemia remains unrecognized until adulthood (Pinney et al., 2008). Children treated with near-total pancreatectomy are at high risk of progression to insulin-dependent diabetes mellitus; incidence rates exceed 90% by early adolescence (Beltrand et al., 2012; Lord et al., 2015). Furthermore, development of both gestational and insulin-dependent diabetes has been reported in non-surgically treated patients. Based upon findings in transgenic KATP-HI mice, hyperglycemia in these cases likely results from increased β-cell apoptosis; however, the pathophysiology in humans has not been fully determined (Huopio et al., 2000).
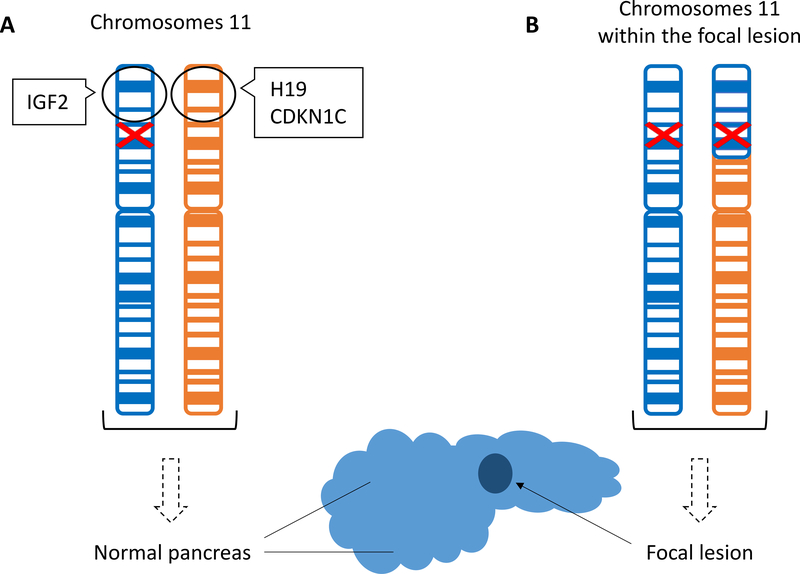
KATP-HI can be classified into two distinct histological forms: a diffuse form with functional abnormality of all pancreatic β-cells and a focal form with localized islet cell adenomatous hyperplasia. Diffuse hyperinsulinism results from monoallelic dominant or biallelic recessive mutations in ABCC8 or KCNJ11. Focal KATP-HI occurs via a “two-hit” mechanism of paternal transmission of a monoallelic recessive loss-of-function mutation in ABCC8 or KCNJ11 followed by a somatic loss of maternal 11p15 compensated by paternal uniparental disomy (Verkarre et al., 1998). Loss of maternally expressed genes involved in tumor suppression results in the histological findings of localized islet hyperplasia, while reduction to homozygosity of the paternally inherited recessive mutation within the lesion results in diazoxide-unresponsiveness (Figure 2). Clinically, focal KATP-HI presents similarly to the diffuse diazoxide-unresponsive KATP forms. While focal disease is more likely to present at an older age and with hypoglycemic seizures than diffuse, the observed differences are sufficiently subtle to preclude clinical differentiation between these histologic forms (Lord, Dzata, Snider, Gallagher, & De Leon, 2013). However, identification of focal cases is extremely important as surgical excision of the discrete lesion results in cure in 97% of patients (Adzick et al., 2018). Conversely, surgery can help ameliorate severe hypoglycemia, but does not cure diffuse disease.
Figure 2. “Two-hit” mechanism of focal hyperinsulinism.
(A) The paternally inherited chromosome is depicted in blue and the maternally inherited chromosome in orange. The paternally inherited mutation in the ABCC8 or KCNJ11 gene (red) is present in all cells. The black circle highlights the imprinted 11p15 region normally containing maternally expressed tumor suppressor genes, H19 and CDKN1C, and paternally expressed growth promoting factor IGF2. (B) Somatic loss of the maternal 11p15 region is compensated by paternal uniparental disomy resulting in the focal pancreatic lesion.
Over 180 unique mutations causing KATP-HI have been identified since the initial description of the molecular basis of the disorder in 1995 with ABCC8 mutations comprising the majority. KATP defects account for approximately 90% of identified mutations in diazoxide-unresponsive HI and 50% of these cases are focal. These findings highlight the paramount importance of mutation analysis in determining therapeutic options and prognostication.
GK-HI
Glucokinase, encoded by GCK on chromosome 7p13, functions as the pancreatic β-cell glucose sensor responsible for regulation of glucose-stimulated insulin secretion. Dominant activating mutations lower the glucose threshold for insulin release resulting in hyperinsulinemic hypoglycemia of varying severity (Glaser et al., 1998). Affected children are often born large-for-gestational age and may present with severe hypoglycemia at birth. However, presentation in adulthood is not uncommon and clinical phenotype may differ markedly, even between affected individuals within the same family (Challis et al., 2014; Martinez et al., 2017). The majority of cases are unresponsive to diazoxide therapy. Among those treated with near-total pancreatectomy, many continue to require medical management to prevent hypoglycemia (Sayed, 2012). In these difficult-to-manage cases, combined therapy with diazoxide and a long-acting somatostatin analogue has been employed with some success (Wabitsch et al., 2007). For patients requiring dietary management, continuous enteric feeds may prove superior to treatment with intragastric dextrose.
GDH-HI
Dominant activating mutations in GLUD1 cause the hyperinsulinism hyperammonemia (HI/HA) syndrome, the second most common form of congenital HI and most common form of diazoxide-responsive HI (Snider et al., 2013; Stanley et al., 1998). GLUD1, located on chromosome 10q23.3, encodes glutamate dehydrogenase (GDH) a mitochondrial matrix enzyme expressed in liver, kidney, brain, and pancreatic ß-cells. Disease-causing mutations in GDH result in impaired sensitivity to allosteric inhibition by guanosine triphosphate (GTP). Consequently, normal leucine-mediated allosteric activation is uninhibited leading to the clinical phenotype of fasting and protein-induced hyperinsulinemic hypoglycemia (Kelly et al., 2001) (Figure 1). In addition to HI, affected patients have persistent hyperammonemia due to the effects of the activating mutation in the kidney (Treberg, Clow, Greene, Brosnan, & Brosnan, 2010). Ammonia levels are typically elevated two to five times the normal range but are not associated with the classical signs of lethargy, headaches, or vomiting observed in other hyperammonemic disorders (Palladino & Stanley, 2010). However, a high rate of neurological abnormalities, including epilepsy, learning disabilities, and behavioral disorders are observed in affected patients (Bahi-Buisson et al., 2008; Kelly & Stanley, 2008). These symptoms are unrelated to hypoglycemia and may reflect GDH overactivity in the brain. HI/HA syndrome is associated with a normal birthweight, milder fasting hypoglycemia, and later age of presentation (median age 4–5 months) as compared to KATP-HI (Kapoor et al., 2009; Stanley et al., 2000). Hypoglycemia is usually easily controlled by diazoxide and dietary modification, including avoiding the consumption of protein without concomitant carbohydrate intake.
SCHAD-HI
Inactivating mutations of short-chain, 3-hydroxyacyl-coA dehydrogenase (SCHAD) encoded by HADH on chromosome 4q25 cause a rare, autosomal recessive form of HI. SCHAD is a mitochondrial enzyme highly expressed in the pancreas with dual roles of catalyzing fatty acid β-oxidation and inhibitory regulation of GDH (Li et al., 2010). Loss of inhibitory regulation of GDH by SCHAD results in a similar phenotype of fasting and protein-induced hypoglycemia as in GDH-HI but without associated hyperammonemia or neurodevelopmental abnormalities (Heslegrave & Hussain, 2013). An abnormal pattern of elevated fatty acid metabolites, 3-hydroxybutyryl-carnitine in plasma and 3-hydroxyglutaric acid in urine, was noted in the first reported cases but has not been universally observed in subsequent reports possibly due to varying degrees of enzyme deficiency (Clayton et al., 2001; Popa et al., 2012). Notably, affected children do not exhibit hepatic, cardiac, or skeletal muscle dysfunction characteristic of fatty acid oxidation disorders. Over 40 cases have been reported in the literature to date with variable clinical presentation ranging from severe neonatal hypoglycemia presenting in the first few days of life to mild infancy-onset hypoglycemia (Camtosun et al., 2015; Flanagan et al., 2011). The majority of cases had normal birth weights and all have been responsive to diazoxide.
HNF4A-HI and HNF1A-HI
Dominant inactivating mutations in HNF4A and HNF1A, the genes encoding transcription factors hepatocyte nuclear factors 4α (HNF4α) and 1α (HNF1α), respectively, cause congenital hyperinsulinism followed by monogenic diabetes later in life (Pearson et al., 2007; Stanescu, Hughes, Kaplan, Stanley, & De Leon, 2012). HNF4A, located on chromosome 20q13.2, and HNF1A, located on chromosome 12q24.31, have been shown to co-regulate expression of each other in addition to forming a regulatory network important for both glucose-stimulated insulin secretion and maintenance of the fully differentiated β-cell phenotype (Mitchell & Frayling, 2002). However, the mechanism by which these mutations result in a biphasic phenotype of hyperinsulinism early in life and diabetes in adulthood has not been elucidated. HI due to mutations in these genes is classically characterized by macrosomia, early neonatal presentation, and diazoxide responsiveness. Importantly, consideration of this diagnosis should not be excluded among those with normal birth weight; large-for-gestational age birth weight was recently reported among 30% of children with HNF1A-HI and 40% with HNF4A-HI in the largest case series to date (Tung, Boodhansingh, Stanley, & De Leon, 2018). The severity and natural history are clinically heterogeneous, ranging from transient hypoglycemia that does not necessitate pharmacologic treatment to the persistence of hyperinsulinism into late childhood (McGlacken-Byrne et al., 2014; Tung et al., 2018). Recently, a mutation-specific phenotype associated with the p.Arg63Trp mutation in HNF4A has been identified. Nine patients with this mutation have been described, all with proximal renal tubular dysfunction (atypical Fanconi syndrome) in addition to the recognized β-cell phenotype (Hamilton et al., 2014; Improda et al., 2016; Stanescu et al., 2012).
MCT1-HI
Dominant mutations in the regulatory regions of SLC16A1 on chromosome 1 encoding the pyruvate transporter monocarboxylate transporter 1 (MCT1) result in exercise-induced hyperinsulinism (EIHI). Normally, pancreatic β-cell expression of MCT1 is suppressed to prevent stimulation of insulin secretion by pyruvate and lactate. Mutations in the promoter region of SLC16A1 lead to aberrant MCT1 expression and inappropriate insulin secretion in response to rising intracellular pyruvate concentrations during anaerobic exercise. Thirteen patients from three families have been identified thus far with severity of symptoms ranging from mild to recurrent hypoglycemic syncope (Meissner et al., 2001; Otonkoski et al., 2007). This disorder is best treated by carbohydrate intake surrounding periods of vigorous exercise because the response to diazoxide is often incomplete.
UCP2-HI
Dominant loss of function mutations in UCP2, located on chromosome 11q13.4, encoding the mitochondrial carrier protein uncoupling protein 2 (UCP2) were first described to result in diazoxide-responsive hyperinsulinism in 2008. Since then, a total of nine patients with this disorder have been identified (Gonzalez-Barroso et al., 2008; Laver et al., 2017; Snider et al., 2013). The majority of affected children were born appropriate for gestational age and presented with hypoglycemic seizure. Profound postprandial hypoglycemia due to amplified β-cell glucose-stimulated insulin secretion has been described as a distinguishing feature (C. T. Ferrara et al., 2017). More recently, the role of UCP2 as a monogenic cause of HI has been questioned based upon the high prevalence of UCP2 variants identified in the general population utilizing data from the Genome Aggregation Database (gnomAD) (Laver et al., 2017).
HK-1
The HK1 gene encodes hexokinase 1 (HK1), a glucose-phosphorylating enzyme with high affinity for glucose. Its expression is normally suppressed in β-cells. Impaired silencing of HK1 expression lowers the glucose threshold for insulin secretion, resulting in hyperinsulinemic hypoglycemia (Henquin et al., 2013). Variants in the noncoding regions of HK1 have been proposed as the cause of a dominant form of diazoxide-responsive HI based upon analysis of a large, four-generation pedigree with congenital hyperinsulinism (Pinney et al., 2013).
Syndromic forms of HI
Hyperinsulinism is a feature of several developmental syndromes (Table 2). The most common syndrome associated with HI is Beckwith-Wiedemann syndrome, which is discussed in detail elsewhere in this issue. In some syndromes, such as Kabuki syndrome, congenital hyperinsulinism may be the presenting feature (Yap et al., 2018). HI is observed in up to 70% of infants with Kabuki syndrome and is often diazoxide-responsive. While the genetic basis for many of these disorders has been discovered, the mechanisms responsible for hyperinsulinism remain unknown.
Table 2.
Syndromic forms of HI:
| GENE | LOCUS | INHERITANCE | ASSOCIATED FEATURES | |
|---|---|---|---|---|
| OVERGROWTH SYNDROMES | ||||
| BECKWITH-WIEDEMANN SYNDROME (KALISH ET AL., 2016) |
IGF2, H19, CDKN1C, KCNQ1 | 11p15.4 | Sporadic | Macrosomia, macroglossia, hemihypertrophy, visceromegaly, abdominal wall defects, ear creases/pits, embryonal tumors |
| SOTOS-LIKE SYNDROMES SOTOS SYNDROME MALAN SYNDROME (GRAND ET AL., 2019; MATSUO ET AL., 2013; TATTON-BROWN ET AL., 2005) |
NSD1 NFIX | 5q35 19p13 | Sporadic or AD | Macrocephaly, distinctive facial features (broad and prominent forehead, sparse frontotemporal hair, long narrow face and chin), learning disability, advanced bone age |
| SIMPSON-GOLABI-BEHMEL (SAJORDA, GONZALEZ-GANDOLFI, HATHAWAY, & KALISH, 1993) |
GPC3 | Xq26 | X-linked | Pre- and postnatal overgrowth, macrocephaly, coarse facial features, macrosomia, macroglossia, palatal abnormalities, mild to severe intellectual disability with or without structural brain anomalies |
| PERLMAN SYNDROME (MORRIS, ASTUTI, & MAHER, 2013) |
DIS3L2 | 2q37.1 | AR | Neonatal macrosomia, polyhydramnios, broad and flat nasal bridge, everted V-shaped upper lip, low-set ears, deep-set eyes, prominent forehead, renal dysplasia, nephroblastomatosis, high neonatal mortality rate |
| POSTNATAL GROWTH FAILURE SYNDROMES | ||||
| KABUKI SYNDROME (ADAM ET AL., 2018) |
KMT2D KDM6A | 12q13.12 Xp11.3 | AR Sporadic | Postnatal growth restriction, long palpebral fissures with eversion of the lateral third of the lower eyelid, arched and broad eyebrows, large, prominent or cupped ears, short columella with depressed nasal tip, persistent fingertip pads, cardiac defects, intellectual disability |
| COSTELLO SYNDROME (GRIPP ET AL., 2016) |
HRAS | 11p15.5 | AD Sporadic | Failure to thrive, short stature; developmental delay or intellectual disability, coarse facial features (full lips, large mouth, full nasal tip), macrocephaly, hypertrophic cardiomyopathy, solid tumor risk |
| TURNER SYNDROME (GIBSON ET AL., 2018) |
KDM6A? | X | Sporadic | Short stature, premature ovarian failure, low posterior hairline, congenital heart defects, renal and skeletal abnormalities, autoimmune thyroiditis |
| CONGENITAL DISORDERS OF GLYCOSYLATION (CDG) | ||||
| CDG 1A (SPARKS & KRASNEWICH, 1993) |
PMM2 | 16p13.2 | AR | Failure to thrive, hypotonia, inverted nipples, abnormal subcutaneous fat distribution, cerebellar hypoplasia, developmental delay |
| CDG 1B (SPARKS & KRASNEWICH, 1993) |
MPI | 15q24.1 | AR | Failure to thrive, cyclic vomiting, hepatic fibrosis, protein-losing enteropathy, coagulopathy |
| PGM-1 CDG (FORMERLY CDG 1T) (WONG ET AL., 2016) |
PGM1 | 1p31.3 | AR | Hepatopathy, cleft palate, bifid uvula, dilated cardiomyopathy, growth retardation, myopathy |
| OTHER | ||||
| FORKHEAD BOX A2 (GIRI ET AL., 2017; VAJRAVELU ET AL., 2018) |
FOXA2 | 20p11.21 | Sporadic | Hypopituitarism, hypertelorism, choroidal coloboma, thin upper lip, low-set ears, widely spaced nipples |
| L-TYPE VOLTAGE-DEPENDENT CALCIUM CHANNEL, ALPHA-1D SUBUNIT (FLANAGAN ET AL., 2017) |
CACNA1D | 3p21.1 | Sporadic | Congenital heart defects (aortic insufficiency, ventricular septal defect, biventricular hypertrophy), hypotonia, seizures, developmental delay, primary hyperaldosteronism |
| TIMOTHY SYNDROME (NAPOLITANO, SPLAWSKI, TIMOTHY, BLOISE, & PRIORI, 1993) |
CACNA1C | 12p13.33 | AD Sporadic | Prolonged QT interval, congenital heart disease, cutaneous syndactyly, low-set ears, depressed nasal bridge, thin vermilion border of the upper lip, developmental delay, autism |
| TRISOMY 13 (TAMAME ET AL., 2004) |
Trisomy 13 | Sporadic | Holoprosencephaly, microphthalmia, cleft lip/palate, postaxial polydactyly, cardiac and urogenital malformations, severe intellectual disability | |
| TYROSINEMIA TYPE 1 (BAUMANN, PREECE, GREEN, KELLY, & MCKIERNAN, 2005) |
FAH | 15q25.1 | AR | Failure to thrive, liver failure, renal Fanconi syndrome, neurologic crises, risk for hepatocellular carcinoma |
| USHER SYNDROME, TYPE 1C (AL MUTAIR ET AL., 2013) |
USH1C-ABCC8† | 11p15.1 | AR | Congenital sensorineural deafness, vestibular dysfunction, retinitis pigmentosa |
AD: autosomal dominant, AR: autosomal recessive
contiguous gene deletion including ABCC8
Mimickers of Congenital HI
Activating mutations in the PI3K-AKT-mTOR insulin signaling pathway genes including AKT2, AKT3, and PIK3CA cause hypoinsulinemic hypoglycemia albeit with a phenotype similar to HI characterized by severe, recurrent hypoketotic hypoglycemia and inappropriately low free fatty acids indicative of excess insulin action. This phenotype results from constitutive, autonomous activity of the downstream signaling pathway in the absence of the normal physiological ligand, insulin (Arya et al., 2014; Hussain et al., 2011). Affected children often require continuous intragastric feedings to maintain euglycemia. All three mutations are associated with asymmetric somatic overgrowth; AKT3 and PIK3CA mutations are additionally associated with megalocephaly (Leiter et al., 2017).
Perinatal Stress-Induced HI
In contrast to the rarity of monogenic congenital HI, prolonged neonatal hyperinsulinism due to perinatal stress is quite common. Risk factors are conditions associated with fetal distress and include intrauterine growth restriction, perinatal asphyxia, meconium aspiration, and maternal toxemia, among others. While most infants have an apparent predisposition based upon birth history, this may not be obvious in all cases. The etiology of perinatal stress-induced HI remains unknown; a persistence of fetal patterns of insulin regulation has been hypothesized. Affected neonates are, with rare exception, diazoxide-responsive and hyperinsulinism typically resolves within the first few weeks of life although may persist for several months (Hoe et al., 2006).
Strategic Approach to Genetic Testing
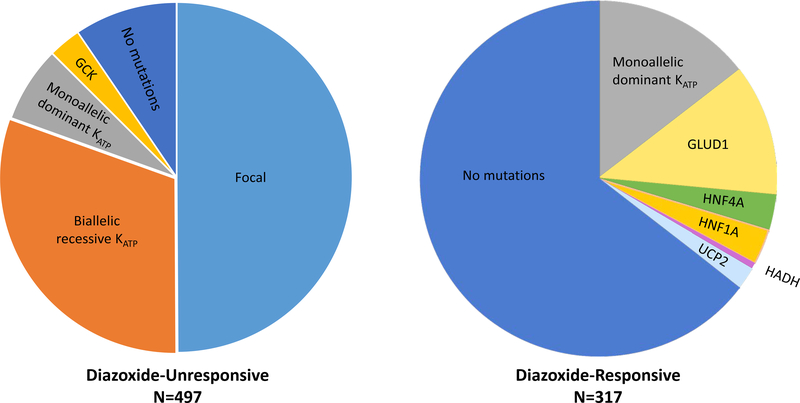
Genetic testing for hyperinsulinism is performed in a two tier approach (Figure 3). Children who are unresponsive to medical therapy with diazoxide undergo Tier 1 testing which includes genes involved in diazoxide-unresponsive HI (ABCC8, KCNJ11, GCK). Tier 1 testing is performed by direct sequencing of exons and flanking introns for all genes. This testing also includes deletion/duplication analysis for all genes as well as screening for the deep intronic mutation in ABCC8 identified as a founder mutation in the Irish population. Tier 1 testing is performed with a rapid turnaround time of five to seven days, and includes parental testing of identified variants. This rapid turnaround time with simultaneous parental testing is crucial for identifying children who likely have a focal lesion and thus can be cured of their hypoglycemia following surgical resection. The presence of a single recessive KATP mutation has been shown to predict focal HI with 97% sensitivity and 90% specificity (Snider et al., 2013).
Figure 3. Genetic etiologies of HI stratified by diazoxide responsiveness.
Data from 814 children with hyperinsulinism treated at the Children’s Hospital of Philadelphia. Among those with diazoxide-unresponsive HI, KATP mutations were the most common etiology identified by genetic testing: focal KATP (50%), biallelic recessive KATP (31%), monoallelic dominant KATP (7%), GCK (3%), no mutation identified (9.5%). In contrast, the most common finding among those with diazoxide-responsive HI was no identified mutation (64.5%), followed by monoallelic dominant KATP (14.5%), GLUD1 (12%), HNF4A (3%), HNF1A (3%), UCP2 (2%), and HADH (0.6%).
Children who are responsive to medical therapy with diazoxide undergo Tier 2 testing which includes testing for 9 genes (ABCC8, KCNJ11, GLUD1, GCK, HADH, UCP2, HNF4A, HNF1A, MCT1). Tier 2 testing is performed by next generation sequencing of exons and flanking introns for all genes. Deletion/duplication analysis for all genes is included in Tier 2 testing. Testing for the deep intronic mutation in ABCC8 identified as a founder mutation in the Irish population, as well as for the deep intronic mutation in HADH identified in the Turkish population, are also included in this testing. The turnaround time for Tier 2 testing is approximately four weeks and includes parental testing for identified mutations.
Reporting laboratories rely on the American College of Medical Genetics guidelines for variant interpretation, published literature, and laboratory-specific internal knowledge to determine whether a given variant detected in a causative gene should be classified as pathogenic (Richards et al., 2015). The most difficult variants are those classified as “unknown significance” where the burden of evidence does not rule in/out a change as diseases causing. In these cases our approach includes evaluation of additional evidence from functional studies, familial segregation, phenotyping of the proband and other family members carrying the variant, which may help to reclassify the changes as pathogenic or non-pathogenic.
Parental testing should be included in the genetic analysis for all cases in which a mutation is identified. This information not only aids in the clinical management for the affected child, but can identify other family members who may be at risk of hypoglycemia. Furthermore, parental testing can aid in counseling the families of recurrence risk in future children.
Our approach has been to consider genetic testing for syndromic forms of HI in cases where a mutation is not detected on the above panels. Others have proposed that routine testing for syndromic HI in patients without suggestive features is not warranted. Laver, et al. recently reported their findings on 82 infants with HI of unknown genetic etiology without a clinical diagnosis of a known syndrome. After genetic evaluation for 20 syndromes associated with HI, a pathologic KMT2D variant was identified in one patient. No pathogenic variants were identified in the remainder of the cohort (Laver et al., 2018). While not included in our congenital HI genetic panel, some panels employed at other institutions include KMT2D and KDM6A, the genes responsible for Kabuki syndrome. We anticipate that our recommended approach may change as our understanding of the mechanisms underlying hyperinsulinism in these syndromic forms develops.
Of note, the approach to genetic testing in other centers around the world may differ to ours. The variety of gene testing arises from various aspects of gene testing. For example, the panel of genes included in the Targeted Panel sequencing ranges from as few as 4 genes (ABCC8, KCNJ11, GCK and GLUD1) to 9 genes (ABCC8, KCNJ11, GCK, GLUD1, SLC16A1, UCP2, HNF1A, HNF4A, and HADH). Some laboratories include syndrome associated HI genes such as KMT2D, KDM6A for Kabuki syndrome. In many cases, parental testing is not included as standard of care. However for diazoxide-unresponsive cases, information on the parent of origin can inform on the possibility of focal HI and therefore, potential benefits of surgical management and can be crucial in clinical decision making process.
It is important to recognize the limitations of genetic testing in cases where a disease-causing mutation is not identified. De novo mutations confined to the pancreas will not be detected by analysis of peripheral blood (Henquin et al., 2013). Deep intronic or promoter mutations may be missed as a consequence of the sequencing methodology employed by a given laboratory. Lastly, mutations in novel causative genes cannot be excluded.
Conclusions
Timely identification of genotype is crucial to aid in prediction of clinical phenotype, including determination of best therapeutic options, to limit exposure to persistent hypoglycemia, and thus reduce risk of permanent brain damage in affected infants and children. Currently, the number of cases without an identified genetic diagnosis remains high. Thus, further research into the molecular genetics of congenital HI including the development and utilization of newer sequencing technologies is needed.
Acknowledgments
The authors thank Kara E. Boodhansingh, BS for reviewing the article. This work was supported by National Institutes of Health grants T32DK063688 (E.R.) and R01DK056268 (D.D.D.L. and A.G.).
Grant support: This work was supported by National Institutes of Health grants R01 DK056268 (D.D.D.L. and A.G.) and T32 DK063688 (E.R.).
Footnotes
Disclosure summary: The authors have nothing to disclose.
References
- Adam MP, Banka S, Bjornsson HT, Bodamer O, Chudley AE, Harris J, … Kabuki Syndrome Medical Advisory, B. (2018). Kabuki syndrome: international consensus diagnostic criteria. J Med Genet. doi: 10.1136/jmedgenet-2018-105625 [DOI] [PubMed] [Google Scholar]
- Adzick NS, De Leon DD, States LJ, Lord K, Bhatti TR, Becker SA, & Stanley CA (2018). Surgical treatment of congenital hyperinsulinism: Results from 500 pancreatectomies in neonates and children. J Pediatr Surg. doi: 10.1016/j.jpedsurg.2018.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Mutair AN, Brusgaard K, Bin-Abbas B, Hussain K, Felimban N, Al Shaikh A, & Christesen HT (2013). Heterogeneity in phenotype of usher-congenital hyperinsulinism syndrome: hearing loss, retinitis pigmentosa, and hyperinsulinemic hypoglycemia ranging from severe to mild with conversion to diabetes. Diabetes Care, 36(3), 557–561. doi: 10.2337/dc12-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya VB, Flanagan SE, Schober E, Rami-Merhar B, Ellard S, & Hussain K (2014). Activating AKT2 mutation: hypoinsulinemic hypoketotic hypoglycemia. J Clin Endocrinol Metab, 99(2), 391–394. doi: 10.1210/jc.2013-3228 [DOI] [PubMed] [Google Scholar]
- Bahi-Buisson N, Roze E, Dionisi C, Escande F, Valayannopoulos V, Feillet F, … de Lonlay P (2008). Neurological aspects of hyperinsulinism-hyperammonaemia syndrome. Dev Med Child Neurol, 50(12), 945–949. doi: 10.1111/j.1469-8749.2008.03114.x [DOI] [PubMed] [Google Scholar]
- Baumann U, Preece MA, Green A, Kelly DA, & McKiernan PJ (2005). Hyperinsulinism in tyrosinaemia type I. J Inherit Metab Dis, 28(2), 131–135. doi: 10.1007/s10545-005-5517-1 [DOI] [PubMed] [Google Scholar]
- Beltrand J, Caquard M, Arnoux JB, Laborde K, Velho G, Verkarre V, … de Lonlay P (2012). Glucose metabolism in 105 children and adolescents after pancreatectomy for congenital hyperinsulinism. Diabetes Care, 35(2), 198–203. doi: 10.2337/dc11-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camtosun E, Flanagan SE, Ellard S, Siklar Z, Hussain K, Kocaay P, & Berberoglu M (2015). A Deep Intronic HADH Splicing Mutation (c.636+471G>T) in a Congenital Hyperinsulinemic Hypoglycemia Case: Long Term Clinical Course. J Clin Res Pediatr Endocrinol, 7(2), 144–147. doi: 10.4274/jcrpe.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier EA, Conti LR, Vandenberg CA, & Shyng SL (2001). Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc Natl Acad Sci U S A, 98(5), 2882–2887. doi: 10.1073/pnas.051499698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis BG, Harris J, Sleigh A, Isaac I, Orme SM, Seevaratnam N, … Semple RK (2014). Familial adult onset hyperinsulinism due to an activating glucokinase mutation: implications for pharmacological glucokinase activation. Clin Endocrinol (Oxf), 81(6), 855–861. doi: 10.1111/cen.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton PT, Eaton S, Aynsley-Green A, Edginton M, Hussain K, Krywawych S, … van den Berg IE (2001). Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of beta-oxidation in insulin secretion. J Clin Invest, 108(3), 457–465. doi: 10.1172/JCI11294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon DD, Li C, Delson MI, Matschinsky FM, Stanley CA, & Stoffers DA (2008). Exendin-(9–39) corrects fasting hypoglycemia in SUR-1−/− mice by lowering cAMP in pancreatic beta-cells and inhibiting insulin secretion. J Biol Chem, 283(38), 25786–25793. doi: 10.1074/jbc.M804372200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon DD, & Stanley CA (2013). Determination of insulin for the diagnosis of hyperinsulinemic hypoglycemia. Best Pract Res Clin Endocrinol Metab, 27(6), 763–769. doi: 10.1016/j.beem.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon DD, Stanley CA (2012). Pathophysiology of Diffuse ATP-Sensitive Potassium Channel Hyperinsulinism In D. D. D. L., Stanley CA (Ed.), Monogenic Hyperinsulinemic Hypoglycemia Disorders (Vol. 21, pp. 18–29). Basel: Karger. [Google Scholar]
- Ferrara C, Patel P, Becker S, Stanley CA, & Kelly A (2016). Biomarkers of Insulin for the Diagnosis of Hyperinsulinemic Hypoglycemia in Infants and Children. J Pediatr, 168, 212–219. doi: 10.1016/j.jpeds.2015.09.045 [DOI] [PubMed] [Google Scholar]
- Ferrara CT, Boodhansingh KE, Paradies E, Fiermonte G, Steinkrauss LJ, Topor LS, … Stanley CA (2017). Novel Hypoglycemia Phenotype in Congenital Hyperinsulinism Due to Dominant Mutations of Uncoupling Protein 2. J Clin Endocrinol Metab, 102(3), 942–949. doi: 10.1210/jc.2016-3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold DN, Stanley CA, & Baker L (1980). Glycemic response to glucagon during fasting hypoglycemia: an aid in the diagnosis of hyperinsulinism. J Pediatr, 96(2), 257–259. [DOI] [PubMed] [Google Scholar]
- Flanagan SE, Patch AM, Locke JM, Akcay T, Simsek E, Alaei M, … Ellard S (2011). Genome-wide homozygosity analysis reveals HADH mutations as a common cause of diazoxide-responsive hyperinsulinemic-hypoglycemia in consanguineous pedigrees. J Clin Endocrinol Metab, 96(3), E498–502. doi: 10.1210/jc.2010-1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SE, Vairo F, Johnson MB, Caswell R, Laver TW, Lango Allen H, … Ellard S (2017). A CACNA1D mutation in a patient with persistent hyperinsulinaemic hypoglycaemia, heart defects, and severe hypotonia. Pediatr Diabetes, 18(4), 320–323. doi: 10.1111/pedi.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CE, Boodhansingh KE, Li C, Conlin L, Chen P, Becker SA, … Stanley CA (2018). Congenital Hyperinsulinism in Infants with Turner Syndrome: Possible Association with Monosomy X and KDM6A Haploinsufficiency. Horm Res Paediatr, 89(6), 413–422. doi: 10.1159/000488347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri D, Vignola ML, Gualtieri A, Scagliotti V, McNamara P, Peak M, … Senniappan S (2017). Novel FOXA2 mutation causes Hyperinsulinism, Hypopituitarism with Craniofacial and Endoderm-derived organ abnormalities. Hum Mol Genet, 26(22), 4315–4326. doi: 10.1093/hmg/ddx318 [DOI] [PubMed] [Google Scholar]
- Glaser B, Kesavan P, Heyman M, Davis E, Cuesta A, Buchs A, … Herold KC (1998). Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med, 338(4), 226–230. doi: 10.1056/NEJM199801223380404 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Barroso MM, Giurgea I, Bouillaud F, Anedda A, Bellanne-Chantelot C, Hubert L, … Ricquier D (2008). Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PLoS One, 3(12), e3850. doi: 10.1371/journal.pone.0003850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand K, Gonzalez-Gandolfi C, Ackermann AM, Aljeaid D, Bedoukian E, Bird LM, … Kalish JM (2019). Hyperinsulinemic hypoglycemia in seven patients with de novo NSD1 mutations. Am J Med Genet A, 179(4), 542–551. doi: 10.1002/ajmg.a.61062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Robbins KM, Sheffield BS, Lee AF, Patel MS, Yip S, … Sol-Church K (2016). Paternal uniparental disomy 11p15.5 in the pancreatic nodule of an infant with Costello syndrome: Shared mechanism for hyperinsulinemic hypoglycemia in neonates with Costello and Beckwith-Wiedemann syndrome and somatic loss of heterozygosity in Costello syndrome driving clonal expansion. Am J Med Genet A, 170(3), 559–564. doi: 10.1002/ajmg.a.37471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Bingham C, McDonald TJ, Cook PR, Caswell RC, Weedon MN, … Hattersley AT (2014). The HNF4A R76W mutation causes atypical dominant Fanconi syndrome in addition to a beta cell phenotype. J Med Genet, 51(3), 165–169. doi: 10.1136/jmedgenet-2013-102066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC, Sempoux C, Marchandise J, Godecharles S, Guiot Y, Nenquin M, & Rahier J (2013). Congenital hyperinsulinism caused by hexokinase I expression or glucokinase-activating mutation in a subset of beta-cells. Diabetes, 62(5), 1689–1696. doi: 10.2337/db12-1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslegrave AJ, & Hussain K (2013). Novel insights into fatty acid oxidation, amino acid metabolism, and insulin secretion from studying patients with loss of function mutations in 3-hydroxyacyl-CoA dehydrogenase. J Clin Endocrinol Metab, 98(2), 496–501. doi: 10.1210/jc.2012-3134 [DOI] [PubMed] [Google Scholar]
- Hill DE (1982). Fetal effects of insulin. Obstet Gynecol Annu, 11, 133–149. [PubMed] [Google Scholar]
- Hoe FM, Thornton PS, Wanner LA, Steinkrauss L, Simmons RA, & Stanley CA (2006). Clinical features and insulin regulation in infants with a syndrome of prolonged neonatal hyperinsulinism. J Pediatr, 148(2), 207–212. doi: 10.1016/j.jpeds.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Hosokawa Y, Kawakita R, Yokoya S, Ogata T, Ozono K, Arisaka O, … Yorifuji T (2017). Efficacy and safety of octreotide for the treatment of congenital hyperinsulinism: a prospective, open-label clinical trial and an observational study in Japan using a nationwide registry. Endocr J, 64(9), 867–880. doi: 10.1507/endocrj.EJ17-0024 [DOI] [PubMed] [Google Scholar]
- Huopio H, Reimann F, Ashfield R, Komulainen J, Lenko HL, Rahier J, … Otonkoski T (2000). Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest, 106(7), 897–906. doi: 10.1172/JCI9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain K, Challis B, Rocha N, Payne F, Minic M, Thompson A, … Semple RK (2011). An activating mutation of AKT2 and human hypoglycemia. Science, 334(6055), 474. doi: 10.1126/science.1210878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Improda N, Shah P, Guemes M, Gilbert C, Morgan K, Sebire N, … Hussain K (2016). Hepatocyte Nuclear Factor-4 Alfa Mutation Associated with Hyperinsulinaemic Hypoglycaemia and Atypical Renal Fanconi Syndrome: Expanding the Clinical Phenotype. Horm Res Paediatr, 86(5), 337–341. doi: 10.1159/000446396 [DOI] [PubMed] [Google Scholar]
- Kalish JM, Boodhansingh KE, Bhatti TR, Ganguly A, Conlin LK, Becker SA, … Deardorff MA (2016). Congenital hyperinsulinism in children with paternal 11p uniparental isodisomy and Beckwith-Wiedemann syndrome. J Med Genet, 53(1), 53–61. doi: 10.1136/jmedgenet-2015-103394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor RR, Flanagan SE, Arya VB, Shield JP, Ellard S, & Hussain K (2013). Clinical and molecular characterisation of 300 patients with congenital hyperinsulinism. Eur J Endocrinol, 168(4), 557–564. doi: 10.1530/EJE-12-0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor RR, Flanagan SE, Fulton P, Chakrapani A, Chadefaux B, Ben-Omran T, … Hussain K (2009). Hyperinsulinism-hyperammonaemia syndrome: novel mutations in the GLUD1 gene and genotype-phenotype correlations. Eur J Endocrinol, 161(5), 731–735. doi: 10.1530/EJE-09-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Ng D, Ferry RJ Jr., Grimberg A, Koo-McCoy S, Thornton PS, & Stanley CA (2001). Acute insulin responses to leucine in children with the hyperinsulinism/hyperammonemia syndrome. J Clin Endocrinol Metab, 86(8), 3724–3728. doi: 10.1210/jcem.86.8.7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, & Stanley CA (2008). Neurological aspects in hyperinsulinism-hyperammonaemia syndrome. Dev Med Child Neurol, 50(12), 888. doi: 10.1111/j.1469-8749.2008.03149.x [DOI] [PubMed] [Google Scholar]
- Laver TW, Wakeling MN, Hua JHY, Houghton JAL, Hussain K, Ellard S, & Flanagan SE (2018). Comprehensive screening shows that mutations in the known syndromic genes are rare in infants presenting with hyperinsulinaemic hypoglycaemia. Clin Endocrinol (Oxf), 89(5), 621–627. doi: 10.1111/cen.13841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver TW, Weedon MN, Caswell R, Hussain K, Ellard S, & Flanagan SE (2017). Analysis of large-scale sequencing cohorts does not support the role of variants in UCP2 as a cause of hyperinsulinaemic hypoglycaemia. Hum Mutat, 38(10), 1442–1444. doi: 10.1002/humu.23289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter SM, Parker VER, Welters A, Knox R, Rocha N, Clark G, … Semple RK (2017). Hypoinsulinaemic, hypoketotic hypoglycaemia due to mosaic genetic activation of PI3-kinase. Eur J Endocrinol, 177(2), 175–186. doi: 10.1530/EJE-17-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Palladino A, Narayan S, Russell LK, Sayed S, … Stanley CA (2010). Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J Biol Chem, 285(41), 31806–31818. doi: 10.1074/jbc.M110.123638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord K, Dzata E, Snider KE, Gallagher PR, & De Leon DD (2013). Clinical presentation and management of children with diffuse and focal hyperinsulinism: a review of 223 cases. J Clin Endocrinol Metab, 98(11), E1786–1789. doi: 10.1210/jc.2013-2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord K, Radcliffe J, Gallagher PR, Adzick NS, Stanley CA, & De Leon DD (2015). High Risk of Diabetes and Neurobehavioral Deficits in Individuals With Surgically Treated Hyperinsulinism. J Clin Endocrinol Metab, 100(11), 4133–4139. doi: 10.1210/jc.2015-2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmullen CM, Zhou Q, Snider KE, Tewson PH, Becker SA, Aziz AR, … Stanley CA (2011). Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the beta-cell sulfonylurea receptor SUR1. Diabetes, 60(6), 1797–1804. doi: 10.2337/db10-1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R, Fernandez-Ramos C, Vela A, Velayos T, Aguayo A, Urrutia I, … Spanish Congenital Hyperinsulinism, G. (2016). Clinical and genetic characterization of congenital hyperinsulinism in Spain. Eur J Endocrinol, 174(6), 717–726. doi: 10.1530/EJE-16-0027 [DOI] [PubMed] [Google Scholar]
- Martinez R, Gutierrez-Nogues A, Fernandez-Ramos C, Velayos T, Vela A, Spanish Congenital Hyperinsulinism G, … Castano L (2017). Heterogeneity in phenotype of hyperinsulinism caused by activating glucokinase mutations: a novel mutation and its functional characterization. Clin Endocrinol (Oxf), 86(6), 778–783. doi: 10.1111/cen.13318 [DOI] [PubMed] [Google Scholar]
- Mathew PM, Young JM, Abu-Osba YK, Mulhern BD, Hammoudi S, Hamdan JA, & Sa’di AR (1988). Persistent neonatal hyperinsulinism. Clin Pediatr (Phila), 27(3), 148–151. doi: 10.1177/000992288802700307 [DOI] [PubMed] [Google Scholar]
- Matsuo T, Ihara K, Ochiai M, Kinjo T, Yoshikawa Y, Kojima-Ishii K, … Hara T (2013). Hyperinsulinemic hypoglycemia of infancy in Sotos syndrome. Am J Med Genet A, 161A(1), 34–37. doi: 10.1002/ajmg.a.35657 [DOI] [PubMed] [Google Scholar]
- McGlacken-Byrne SM, Hawkes CP, Flanagan SE, Ellard S, McDonnell CM, & Murphy NP (2014). The evolving course of HNF4A hyperinsulinaemic hypoglycaemia--a case series. Diabet Med, 31(1), e1–5. doi: 10.1111/dme.12259 [DOI] [PubMed] [Google Scholar]
- Meissner T, Otonkoski T, Feneberg R, Beinbrech B, Apostolidou S, Sipila I, … Mayatepek E (2001). Exercise induced hypoglycaemic hyperinsulinism. Arch Dis Child, 84(3), 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SM, & Frayling TM (2002). The role of transcription factors in maturity-onset diabetes of the young. Mol Genet Metab, 77(1–2), 35–43. [DOI] [PubMed] [Google Scholar]
- Modan-Moses D, Koren I, Mazor-Aronovitch K, Pinhas-Hamiel O, & Landau H (2011). Treatment of congenital hyperinsulinism with lanreotide acetate (Somatuline Autogel). J Clin Endocrinol Metab, 96(8), 2312–2317. doi: 10.1210/jc.2011-0605 [DOI] [PubMed] [Google Scholar]
- Morris MR, Astuti D, & Maher ER (2013). Perlman syndrome: overgrowth, Wilms tumor predisposition and DIS3L2. Am J Med Genet C Semin Med Genet, 163C(2), 106–113. doi: 10.1002/ajmg.c.31358 [DOI] [PubMed] [Google Scholar]
- Napolitano C, Splawski I, Timothy KW, Bloise R, & Priori SG (1993). Timothy Syndrome. In Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, & Amemiya A (Eds.), GeneReviews((R)) Seattle (WA). [Google Scholar]
- Otonkoski T, Ammala C, Huopio H, Cote GJ, Chapman J, Cosgrove K, … Thomas PM (1999). A point mutation inactivating the sulfonylurea receptor causes the severe form of persistent hyperinsulinemic hypoglycemia of infancy in Finland. Diabetes, 48(2), 408–415. [DOI] [PubMed] [Google Scholar]
- Otonkoski T, Jiao H, Kaminen-Ahola N, Tapia-Paez I, Ullah MS, Parton LE, … Kere J (2007). Physical exercise-induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic beta cells. Am J Hum Genet, 81(3), 467–474. doi: 10.1086/520960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino AA, Bennett MJ, & Stanley CA (2008). Hyperinsulinism in infancy and childhood: when an insulin level is not always enough. Clin Chem, 54(2), 256–263. doi: 10.1373/clinchem.2007.098988 [DOI] [PubMed] [Google Scholar]
- Palladino AA, & Stanley CA (2010). The hyperinsulinism/hyperammonemia syndrome. Rev Endocr Metab Disord, 11(3), 171–178. doi: 10.1007/s11154-010-9146-0 [DOI] [PubMed] [Google Scholar]
- Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, … Hattersley AT (2007). Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med, 4(4), e118. doi: 10.1371/journal.pmed.0040118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney SE, Ganapathy K, Bradfield J, Stokes D, Sasson A, Mackiewicz K, … Stanley CA (2013). Dominant form of congenital hyperinsulinism maps to HK1 region on 10q. Horm Res Paediatr, 80(1), 18–27. doi: 10.1159/000351943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney SE, MacMullen C, Becker S, Lin YW, Hanna C, Thornton P, … Stanley CA (2008). Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest, 118(8), 2877–2886. doi: 10.1172/JCI35414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa FI, Perlini S, Teofoli F, Degani D, Funghini S, La Marca G, … Camilot M. (2012). 3-hydroxyacyl-coenzyme a dehydrogenase deficiency: identification of a new mutation causing hyperinsulinemic hypoketotic hypoglycemia, altered organic acids and acylcarnitines concentrations. JIMD Rep, 2, 71–77. doi: 10.1007/8904_2011_50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, … Committee, A. L. Q. A. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med, 17(5), 405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajorda BJ, Gonzalez-Gandolfi CX, Hathaway ER, & Kalish JM (1993). Simpson-Golabi-Behmel Syndrome Type 1. In Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, & Amemiya A (Eds.), GeneReviews((R)) Seattle (WA). [PubMed] [Google Scholar]
- Sayed S, Matschinsky FM, Stanley CA (2012). Hyperinsulinism Due to Activating Mutations of Glucokinase In Stanley CA, De Leon DD (Ed.), Monogenic Hyperinsulinemic Hypoglycemia Disorders (Vol. 21): Karger. [Google Scholar]
- Snider KE, Becker S, Boyajian L, Shyng SL, MacMullen C, Hughes N, … Ganguly A (2013). Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab, 98(2), E355–363. doi: 10.1210/jc.2012-2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks SE, & Krasnewich DM (1993). Congenital Disorders of N-Linked Glycosylation and Multiple Pathway Overview. In Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, & Amemiya A (Eds.), GeneReviews((R)) Seattle (WA). [PubMed] [Google Scholar]
- Stanescu DE, Hughes N, Kaplan B, Stanley CA, & De Leon DD (2012). Novel presentations of congenital hyperinsulinism due to mutations in the MODY genes: HNF1A and HNF4A. J Clin Endocrinol Metab, 97(10), E2026–2030. doi: 10.1210/jc.2012-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley CA, Fang J, Kutyna K, Hsu BY, Ming JE, Glaser B, & Poncz M (2000). Molecular basis and characterization of the hyperinsulinism/hyperammonemia syndrome: predominance of mutations in exons 11 and 12 of the glutamate dehydrogenase gene. HI/HA Contributing Investigators. Diabetes, 49(4), 667–673. [DOI] [PubMed] [Google Scholar]
- Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, … Poncz M (1998). Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med, 338(19), 1352–1357. doi: 10.1056/NEJM199805073381904 [DOI] [PubMed] [Google Scholar]
- Tamame T, Hori N, Homma H, Yoshida R, Inokuchi M, Kosaki K, … Hasegawa T (2004). Hyperinsulinemic hypoglycemia in a newborn infant with trisomy 13. Am J Med Genet A, 129A(3), 321–322. doi: 10.1002/ajmg.a.30147 [DOI] [PubMed] [Google Scholar]
- Tatton-Brown K, Douglas J, Coleman K, Baujat G, Cole TR, Das S, … Childhood Overgrowth, C. (2005). Genotype-phenotype associations in Sotos syndrome: an analysis of 266 individuals with NSD1 aberrations. Am J Hum Genet, 77(2), 193–204. doi: 10.1086/432082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Ye Y, & Lightner E (1996). Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet, 5(11), 1809–1812. [DOI] [PubMed] [Google Scholar]
- Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, … Bryan J (1995). Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science, 268(5209), 426–429. [DOI] [PubMed] [Google Scholar]
- Thornton PS, Stanley CA, De Leon DD, Harris D, Haymond MW, Hussain K, … Pediatric Endocrine, S. (2015). Recommendations from the Pediatric Endocrine Society for Evaluation and Management of Persistent Hypoglycemia in Neonates, Infants, and Children. J Pediatr, 167(2), 238–245. doi: 10.1016/j.jpeds.2015.03.057 [DOI] [PubMed] [Google Scholar]
- Treberg JR, Clow KA, Greene KA, Brosnan ME, & Brosnan JT (2010). Systemic activation of glutamate dehydrogenase increases renal ammoniagenesis: implications for the hyperinsulinism/hyperammonemia syndrome. Am J Physiol Endocrinol Metab, 298(6), E1219–1225. doi: 10.1152/ajpendo.00028.2010 [DOI] [PubMed] [Google Scholar]
- Tung JY, Boodhansingh K, Stanley CA, & De Leon DD (2018). Clinical heterogeneity of hyperinsulinism due to HNF1A and HNF4A mutations. Pediatr Diabetes, 19(5), 910–916. doi: 10.1111/pedi.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajravelu ME, Chai J, Krock B, Baker S, Langdon D, Alter C, & De Leon DD (2018). Congenital Hyperinsulinism and Hypopituitarism Attributable to a Mutation in FOXA2. J Clin Endocrinol Metab, 103(3), 1042–1047. doi: 10.1210/jc.2017-02157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkarre V, Fournet JC, de Lonlay P, Gross-Morand MS, Devillers M, Rahier J, … Junien C (1998). Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest, 102(7), 1286–1291. doi: 10.1172/JCI4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabitsch M, Lahr G, Van de Bunt M, Marchant C, Lindner M, von Puttkamer J, … Gloyn AL (2007). Heterogeneity in disease severity in a family with a novel G68V GCK activating mutation causing persistent hyperinsulinaemic hypoglycaemia of infancy. Diabet Med, 24(12), 1393–1399. doi: 10.1111/j.1464-5491.2007.02285.x [DOI] [PubMed] [Google Scholar]
- Wong SY, Beamer LJ, Gadomski T, Honzik T, Mohamed M, Wortmann SB, … Morava E. (2016). Defining the Phenotype and Assessing Severity in Phosphoglucomutase-1 Deficiency. J Pediatr, 175, 130–136 e138. doi: 10.1016/j.jpeds.2016.04.021 [DOI] [PubMed] [Google Scholar]
- Yap KL, Johnson AEK, Fischer D, Kandikatla P, Deml J, Nelakuditi V, … Del Gaudio D (2018). Congenital hyperinsulinism as the presenting feature of Kabuki syndrome: clinical and molecular characterization of 10 affected individuals. Genet Med. doi: 10.1038/s41436-018-0013-9 [DOI] [PubMed] [Google Scholar]