Abstract
The aim of this study was to investigate the effects of diet complexity and l-Thr supplementation level on the growth performance, immune response, intestinal barrier function, and microbial metabolites in nursery pigs. Thirty-two weaned pigs (body weight 7.23 ± 0.48 kg) were randomly assigned to dietary treatments in a 2 × 2 factorial arrangement based on diet complexity (complex or simple) and dietary Thr content. The complex diet contained fish meal, plasma protein, and dried whey to mimic a conventional nursery diet. The simple diet was formulated with corn, wheat, and soybean meal and did not contain any animal products. l-Thr was supplemented to each diet to supply either 100% (STD Thr) or 115% (SUP Thr) of the NRC (2012) requirement for standardized ileal digestible Thr. Pigs were individually housed and fed experimental diets ad libitum for 14 d. Diet complexity, dietary Thr content, and their interactions were considered the main effects. Pigs fed the simple diet had greater (P < 0.05) plasma interleukin (IL)-10 and IL-6 concentrations compared with those fed the complex diet on days 7 and 14, respectively. Simple diet-fed pigs tended to show greater (P < 0.10) expression of genes encoding for tumor necrosis factor-α, claudin-1, and zonula occludens-1 in the jejunum compared with complex diet-fed pigs. The simple diet-fed pigs had greater (P < 0.05) concentrations of NH3-N in the jejunum digesta than did complex diet-fed pigs. The SUP Thr increased (P < 0.05) villus height and goblet cell (GC) density in villi and crypts in the jejunum and deepened (P < 0.05) crypts in the proximal colon. The SUP Thr resulted in the upregulation (P < 0.05) of occludin gene expression and a tendency toward the downregulation (P = 0.10) of IL-6 gene expression in the jejunum. Interactions (P < 0.05) between diet complexity and l-Thr supplementation level were observed in GC density in the crypt, NH3-N concentration in the jejunum, and the contents of acetate, propionate, and total volatile fatty acids in the colon. In conclusion, feeding a simple diet to nursery pigs resulted in systemic and intestinal inflammation. The SUP Thr diet did not normalize the simple diet-induced inflammation but improved gut integrity. SUP Thr seems to have greater benefits with a simple diet than with a complex diet. Therefore, SUP Thr in a simple diet could be a beneficial nutritional strategy for enhancing gut health.
Keywords: diet complexity, immune response, intestinal barrier function, nursery pigs, threonine
Introduction
Nursery pig diets have been conventionally formulated with digestible and palatable ingredients that include animal protein sources and dairy products (Mahan et al., 2004). However, this has resulted in a highly complex diet composition and, consequently, high feed costs. Many attempts have, therefore, been made to simplify the conventional complex diet by increasing the proportion of soybean meal as a way to save on feed costs in nursery pig production. Previous studies have confirmed that simple diets do not compromise the growth performance at the end of the nursery phase (Skinner et al., 2014; Koo et al., 2017). However, concerns remain that the large amounts of non-starch polysaccharides (NSP) and antigenic compounds in soybean meal may stimulate the pig’s immune system and impair intestinal integrity (Koo et al., 2017), thereby leading to poor growth performance in pigs raised in a commercial swine barn environment (Pastorelli et al., 2012). These concerns have restricted the actual introduction of simple diets in nursery production and have stressed the need to find an economic nutritional strategy that can fortify the gut barrier function of pigs to allow a practical introduction of a simple diet in nursery production. This might be possible by focusing on dietary supplementation with Thr, a major component of mucins (MUCs) and γ-globulins, including immunoglobulin (Wang et al., 2009). Dietary Thr is mostly metabolized in the intestine for incorporation into the functional proteins (Floc’h and Sève, 2005; Schaart et al., 2005). When pigs are immunologically challenged, body proteins are mobilized to supply the intestine with additional Thr, which suggests that supplementation with dietary Thr above the requirement is necessary for optimal growth and immune system function in animals (Faure et al., 2007; Jayaraman et al., 2015). In this regard, previous studies (Mao et al., 2014; Ren et al., 2014) have reported that dietary l-Thr supplementation over the NRC (2012) recommended levels improved intestinal morphology and immune status by regulating immunoglobulins and cytokines in pigs. However, although diet composition is one of the major factors affecting gut integrity, immune system, and gut microbiota during the post-weaning period (Pluske et al., 2018), most pig studies (Mao et al., 2014; Ren et al., 2014) on Thr supplementation have focused on the effects of dietary Thr content in response to pathogen infection. In the present study, we hypothesized that pigs fed a simple diet would elicit systemic and gut inflammation and show impairment in their gut integrity and barrier function when compared with pigs fed a complex diet and that l-Thr supplementation would ameliorate the inflammation and the gut impairment. The objective of this study was to investigate the effects of diet complexity and l-Thr supplementation levels on the growth performance, immune response, intestinal barrier function, and microbial metabolites in nursery pigs.
Materials and Methods
All experimental procedures were reviewed and approved by the University of Manitoba Animal Care Committee (AC11414), and the pigs were cared for according to the guidelines of the Canadian Council on Animal Care (CCAC, 2009).
Animals, housings, diets, and experimental design
Thirty-two male piglets (TN 70 × TN Tempo; Topigs Norsvin, Winnipeg, MB, Canada) with an initial body weight of 7.23 ± 0.48 kg were obtained from the Glenlea Research Station at the University of Manitoba. The pigs were weaned at 21 d of age and fed a commercial diet for 1 wk before the experiment commenced. The pigs were randomly assigned to a 2 × 2 factorial arrangement based on diet complexity and the levels of dietary l-Thr supplementation to give eight replicates per treatment. Pigs were individually housed for 14 d in adjustable metabolism crates (1.80 × 0.60 m) with smooth, transparent plastic sides and plastic covered expanded sheet-metal flooring. Each crate was equipped with a stainless-steel feeder and a nipple drinker, allowing the pigs ad libitum access to feed and water. The complex diet contained animal protein sources (e.g., fish meal, spray-dried animal plasma) and a dairy product (e.g., dried whey) to mimic a conventional nursery diet (Table 1). These animal protein sources and dairy product were replaced with soybean meal to make the simple diet. The two respective diets were supplemented with l-Thr to supply the standard NRC (2012) level of Thr (STD Thr) or 15% over the standardized ileal digestible (SID) Thr requirement (SUP Thr) for pigs weighing 9 kg. All the experimental diets were formulated to meet or exceed the requirements for essential amino acids (AA), Ca, and standardized total tract digestible P. Room temperature was maintained at 29 ± 1 °C during week 1 and 28 ± 1 °C during week 2.
Table 1.
Diet composition and nutrients contents of experimental diets, % (as-fed basis)1
| Complex | Simple | |||
|---|---|---|---|---|
| Item | STD Thr | SUP Thr | STD Thr | SUP Thr |
| Ingredients | ||||
| Corn | 33.123 | 33.002 | 33.175 | 33.055 |
| Wheat | 30.00 | 30.00 | 30.00 | 30.00 |
| Soybean meal | 13.00 | 13.00 | 30.30 | 30.30 |
| Spray-dried animal plasma | 5.00 | 5.00 | — | — |
| Fish meal | 5.00 | 5.00 | — | — |
| Dried whey | 10.00 | 10.00 | — | — |
| Vegetable oil | 0.80 | 0.80 | 1.80 | 1.80 |
| Limestone | 1.09 | 1.09 | 1.30 | 1.30 |
| Monocalcium phosphate | 0.20 | 0.20 | 1.05 | 1.05 |
| Salt | 0.35 | 0.35 | 0.35 | 0.35 |
| Vitamin–mineral premix2 | 1.00 | 1.00 | 1.00 | 1.00 |
| l-Lys∙HCl | 0.322 | 0.322 | 0.552 | 0.552 |
| dl-Met | 0.097 | 0.098 | 0.185 | 0.185 |
| l-Thr | 0.018 | 0.138 | 0.162 | 0.282 |
| l-Trp | — | — | 0.012 | 0.012 |
| l-Val | — | — | 0.114 | 0.114 |
| Calculated nutrients, % | ||||
| Metabolizable energy, Mcal/kg | 3.30 | 3.30 | 3.30 | 3.30 |
| Total calcium | 0.80 | 0.80 | 0.80 | 0.80 |
| Standardized total tract digestible phosphorus | 0.40 | 0.40 | 0.40 | 0.40 |
| SID crude protein | 20.4 | 20.4 | 20.5 | 20.5 |
| SID Lys | 1.35 | 1.35 | 1.35 | 1.35 |
| SID Thr | 0.79 | 0.91 | 0.79 | 0.91 |
1STD Thr, the standard NRC (2012) level of SID Thr (0.79%); SUP Thr, 15% over the SID Thr requirement.
2Supplied per kilogram of diet: vitamins A, 2,200 IU; vitamin D3, 220 IU; vitamin E, 16 IU; vitamin K, 0.5 mg; thiamine, 1.5 mg; riboflavin, 4 mg; niacin, 30 mg; pantothenic acid, 12 mg; vitamin B12, 0.02 mg; folic acid, 0.3 mg; Cu, 6 mg as copper sulfate; I, 0.14 mg as calcium iodate; Fe, 100 mg as ferrous sulfate; Mn, 4 mg as manganese oxide; Se, 0.3 mg as sodium selenite; Zn, 100 mg as zinc oxide; biotin 0.2 mg.
Sampling and measurements
Freshly voided feces were grab-sampled, weighed, and immediately frozen at –20 °C for dry matter determination as an indicator of fecal consistency. This procedure was performed every morning and evening from day 2 to 14. On days 7 and 14, the body weights of the pigs and the feed disappearance were recorded to determine the average daily gain, average daily feed intake, and gain to feed ratios. On the same days, blood samples (10 mL) from all the pigs were collected from the jugular vein into vacutainer tubes (Becton Dickson, Rutherford, NJ).
On day 14, all pigs were euthanized using captive bolt following sedation with stresnil/xylazine (2:4 mg/kg) sedation in order to collect tissue sample collection. The abdominal cavity was opened from the sternum to the pubis to expose the entire gastrointestinal tract. A sample of jejunum and colon was taken 2 m away from the ileocecal junction and 20 cm away from cecum, respectively. The jejunum sample was rinsed with Krebs Ringer Buffer (KRB), immersed in KRB, and then immediately transported to the laboratory for ex vivo Ussing chamber analysis. Samples for messenger RNA (mRNA) gene expression were washed with 1 × phosphate-buffered saline, immediately snap-frozen in liquid nitrogen, and then stored in a –80 °C freezer. Samples for histomorphology were immediately stored in 10% buffered formalin to fix the villi, crypts, and goblet cells (GC). The contents of the jejunum and proximal colon were collected for analysis of short chain fatty acids (SCFA) and immediately snap-frozen and transferred into a –80 °C freezer. The pH of the jejunum and colon contents was measured by direct submergence of the pH probe (AB15 plus, Fisher Scientific, Toronto, ON, Canada).
Total RNA extraction, complementary DNA synthesis, and real-time polymerase chain reaction (PCR)
Jejunum samples for mRNA gene expression were immersed in liquid nitrogen in a mortar and ground with a pestle. Total RNA was extracted from 80 mg ground jejunum samples using a TRIzol Plus RNA Purification Kit (Invitrogen Canada Inc., Burlington, ON, Canada) according to the manufacturer’s protocol. The quantity and quality of the isolated RNA were determined using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE). The integrity of the total RNA was confirmed by agarose gel electrophoresis. From the extracted RNA, first-strand complementary DNA was synthesized using a high-capacity complementary DNA synthesis kit (Applied Biosystems, Burlington, ON, Canada) following the supplier’s protocol. Quantitative real-time polymerase chain reaction (qPCR) was performed in duplicate reactions, using a CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories, Mississauga, ON, Canada), as described by Waititu et al. (2017). For the qPCR amplification, negative controls were prepared by replacing the complementary DNA with nuclease-free water. A melt curve analysis was performed with a temperature gradient of 0.1 °C/s from 70 to 95 °C to confirm that only specific products were amplified. Pairs of primers for each gene were designed with Primer-Blast based on the published complementary DNA sequences in the gene bank of the National Center for Biotechnology Information (Table 2). The specificity of all primers was confirmed by a melting curve analysis, and all assays had efficiency between 90% and 110%.
Table 2.
Primers used for qPCR analyses of immune cytokines, tight junction protein, and MUC 2
| Target | Amplicon size, bp | Primer sequence (5′ to 3′) | Reference1 |
|---|---|---|---|
| GAPDH | 142 | F: GTGAACGGATTTGGCCGC | NM_001206359.1 |
| R: AAGGGGTCATTGATGGCGAC | |||
| IL-1β | 91 | F: TGGCTAACTACGGTGACAACA | NM_214055 |
| R: CCAAGGTCCAGGTTTTGGGT | |||
| IL-4 | 243 | F: TCCACGGACACAAGTGCGAC | NM_214123 |
| R: TGTTTGCCATGCTGCTCAGG | |||
| IL-6 | 151 | F: AAGGTGATGCCACCTCAGAC | M86722 |
| R: TCTGCCAGTACCTCCTTGCT | |||
| IL-8 | 126 | F: AGAGGTCTGCCTGGACCCCA | NM_213867 |
| R: GGGAGCCACGGAGAATGGGT | |||
| IL-10 | 220 | F: CATCCACTTCCCAACCAGCC | NM_214041 |
| R: CTCCCCATCACTCTCTGCCTTC | |||
| TNF-α | 151 | F: ATGGATGGGTGGATGAGAAA | X54001 |
| R: TGGAAACTGTTGGGGAGAAG | |||
| CLDN1 | 93 | F: CTGTGGATGTCCTGCGTGT | NM_001244539.1 |
| R: GGTTGCTTGCAAAGTGGTGTT | |||
| OCLN | 163 | F: GAGAGAGTGGACAGCCCCAT | NM_001163647 |
| R: TGCTGCTGTAATGAGGCTGC | |||
| ZO-1 | 200 | F: GATCCTGACCCGGTGTCTGA | XM_021098856 |
| R: TTGGTGGGTTTGGTGGGTTG | |||
| MUC2 | 90 | F: CCAGGTCGAGTACATCCTGC | XM_021082584.1 |
| R: GTGCTGACCATGGCCCC |
1GenBank accession reference number.
Histomorphology measurement
After fixation in 10% buffered formalin, the specimens were embedded in paraffin and cut into 5 µm sections. Each section was dewaxed and immersed sequentially in xylene, 95% ethanol, and 100% ethanol for 5 min; this sequential immersion was repeated twice. After rinsing with water, the sections were stained with 0.5% periodic acid solution for 5 min, followed by Schiff reagent staining for 10 min. The sections were counterstained with hematoxylin for 10 s and then dehydrated in alcohol, cleared, and mounted on slides for viewing with an Axio Scope A1 microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany) coupled with an Infinity 2 digital camera (Lumenera Corporation, Ottawa, ON, Canada). Images from all the circular basolateral membranes of the specimens were captured for evaluation of villi and crypts. Villus heights (VH) and crypt depths (CD) were measured for all the distinguishable villi and crypts in the captured images using ImageJ software (National Institutes of Health, MD). The numbers of GC on the corresponding villi and crypts were counted manually.
Ex vivo Ussing chamber analyses
A modified Ussing chamber (VCC-MC6; Physiologic Instruments Inc., San Diego, CA) was used to study gut permeability across the jejunal epithelial tissues. The serosal and muscle layers of the jejunum were removed using microforceps, and the epithelial tissues were placed in the tissue holder with an aperture of 1 cm2. Each holder was mounted in a two-coupled chamber containing pairs of current (Ag wire) and voltage (Ag/AgCl pellet) electrodes housed in 3% agar bridges and filled with 3 M KCl. The samples for Ussing chamber analysis were processed within 15 min postmortem to ensure the retention of the electrophysiological properties of the jejunal samples. Both mucosal and serosal chambers were bathed in 5 mL of KRB containing 115 mM NaCl, 2.4 mM K2HPO4, 0.4 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 1 µm indomethacin, and 10 mM d-glucose. The bathing medium in the chambers was continuously aerated with a mixture of 95% O2 and 5% CO2 and maintained at 37 °C in a water bath. The electrode potential and the solution resistance were corrected before the tissues were mounted in the chamber. The clamps were connected to the software (Physiological Instruments) for automatic data collection and calculation. After a 10-min period to allow establishment of electrophysiological equilibrium, the transepithelial electrical resistance (TEER) was recorded at 5 s intervals over a 1-h period. In addition, the flux of 4 kDa fluorescein isothiocyanate dextran (FD4) was measured by adding 0.1 mg/mL of FD4 (Sigma-Aldrich, St. Louis, MO) to the mucosal chamber at the end of the equilibrium period, and 2 mL of the serosal buffer was sampled at 60 min and transferred to a light protection tube. The optical density of the samples was read at 450 nm with the emission wavelength set at 540 nm.
Sample preparation and chemical analyses
Diet samples were finely ground with a grain miller (50 to 200 µm fineness; HC-700, Boshi Electronic Instrument, Guangzhou, China) and analyzed for AA. Fecal samples were oven-dried at 60 °C for 3 d and weighed. Blood samples were centrifuged at 2,000 × g for 10 min at 4 °C to recover plasma, which was immediately stored at –80 °C until required for cytokine analyses. Plasma samples were used to measure the concentration of interleukin (IL)-6 and IL-10 with a quantitative sandwich enzyme-linked immunosorbent assay technique using porcine IL-6 and IL-10 immunoassay kits (Porcine IL-6 ELISA Kit and Porcine IL-10 ELISA Kit; Sigma-Aldrich) according to the manufacturer’s instructions. The optical densities were read on a spectrophotometer (SoftMax Pro; Molecular Devices, Abingdon, Oxfordshire, UK) at 450 nm with the emission wavelength set at 540 nm. The SCFA concentrations were determined by gas chromatography (Varian Chromatography System, model Star 3400; Varian Medical Systems, Palo Alto, CA) with a capillary column (30 × 0.5 mm; Restek Corp., Bellefonte, PA), according to the method described by Erwin et al. (1961). Briefly, 1 mL of 25% metaphosphoric acid was mixed with 5 mL of digesta fluid in a 15-mL centrifuge tube, and the mixture was frozen overnight. The acidified samples were then thawed, neutralized with 0.4 mL of 25% NaOH, and vortexed. A 0.65 mL volume of 0.3 M oxalic acid was then added and the samples were vortexed again. The samples were then centrifuged for 20 min at 3,000 × g at 4 °C, and 2 mL of the supernatant was transferred to a gas chromatography vial.
The NH3-N concentration in the jejunum and colon digesta samples were determined using the method described by Novozamsky et al. (1974). Briefly, 1.5 mL of a reagent containing 200 mL of 0.05% sodium nitroprusside and 10 mL of 4% ethylenediaminetetraacetic acid was added to 50 µL of sample in a 10-mL test tube. A solution containing 10% sodium hypochlorite (2.5 mL) was then added to the mixture. Test tubes containing the resulting mixture were placed in a test tube rack and incubated in complete darkness for 30 min, and then the optical density of the mixture was immediately read at 630 nm using a spectrophotometer (SoftMax Pro).
Calculations and statistical analyses
The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was a control gene and used to normalize the transcriptional levels for immune cytokines, tight junction protein, and MUC protein. The relative expression was expressed as a ratio of the target gene to the simple diet (STD Thr)-fed group gene, using the formula 2−ΔΔCt according to Livak and Schmittgen (2001), where ΔΔCt = (Cttarget – CtGAPDH)treatment – (Cttarget – CtGAPDH)simple diet (STU Thr).
The concentrations of IL-6, IL-10, FD4, and ammonia-N were determined by calculating the concentration from a regression equation of the standard curves with R2 values of 0.963, 0.998, 0.999, and 0.998, respectively.
All data were analyzed using the MIXED procedure of SAS (version 9.4; SAS Inst. Inc., Cary, NC) with each animal used as the experimental unit. The model included diet complexity, the level of l-Thr supplementation, and their interaction. The LSMEANS statement with the Tukey-adjusted PDIFF option was used to calculate and separate the mean values for each treatment. Results were considered significant at P < 0.05 and tendencies were observed at 0.05 < P ≤ 0.10.
Results
Growth performance and fecal dry matter
Dietary treatment did not affect the average daily gain, average feed intake, or gain to feed ratio throughout the experimental period (Table 3). Pigs fed a complex diet had lower (P < 0.05) fecal dry matter than those fed the simple diet for the first week of the experimental period, whereas no difference was observed during the second week.
Table 3.
Effect of diet complexity and threonine supplementation on growth performance and fecal dry matter1
| Complex | Simple | P-value2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | STD Thr | SUP Thr | STD Thr | SUP Thr | SEM | Diet | Thr | Diet × Thr |
| No. of animals | 8 | 8 | 9 | 7 | ||||
| Body weight, kg | ||||||||
| Day 0 | 7.2 | 7.2 | 7.2 | 7.3 | ||||
| Day 7 | 8.9 | 8.7 | 8.4 | 9.0 | 0.35 | 0.863 | 0.536 | 0.235 |
| Day 14 | 12.7 | 12.8 | 11.8 | 13.0 | 0.65 | 0.507 | 0.286 | 0.373 |
| Average daily gain, g/d | ||||||||
| Day 0 to 7 | 241 | 213 | 172 | 239 | 32.2 | 0.469 | 0.529 | 0.120 |
| Day 7 to 14 | 548 | 591 | 477 | 563 | 49.7 | 0.288 | 0.172 | 0.644 |
| Day 0 to 14 | 395 | 402 | 324 | 401 | 38.1 | 0.318 | 0.246 | 0.334 |
| Average daily feed intake, g/d | ||||||||
| Day 0 to 7 | 320 | 294 | 247 | 307 | 32.4 | 0.316 | 0.579 | 0.164 |
| Day 7 to 14 | 630 | 651 | 542 | 604 | 52.3 | 0.172 | 0.404 | 0.677 |
| Day 0 to 14 | 475 | 473 | 395 | 455 | 40.3 | 0.198 | 0.445 | 0.404 |
| Gain to feed ratio, g/g | ||||||||
| Day 0 to 7 | 0.74 | 0.71 | 0.69 | 0.77 | 0.053 | 0.988 | 0.628 | 0.278 |
| Day 7 to 14 | 0.87 | 0.90 | 0.88 | 0.94 | 0.032 | 0.461 | 0.171 | 0.721 |
| Day 0 to 14 | 0.83 | 0.84 | 0.82 | 0.88 | 0.029 | 0.539 | 0.186 | 0.500 |
| Fecal dry matter, % | ||||||||
| Day 0 to 7 | 31.8 | 31.6 | 33.2 | 37.6 | 1.55 | 0.017 | 0.161 | 0.137 |
| Day 7 to 14 | 44.3 | 42.0 | 44.1 | 45.4 | 2.90 | 0.551 | 0.861 | 0.513 |
| Day 0 to 14 | 37.9 | 36.5 | 38.7 | 41.4 | 1.90 | 0.125 | 0.718 | 0.253 |
1STD Thr, the standard NRC (2012) level of SID Thr (0.79%); SUP Thr, 15% over the SID Thr requirement.
2Diet, main effect of diet complexity; Thr, main effect of the levels of dietary Thr supplementation; Diet × Thr, interactive effect of diet complexity and Thr supplementation.
Plasma cytokine contents and intestinal histomorphology
Although no difference in IL-6 was observed across dietary treatments on day 7, the plasma IL-6 contents were higher (P < 0.05) in the simple diet-fed pigs than in the complex diet-fed pigs on day 14, as shown in Table 4. Conversely, plasma IL-10 contents were higher in the pigs fed simple diets than those fed complex diets (P < 0.05), but no difference was observed on day 14. The SUP Thr did not change plasma cytokine contents on either day 7 or 14. However, SUP Thr increased (P < 0.05) the VH, VH:CD, and GC density of the villi (n/100 µm of villi and n/villi) and crypts (n/100 µm of crypts) in the jejunum. However, SUP Thr did not alter the GC density in the colon, although it deepened (P < 0.01) the CD. Diet complexity did not affect the histomorphology in either the jejunum or the colon. However, interactive effects (P < 0.05) were observed for diet complexity and the level of dietary l-Thr supplementation on GC density (n/villi and n/100 µm of villi). In addition, trends toward an interaction were observed in VH:CD (P = 0.07) and GC density (n/100 µm villi; P = 0.06).
Table 4.
Effect of diet complexity and threonine supplementation on plasma cytokine contents and histomorphology1
| Complex | Simple | P-value2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | STD Thr | SUP Thr | STD Thr | SUP Thr | SEM | Diet | Thr | Diet × Thr |
| IL-6, pg/mL | ||||||||
| Day 7 | 1,309 | 1,556 | 1,697 | 2,316 | 549.1 | 0.274 | 0.408 | 0.722 |
| Day 14 | 555 | 945 | 2,297 | 2,211 | 688.6 | 0.040 | 0.826 | 0.730 |
| IL-10, pg/mL | ||||||||
| Day 7 | 112 | 45 | 239 | 245 | 74.7 | 0.027 | 0.663 | 0.602 |
| Day 14 | 70 | 34 | 144 | 106 | 53.3 | 0.181 | 0.488 | 0.987 |
| Jejunum | ||||||||
| VH, μm | 345 | 375 | 347 | 393 | 17.9 | 0.547 | 0.024 | 0.591 |
| CD, μm | 229 | 245 | 247 | 227 | 14.6 | 0.985 | 0.885 | 0.172 |
| VH:CD | 1.5xy | 1.6xy | 1.4y | 1.8x | 0.09 | 0.520 | 0.031 | 0.074 |
| GCs/100 μm villi | 2.5y | 2.8xy | 2.2y | 3.8x | 0.35 | 0.312 | 0.005 | 0.056 |
| GC/villi | 8.7y | 10.5xy | 7.7y | 15.0x | 1.46 | 0.191 | 0.002 | 0.041 |
| GC/100 μm crypt | 6.7y | 6.8xy | 6.1y | 8.0x | 0.35 | 0.323 | 0.004 | 0.011 |
| GC/crypt | 15.1 | 16.4 | 15.3 | 18.2 | 1.34 | 0.434 | 0.086 | 0.511 |
| Colon | ||||||||
| CD, μm | 167xy | 191x | 150y | 197x | 9.9 | 0.578 | 0.001 | 0.243 |
| GC/100 μm crypt | 9.2 | 16.0 | 5.9 | 8.8 | 4.36 | 0.210 | 0.246 | 0.641 |
| GC/crypt | 15.5 | 28.7 | 8.8 | 17.9 | 7.55 | 0.227 | 0.127 | 0.779 |
1STD Thr, the standard NRC (2012) level of SID Thr (0.79%); SUP Thr, 15% over the SID Thr requirement. Data represented as least square means of 7 to 9 pigs.
2Diet, main effect of diet complexity; Thr, main effect of the levels of dietary Thr supplementation; Diet × Thr, interactive effect of diet complexity and Thr supplementation.
x,yWithin a row, means with different superscripts differ (P < 0.05).
Relative mRNA gene expression
The expression of genes encoding tumor necrosis factor alpha (TNF-α; P = 0.06), claudin 1 (CLDN1; P = 0.08), and zonula occludens (ZO)-1 tended to be higher (P = 0.07) in pigs fed the simple diet than in those fed the complex diet (Table 5). SUP Thr upregulated (P < 0.05) occludin (OCLN) gene expression in the jejunum. Furthermore, a tendency (P = 0.07) was observed for an increase in IL-6 gene expression in the jejunum of pigs fed the SUP Thr diet than those fed the STD Thr. An interactive effect (P = 0.10) was observed between diet complexity and Thr supplementation level on the expression of the IL-6 gene in the jejunum.
Table 5.
Effect of diet complexity and threonine supplementation on relative mRNA gene expression (2−∆∆Ct) in jejunum1,2
| Complex | Simple | P-value3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | STD Thr | SUP Thr | STD Thr | SUP Thr | SEM | Diet | Thr | Diet × Thr |
| IL-1β | 1.33 | 1.06 | 1.00 | 2.14 | 0.435 | 0.401 | 0.326 | 0.120 |
| IL-4 | 1.22 | 0.84 | 1.00 | 0.59 | 0.394 | 0.530 | 0.297 | 0.959 |
| IL-6 | 0.46 | 0.44 | 1.00 | 0.40 | 0.182 | 0.143 | 0.069 | 0.097 |
| IL-8 | 0.47 | 0.86 | 1.00 | 0.76 | 0.299 | 0.450 | 0.796 | 0.278 |
| IL-10 | 0.66 | 0.66 | 1.00 | 0.96 | 0.252 | 0.170 | 0.930 | 0.943 |
| TNF-α | 0.62 | 0.43 | 1.00 | 1.15 | 0.306 | 0.060 | 0.943 | 0.546 |
| CLDN1 | 0.61 | 0.55 | 1.00 | 0.90 | 0.222 | 0.076 | 0.709 | 0.924 |
| OCLN | 0.70 | 1.10 | 1.00 | 1.11 | 0.133 | 0.211 | 0.042 | 0.233 |
| ZO-1 | 0.79 | 0.81 | 1.00 | 0.95 | 0.094 | 0.070 | 0.867 | 0.706 |
| MUC2 | 0.94 | 0.95 | 1.00 | 0.77 | 0.169 | 0.692 | 0.489 | 0.450 |
1STD Thr, the standard NRC (2012) level of SID Thr (0.79%); SUP Thr, 15% over the SID Thr requirement. Data represented as least square means of 7 to 9 pigs.
2The relative data were expressed as a ratio of the target gene to the Simple (STD Thr) gene, using the formula 2−∆∆Ct (Livak and Schmittgen, 2001), where ∆∆Ct = (Cttarget - CtGAPDH)treatment – (Cttarget − CtGAPDH)Simple (STD Thr).
3Diet, main effect of diet complexity; Thr, main effect of the levels of dietary Thr supplementation; Diet × Thr, interactive effect of diet complexity and Thr supplementation.
Concentrations of microbial metabolites and pH in the jejunum and colon contents
Diet complexity did not affect the SCFA contents or the pH value of the jejunum (Table 6). However, the NH3-N concentration in the jejunum tended to be higher (P = 0.07) in the simple diet-fed pigs than in the complex diet-fed pigs. The butyrate concentration in the colon was higher (P < 0.05) in the simple diet-fed pigs than in those fed the complex diet. Interactive effects (P < 0.05) were observed for diet complexity and level of l-Thr supplementation for the NH3-N and pH levels in the jejunum and the acetate, propionate, total volatile fatty acids (VFA), and total SCFA levels in the colon. A trend for the interaction was also found for valerate content (P = 0.08) and pH (P = 0.09) in the colon.
Table 6.
Effect of diet complexity and threonine supplementation on microbial metabolites and pH in jejunum and colon digesta1
| Complex | Simple | P-value2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | STD Thr | SUP Thr | STD Thr | SUP Thr | SEM | Diet | Thr | Diet × Thr |
| Jejunal SCFA3, mmol/L3 | ||||||||
| Acetate | 15.8 | 18.8 | 16.0 | 11.9 | 4.09 | 0.389 | 0.897 | 0.362 |
| Propionate | 0.6 | 0.3 | 0.7 | 1.1 | 0.38 | 0.156 | 0.874 | 0.323 |
| Butyrate | 0.5 | 0.5 | 0.4 | 0.3 | 0.13 | 0.155 | 0.738 | 0.598 |
| Isovalerate | 1.3 | 1.2 | 1.4 | 0.8 | 0.28 | 0.600 | 0.177 | 0.309 |
| Valerate | 0.1 | 0.1 | 0.1 | 0.1 | 0.05 | 0.643 | 0.739 | 0.255 |
| Lactate | 11.1 | 8.2 | 10.9 | 11.2 | 2.79 | 0.598 | 0.628 | 0.564 |
| BCFA4 | 1.4 | 1.4 | 1.6 | 0.9 | 0.32 | 0.567 | 0.208 | 0.286 |
| Total VFA | 18.2 | 20.9 | 18.6 | 14.2 | 4.43 | 0.450 | 0.836 | 0.395 |
| Total SCFA | 29.3 | 29.2 | 29.5 | 25.3 | 4.20 | 0.549 | 0.589 | 0.607 |
| Colonic SCFA, mmol/L | ||||||||
| Acetate | 113.6x | 87.1y | 106.8xy | 110.2xy | 7.17 | 0.238 | 0.097 | 0.036 |
| Propionate | 72.6 | 52.9 | 64.7 | 74.7 | 6.38 | 0.254 | 0.422 | 0.020 |
| Isobutyrate | 1.5 | 2.0 | 1.9 | 1.2 | 0.51 | 0.651 | 0.842 | 0.242 |
| Butyrate | 35.9 | 32.9 | 37.8 | 46.7 | 3.94 | 0.043 | 0.430 | 0.120 |
| Isovalerate | 2.6 | 3.4 | 3.0 | 2.1 | 0.77 | 0.515 | 0.921 | 0.224 |
| Valerate | 12.8 | 10.0 | 10.1 | 14.4 | 2.06 | 0.670 | 0.692 | 0.082 |
| Lactate | 21.5 | 28.3 | 9.7 | 20.4 | 11.71 | 0.376 | 0.434 | 0.859 |
| BCFA4 | 16.9 | 15.5 | 15.0 | 17.6 | 2.27 | 0.947 | 0.778 | 0.348 |
| Total VFA | 239.0xy | 188.4y | 224.3xy | 249.2x | 14.69 | 0.106 | 0.359 | 0.011 |
| Total SCFA | 260.5 | 216.7 | 234.0 | 269.5 | 20.22 | 0.493 | 0.829 | 0.046 |
| Ammonia N, mg/L | ||||||||
| Jejunum | 73.6y | 93.8xy | 158.5x | 76.3y | 18.82 | 0.067 | 0.091 | 0.007 |
| Colon | 254.2 | 320.3 | 271.0 | 301.4 | 41.89 | 0.979 | 0.231 | 0.653 |
| pH | ||||||||
| Jejunum | 6.42 | 6.05 | 6.02 | 6.28 | 0.151 | 0.561 | 0.705 | 0.033 |
| Colon | 5.79 | 5.95 | 5.83 | 5.65 | 0.107 | 0.216 | 0.927 | 0.090 |
1STD Thr, the standard NRC (2012) level of SID Thr (0.79%); SUP Thr, 15% over the SID Thr requirement. Data represented as least square means of 7 to 9 pigs.
2Diet, main effect of diet complexity; Thr, main effect of the levels of dietary Thr supplementation; Diet × Thr, interactive effect of diet complexity and Thr supplementation.
3Isobutyrate was not detected.
4Branched chain fatty acids = isobutyrate + isovalerate + valeric acid.
x,yWithin a row, means with different superscripts differ (P < 0.05).
Organ weights and gut permeability
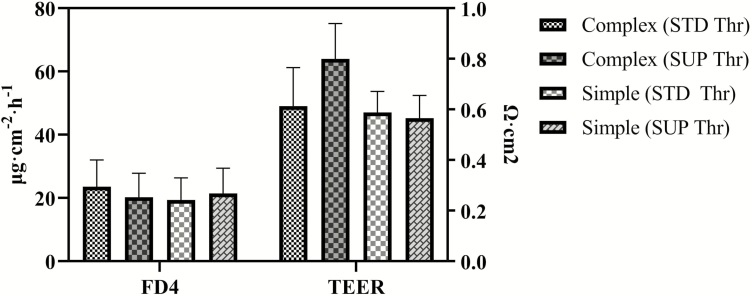
The kidney weights (% of empty body weight) were greater in the pigs fed the simple diet than in those fed the complex diet (Table 7). Although SUP Thr did not change organ weights, a trend (P = 0.062) was observed for an interaction between diet complexity and the level of l-Thr supplementation for the liver weight. Dietary treatment did not affect TEER or FD4 flux in the jejunum (Figure 1).
Table 7.
Effect of diet complexity and threonine supplementation on organ weights1
| Complex | Simple | P-value2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Item | STD Thr | SUP Thr | STD Thr | SUP Thr | SEM | Diet | Thr | Diet × Thr |
| Empty BW | 12.13 | 12.33 | 11.24 | 12.23 | 0.641 | 0.419 | 0.331 | 0.518 |
| % of empty BW | ||||||||
| Liver | 4.15 | 3.58 | 3.40 | 3.65 | 0.223 | 0.116 | 0.454 | 0.062 |
| Spleen | 0.27 | 0.29 | 0.29 | 0.26 | 0.036 | 0.796 | 0.819 | 0.539 |
| Kidneys | 0.74 | 0.76 | 0.66 | 0.72 | 0.029 | 0.027 | 0.155 | 0.388 |
| Stomach | 0.88 | 0.80 | 0.81 | 0.85 | 0.051 | 0.893 | 0.644 | 0.199 |
| Small intestine | 4.26 | 4.42 | 4.33 | 4.59 | 0.203 | 0.562 | 0.281 | 0.790 |
| Large intestine | 1.66 | 1.73 | 1.96 | 1.91 | 0.168 | 0.140 | 0.945 | 0.714 |
| Gastrointestinal tract3 | 6.81 | 6.95 | 7.09 | 7.35 | 0.283 | 0.205 | 0.462 | 0.834 |
1STD Thr, the standard NRC (2012) level of SID Thr (0.79%); SUP Thr, 15% over the SID Thr requirement. Data represented as least square means of 7 to 9 pigs.
2Diet, main effect of diet complexity; Thr, main effect of the levels of dietary Thr supplementation; Diet × Thr, interactive effect of diet complexity and Thr supplementation.
3Stomach + small and large intestine.
Figure 1.
Effect of diet complexity and threonine supplementation on FD4 flux and TEER in the jejunum. Complex = animal protein sources containing complex diet, Simple = corn–wheat–soybean meal-based simple diet, STD Thr = the standard NRC (2012) level of SID Thr (0.79%), SUP Thr = 15% over the SID Thr requirement. No significant effects (P > 0.10) of dietary complexity, Thr supplementation, and their interaction were observed.
Discussion
The conventional complex diet for pigs was mimicked in the present study by including spray-dried animal plasma, fish meal, and dried whey. These ingredients were replaced in the simple diet with 17.3% soybean meal, bringing its dietary content to 30.3%. Each diet was supplemented with 0.12% l-Thr to supply 115% of the NRC (2012) SID Thr requirement. Our analysis showed that SUP Thr diets contained 0.13% greater total Thr content when compared with the STD Thr diets, confirming the successful mixing of the added l-Thr in the diets. The SID Lys contents remained constant across the diets, at 1.35%, to maintain the SID Lys:SID Thr ratio in the STD Thr and SUP Thr diets. The level of SUP Thr was based on our previous study (Jayaraman et al., 2015) which showed 15% more dietary Thr is required for nursery pigs when their immune system is stimulated.
In general, the major limitation regarding the use of soybean meal in a weaner pig diet is the presence of antigenic compounds, such as glycinin and β-conglycinin (Li et al., 1990). These antigens cause a hypersensitivity that elicits overreaction of the immune system and commonly results in inflammation and proliferation of immune cells, particularly in nursery pigs, as their immune systems are still immature (Hao et al., 2009). The process of hypersensitivity appears to be modulated by cytokines, which play roles in cell signaling among several immune cell types, including macrophages, lymphocytes, and mast cells. In fact, previous studies (Guo et al., 2007; Hao et al., 2009) have reported that oral gavage with β-conglycinin, one of antigenic compounds in soybean meal, significantly increased plasma IL-4, IL-5, TNF-α, and interferon-γ in both piglets and rats. Therefore, our findings of greater IL-6 and TNF-α gene expression in response to the simple diet may be a consequence of hypersensitivity to antigenic compounds provided by the simple diet. However, one point to note is that the inflammation state appears to be maintained by IL-10, a typical anti-inflammatory cytokine, which functions by suppressing the action of pro-inflammatory cytokines such as IL-6 during the first week of the experimental period. This may be associated with the quantitative threshold of antigens that allows nursery pigs to maintain homeostasis. In fact, the pigs fed simple diets consumed approximately 2.5 times more feed in week 2 than in week 1, indicating more consumption of antigens from soybean meal.
Intestinal permeability is associated with the functioning of the intramembrane multiprotein complexes known as tight junctions (Vereecke et al., 2011). Two transmembrane proteins of tight junctions, OCLN and CLDN, create the link between two adjacent epithelial cells, whereas ZO-1, a cytoplasmic adaptor protein, is associated with cytoskeletal tethering and binding of the transmembrane proteins (Cummins, 2012). Intestinal tight junctions rely on cytokines for modulation of their function (Capaldo and Nusrat, 2009), with TNF-α serving as a key mediator of intestinal permeability and inflammation by activating the myosin light chain kinase gene, thereby promoting actomyosin contractility (Capaldo and Nusrat, 2009). Furthermore, Yang et al. (2003) reported a modulation of ZO-1 stability in the tight junction complex in mice in response to IL-6. In the present study, the intestinal tight junction permeability was assessed by measuring TEER and FD4 flux in the jejunum. The pigs fed the simple diet showed a greater expression of IL-6 and TNF-α than those fed the complex diet, but they maintained their TEER and FD4 fluxes in the jejunum at levels comparable to those of the pigs fed the complex diet. This finding contrasts with those of previous studies (Zhao et al., 2014; Wu et al., 2016), in which the permeability of epithelial cells was dose-dependently compromised by sensitization to soy protein antigens. Our findings indicated that the jejunal permeability may have been maintained by transcriptional regulation of tight junction proteins. In fact, simple diet fed pigs showed upregulated genes encoding CLDN1 and ZO-1 than complex diet-fed pigs.
Soy antigens are well known to cause villus atrophy and crypt proliferation (Dréau et al., 1994; Hao et al., 2009). In this regard, we previously showed that simple diet-fed pigs had deeper CD and shorter VH in the duodenum and ileum than complex diet-fed pigs (Koo et al., 2017). However, no differences in VH and CD were noted in the jejunum between complex-fed and simple-fed pigs in the present study. Various factors such as different ages, feed intakes, diet compositions, and feeding regimens may have resulted in the discrepancy (Pluske et al., 1997). The effects of the simple diet on intestinal morphology may also occur in a segment-dependent manner, as no difference was observed in jejunal morphology in our previous study (Koo et al., 2017).
A disruption in gut health could be caused by ammonia produced in the gut lumen by microbial fermentation of nitrogenous compounds. The produced NH3, as a toxic compound, can irritate intestinal mucosa and be absorbed into the bloodstream via nonionic diffusion (Williams et al., 2001). Our assessment of NH3-N content in digesta was, therefore, as an indicator of protein fermentation as well as gut health. Because the diets were formulated to contain identical CP content, the NH3-N content in the jejunum indicated a higher content of indigestible protein and greater protein fermentation with the simple diet than in the complex diet. Notably, urea is utilized and converted into NH3 by the urease of ureolytic bacteria (Vince et al., 1973). Considering that approximately 25% of the circulating urea is excreted into the gut lumen (Barrett, 2014), the N balance in the body may partially contribute to the NH3 concentration found in the gut. In fact, a 39% greater plasma urea nitrogen content was observed in the pigs fed the simple diet when compared with those fed the complex diet.
In contrast to NH3 production in the jejunum, the simple diet appeared to have a beneficial effect on microbial fermentation in the proximal colon by promoting butyrate production. Butyrate is a well-documented microbial metabolite that plays a trophic role in colonocytes as a major source of energy for their metabolic activities and as a stimulant of epithelial proliferation in pigs (Montagne et al., 2003). This may be associated with the higher NSP content in the simple diet than in the complex diet. The simple diet had a 24% greater NSP content (8.8%), which originated from the xylogalacturonan, hemicellulose, and pectin known to occur in soybean meal (Knudsen, 2014). This potential association is partially supported by previous studies (Levrat et al., 1991; Jiménez-Escrig et al., 2008) in which inclusion of soy fiber in a rat diet significantly promoted butyrate production in the gut. The presence of butyrate in the large intestine is reported to promote water and sodium absorption; thus, butyrate has the ability to limit osmotic diarrhea (Montagne et al., 2003). A butyrate effect may, therefore, partially explain the greater fecal dry matter in the pigs fed the simple diet than those fed the complex diet. In addition, the kidney is a major osmoregulatory organ in the body (Strange, 1992), so a modification of electrolyte absorption could explain the heavier kidneys in the pigs fed the complex diet than those fed the simple diet.
Threonine is of great importance in maintaining the immune system because it is a major AA component of MUCs and immunoglobulin (Li et al., 2007). Studies using isotope tracers revealed that dietary Thr is mainly metabolized in the intestine by incorporation into newly synthesized proteins (Floc’h and Sève, 2005; Schaart et al., 2005). In the present study, SUP Thr improved the VH and GC density in the jejunum. This is in agreement with previous findings (Ren et al., 2014; Min et al., 2017; Chen et al., 2018) where dietary supplementation with l-Thr above the requirement enhanced intestinal morphology or GC density in pigs and broiler chickens. The interactive effects of diet complexity and l-Thr supplementation level on gut integrity showed the patterns that were similar to those seen for microbial metabolites. This suggests that SUP Thr may have enhanced intestinal morphology and GC density through an interplay with microbial metabolites. This is because intestinal NH3 and VFA contents are closely associated with enterocyte atrophy and proliferation, respectively (Williams et al., 2001).
The GC are propagated from stem cells at the base of the crypt, and as they mature, they migrate up to the villus tip to condense with MUC granules (Specian and Oliver, 1991). Therefore, the increased GC density in the crypts could reflect a promotion of GC proliferation by SUP Thr. However, SUP Thr did not alter MUC2 gene expression, indicating that the STD Thr level may have not been sufficient for optimum GC proliferation and the synthesis of MUC2. The improved GC density in response to SUP Thr seems to parallel the improved VH, because GC secrete MUC2 as well as trefoil factor 2 and resistin-like molecule β, which are responsible for maintaining intestinal integrity (McGuckin et al., 2009).
Evidence is growing that dietary Thr supplementation beneficially modifies the gut microbiota composition in poultry (Chen et al., 2017; Dong et al., 2017) and in rats (Faure et al., 2006). The authors of these previous studies speculated that dietary Thr supplementation promoted saccharolytic bacteria by stimulating the secretion of MUC (glycoconjugates) that serve as substrates for these microorganisms. However, the present results indicate that SUP Thr seems to modify intestinal microbial fermentation in an interactive manner with diet composition. Interestingly, SUP Thr in simple diet decreased NH3 content to the level observed with the complex diet. Conversely, SUP Thr in the simple diet increased VFA production compared with that in the complex diet. Therefore, the promotion of saccharolytic bacteria by SUP Thr may have led to a preferential fermentation of carbohydrates over protein, thereby increasing the VFA production and decreasing NH3 production. However, we found no benefits of SUP Thr with the complex diet in terms of microbial metabolite production, suggesting a requirement for adequate dietary carbohydrates to generate benefits with respect to microbial metabolites.
The microbial production of VFA in the gut lumen is known to have gut anti-inflammatory activity through regulation of T cell differentiation (Kim et al., 2014), whereas microbial production of NH3 elicits inflammation in the host (Williams et al., 2001). In this regard, our findings on IL-6 gene expression paralleled the changes in gut microbial metabolites (e.g., VFA and NH3-N). IL-6 gene expression may also have been associated with enhanced GC density, as the GC secrete MUC2, a major component of the mucus that coats intestinal epithelium and protects the gut from immune stimulants, such as microorganisms, toxins, and antigens (McGuckin et al., 2009).
As mentioned earlier, the production of pro-inflammatory molecules is a key modulator affecting the function of tight junction proteins in the intestine. The reduction in IL-6 gene expression by SUP Thr may be a contributing factor to the upregulation of OCLN gene expression. This is partially supported by work of Chen et al. (2018) which showed that SUP Thr downregulated IL-1β and upregulated ZO-1 gene expression in the ileum of broiler chickens challenged with Escherichia coli lipopolysaccharides. However, as was seen in the present study regarding the effects of diet complexity on gut permeability, the upregulation of the OCLN gene expression by SUP Thr did not extend to improvement in TEER and FD4 flux. This indicated that the expression of tight junction protein-encoding genes does not translate into modification of the intestinal barrier function. Intestinal permeability is maintained in a systematic way (Wijtten et al., 2011); therefore, the pigs in the present study may have maintained an optimal gut permeability without a requirement for additional tight junction proteins. However, as Wang et al. (2015) pointed out, the increase in intestinal permeability post-weaning is associated with a significant reduction in the expression of genes encoding OCLN, CLDN1, ZO-2, and ZO-3. Therefore, the upregulation of OCLN expression in the jejunum by SUP Thr may be beneficial for maintaining intestinal permeability when intestinal homeostasis is compromised.
The present understanding of the cellular function of the colonic crypt remains incomplete. To date, the existing evidence supports a role for the colonocyte in vectorial transport, mostly of electrolytes, and in innate immunity by serving as a barrier against various pathogens and their toxins (Kiela and Ghishan, 2016; Litvak et al., 2018). Therefore, crypt development seems beneficial for maintaining immunity and ion transport by increasing cell renewal and maturation. In the present study, SUP Thr deepened the crypts in the colon. Supplementary l-Thr is mostly absorbed in the small intestine, so it may be indirectly involved in alterations of CD, but additional studies are required to confirm this possibility.
Conclusions
Feeding a simple diet to nursery pigs resulted in systemic and intestinal inflammation, possibly due to a higher consumption of the antigenic compounds present in soybean meal and a greater NH3 production in the jejunum when compared with feeding a complex diet. The SUP Thr diet, which included 115% of the NRC (2012) requirement for SID Thr, did not normalize the simple diet-induced inflammation. However, SUP Thr did normalize NH3 production in the jejunum and resulted in the downregulation of IL-6 gene expression in the jejunum. SUP Thr also improved gut integrity and architecture, as indicated by greater VH, GC density, and OCLN gene expression in the jejunum. SUP Thr seems to have greater benefits with a simple diet than with a complex diet in terms of production of gut microbial metabolites (e.g., NH3, VFA), intestinal morphology, and inflammatory status in the jejunum. Therefore, SUP Thr in a simple diet could be a beneficial nutritional strategy for enhancing gut health. However, additional strategies should be combined with SUP Thr to maintain the systemic inflammatory state of pigs fed a simple diet. In addition, further study with large numbers of animals is required to investigate whether the benefits of SUP Thr in the simple diet can lead to superior growth performance in nursery pig production. Further research on gut microbiota is also warranted to understand the mode of action of SUP Thr on microbial metabolites.
Acknowledgments
We thank R. Stuski for animal care and A. Karamanov for technical assistance. Financial support for this research was provided by the Swine Innovation Porc through the Canadian Swine Research and Development Cluster.
Glossary
Abbreviations
- AA
amino acids
- BCFA
branched fatty acids
- CD
crypt depth
- CLDN
claudin
- FD4
fluorescein isothiocyanate dextran – 4kDa
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GC
goblet cell
- IL
interleukin
- KRB
Krebs Ringer buffer
- MUC
mucin
- NSP
non-starch polysaccharides
- OCLN
occludin
- PCR
polymerase chain reaction
- qPCR
quantitative real-time PCR
- SCFA
short-chain fatty acids
- SID
standardized ileal digestible
- STD Thr
standard level of Thr requirement
- SUP Thr
15% over Thr requirement
- TEER
transepithelial electrical resistance
- TNF-α
tumor necrosis factor alpha
- VFA
volatile fatty acids
- VH
villus height
- ZO
zonula occludens
Conflict of interest statement
The authors declare that there is no real or perceived conflict of interest.
Literature Cited
- Barrett K. E. 2014. Gastrointestinal physiology. 2nd ed. New York (NY): McGraw-Hill Education. [Google Scholar]
- Capaldo C. T., and Nusrat A.. . 2009. Cytokine regulation of tight junctions. Biochim. Biophys. Acta 1788:864–871. doi: 10.1016/j.bbamem.2008.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCAC. 2009. CCAC guidelines on: the care and use of farm animals in research, teaching and testing. vol. II Ottawa (ON, Canada): Canadian Council on Animal Care; p. 103–125. [Google Scholar]
- Chen Y. P., Cheng Y. F., Li X. H., Yang W. L., Wen C., Zhuang S., and Zhou Y. M.. . 2017. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. Poult. Sci. 96:405–413. doi: 10.3382/ps/pew240 [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Cheng Y., Li Y., Wen C., and Zhou Y.. . 2018. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 119:1254–1262. doi: 10.1017/S0007114518000740 [DOI] [PubMed] [Google Scholar]
- Cummins P. M. 2012. Occludin: one protein, many forms. Mol. Cell. Biol. 32:242–250. doi: 10.1128/MCB.06029-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. Y., Azzam M. M. M., and Zou X. T.. . 2017. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 96:3654–3663. doi: 10.3382/ps/pex185 [DOI] [PubMed] [Google Scholar]
- Dréau D., Lallès J. P., Philouze-Romé V., Toullec R., and Salmon H.. . 1994. Local and systemic immune responses to soybean protein ingestion in early-weaned pigs. J. Anim. Sci. 72:2090–2098. doi: 10.2527/1994.7282090x [DOI] [PubMed] [Google Scholar]
- Erwin E. S., Marco G. J., and Emery E. M.. . 1961. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6 [DOI] [Google Scholar]
- Faure M., Choné F., Mettraux C., Godin J. P., Béchereau F., Vuichoud J., Papet I., Breuillé D., and Obled C.. . 2007. Threonine utilization for synthesis of acute phase proteins, intestinal proteins, and mucins is increased during sepsis in rats. J. Nutr. 137:1802–1807. doi: 10.1093/jn/137.7.1802 [DOI] [PubMed] [Google Scholar]
- Faure M., Mettraux C., Moennoz D., Godin J. P., Vuichoud J., Rochat F., Breuillé D., Obled C., and Corthésy-Theulaz I.. . 2006. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J. Nutr. 136:1558–1564. doi: 10.1093/jn/136.6.1558 [DOI] [PubMed] [Google Scholar]
- Floc’h N. L., and Sève B.. . 2005. Catabolism through the threonine dehydrogenase pathway does not account for the high first-pass extraction rate of dietary threonine by the portal drained viscera in pigs. Br. J. Nutr. 93:447–456. doi: 10.1079/BJN20051375 [DOI] [PubMed] [Google Scholar]
- Guo P., Piao X., Ou D., Li D., and Hao Y.. . 2007. Characterization of the antigenic specificity of soybean protein beta-conglycinin and its effects on growth and immune function in rats. Arch. Anim. Nutr. 61:189–200. doi: 10.1080/17450390701318358 [DOI] [PubMed] [Google Scholar]
- Hao Y., Zhan Z., Guo P., Piao X., and Li D.. . 2009. Soybean β-conglycinin-induced gut hypersensitivity reaction in a piglet model. Arch. Anim. Nutr. 63:188–202. doi: 10.1080/17450390902860026 [DOI] [Google Scholar]
- Jayaraman B., Htoo J., and Nyachoti C. M.. . 2015. Effects of dietary threonine:lysine ratios and sanitary conditions on performance, plasma urea nitrogen, plasma-free threonine and lysine of weaned pigs. Anim. Nutr. 1:283–288. doi: 10.1016/j.aninu.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Escrig A., Tenorio M. D., Espinosa-Martos I., and Rupérez P.. . 2008. Health-promoting effects of a dietary fiber concentrate from the soybean byproduct okara in rats. J. Agric. Food Chem. 56:7495–7501. doi: 10.1021/jf800792y [DOI] [PubMed] [Google Scholar]
- Kiela P. R., and Ghishan F. K.. . 2016. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 30:145–159. doi: 10.1016/j.bpg.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. H., Park J., and Kim M.. . 2014. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 14:277–288. doi: 10.4110/in.2014.14.6.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K. E. B. 2014. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 93:2380–2393. doi: 10.3382/ps.2014-03902 [DOI] [PubMed] [Google Scholar]
- Koo B., Kim J. W., de Lange C. F. M., Hossain M. M., and Nyachoti C. M.. . 2017. Effects of diet complexity and multicarbohydrase supplementation on growth performance, nutrient digestibility, blood profile, intestinal morphology, and fecal score in newly weaned pigs. J. Anim. Sci. 95:4060–4071. doi: 10.2527/jas2017.1760 [DOI] [PubMed] [Google Scholar]
- Levrat M. A., Behr S. R., Rémésy C., and Demigné C.. . 1991. Effects of soybean fiber on cecal digestion in rats previously adapted to a fiber-free diet. J. Nutr. 121:672–678. doi: 10.1093/jn/121.5.672 [DOI] [PubMed] [Google Scholar]
- Li D. F., Nelssen J. L., Reddy P. G., Blecha F., Hancock J. D., Allee G. L., Goodband R. D., and Klemm R. D.. . 1990. Transient hypersensitivity to soybean meal in the early-weaned pig. J. Anim. Sci. 68:1790–1799. doi: 10.2527/1990.6861790x [DOI] [PubMed] [Google Scholar]
- Li P., Yin Y. L., Li D., Kim S. W., and Wu G.. . 2007. Amino acids and immune function. Br. J. Nutr. 98:237–252. doi: 10.1017/S000711450769936X [DOI] [PubMed] [Google Scholar]
- Litvak Y., Byndloss M. X., and Baumler A. J.. . 2018. Colonocyte metabolism shapes the gut microbiota. Science 362:1017–1024. doi: 10.1126/science.aat9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Mahan D. C., Fastinger N. D., and Peters J. C.. . 2004. Effects of diet complexity and dietary lactose levels during three starter phases on postweaning pig performance. J. Anim. Sci. 82:2790–2797. doi: 10.2527/2004.8292790x [DOI] [PubMed] [Google Scholar]
- Mao X., Lai X., Yu B., He J., Yu J., Zheng P., Tian G., Zhang K., and Chen D.. . 2014. Effects of dietary threonine supplementation on immune challenge induced by swine pseudorabies live vaccine in weaned pigs. Arch. Anim. Nutr. 68:1–15. doi: 10.1080/1745039X.2013.869988 [DOI] [PubMed] [Google Scholar]
- McGuckin M. A., Eri R., Simms L. A., Florin T. H., and Radford-Smith G.. . 2009. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis. 15:100–113. doi: 10.1002/ibd.20539 [DOI] [PubMed] [Google Scholar]
- Min Y. N., Liu S. G., Qu Z. X., Meng G. H., and Gao Y. P.. . 2017. Effects of dietary threonine levels on growth performance, serum biochemical indexes, antioxidant capacities, and gut morphology in broiler chickens. Poult. Sci. 96:1290–1297. doi: 10.3382/ps/pew393 [DOI] [PubMed] [Google Scholar]
- Montagne L., Pluske J. R., and Hampson D. J.. . 2003. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 108:95–117. doi: 10.1016/S0377-8401(03)00163-9 [DOI] [Google Scholar]
- Novozamsky I., Eck R., and van Schouwenburg J. C.. . 1974. Total nitrogen determination in plant material by means of the indophenol-blue method. Neth. J. Agric. Sci. 22:3–5. [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC):The National Academies Press. [Google Scholar]
- Pastorelli H., van Milgen J., Lovatto P., and Montagne L.. . 2012. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 6:952–961. doi: 10.1017/S175173111100228X [DOI] [PubMed] [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. . 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Pluske J. R., Turpin D. L., and Kim J. C.. . 2018. Gastrointestinal tract (gut) health in the young pig. Anim. Nutr. 4:187–196. doi: 10.1016/j.aninu.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Liu X. T., Wang X., Zhang G. J., Qiao S. Y., and Zeng X. F.. . 2014. Increased levels of standardized ileal digestible threonine attenuate intestinal damage and immune responses in Escherichia coli K88+ challenged weaned piglets. Anim. Feed Sci. Technol. 195:67–75. doi: 10.1016/j.anifeedsci.2014.05.013 [DOI] [Google Scholar]
- Schaart M. W., Schierbeek H., van der Schoor S. R., Stoll B., Burrin D. G., Reeds P. J., and van Goudoever J. B.. . 2005. Threonine utilization is high in the intestine of piglets. J. Nutr. 135:765–770. doi: 10.1093/jn/135.4.765 [DOI] [PubMed] [Google Scholar]
- Skinner L. D., Levesque C. L., Wey D., Rudar M., Zhu J., Hooda S., and de Lange C. F.. . 2014. Impact of nursery feeding program on subsequent growth performance, carcass quality, meat quality, and physical and chemical body composition of growing-finishing pigs. J. Anim. Sci. 92:1044–1054. doi: 10.2527/jas.2013-6743 [DOI] [PubMed] [Google Scholar]
- Specian R. D., and Oliver M. G.. . 1991. Functional biology of intestinal goblet cells. Am. J. Physiol. 260(2 Pt 1):C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183 [DOI] [PubMed] [Google Scholar]
- Strange K. 1992. Regulation of solute and water balance and cell volume in the central nervous system. J. Am. Soc. Nephrol. 3:12–27. [DOI] [PubMed] [Google Scholar]
- Vereecke L., Beyaert R., and van Loo G.. . 2011. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol. Med. 17:584–593. doi: 10.1016/j.molmed.2011.05.011 [DOI] [PubMed] [Google Scholar]
- Vince A., Dawson A. M., Park N., and O’Grady F.. . 1973. Ammonia production by intestinal bacteria. Gut 14:171–177. doi: 10.1136/gut.14.3.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waititu S. M., Yin F., Patterson R., Yitbarek A., Rodriguez-Lecompte J. C., and Nyachoti C. M.. . 2017. Dietary supplementation with a nucleotide-rich yeast extract modulates gut immune response and microflora in weaned pigs in response to a sanitary challenge. Animal 11:2156–2164. doi: 10.1017/S1751731117001276 [DOI] [PubMed] [Google Scholar]
- Wang W. W., Qiao S. Y., and Li D. F.. . 2009. Amino acids and gut function. Amino Acids 37:105–110. doi: 10.1007/s00726-008-0152-4 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang C., Wu G., Sun Y., Wang B., He B., Dai Z., and Wu Z.. . 2015. Glutamine enhances tight junction protein expression and modulates corticotropin-releasing factor signaling in the jejunum of weanling piglets. J. Nutr. 145:25–31. doi: 10.3945/jn.114.202515 [DOI] [PubMed] [Google Scholar]
- Wijtten P. J. A., Meulen J. V. D., and Verstegen M. W. A.. . 2011. Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 105:967–981. doi: 10.1017/S0007114510005660 [DOI] [PubMed] [Google Scholar]
- Williams B. A., Verstegen M. W., and Tamminga S.. . 2001. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 14:207–228. doi: 10.1079/NRR200127 [DOI] [PubMed] [Google Scholar]
- Wu J. J., Cao C. M., Ren D. D., Zhang Y., Kou Y. N., Ma L. Y., Feng S. B., Li Y., and Wang X. C.. . 2016. Effects of soybean antigen proteins on intestinal permeability, 5-hydroxytryptamine levels and secretory IgA distribution in the intestine of weaned piglets. Ital. J. Anim. Sci. 15:174–180. doi: 10.1080/1828051x.2016.1148559 [DOI] [Google Scholar]
- Yang R., Han X., Uchiyama T., Watkins S. K., Yaguchi A., Delude R. L., and Fink M. P.. . 2003. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G621–G629. doi: 10.1152/ajpgi.00177.2003 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Qin G., Han R., Wang J., Zhang X., and Liu D.. . 2014. β-Conglycinin reduces the tight junction occludin and ZO-1 expression in IPEC-J2. Int. J. Mol. Sci. 15:1915–1926. doi: 10.3390/ijms15021915 [DOI] [PMC free article] [PubMed] [Google Scholar]