Abstract
Background and Aims
There is a lack of consensus on which GI endoscopic procedures should be performed during the COVID-19 pandemic, and which procedures could be safely deferred without having a significant impact on outcomes.
Methods
We selected a panel of 14 expert endoscopists. We identified 41 common indications for advanced endoscopic procedures from the ASGE Appropriate Use of GI Endoscopy guidelines. Using a modified Delphi method, we first achieved consensus on the patient-important outcome for each procedural indication. Panelists prioritized consensus patient-important outcome when categorizing each indication into one of the following 3 procedural time periods: (1) time-sensitive emergent (schedule within 1 week), (2) time-sensitive urgent (schedule within 1 to 8 weeks), and (3) non-time sensitive (defer for >8 weeks and then reassess the timing). Three anonymous rounds of voting were allowed before attempts at consensus were abandoned.
Results
All 14 invited experts agreed to participate in the study. The prespecified consensus threshold of 51% was achieved for assigning patient-important outcome(s) to each advanced endoscopy indication. The prespecified consensus threshold of 66.7% was achieved for 40 of 41 advanced endoscopy indications in stratifying them into 1 of 3 procedural time periods. For 12 of 41 indications, 100% consensus was achieved; for 20 of 41 indications, 75% to 99% consensus was achieved.
Conclusions
By using a Modified Delphi method that prioritized patient-important outcomes, we developed consensus recommendations on procedural timing for common indications for advanced endoscopy. These recommendations and the structured decision framework provided by our study can inform decision making as endoscopy services are reopened.
Abbreviations: AGA, American Gastroenterological Association; ASGE, American Society for Gastrointestinal Endoscopy
Introduction
In March 2020, the World Health Organization declared COVID-19 a global pandemic. Worldwide, almost 2 million people have been infected with this virus and more than 120,000 deaths have been reported.1 In anticipation of the surge of COVID-19 cases in the United States, the Surgeon General of the United States advised hospitals to cancel all elective procedures.2 The American College of Surgeons and the 4 national gastroenterology organizations similarly recommended that elective procedures should be rescheduled to mitigate the spread of COVID-19 and preserve personal protective equipment.3 , 4 Subsequently, the American Gastroenterological Association (AGA) issued recommendations suggesting that only time-sensitive GI endoscopy procedures should be performed.5 A joint GI society statement and recommendations from a group of New York physicians were also published, providing limited advice regarding which procedures should be performed during the pandemic.6 , 7
Despite these recommendations, there continues to be ambiguity among practicing gastroenterologists regarding which endoscopic procedures should be performed during the COVID-19 pandemic, and which ones could be safely deferred.8 To provide more specific guidance on triaging endoscopic procedures, we used a modified Delphi methodology to attain expert consensus regarding procedural timing for advanced endoscopic procedures. The Delphi method is a validated and structured technique to obtain expert consensus, and it is particularly well suited for the present situation where there is limited outcome data, and guidance for procedural timing is urgently needed.9 , 10 Conducting new studies to assess outcomes related to delaying procedures amidst the ongoing pandemic is impractical. The Delphi method allows for timely formulation of expert consensus in a rigorous and systematic manner. We also recognized that delaying procedures not only has clinical implications but also moral and ethical ones. We therefore designed our study to emphasize patient-important outcomes while considering procedural timing.
Methods
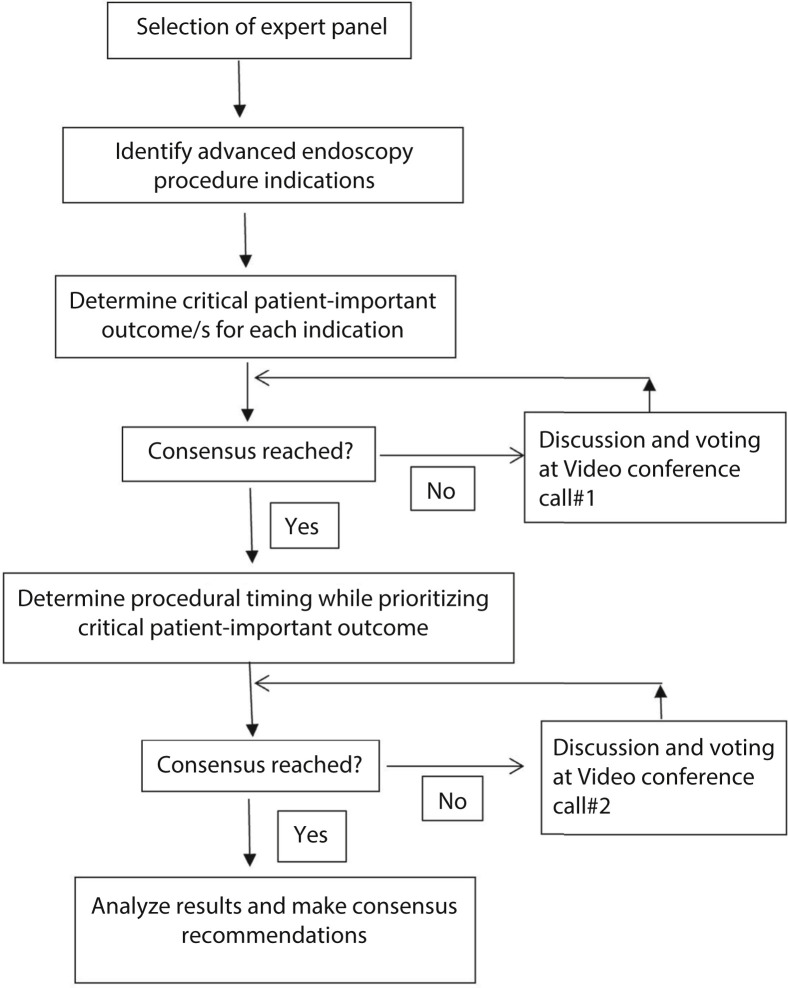
Our study overview is shown in Figure 1 and was as follows: our initial step was to achieve consensus on the patient-important outcome(s) for each advanced endoscopy indication. Experts were then asked to determine the timing of the advanced endoscopy procedure for each indication while strongly prioritizing patient-important outcomes in their decision making. Detailed study steps were as follows:
-
(1)
Selection of expert panel. An expert panel of 14 gastroenterologists was invited such that diversity was achieved in geography, practice location (academic, private practice, and Veterans Administration), and practice type (general gastroenterology and advanced endoscopy).
-
(2)
Selecting advanced endoscopy procedure indications (survey no. 1). American Society for Gastrointestinal Endoscopy (ASGE) guidelines on the “Appropriate Use of GI Endoscopy” were reviewed, and advanced endoscopy procedure indications were identified.11 Advanced endoscopy procedures were defined as those that required training in addition to what is typically provided during a general gastroenterology fellowship. These procedures included but were not limited to ERCP and EUS. Indications identified in the ASGE guideline were then adapted for inclusion in this survey.
-
(3)
An affinity chart was created using patient-important outcomes extracted from several clinical studies.12 , 13 Using a grouping process, we identified 4 major groups of patient-important outcomes that were relevant to our study: (1) avoidance of death/prolongation of life, (2) avoidance of cancer/avoidance of cancer progression, (3) avoidance of major surgery and/or hospitalization, and (4) improvement or palliation of symptoms.
-
(4)
Panelists were asked to choose up to 2 critical patient-important outcomes from the categories mentioned in point (3) for each indication (survey no. 1). Panelists were also allowed to add other patient-important outcomes.
-
(5)
Panelists were also asked to suggest additional indications for commonly performed advanced endoscopy procedures that were not already listed in survey no. 1.
Figure 1.
Study overview.
The consensus threshold was set at >51% and responses were kept anonymous.
-
(6)
Procedure indications for which patient-important outcomes failed to reach the consensus threshold, and new procedure indications suggested by panelists were discussed in video conference call no. 1.
-
(7)
Discussion followed by voting for each indication was undertaken in keeping with the Delphi technique (video conference call no. 1). If despite 3 rounds of discussion and voting, consensus could not be reached, attempts at further consensus were abandoned.
-
(8)
A panel of experts from our previous study on triaging general endoscopy procedures had achieved consensus that procedure timing should be categorized into the following blocks: (1) time-sensitive emergent (schedule within 1 week), (2) time-sensitive urgent (schedule within 1 to 8 weeks), (3) non-time sensitive (defer for >8 weeks and then reassess the timing). We used the same categorization for our present study.
-
(9)
Panelists were asked to select one of 3 timing categories described in point (8) for each procedure indication (survey no. 2). The consensus threshold was set at >66.7% and responses were kept anonymous.
-
(10)
Procedure indications that failed to reach the consensus threshold were identified. Video conference call no. 2 took place, and rules similar to conference call no. 1 were applied.
Results
Expert panel
The expert panel comprised 14 gastroenterologists, and all those who were invited to participate in the survey agreed at the first invitation. There were 12 advanced endoscopists, 1 general gastroenterologist, and 1 advanced endoscopy fellow. The average years in practice was 12 years (range, 1-27 years). Thirteen of the panelists worked in academic teaching hospitals, 1 at a Veterans Administration hospital, and 1 in private/community practice. Ten of the panelists only performed endoscopy in hospital-based endoscopy units, whereas 4 performed endoscopy in both ambulatory surgical centers and hospital-based endoscopy units. Seven panelists were from northeastern, 2 from western, 3 from midwestern, and 2 from southern United States. All panelists were currently performing endoscopy at their institutions. The average proportion of procedures being performed now compared with before the pandemic was 16.4% (range, 5%-30%).
Consensus on patient-important outcomes
Thirty-seven advanced endoscopy indications were adapted from the ASGE “Appropriate Use of GI Endoscopy” guidelines.11 During survey no. 1, panelists added 4 indications. These were all related to EGD procedures and are shown as indications 9 to 12 in Table 1 . This resulted in a total of 41 advanced endoscopy indications. We defined the consensus threshold a priori to be 51%. Consensus on patient-important outcome was achieved at survey no. 1 for 35 of 41 indications. Consensus on the remaining 6 indications was achieved during discussion and voting during video conference call no. 1.
Table 1.
Indications related to upper endoscopy
| Procedural indication | Critical patient-important outcome(s) | Consensus time interval | Consensus reached (%) |
|---|---|---|---|
|
Avoidance of cancer/cancer progression | Defer >8 weeks, and reassess timing | 100 |
|
Improvement or palliation of symptoms | Within 1 week | 69.2 |
|
Improvement or palliation of symptoms | Within 1 week | 84.6 |
|
Improvement or palliation of symptoms | Defer >8 weeks, and reassess timing | 100 |
|
Avoidance of cancer/cancer progression | Defer >8 weeks, and reassess timing | 100 |
| Avoidance of cancer/cancer progression | Defer >8 weeks, and reassess timing | 100 | |
| Avoidance of cancer/cancer progression; avoidance of major surgery and/or hospitalization | Defer >8 weeks, and reassess timing | 75 | |
|
Avoidance of major surgery and/or hospitalization | Within 1 week | 100 |
|
Improvement or palliation of symptoms | Within 1-8 weeks | 69.2 |
|
Avoidance of major surgery and/or hospitalization | Within 1-8 weeks | 75 |
|
Avoidance of cancer/cancer progression; avoidance of major surgery and/or hospitalization | Defer >8 weeks, and reassess timing | 100 |
No consensus achieved on the first round of voting.
No consensus achieved on the second round of voting.
Consensus on procedural timing
Survey no. 2 was considered the first round of voting, and the consensus threshold of 66.7% defined a priori was achieved for 23 of 41 indications. For the remaining 18 indications, consensus was achieved for 13 indications during the second round of voting. The expert panel voted to modify the remaining 5 indications. These indications are delineated in Table 1, Table 2, Table 3, Table 4 . After these modifications, a third round of voting was conducted, and consensus was achieved for 4 of the 5 indications. In aggregate, consensus on procedural timing for 40 of 41 indications was achieved. The experts achieved 100% consensus for 12 of 41 indications, 75% to 99% consensus for 20 of 41 indications, and 67% to 74% consensus for 8 of 41 indications (Table 1, Table 2, Table 3, Table 4). The only indication for which consensus could not be achieved despite modification and 3 voting rounds was “Incidentally found pancreatic duct dilation >6 mm and common bile duct dilation >10 mm on CT scan or magnetic resonance imaging (with normal results for liver function tests).”
Table 2.
Indications related to colonoscopy
| Procedural indication | Critical patient-important outcome(s) | Consensus time interval | Consensus reached (%) |
|---|---|---|---|
|
Avoidance of cancer/cancer progression; avoidance of major surgery and/or hospitalization | Defer >8 weeks, and reassess timing | 84.6 |
| Avoidance of cancer/cancer progression; avoidance of major surgery and/or hospitalization | Defer >8 weeks, and reassess timing | 82 | |
|
Improvement or palliation of symptoms; avoidance of major surgery and/or hospitalization | Within 1 week | 100 |
No consensus achieved on the first round of voting.
No consensus achieved on the second round of voting.
Consensus was achieved on the third round of voting.
Table 3.
Indications related to ERCP
| Procedural indication | Critical patient-important outcome(s) | Consensus time interval | Consensus reached (%) |
|---|---|---|---|
|
Improvement/palliation of symptoms | Within 1-8 weeks | 91 |
|
Improvement/palliation of symptoms; avoidance of major surgery/hospitalization | Within 1 week | 69.2 |
|
Avoidance of death/prolongation of life | Within 1 week | 100 |
|
Avoidance of major surgery and/or hospitalization | Within 1 week | 70 |
| Avoidance of major surgery and/or hospitalization | Defer >8 weeks, and reassess timing | 83 | |
|
Avoidance of major surgery and/or hospitalization | Defer >8 weeks, and reassess timing | 75 |
|
Avoidance of major surgery and/or hospitalization | Defer >8 weeks, and reassess timing | 67 |
|
Avoidance of major surgery and/or hospitalization | Within 1 week | 100 |
|
Avoidance of cancer/avoidance of cancer progression; avoidance of major surgery and /or hospitalization | Defer >8 weeks, and reassess timing | 84.6 |
|
Avoidance of cancer/avoidance of cancer progression; avoidance of major surgery and/or hospitalization | Defer >8 weeks, and reassess timing | 67 |
|
Avoidance of major surgery and/or hospitalization and Improvement or palliation of symptoms | Defer >8 weeks, and reassess timing | 92 |
|
Avoidance of major surgery and/or hospitalization | Within 1-8 weeks | 75 |
No consensus achieved on the first round of voting.
No consensus achieved on the second round of voting.
Consensus was achieved on the second round of voting.
Table 4.
Indications related to EUS and enteroscopy
| Procedural indication | Critical patient-important outcome(s) | Consensus time interval | Consensus reached (%) |
|---|---|---|---|
|
Avoidance of cancer/cancer progression | Within 1-8 weeks | 69.3 |
|
Avoidance of cancer/cancer progression | Defer >8 weeks, and reassess timing | 100 |
|
Avoidance of cancer/cancer progression | Defer >8 weeks, and reassess timing | 76.9 |
|
Avoidance of cancer/cancer progression | Within 1-8 weeks | 83 |
|
Avoidance of cancer/cancer progression | Defer >8 weeks, and reassess timing | 84.6 |
|
Avoidance of cancer/cancer progression | Defer >8 weeks, and reassess timing | 67 |
|
Avoidance of cancer/cancer progression | Defer >8 weeks, and reassess timing | 84.6 |
| Avoidance of cancer/cancer progression | No consensus was achieved | ||
|
Avoidance of cancer/cancer progression | Within 1-8 weeks | 92 |
|
Improvement/ palliation of symptoms; avoidance of major surgery/hospitalization | Within 1-8 weeks | 76.9 |
|
Avoidance of death/prolongation of life; avoidance of major surgery/hospitalization | Within 1 week | 100 |
|
Improvement or palliation of symptoms | Within 1-8 weeks | 75 |
|
Avoidance of major surgery/hospitalization | Defer >8 weeks, and reassess timing | 84.6 |
|
Avoidance of cancer/cancer progression | Defer >8 weeks, and reassess timing | 100 |
|
Avoidance of major surgery and/or hospitalization | Defer >8 weeks, and reassess timing | 83 |
No consensus achieved on the first round of voting.
No consensus achieved on the second round of voting.
Discussion
We used a modified Delphi method to achieve consensus among experts in categorizing 40 of 41 advanced endoscopy procedure indications into 1 of 3 timing categories: (1) time-sensitive emergent (schedule within 1 week), (2) time-sensitive urgent (schedule within 1 to 8 weeks), or (3) non-time sensitive (defer for >8 weeks and then reassess the timing). We placed patient priorities at the center of this decision-making process by prioritizing patient-important outcomes. This study provides a decision-making framework by which endoscopists may determine scheduling timing for endoscopic procedures as they start to reopen their endoscopy suites.
Several guidelines have been published on procedural timing during the COVID-19 pandemic.4 , 5 , 7 An expert panel previously constituted by our study group to triage general endoscopic procedures had failed to reach consensus on any of these pre-existing categorizations (unpublished data). Instead, by consensus, the expert panel modified the AGA recommendations into 3 time categories for procedural timing: (1) time-sensitive emergent (schedule within 1 week), (2) time-sensitive urgent (schedule within 1 to 8 weeks), and (3) non-time sensitive (defer for >8 weeks and then reassess timing).5 The panel felt that the AGA “time-sensitive category” (schedule within 0 to 8 weeks) was too broad and did not adequately differentiate between emergent procedures, such as acute cholangitis, and urgent procedures that could be delayed a few weeks, such as cancer staging. We chose to adopt these three-tier timing categories for our present study. In our previous study, we also prioritized patient-important outcomes during decision making. Patient-important outcomes are defined as characteristics or variables that reflect how a patient feels, functions, or survives.14 , 15 These are outcomes that patients’ value and are related to death and quality of life (morbidity, pain, function). This structure was relevant for our present study, because it placed patient preferences at the center of decision making, avoided a multistep decision tree, and could be adapted to iterative improvements using the Delphi technique.
Some indications required significant discussion to achieve consensus.
Indications 5 to 7
For radiofrequency ablation for low- and high-grade dysplasia, 100% consensus was achieved that ablation could be deferred for >8 weeks given the low short-term risk of disease progression, estimated at 1.7%/year for progression of low-grade dysplasia to either high-grade dysplasia or cancer, and 7%/year for malignant transformation of high-grade dysplasia.16 However, for EMR to treat nodular high-grade dysplasia (confirmed by expert pathologist review), a lower consensus of 75% was achieved on concerns regarding the accuracy of biopsies in detecting the most advanced pathology present, given data indicating that EMR can often upstage a biopsy diagnosis of high-grade dysplasia.17 , 18
Indications 12 and 13
Deferring EMR of large (≥20 mm) colorectal polyps for 8 weeks may increase the risk of transition to cancer or progression of unrecognized cancer to more invasive cancer. The risk of covert prevalent cancer in such polyps ranges from 3% to 7%,19, 20, 21 with a lower risk where biopsy specimens do not indicate cancer. Although covert cancer may be missed due to sampling error, the likelihood of progression to an unresectable stage within 8 weeks is unlikely. Moreover, with EMR, the risk of adverse events requiring hospital admission is 5% to 10%,22 which could put the patient and others at increased risk for COVID-19 exposure. In addition, polyp characteristics maybe helpful in characterizing the risk of prevalent cancer, including location in the rectum, nongranular appearance, and very large size,21 and should be considered when making individual recommendations.
Indications 15 and 30 to 34
Early diagnosis is essential in improving survival in patients with a high suspicion of pancreaticobiliary malignancy,23 including patients with painless obstructive jaundice, or with cross-sectional imaging demonstrating a malignant-appearing solid mass in the pancreas. In the absence of symptoms, consensus was reached that endoscopic intervention should be performed in 1 to 8 weeks. Main pancreatic duct dilation >6 mm may precede the diagnosis of pancreatic cancer by several months.23, 24, 25 In the absence of additional imaging abnormalities, such as a stricture, the consensus was that evaluation with endoscopic ultrasonography could be deferred by 8 weeks, then reassessed. For isolated biliary dilation without symptoms or biochemical derangements, the likelihood of significant biliary pathology is low,26, 27, 28 and the consensus was that evaluation could be deferred by 8 weeks, then reassessed When this finding co-existed with pancreatic duct dilation >6 mm, no consensus was reached for procedural timing.
Indications 16 to 19
Although some studies have shown that endoscopic stone removal in asymptomatic patients has little effect in preventing biliary adverse events,29 a large Swedish registry cohort analysis suggested that stone removal resulted in improved outcomes.30 Although ERCP is usually undertaken in patients with asymptomatic choledocholithiasis, early ERCP is not warranted because the short-term risk of biliary adverse events is low,29 and the consensus was to defer ERCP for >8 weeks, then reassess. There was broad consensus that symptomatic patients with known/suspected choledocholithiasis required ERCP within 1 week and that urgent ERCP was warranted in patients with acute cholangitis, because this is associated with lower in-hospital mortality, 30-day mortality, length of hospital stay, and organ failure.31 , 32
Indications 20 and 21
The risk of permanent duct changes and pancreatitis is low with small-caliber pancreatic stents, and given the high spontaneous migration rate (>85% by 100 days), deferring ERPC for >8 weeks was deemed appropriate.33 In young patients, women, those with a history of recurrent acute pancreatitis, small pancreatic duct diameter, or 5F stent placement, removal in <8 weeks may be appropriate.34 For patients with biliary stent in situ for 3 months, there was agreement among only 67% of panelists to defer stent exchange for >8 weeks. Most panelists felt that routine stent changes could be deferred during this pandemic as long as close clinical follow-up of patients was undertaken, and an individualized approach was undertaken to balance the risk of stent occlusion with that of exposure to COVID-19. Thus, some patients are at relatively low risk for biliary stent occlusion; for example, stent placement after incomplete clearance of bile duct stones, or treatment of postoperative bile leak, or those with multiple stents in place for stricture management.35 In contrast, patients with malignant hilar strictures or distal biliary strictures may be at a higher risk of stent occlusion.36 , 37
Indication 27
For EUS staging of GI cancer, there was 69% consensus that procedures should occur within 1 to 8 weeks. Differing opinions over timing centered around 2 issues: (1) unpredictability regarding the possibility of clinically relevant tumor progression over an 8-week time period, owing to significant variability of tumor doubling times and (2) patient anxiety surrounding a new diagnosis of malignancy and the importance of establishing a treatment plan.38
Some aspects and limitations of our study warrant further discussion. First, our recommendations are based on expert opinion. The decision to perform endoscopy during the COVID-19 pandemic needs to balance the risks associated with delaying the procedure with the risk of viral exposure to patients and health care providers. Literature on outcomes when procedures are delayed, especially for short duration of up to 8 weeks, and on the likelihood of acquiring COVID-19 infection during endoscopy, are extremely limited. Furthermore, scheduling timing decisions also needs to take into consideration factors, such as saving personal protective equipment in a time of dire shortage and avoiding diversion of hospital resources away from the direct care of patients infected with COVID-19. Complex nonlinear decisions that require subjective judgment are often unsuitable for traditional methods of guideline development. However, the Delphi method is well suited for such situations because it is a validated methodology that provides a framework whereby conflicting values and differing opinions can be systematically incorporated to achieve consensus.39 Anonymity is an important aspect of the Delphi method and reduces the likelihood of personality conflicts and status relations and helps preserve constructive group dynamics. In our study, results of the written survey and voting during video conferences were anonymous. However, panelists were able to see and hear each other during the video conferences, and this may have potentially biased their responses. Second, in the absence of a universally accept threshold, we chose an arbitrary value of 66.7% agreement to declare expert consensus when determining procedural timing. Not all recommendations achieved the same level of expert consensus, and this should also be taken into consideration when using an individual recommendation. Third, we fully recognize that in addition to procedural indications, factors including severity of symptoms and patient co-morbidities should be considered when determining procedural timing. We hope that our recommendations will serve as a starting point for such difficult decision making, and that endoscopists will adapt these on a case-by-case basis to reach their final recommendation. We are entering a new phase of the COVID-19 pandemic where there is even more heterogeneity in the prevalence of infection across the country. Our system of categorizing procedures into broad time periods allows endoscopists the flexibility to take these local circumstances as well as local resource availability into consideration.
In conclusion, using a structured decision framework that prioritized patient-important outcomes, we were successful in achieving consensus on procedural timing for 40 of 41 common indications for advanced endoscopy procedures. We chose to classify indications within a 3-tier system that provided specific guidance while allowing gastroenterologists the additional flexibility in scheduling procedures. We believe it will take many months before endoscopy capacity returns close to prepandemic levels. It is our hope that these guidelines will serve as a useful instrument for endoscopists in planning their strategy as they reopen and ramp up endoscopy at their institutions.
Footnotes
If you would like to chat with an author of this article, you may contact Dr Sawhney at msawhney@bidmc.harvard.edu.
DISCLOSURE: Dr Khashab is a consultant for Boston Scientific, Olympus and Medtronic. Dr Schulman is a consultant for Apollo Endosurgery, Boston Scientific, Microtech, and receives research funding from GI Dynamics. Dr Berzin is a consultant for Boston Scientific and Medtronic. Dr Muthusamy has received research support fromMedtronicandBoston Scientific, is a consultant for Medtronic, Boston Scientific,Interpace Diagnostics, Medivators, a stockholder in Capsovision, and receives honoraria from Torax Medical/Ethicon; Dr Pohl received research grants from Steris and Cosmo Pharmaceuticals. All other authors disclosed no financial relationships.
See CME section; p. 754.
References
- 1.WHO Coronavirus Disease 2019 (COVID-19) Situation Report – 86. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200415-sitrep-86-covid-19.pdf?sfvrsn=c615ea20_6 Available at:
- 2.Luthi S. Surgeon General advises hospitals to cancel elective surgeries. Politico. March 14 2020. Available at: https://www.politico.com/news/2020/03/14/surgeon-general-elective-surgeriescoronavirus-129405. Accessed April 14, 2014.
- 3.COVID-19: recommendations for management of elective surgical procedures. American College of Surgeons. March 19, 2020. Available at: https://www.facs.org/about-acs/covid-19/information-forsurgeons/elective-surgery. Accessed April 14, 2020.
- 4.Joint GI Society Message on COVID-19. https://gi.org/2020/03/15/joint-gi-society-message-on-covid-19/ Available at:
- 5.Sultan S., Lim J.K., Altayar O. AGA institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology. Epub 2020 Mar 31 doi: 10.1053/j.gastro.2020.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross S.A., Robbins D., Greenwald D. Preparation in the Big Apple: New York City, a new epicenter of the COVID-19 pandemic. Am J Gastroenetrol. 2020;115:801–804. doi: 10.14309/ajg.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gastroenterology Professional Society guidance on endoscopic procedures during the COVID-19 pandemic. https://webfiles.gi.org/links/media/Joint_GI_Society_Guidance_on_Endoscopic_Procedure_During_COVID19_FINAL_impending_3312020.pdf Available at:
- 8.Bilal M., Simons M., Rahman A.U. What constitutes urgent endoscopy? A social media snapshot of gastroenterologists’ views during the COVID-19 pandemic. Endosc Int Open. 2020;8:E693–E698. doi: 10.1055/a-1153-9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalkey N., Helmer O. An experimental application of the DELPHI method to the use of experts. Manag Sci. 1963;9:458–467. [Google Scholar]
- 10.Hsu C.-C., Sandford B.A. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12:1–8. [Google Scholar]
- 11.Early D.S., Ben-Menachem T., Decker G.A. Appropriate use of GI endoscopy. Gastrointest Endosc. 2012;75:1127–1131. doi: 10.1016/j.gie.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi G.Y., Murad M.H., Fujiyoshi A. Patient-important outcomes in registered diabetes trials. JAMA. 2008;299:2543–2549. doi: 10.1001/jama.299.21.2543. [DOI] [PubMed] [Google Scholar]
- 13.Moller M.H. Patient-important outcomes and core outcome sets: increased attention needed! Br J Anaesth. 2019;122:408–410. doi: 10.1016/j.bja.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudry S., Messika J., Ricard J.D. Patient-important outcomes in randomized controlled trials in critically ill patients: a systematic review. Ann Intensive Care. 2017;7:28. doi: 10.1186/s13613-017-0243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal A., Johnston B.C., Vernooij R.W. Authors seldom report the most patient-important outcomes and absolute effect measures in systematic review abstracts. J Clin Epidemiol. 2017;81:3–12. doi: 10.1016/j.jclinepi.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Shaheen N.J., Falk G.W., Iyer P.G. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol. 2016;111:30–50. doi: 10.1038/ajg.2015.322. quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wani S., Abrams J., Edmundowicz S.A. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett's esophagus patients with visible and flat neoplasia: a multicenter cohort study. Dig Dis Sci. 2013;58:1703–1709. doi: 10.1007/s10620-013-2689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thota P.N., Sada A., Sanaka M.R. Correlation between endoscopic forceps biopsies and endoscopic mucosal resection with endoscopic ultrasound in patients with Barrett's esophagus with high-grade dysplasia and early cancer. Surg Endosc. 2017;31:1336–1341. doi: 10.1007/s00464-016-5117-1. [DOI] [PubMed] [Google Scholar]
- 19.Pohl H., Grimm I.S., Moyer M.T. Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pohl H., Grimm I.S., Moyer M.T. Clip closure prevents bleeding after endoscopic resection of large colon polyps in a randomized trial. Gastroenterology. 2019;157:977–984.e3. doi: 10.1053/j.gastro.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess N.G., Hourigan L.F., Zanati S.A. Risk stratification for covert invasive cancer among patients referred for colonic endoscopic mucosal resection: a large multicenter cohort. Gastroenterology. 2017;153:732–742.e1. doi: 10.1053/j.gastro.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 22.Kaltenbach T., Anderson J.C., Burke C.A. Endoscopic removal of colorectal lesions-recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;158:1095–1129. doi: 10.1053/j.gastro.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Wang W., Shpaner A., Krishna S.G. Use of EUS-FNA in diagnosing pancreatic neoplasm without a definitive mass on CT. Gastrointest Endosc. 2013;78:73–80. doi: 10.1016/j.gie.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S., Nakaizumi A., Ioka T. Main pancreatic duct dilatation: a sign of high risk for pancreatic cancer. Jpn J Clin Oncol. 2002;32:407–411. doi: 10.1093/jjco/hyf093. [DOI] [PubMed] [Google Scholar]
- 25.Gangi S., Fletcher J.G., Nathan M.A. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol. 2004;182:897–903. doi: 10.2214/ajr.182.4.1820897. [DOI] [PubMed] [Google Scholar]
- 26.D'Souza L.S., Buscaglia J.M. The use of endoscopic ultrasound in the evaluation of unexplained biliary dilation. Gastrointest Endosc Clin N Am. 2019;29:161–171. doi: 10.1016/j.giec.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Bruno M., Brizzi R.F., Mezzabotta L. Unexplained common bile duct dilatation with normal serum liver enzymes: diagnostic yield of endoscopic ultrasound and follow-up of this condition. J Clin Gastroenterol. 2014;48:e67–e70. doi: 10.1097/MCG.0b013e3182a8848a. [DOI] [PubMed] [Google Scholar]
- 28.Kwok A., Lau J., Jones D.B. Role of endoscopic ultrasound in evaluation of unexplained common bile duct dilation [abstract] Gastrointest Endosc. 2009;69:AB250. [Google Scholar]
- 29.Hakuta R., Hamada T., Nakai Y. Natural history of asymptomatic bile duct stones and association of endoscopic treatment with clinical outcomes. J Gastroenterol. 2020;55:78–85. doi: 10.1007/s00535-019-01612-7. [DOI] [PubMed] [Google Scholar]
- 30.Moller M., Gustafsson U., Rasmussen F. Natural course vs interventions to clear common bile duct stones: data from the Swedish Registry for Gallstone Surgery and Endoscopic Retrograde Cholangiopancreatography (GallRiks) JAMA Surg. 2014;149:1008–1013. doi: 10.1001/jamasurg.2014.249. [DOI] [PubMed] [Google Scholar]
- 31.Iqbal U., Khara H.S., Hu Y. Emergent versus urgent ERCP in acute cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2020;91:753–760.e4. doi: 10.1016/j.gie.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 32.Khashab M.A., Tariq A., Tariq U. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin Gastroenterol Hepatol. 2012;10:1157–1161. doi: 10.1016/j.cgh.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Pahk A., Rigaux J., Poreddy V. Prophylactic pancreatic stents: does size matter? A comparison of 4-Fr and 5-Fr stents in reference to post-ERCP pancreatitis and migration rate. Dig Dis Sci. 2011;56:3058–3064. doi: 10.1007/s10620-011-1695-x. [DOI] [PubMed] [Google Scholar]
- 34.Bakman Y.G., Safdar K., Freeman M.L. Significant clinical implications of prophylactic pancreatic stent placement in previously normal pancreatic ducts. Endoscopy. 2009;41:1095–1098. doi: 10.1055/s-0029-1215317. [DOI] [PubMed] [Google Scholar]
- 35.Tohda G., Dochin M. Management of endoscopic biliary stenting for choledocholithiasis: evaluation of stent-exchange intervals. World J Gastrointest Endosc. 2018;10:45–50. doi: 10.4253/wjge.v10.i1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khashab M.A., Kim K., Hutfless S. Predictors of early stent occlusion among plastic biliary stents. Dig Dis Sci. 2012;57:2446–2450. doi: 10.1007/s10620-012-2178-4. [DOI] [PubMed] [Google Scholar]
- 37.Dumonceau J.M., Tringali A., Papanikolaou I.S. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - updated October 2017. Endoscopy. 2018;50:910–930. doi: 10.1055/a-0659-9864. [DOI] [PubMed] [Google Scholar]
- 38.Luebeck E.G., Curtius K., Jeon J. Impact of tumor progression on cancer incidence curves. Cancer Res. 2013;73:1086–1096. doi: 10.1158/0008-5472.CAN-12-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donohoe H., Stellefson M., Tennant B. Advantages and limitations of the e-Delphi technique: implications for health education researchers. Am J Health Educ. 2012;43:38–46. [Google Scholar]