Dear Editor,
According to the report by Chen et al. published in this journal,1 a median duration of 11 days was observed between positive to negative SARS-CoV-2 PCR tests of upper respiratory tract samples from COVID-19 patients. COVID-19 morbidity and mortality are higher among older individuals.2 However, no breakdown of recovery periods is available for different patient age groups in large population cohorts. Such knowledge may improve our understanding of COVID-19 and assist healthcare system readiness for future coronavirus epidemics or pandemics. Our study aimed at assessing the effects of age and sex of Israeli COVID-19 patients on their rate of recovery, applying datamining of a large dataset of 5769 recovered Israeli patients released to the public on April 28, 2020 by the Israel Ministry of Health.
A consortium study of 1420 mild-to-moderate recovered COVID-19 patients reported mean disease duration of 11.5 ± 5.7 days.3 A single center Chinese study of 221 discharged COVID‐19 patients observed an average time to recovery of 10.63±1.93 days for mild to moderate patients, compared with 18.70±2.50 for severe patients.4 Two other single center Chinese studies (127 and 225 recovered patients) reported a mean recovery time or median time of 20 and 21 days, respectively.5 , 6 None of the above studies reported on time to recovery according to patient age or sex.
We analyzed a public dataset released on April 28th 2020 by the Israel Ministry of Health (https://data.gov.il/dataset/covid-19). The dataset listing recovered patient demographics includes anonymized records of 5769 Israeli patients (3370 men and 2399 women). Age groups are shown in this public dataset according to decades for the age range of 20 to 59 years, while for younger or older patients age groups are assigned as 0–19 years or as over 60 years. Recovery is defined by two consecutive negative SARS-CoV-2 throat swab test results. Time from infection to recovery is measured as number of days from first positive to first negative SARS-CoV-2 PCR test result. Statistical significance of differences in the recovery times between patient groups were compared using two-way ANOVA as described in the Figure and Table legends. Group differences with p<0.05 were considered significant.
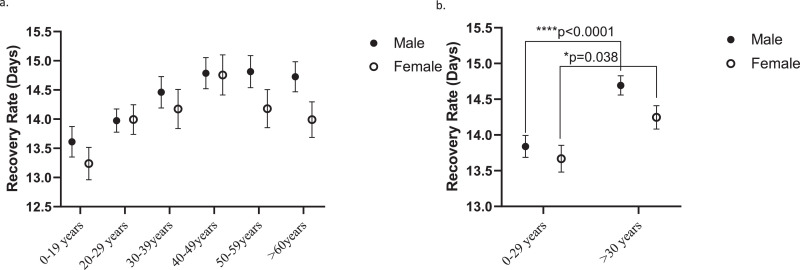
Table 1 shows the time for recovery by patient sex and age group according to the Israel Ministry of Health dataset of recovered patients released on April 28, 2020 and the differences (days) between group recovery periods. Fig. 1 presents (a) average recovery times in each age group for male and female patients and (b) average recovery times for male and female patients aged 0–29 years or >30 years. As shown, male and female patients aged >30 years had significantly longer recovery periods compared with younger patients (FD=0.95, p<0.0001 and FD=0.97 days, p = 0.038, for men and women, respectively). These differences, while statistically significant, may seem small. Yet, they allow a conclusion that younger individuals, in addition to be less likely to have severe COVID-19 symptoms requiring intensive care unit hospitalization, are also recovering on average faster from SARS-CoV-2 infection.
Table 1.
Recovery rates from SARS-CoV-2 infection among Israeli male and female COVID-19 patients by age groups. (a) Recovery periods (days) calculated from an anonymized dataset of recovered COVID-19 patients released to the public by the Israel Ministry of Health on April 28 2020 (https://data.gov.il/dataset/covid-19). This dataset includes time (days) from first positive to first negative SARS-CoV-2 PCR test for 5769 Israelis (3370 men and 2399 women). (b) Analysis comparing patients aged 0–29 years and >30 years.
| a. | ||||||
|---|---|---|---|---|---|---|
| Recovery Rate (Days±SD) | ||||||
| Age (%Male)\Sex | 0–19 years (54.6%) |
20–29 years (60.7%) |
30–39 years (59.9%) |
40–49 years (59.5%) |
50–59 years (54.5%) |
>60 years (56.7%) |
| Male | 13.61±5.89 N = 510 |
13.97±5.81 N = 859 |
14.46±6.0 N = 502 |
14.79±5.72 N = 460 |
14.81±5.90 N = 457 |
14.73±5.896 N = 582 |
| Female | 13.24±5.70 N = 423 |
13.99±5.9 N = 556 |
14.17±6.14 N = 335 |
14.76±5.90 N = 313 |
14.18±5.90 N = 328 |
13.99±6.44 N = 444 |
| b. | ||||||
|---|---|---|---|---|---|---|
| Sex | Age | Recovery Rate (Days±SD) | ||||
| Male | 0–29 years N = 1369 |
13.84 ± 5.67 | ||||
| >30 years N = 2001 |
14.69±6.0 | |||||
| Female | 0–29 years N = 979 |
13.66±5.87 | ||||
| >30 years N = 1420 |
14.24±6.16 | |||||
Fig. 1.
Comparative recovery rates from SARS-CoV-2 infection among Israeli male and female COVID-19 patients by age groups. (a) Statistics analysis by age decades (two-way ANOVA test) indicated statistically significant effects of age (****p<0.0001, F (5, 5757) = 5.406) and sex (*p = 0.037, F (1, 5757) = 4.353). (b) Statistics analysis comparing patients aged 0–20 years and >30 years indicated a significant effects of age (****p<0.0001, F (1, 5765) = 19.65). Sidak's multiple comparisons test showed a significantly slower recovery rate in male patients over 30-year-old (****p<0.001) and in female patients (*p = 0.038), compared to patients aged 0–29 years. Values signify average recovery rate (days) ± SEM.
Of note, severity of COVID-19 or comorbidities are not included in the Israel Ministry of Health dataset of recovered patients applied for this analysis. However, given the average recovery times of between 13.239 to 14.814 days (Table 1; lowest for women under 19 years and highest for men aged 50–59 years, respectively) it is clear that severe COVID-19 cases are not included in this dataset. Indeed, severe cases were reported to be discharged from hospital on average 8 days longer than mild-to-moderate patients requiring hospitalization.4 A separate Israel Ministry of Health public dataset shows symptoms severity at first positive SARS-CoV-2 test result; however, as all records are anonymized there is no option for comparing individual patient symptoms at first positive test with their recovery periods.
Women are less likely than men to have severe acute respiratory distress (ARDS) or fatal outcome following SARS-CoV-2 infection.7 , 8 The reasons for this sex difference remain unclear, and it has been suggested that this may reflect the fact that androgen hormones, which have higher plasma levels in men compared with women, drive the transcription of TMPRSS2, the gene coding for the protease essential for SARS-CoV-2 cell entry following the biding of its spike protein to cell membrane ACE2.9
A key confounder of our analysis is that the first positive SARS-CoV-2 PCR test results do not affect the time of a patient becoming symptomatic, in particular due to heavy workload and shortage of throat swab collection teams during late March and early April 2020, when the new infections in Israel were rapidly increasing. Yet, it seems reasonable to assume that the heavy workload during this period did not affect the observed between group differences, so that the recorded times between the first positive to first negative SARS-CoV-2 PCR test results may serve as proxy of time to recovery. In favor of this, all SARS-CoV-2 PCR testing in Israel (at time of writing this report) is done by the same RNA extraction and PCR specifications as coordinated by the Israel Ministry of Health COVID-19 emergency team.
The Israel Ministry of Health keeps updating the dataset for recovering COVID-19 patients. As the dataset applied for this report includes 5769 recovered COVID-19 patients, the findings on age group differences in time to recovery are unlikely to change for larger Israeli cohorts.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We are grateful to the Israel Ministry of Health for providing public access to anonymized COVID-19 patient records without need to obtain specific permits. This is a fine example of health records data sharing for improving human health.
References
- 1.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.6130. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 doi: 10.1111/joim.13089. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020 doi: 10.1111/joim.13063. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Yu F., Yan L., Wang N., Yang S., Wang L., Tang Y., Gao G., Wang S., Ma C., Xie R., Wang F., Tan C., Zhu L., Guo Y., Zhang F. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa345. pii: ciaa345[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z., Liu X., Wei L., Truelove S.A., Zhang T., Gao W., Cheng C., Tang X., Wu X., Wu Y., Sun B., Huang S., Sun Y., Zhang J., Ma T., Lessler J., Feng T. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30287-5. pii: S1473-3099(20)30287-5[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34(2) doi: 10.23812/Editorial-Conti-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Gudbjartsson D.F., Helgason A., Jonsson H., Magnusson OT… Stefansson K. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020 doi: 10.1056/NEJMoa2006100. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wambier C.G., Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.032. pii: S0190-9622(20)30608-3[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]