Graphical abstract
Keywords: Thrombosis, COVID-19, Clot in transit, Pulmonary embolism, Transesophageal echocardiography, Prone position
Highlights
-
•
The risk for thromboembolic events in COVID-19 is substantial.
-
•
PE should be considered in cases of clinical deterioration.
-
•
Management of CIT is controversial.
Introduction
Although a relatively infrequent entity, clot in transit (CIT) carries a considerable mortality; the ideal management for CIT remains controversial. We present the case of a patient admitted to the intensive care unit with coronavirus disease 2019 (COVID-19) acute respiratory distress syndrome (ARDS) with deteriorating oxygenation and hemodynamics. Emergent transesophageal echocardiography (TEE) was performed to better assess for etiologies of deterioration and demonstrated a CIT in the right ventricle. In this case, we highlight the utility of TEE in the evaluation of an unstable patient while in the prone position and further demonstrate the consequences of the potential hypercoagulable state of COVID-19.
Case Presentation
A 62-year-old man without a significant medical history presented to the emergency department with fevers, malaise, and dyspnea. His temperature was measured at 100.4°F (38°C), and he was found to be hypoxemic with 70% oxygen saturation on room air. Chest radiography showed extensive patchy bilateral mid and lower lung airspace opacities. Laboratory data demonstrated serum creatinine of 2.75 mg/dL, a white blood cell count of 22.6 × 103/μL with lymphopenia (6% lymphocytes), and d-dimer of 356 ng/mL (upper limit of normal <230 ng/mL). He was placed on a high-flow nasal cannula and admitted to the general medicine service, where he was found to be positive for severe acute respiratory syndrome coronavirus-2 by polymerase chain reaction.
The patient was started on low–molecular weight heparin at a dose of 40 mg every 24 hours as prophylaxis for venous thromboembolism. Lower extremity ultrasound on admission, ordered as a result of an elevated d-dimer level, did not reveal acute deep venous thrombosis. d-dimer rose to 4,070 mg/mL, and the patient was transitioned to therapeutic low–molecular weight heparin at a dose of 1 mg/kg every 12 hours given the suspected increased risk for thrombosis among patients with COVID-19. The following day, he became progressively hypoxemic, with increased work of breathing and worsening bilateral opacities on chest radiography, leading to emergent endotracheal intubation and admission to the intensive care unit (Figure 1). The diagnosis of COVID-19 ARDS was made. The patient had refractory hypoxemia with oxygen saturation of 84% despite inhaled nitric oxide and prone positioning. He was hemodynamically unstable requiring vasopressor support. d-dimer rose further to >10,000 ng/mL. The possibility of extracorporeal membrane oxygenation was discussed, but the patient was not deemed a candidate, in part related to significant acute renal failure.
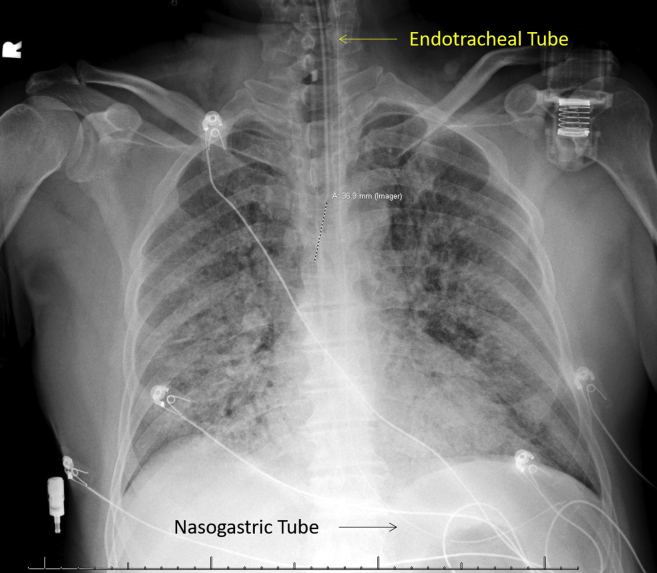
Figure 1.
Postintubation chest radiograph demonstrates hazy airspace opacities in the lungs bilaterally consistent with COVID-19 viral pneumonia and developing ARDS. Endotracheal tube projects 3.2 cm above the carina. Nasogastric tube terminates in the stomach.
Given rapid hemodynamic and respiratory decompensation with a significantly elevated d-dimer level, emergent TEE (Philips Affinity, X7 probe; Philips Medical Systems, Andover, MA) was performed to exclude proximal pulmonary embolism (PE) and to evaluate for alternative causes of deterioration while the patient remained in the prone position (Figure 2). For the purpose of provider safety, all team members donned N-95 masks, face shields, gowns, and two sets of gloves, per our hospital protocol. Transthoracic echocardiography was considered, but because of the emergent clinical status of the patient, TEE was selected as the initial imaging modality to obtain a greater amount of information up front. Transportation for alternative imaging modalities, such as computed tomographic or ventilation/perfusion scanning, was not a safe option at that time. To facilitate probe insertion, the patient's head was positioned to the left side, and an assisted head lift allowed passage of the probe in one attempt without resistance. TEE demonstrated right ventricular dilatation with a 1.2 × 1.2 cm serpiginous mobile echodensity in the right ventricle entangled within the tricuspid valve chordae consistent with CIT visualized in transgastric (Figure 3, Video 1) and midesophageal (Figure 4A, Video 2) views. Severe tricuspid regurgitation was noted as well, possibly related to the displacement of the chordae as a result of the entangled clot (Figure 4B, Video 3). The main, right, and left pulmonary arteries were visualized without a clot identified (Figure 5). Tissue plasminogen activator was administered given high suspicion for massive PE in the setting of a CIT, right ventricular dilatation, and shock, with a total dose of 100 mg over 2 hours. Repeat TEE performed 3 hours later demonstrated complete resolution of the thrombus (Figure 6, Video 4). The two working hypotheses were that the clot dissolved or embolized to the pulmonary artery.
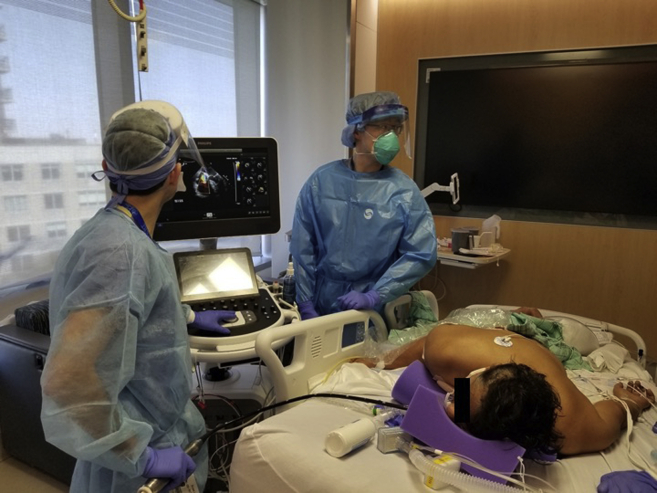
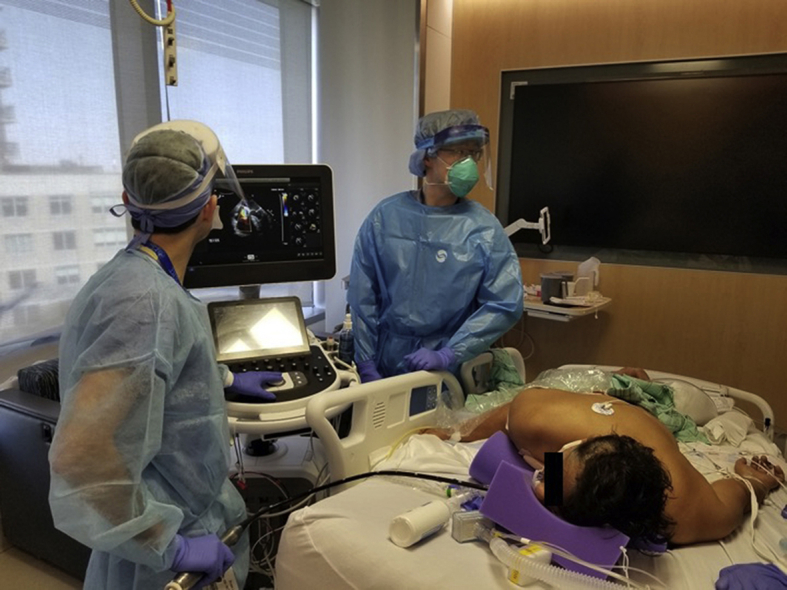
Figure 2.
Performing TEE in this patient, who is mechanically ventilated in a prone position.
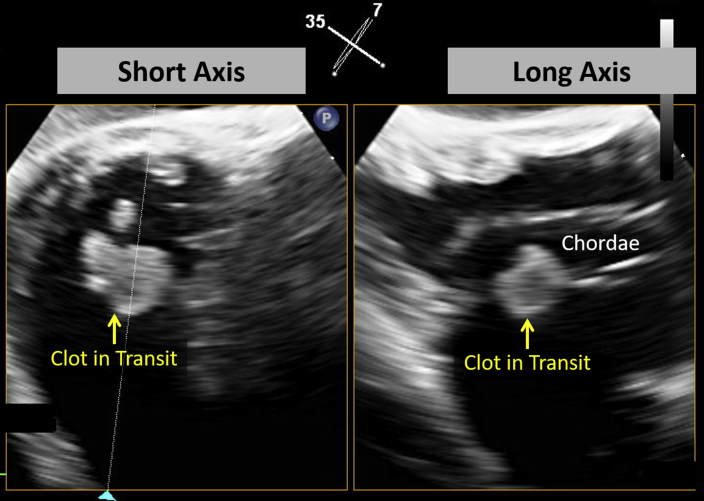
Figure 3.
Simultaneous biplane transgastric TEE demonstrates a CIT entrapped in the subvalvular apparatus in short- and long-axis views of the tricuspid valve. See Video 1.
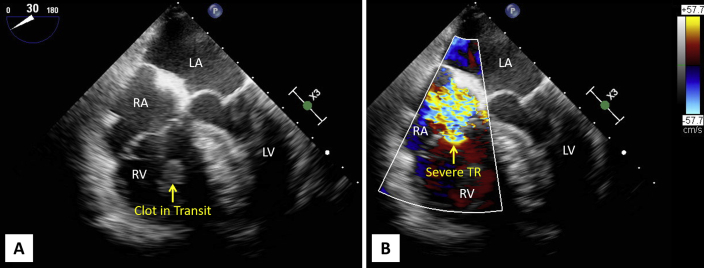
Figure 4.
(A) Midesophageal four-chamber view demonstrates a markedly dilated right ventricle (RV) with CIT entrapped in the subvalvular tricuspid valve apparatus. See Video 2. (B) Midesophageal four-chamber view demonstrates severe tricuspid regurgitation (TR). See Video 3. LA, Left atrium; LV, left ventricle; RA, right atrium.
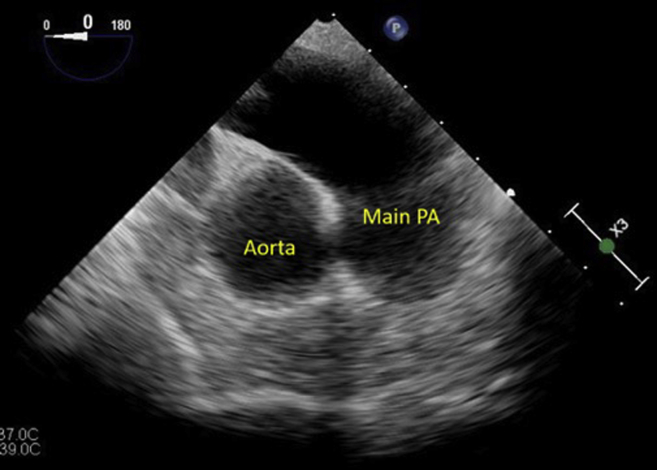
Figure 5.
Transesophageal echocardiographic view of the main pulmonary artery (PA).
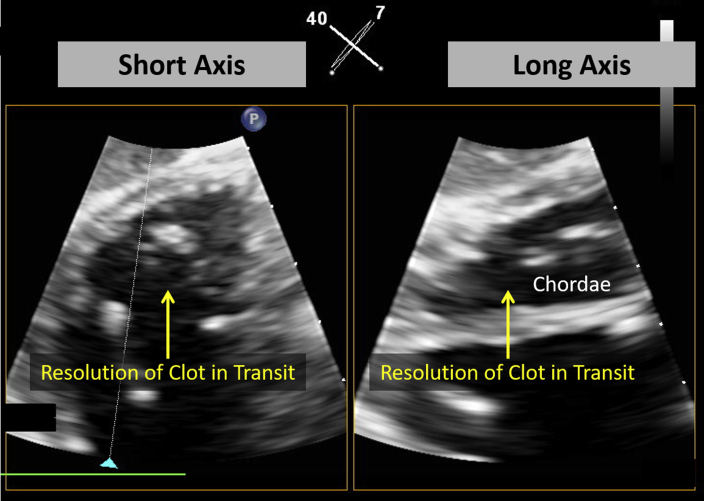
Figure 6.
Simultaneous biplane transgastric transesophageal echocardiographic views demonstrate complete resolution of CIT after tissue plasminogen activator administration. See Video 4.
A heparin infusion was initiated, and the patient remained on mechanical ventilatory support for ARDS with persistent severe hypoxemia as well as worsening shock requiring escalating vasopressor therapies over the next 16 hours. While being transitioned to a supine position, he sustained asystolic cardiac arrest thought to be related to recurrent PE (i.e., CIT embolization) or acute hypoxemia during repositioning from ARDS. Despite resuscitative efforts, the patient expired. An autopsy was offered to the family but declined.
Discussion
Venous thromboembolic events in patients with COVID-19 are thought to be the result of inflammation, hypoxia, and immobilization. Emerging pathologic evidence suggests that endothelial dysfunction, dysregulated inflammation, and thrombotic microangiopathy contribute to the pathologic process.1 The incidence of thrombotic complications among patients with COVID-19 is high. In a study of 184 intensive care unit patients with COVID-19 pneumonia, 31% had thrombotic events despite prophylactic dose anticoagulation, with PE being the most frequent (81% of thrombotic events).2 In case reports, PE has been noted among patients with COVID-19 pneumonia in the absence of other predisposing factors.3,4 Leonard-Lorant et al.5 recently demonstrated the incidence of PE among COVID-19 patients to significantly exceed the rate of PE among critically ill patients without COVID-19 (30% vs 1.3%).
d-dimer, a marker of fibrin formation and degradation, is elevated in conditions associated with thrombosis. Elevated d-dimer has been strongly linked with increased mortality among patients with COVID-19 infection.6, 7, 8 It is postulated that elevated d-dimer levels may identify individuals at highest risk for embolic events. Therefore, it is common practice at many institutions to initiate therapeutic anticoagulation for patients with COVID-19 pneumonia and significantly elevated d-dimer levels. How best to identify those at the highest risk for venous thromboembolism remains unknown.
The constellation of findings of elevated d-dimer, worsening hypoxemia, and shock led to a strong suspicion for high-risk PE. Although computed tomography would have been the optimal imaging modality to further evaluate for this concern, the patient's respiratory and hemodynamic instability created an unsafe scenario for patient transport. Prior studies have demonstrated the feasibility of prone TEE; in a report of 34 patients undergoing TEE in prone position in the setting of ARDS, standard views could be obtained in all but one patient.9
CIT is uncommon, occurring in about 4% of unselected patients with PE, but is associated with a considerable mortality rate of 27% to 45%.10,11 In patients with right ventricular dysfunction, CIT raises significant suspicion for PE. Although TEE is not the modality of choice for the diagnosis of PE, our choice of imaging modalities was limited, and TEE was further used to characterize chamber size and function to assist with the diagnosis. TEE can be instrumental in differentiating suspected CIT from other structures, such as a Chiari network, a Eustachian valve, or intracardiac tumors.12 Optimal therapy for CIT is not defined, as most data are based on case series or registry results. However, data from a meta-analysis suggest superior results with thrombolytic therapy in these patients compared with alternative treatment modalities such as systemic anticoagulation or surgical embolectomy.13 Our patient was hemodynamically unstable with a presumed diagnosis of PE, and treatment with tissue plasminogen activator was indicated. In general, thrombolytic therapy has been reported to lead to complete dissolution of CIT in <2 hours in 50% of cases, while the remaining reach full resolution within 24 hours of treatment.14 In an analysis of 177 patients with CIT, the mortality rate was 11.3% among patients receiving thrombolytics compared with 27.1% among the entire cohort treated with other therapeutic modalities such as heparin or surgical embolectomy.13 We hypothesize that the rapid deterioration and death of our patient was related to recurrent PE or acute hypoxemia related to ARDS.
Conclusion
Although the incidence of thrombotic complications among patients with COVID-19 is high, how best to identify the most high-risk individuals remains unknown. Our case highlights the need to maintain a high index of suspicion for PE in cases of COVID-19 with unexplained hemodynamic instability or respiratory decompensation. TEE can be performed emergently in such scenarios to gather a plethora of information, such a chamber size and function, and to evaluate for severe valvular lesions. Clear visualization of the pulmonary artery and rapid definition of the CIT were added benefits of this imaging modality in our case.
Footnotes
Conflicts of interest: Dr. Horowitz receives funding from Inari Medical.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2020.05.007.
Supplementary Data
Simultaneous biplane transgastric transesophageal echocardiographic views demonstrate a CIT entrapped in the subvalvular apparatus.
Midesophageal four-chamber view demonstrates a markedly dilated right ventricle with CIT entrapped in the subvalvular tricuspid valve apparatus.
Midesophageal four-chamber view demonstrates severe tricuspid regurgitation.
Simultaneous biplane transgastric transesophageal echocardiographic views demonstrate complete resolution of CIT after tissue plasminogen activator administration.
References
- 1.Leisman D.E., Deutschman C.S., Legrand M. Facing COVID-9 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. https://link.springer.com/article/10.1007/s00134-020-06059-6 Intensive Care Med. Available at: [DOI] [PMC free article] [PubMed]
- 2.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. https://www.thrombosisresearch.com/article/S0049-3848(20)30120-1/pdf Thromb Res. Available at: [DOI] [PMC free article] [PubMed]
- 3.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imaging. 2020;2:e200067. doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to d-dimer levels. https://pubs.rsna.org/doi/10.1148/radiol.2020201561 Radiology. Available at: [DOI] [PMC free article] [PubMed]
- 6.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Bai H., Chen X., Gong J., Dengju L., Sun Z. Anticoagulation treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mekontso-Dessap A., Proost O., Boissier F., Louis B., Roche-Campo F., Brochard L. Transesophageal echocardiography in prone position during severe acute respiratory distress syndrome. Intensive Care Med. 2011;37:430–434. doi: 10.1007/s00134-010-2114-z. [DOI] [PubMed] [Google Scholar]
- 10.Lai E., Alishetti S., Wong J.M., Delic L., Egrie G., Rosenblatt A. Right ventricular thrombus in transit: raising the stakes in the management of pulmonary embolism. CASE (Phila) 2019;3:272–276. doi: 10.1016/j.case.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konstantinides S.V., Myer G., Becattini C., Bueno H., Geersing G.J., Huisman M.V. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 12.Saric M., Armour A.C., Arnaout S., Chaudhry F.A., Grimm R.A., Kronzon I. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr. 2016;29:1–42. doi: 10.1016/j.echo.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Rose P.S., Pubjabi N.M., Pearse D.B. Treatment of right heart thromboemboli. Chest. 2002;121:806–814. doi: 10.1378/chest.121.3.806. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari E., Benhamou M., Berthier F., Baudouy M. Mobile thrombi of the right heart in pulmonary embolism: delayed disappearance after thrombolytic treatment. Chest. 2005;127:1051–1053. doi: 10.1378/chest.127.3.1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Simultaneous biplane transgastric transesophageal echocardiographic views demonstrate a CIT entrapped in the subvalvular apparatus.
Midesophageal four-chamber view demonstrates a markedly dilated right ventricle with CIT entrapped in the subvalvular tricuspid valve apparatus.
Midesophageal four-chamber view demonstrates severe tricuspid regurgitation.
Simultaneous biplane transgastric transesophageal echocardiographic views demonstrate complete resolution of CIT after tissue plasminogen activator administration.