Abstract
Spring and Summer 2020 are unique in that the challenges of care for those suffering from pollen allergy coincide with the COVID-19 pandemic. Several considerations are important to allow optimal care of allergic rhinitis (AR) and asthma and hence prevention of coronavirus spread through sneezing, rhinorrhoea, and coughing.
This compact overview of recommendations by the EUFOREA expert teams on allergic airway diseases and allergen-specific immunotherapy (AIT) is based on investigation of the current COVID-19 literature in association with the key words above and shared clinical experience of the experts involved. It deals with similarities and differences between AR and coronavirus infection, specific recommendations for allergic disease care in the COVID-19 era, including guidance on AIT.
Keywords: Allergy, Rhinitis, Asthma, Treatment, Control, COVID-19, Symptoms, Smell and taste, Allergen-specific immunotherapy
Abbreviations: EUFOREA, European Forum for Research and Education in Allergy and Airway Diseases; COVID-19, coronavirus disease 2019; AR, allergic rhinitis; SAR, seasonal allergic rhinitis; INS, intranasal corticosteroids; AIT, allergen-specific immunotherapy; NSAID, non-steroidal anti-inflammatory drug
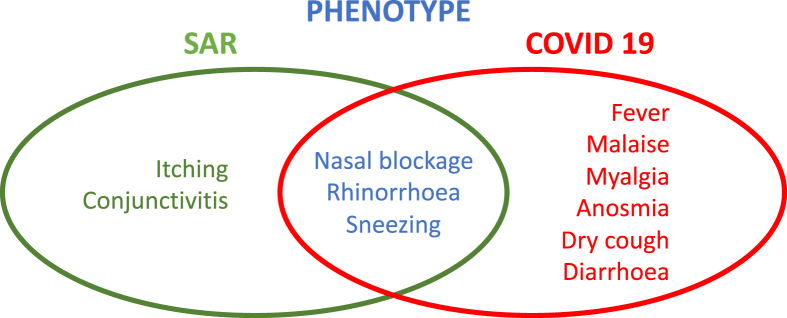
Acute COVID-19 infection and onset of seasonal allergic rhinitis (SAR) share a few similarities in their phenotype, but also differences. Whereas COVID-19 commonly presents as a flu-like illness with fever and persistent cough as its main symptoms, there is evidence of milder disease, especially in younger people. According to the World Health Organisation (WHO), some patients may also have a runny nose, sore throat, nasal congestion, and aches and pains or diarrhoea. Some suffer profound sudden and complete loss of smell and taste.1 About 80% of COVID-19 sufferers experience a mild case – of similar severity to a common cold – and recover without needing any special treatment.2 This clinical presentation might be confused with SAR, especially in those new to such symptoms. Fig. 1 shows the overlap and the major differentiating features. Cough and fever are the most prominent symptoms of COVID-19, whereas conjunctivitis and itching point to allergic rhinitis (AR) as the diagnosis.
Fig. 1.
Similarities and differences between seasonal allergic rhinitis and COVID- 19 symptoms
Given the global COVID-19 threat to humankind in spring 2020 and the fact that 44% of all transmission occurs from asymptomatic people,3 it is very important to keep SAR under the best possible control so as to diminish symptoms, particularly those of sneezing, rhinorrhoea, and coughing, which could be responsible for viral spread to others via aerosol formation in those who do not realise that they also have COVID-19. SAR patients are strongly recommended to keep social distancing, to wear a mask to avoid aerosol formation when sneezing or speaking, to carry spare masks and tissues, and bin them safely after use. It would be sensible, where possible, for SAR patients to be tested for the SARS-CoV-2 virus, since they could be potent viral spreaders.
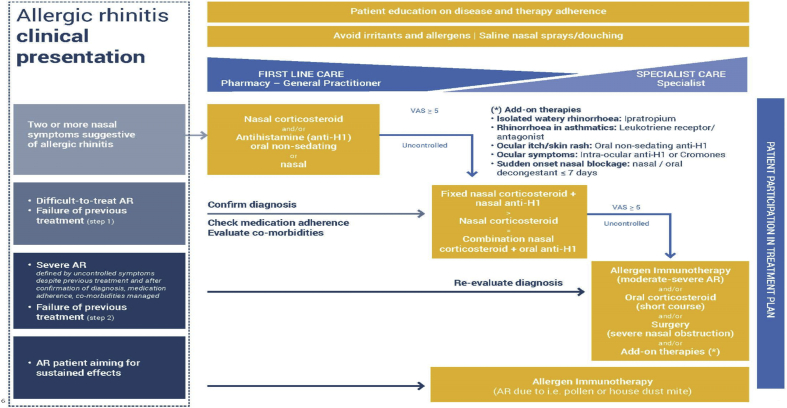
Therapy for SAR is best started early and used regularly throughout the relevant pollen season. None of the recommended treatments for SAR are contra-indicated, other than systemic corticosteroids. The EUFOREA treatment algorithm (Fig. 2) provides useful guidance which remains relevant. There is no contraindication to the use of intranasal corticosteroids (INS). This is the concerted view of over 90% of experts.4 INS do not reduce immunity; in fact they normalize the structure and function of the nasal mucosa5 and do not in vivo adversely affect mucociliary clearance.6 There are even preliminary data indicating that some corticosteroids, e.g., ciclesonide and mometasone, may suppress coronavirus replication.7 However, systemic corticosteroids should be avoided, as they may suppress the human immune system.
Fig. 2.
Treatment algorithm for AR as proposed by EUFOREA, taking into account the reality of patient phenotypes and existing international guidelines. www.euforea.org. Treatments suggested are possible during the COVID-19 pandemic with the exception of the use of systemic corticosteroids which should be avoided as they may be immunosuppressive. The symbol ">" means “more than”
Similarly, asthma inhalers should be continued as before and taken regularly, increasing the dose if needed to maintain control in the pollen season. Inhaled corticosteroids (ICS) and ICS combinations with bronchodilators, long acting beta agonists (LABA), are known to protect against virally-induced asthma exacerbations and may be beneficial in COVID-19.8 Also, treatment with biologicals for patients with more severe asthma and severe chronic rhinosinusitis with nasal polyps should be continued to avoid aggravation of these diseases as per guidelines.9 Although the U.S. Centers for Disease Conrol and Prevention (CDC) advises that, “People with moderate to severe asthma may be at higher risk of getting very sick from COVID-19. COVID-19 can affect your respiratory tract (nose, throat, lungs), cause an asthma attack, and possibly lead to pneumonia and acute respiratory disease”,10 asthma may not be a potent risk factor.8 It is already known that children, in whom respiratory allergy is common, often have very mild disease. In the first report from Wuhan, on 140 patients with severe COVID-disease, none of the patients reported AR or asthma despite a prevalence of AR of nearly 20% in China.11,12 More recently, an Italian report on 3200 COVID-19 positive deceased patients did not mention asthma among coexisting diseases.13
Ongoing allergen-specific immunotherapy (AIT) should be continued, if feasible within the health care system, as long as no COVID-19 infection has been diagnosed. New AIT treatment with subcutaneous injections, subcutaneous immunotherapy (SCIT), is not advised because of the necessity for repeated visits to a physician or hospital; however, the initiation of sublingual immunotherapy (SLIT), which mandates only one initial dose under supervision,14 should be preferred, or a switch to SLIT from SCIT considered, when there is a suitable alternative to SCIT for the allergen in question. AIT should be performed only with registered allergen products which have been proven to be efficacious.15
Management of COVID-19 is supportive, with experimental use of drugs, mainly in clinical trials which may include patients with allergic airway disease. Oral corticosteroids may be needed under supervision in COVID-infected asthma patients for severe virus-evoked exacerbations. Avoidance of non-steroidal anti-inflammatory drugs (NSAIDs), which was suggested for all possible patients,16 then revoked,17 remains vital in NSAID- sensitive asthma.
Take home messages
-
•
Early mild COVID-19 symptoms may be confused with or co-occurrent with AR.
-
•
Proper treatment of AR is very important at this time, as uncontrolled hay fever may increase the risk of viral dissemination.
-
•
Such therapy (including AIT) is not immunosuppressive and does not represent a risk factor for more severe COVID-19-induced disease.
-
•
Topical and inhaled corticosteroids may even be beneficial or preventative for COVID-19 infection.
-
•
Allergic airway disease is probably not a risk factor for more severe COVID-19 disease; however, asthma control can worsen with viral infections.
-
•
Sudden and complete anosmia may be an early sign of COVID-19 infection, differentiating it from AR.
The latest updates on treatment recommendations for SAR, AIT, and smell loss are summarized in expert videos disseminated via the EUFOREA social media channels (https://twitter.com/euforea), allowing the medical community to be updated on medical consensus and expert opinion regarding COVID-19 and respiratory allergy care.
Author contributions
PH had the original idea; the first draft was written by GS who then incorporated suggestions from all other authors.
Declarations of Competing Interest
Glenis Scadding has received fees for lectures and a rhinitis course from EUFOREA and from ALK- Abello, Bayer, GSK, Mylan.
The ENT department at Odense University Hospital has participated in a randomised controlled trial conducted by ASTRA-Zeneca: A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Phase 3 Efficacy and Safety Study Of Benralizumab in Patients with Severe Nasal Polyposis (OSTRO).
Funding
Not applicable.
Consent to Publish
All authors approve the ethics of this paper and give consent for publication.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Carol H., YanFaraji F., Prajapti D.P., Boone C.E., DeConde A.S. International Forum of Allergy & Rhinology; 2020. Association of Chemosensory Dysfunction and Covid-19 in Patients Presenting with Influenza-like Symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC COVID-19 Response Team Coronavirus disease 2019 in children - United States, february 12-april 2, 2020. MMWR Morb Mortal Wkly Rep. 2020 Apr 10;69(14):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He X., Lau E.H.Y., Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J., Akdis C., Jutel M. 2020. Intranasal Corticosteroids in Allergic Rhinitis in COVID-19 Infected Patients: An ARIA-EAACI Statement Allergy. [DOI] [PubMed] [Google Scholar]
- 5.Minshall E., Ghaffar O., Cameron L. Assessment by nasal biopsy of long-term use of mometasone furoate aqueous nasal spray (Nasonex) in the treatment of perennial rhinitis. Otolaryngol Head Neck Surg. 1998 May;118(5):648–654. doi: 10.1177/019459989811800514. PubMed PMID: 9591864. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg K., Pipkorn U. Influence of topical beclomethasone dipropionate suspension on human nasal mucociliary activity. Eur J Clin Pharmacol. 1986;30:625. doi: 10.1007/BF00542425. [DOI] [PubMed] [Google Scholar]
- 7.Halpin D.M.G., Singh D., Hadfield R.M. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020 doi: 10.1183/13993003.01009-2020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halpin D.M.G., Faner R., Sibila O., Badia J.R., Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8(5):436–438. doi: 10.1016/S2213-2600(20)30167-3. Published Online April 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.https://ginasthma.org/covid-19-gina-answers-to-frequently-asked-questions-on-asthma-management/
- 10.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/asthma.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fspecific-groups%2Fasthma.html accessed 15.4.20.
- 11.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China [published online ahead of print, 2020 Feb 19] Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Zhang L. Prevalence of allergic rhinitis in China allergy asthma. Immunol Res. 2014 March;6(2):105–113. doi: 10.4168/aair.2014.6.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Istituto Superiore di Sanità Characteristics of COVID-19 patients dying in Italy Report based on available data on March 20th 2020. https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20
- 14.Canonica W., Cox L., Pawankar R. Sublingual immunotherapy: world Allergy Organization position paper 2013 update. World Allergy Org J. 2014;7:6. doi: 10.1186/1939-4551-7-6. http://www.waojournal.org/content/7/1/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts G., Pfaar O., Akdis C.A. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018 Apr;73(4):765–798. doi: 10.1111/all.13317. [DOI] [PubMed] [Google Scholar]
- 16.https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Anti-inflammatoires-non-steroidiens-AINS-et-complications-infectieuses-graves-Point-d-Information
- 17.https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19