Highlights
-
•
We constructed a tool to estimate healthcare demand stemming from the COVID19 pandemic.
-
•
The tool can help local authorities examine the impacts of intervention strategies.
-
•
We apply the model to three different regions in Chile to illustrate its use.
-
•
A surge in COVID-19 patients could overwhelm Chilean hospitals by June.
-
•
Immediate aggressive action can avert much of the morbidity and mortality
Keywords: Model, COVID, Hospital, Capacity, Intervention, Social distancing
Abstract
Objectives
Public health officials need tools to assist in anticipating the healthcare resources required to confront the SARS-COV-2 pandemic. We constructed a modeling tool to aid active public health officials to estimate healthcare demand from the pandemic in their jurisdictions and to evaluate the potential impact of population-wide social-distancing interventions.
Methods
The tool uses an SEIR compartmental model to project the pandemic’s local spread. Users input case counts, healthcare resources, and select intervention strategies to evaluate. Outputs include the number of infections and deaths with and without intervention, and the demand for hospital and critical care beds and ventilators relative to existing capacity. We illustrate the tool using data from three regions of Chile.
Results
Our scenarios indicate a surge in COVID-19 patients could overwhelm Chilean hospitals by June, peaking in July or August at six to 50 times the current supply of beds and ventilators. A lockdown strategy or combination of case isolation, home quarantine, social distancing of individuals >70 years, and telework interventions may keep treatment demand below capacity.
Conclusions
Aggressive interventions can avert substantial morbidity and mortality from COVID-19. Our tool permits rapid evaluation of locally-applicable policy scenarios and updating of results as new data become available.
Introduction
On December 31, 2019, the regional office of the World Health Organization (WHO) was notified of a cluster of pneumonia cases of unknown origin associated with a market in Wuhan, China (Zhu et al., 2020). A novel coronavirus (SARS-COV-2) was identified as the cause of the infections (Zhu et al., 2020) and has since spread worldwide. As of May 7, 2020, more than 3.6 million cases of COVID-19 (illness caused by SARS-COV-2) have been reported in 184 countries and territories, including ∼250,000 deaths (Dong et al., 2020, World Health Organization, 2020). The pandemic has overwhelmed both national and local healthcare capacity in several countries (Ferguson et al., 2020, Kissler et al., 2020), and is projected to do so in many others. Low- and middle-income countries are particularly vulnerable (Walker et al., 2020), since financial and logistical challenges may hinder their ability to augment treatment capacity. As such, many countries have resorted to societal-wide social distancing interventions in the hopes of reducing morbidity and delaying the demand for healthcare resources, to gain time to increase treatment capacity.
Numerous modeling efforts have forecast the spread of the outbreak and examined the potential benefits of social-distancing interventions (Ferguson et al., 2020, Flaxman et al., 2020, Kissler et al., 2020, Walker et al., 2020). While informative, these efforts have been limited to specific nations and snapshots in time; public health officials are reliant on the authors for updated estimates as the pandemic evolves. Other internet-based tools offer public health users the ability to generate estimates on their own; however, these are limited in their practical utility because their assumptions and desired results may not match the specific needs of jurisdictions and public health decision-makers, or they require coding knowledge to access or modify the calculations (Penn Healthcare, 2020, Henderson, 2020). These considerations are more critical in low and middle-income countries, which may not have the resources to complete or modify such analyses on their own.
Therefore, we developed a modeling tool for use by practicing public health officials to estimate the future impact of the COVID-19 outbreak on demand for healthcare resources in their jurisdictions and to examine the costs and benefits of various intervention strategies. Once downloaded, the model can be used without an internet connection to assist public health officials in choosing locally appropriate intervention strategies and by how much to increase hospital treatment capacity. For illustration, we apply the model to Chile, a Southern Hemisphere country where the virus is generating local transmission and compare various intervention options in the three most affected regions of the country.
Methods
Tool overview
We created a spreadsheet-based tool (Supplementary Material S1) that uses a Susceptible-Latent-Infectious-Recovered (SEIR) Compartmental Model to project the future impact of a COVID-19 epidemic among any population of interest. The model requires information that is typically available to public health officials, including the number of cases in their jurisdiction, the size and demographics of their at-risk population, healthcare capacity, expectations for healthcare use, and societal-wide choices of social-distancing mitigation strategies that users wish to evaluate. Model outputs reflect the potential demand on the healthcare system due to severely ill individuals with and without user-specified mitigation strategies, as well as deaths averted through treatment and excess deaths due to healthcare demand exceeding capacity. The demand for healthcare resources is measured as the estimated number of COVID-19 patients requiring critical-care or Intensive Care Unit (ICU) beds, hospital beds (non-ICU), and mechanical ventilators throughout the outbreak and the maximum occupancy at the outbreak's peak. The tool offers users the ability to evaluate various intervention strategies currently under consideration or in use worldwide (Ferguson et al., 2020, Kissler et al., 2020, Willem et al., 2020). There are five mitigation-type interventions which focus on slowing the epidemic spread and reducing its burden on the healthcare system, and one suppression-type strategy, which employs aggressive interventions aimed at reversing epidemic growth (Table 1 ).
Table 1.
Intervention strategies and effects on onward transmission.
| Strategy name | Reduction in R0a |
||
|---|---|---|---|
| (Strategy type) | Description | Lowb | Highb |
| Case isolation (mitigation) | Symptomatic cases stay at home for 7 days, reducing non-household contacts during this period. Household contacts remain unchanged. | 15.8% | 18.6% |
| Closing schools and universities + telework (mitigation) | Closing Schools/Universities: Physical closure of all schools and universities (or move to a virtual learning environment). Assumes some increase in contacts in the household and the community during the closure, partially offsetting reductions in transmission at schools and universities. | 15.8% | 16.8% |
| Telework: All government switches to telework to the maximum extent possible, and private businesses are encouraged to telework, resulting in 50% of the working population teleworking. | |||
| Case isolation + household quarantine (mitigation) | Case isolation: same as above | 25.4% | 30.0% |
| Household quarantine: After identifying a symptomatic case in the household, all household members voluntarily remain at home for 14 days. Increased transmission between household members during the quarantine period will partially offset transmission reductions in the community. | |||
| Case isolation + household quarantine + social distancing of >70 s + telework (mitigation) | Case isolation: same as above | 41.9% | 47.7% |
| Household quarantine: same as above | |||
| Social Distancing of >70 s: Reduce contacts among older individuals (>70 years of age) because of their increased risk for severe outcomes and healthcare resource requirements. These individuals reduce contacts outside the home by 50%. | |||
| Telework: same as above | |||
| Lockdown (suppression) | Population-wide social distancing by forced quarantine of all households and workplaces and the border closed to travel. Only essential outings from home are permitted (e.g., food/supplies purchases) and for employees working at businesses deemed essential for continued operation. | 57.7% | 68.2% |
R0 = basic reproduction number. It represents the average number of people who will be infected by any given infected person at the early stages of disease spread when there are no control measures.
High and Low values of the reduction in transmission associated with each strategy were used to account for uncertainty in societal compliance and strategy effectiveness. These reductions were based on equivalent reductions in Critical Care Bed Occupancy published in Ferguson et al. (2020) (Supplementary Material S2). We added 10 percentage points to reduction values for strategies including telework, based on Willem et al. (2020).
Users can readily update all input values as new data become available or reflect a jurisdiction’s specific epidemiologic profile of disease and policy considerations. All calculations can be readily modified by users (although no modifications are necessary for tool use).
Calculations
Transmission with and without intervention
Our SEIR model tracks the number of individuals transitioning between disease states every day of the outbreak. The initial number of susceptible individuals is set as the population minus the cumulative number infected since the outbreak’s start. Transmission occurs through contacts between susceptible and infectious individuals, and we assume an equal probability any one person has contact with another (“homogenous mixing”). We also assume transmission chains generated by infected travelers entering the population are minimal compared to existing transmission in the community. As a result, the number of new infections each day is the product of the proportion of the population that is susceptible, the number of infectious persons on a given day, and the average number of new infections each infected person causes over the course of their illness (the reproduction number; hereafter, "R") divided by the duration (in days) of the average infectious period. Infectiousness is assumed to occur five days after infection (Lauer et al., 2020) and lasts 11 days (You et al., 2020). Upon recovery from infection, individuals are assumed immune to re-infection during the timespan modeled (through December 2020). In the absence of intervention R is 2.0 (low estimate) and 2.8 (high estimate), approximately spanning the middle 50% of the gamma distribution of R (95% intervals: 1.4-3.8) estimated from the initial growth rate of the epidemic in Wuhan (Li et al., 2020, Riou and Althaus, 2020). To account for uncertainty in R, all results are depicted as a range based on these low and high estimates for R. During periods when interventions are applied, we reduce R's low and high estimates by the values in Table 1. Upon mitigation concluding, R returns to pre-mitigation levels to illustrate the potential consequences of shorter duration interventions. However, advanced users can alter the tool so that when one mitigation strategy concludes, another begins. Finally, we do not account for any vaccine, as it is only likely to be available beyond the modeled time frame (Li and De Clercq, 2020, Nature, 2020). All equations governing the dynamics of the system are provided in the supplementary material.
Hospitalizations and ICU admissions with and without intervention
In our model, all symptomatic persons with an illness severe enough to warrant hospitalization will seek healthcare, and the risk for hospitalization is age-dependent (Table 2 ) (Verity et al., 2020). Similarly, the percentages of individuals admitted to the hospital requiring ICU care and fatality are also age-dependent (Table 2), while the likelihood of patients admitted to the ICU who require mechanical ventilation is assumed the same (63.2%) for all ages (ICNARC, 2020).
Table 2.
Risk of healthcare use and outcomes among infected.
| Age group | % Infected, Hospitalizeda | % of Hospitalized, Admitted to ICUa | % ICU patients needing ventilationb | Infection fatality ratio (IFR)a | Fatality increase if demand > capacityc |
|---|---|---|---|---|---|
| 0–9 | 0.01% | 5.0% | 63.2% | 0.002% | 1.000% |
| 10–19 | 0.04% | 5.0% | 63.2% | 0.006% | 1.000% |
| 20–29 | 1.10% | 5.0% | 63.2% | 0.030% | 1.000% |
| 30–39 | 3.40% | 5.0% | 63.2% | 0.080% | 1.000% |
| 40–49 | 4.30% | 6.3% | 63.2% | 0.150% | 1.000% |
| 50–59 | 8.20% | 12.2% | 63.2% | 0.600% | 1.000% |
| 60–69 | 11.80% | 27.4% | 63.2% | 2.200% | 1.000% |
| 70–79 | 16.60% | 43.2% | 63.2% | 5.100% | 1.000% |
| 80+ | 18.40% | 70.9% | 63.2% | 9.300% | 1.000% |
Based on ICNARC (2020). Alternative estimates include 60% (Meltzer et al., 2015) and 71.1% (Yang et al., 2020).
Percentage points increase in fatalities when hospitals are overwhelmed. We assumed a 1% increase in the IFR to approximately double the population-weighted age-based IFR in Chile, based on data from COVID19 in China (Zhang et al., 2020).
Based on observations for COVID19, we assume individuals seeking hospital care do so 11 days after infection (five days incubation + six days of symptoms) (Chen et al., 2020, Li et al., 2020, Linton et al., 2020; Zhang et al., 2020). We calculate hospital (non-ICU) bed occupancy based on a ten-day length of stay for patients treated entirely in non-critical hospital wards (Deng et al., 2020, Wang et al., 2020) and ICU bed occupancy based on a ten-day length of stay when critical care is required (Verity et al., 2020; Zhang et al., 2020). We assume a four day lag from hospital admission to ICU admission (Wang et al., 2020; Zhang et al., 2020). When mechanical ventilation is required, we assume the duration of use is nine days, based on expert clinical opinion that ventilation is necessary for the length of ICU stays other than two days (one-day lag post ICU admission to initiate ventilator use plus 1 day in the ICU post-use) and another day required for ventilator cleaning/re-equipping.
To estimate the impact of interventions on hospital resource requirements we calculate two measures for each of the three resources tracked in the model: 1) the reduction in peak occupancy between the projected outbreak without intervention and when interventions are employed, and 2) the number of weeks peak occupancy is delayed due to employed interventions.
Deaths with and without intervention
We assume all deaths occur in the hospital unless treatment capacity is overwhelmed and that it takes the same time for an individual to recover as to die, despite some preliminary evidence that deaths occur faster (Deng et al., 2020, Linton et al., 2020). As such, we might be overestimating the healthcare resources needed to treat the most critical patients (namely ventilators). Given the limited evidence for outcome-based durations of resource use, we took a more conservative approach, assuming planners would prefer to overestimate resources needs than under-prepare.
With treatment, fatality among infected (IFR) is age-dependent (Verity et al., 2020) (Table 2). When hospital capacity is overwhelmed, we assume a 1% increase in the IFR, chosen to approximately double the IFR in Chile, based on the observed reduction in IFR in China after treatment capacity was augmented to meet demand (Zhang et al., 2020). We also chose to base our mortality increase for untreated COVID19 patients on hospital bed availability (versus critical care beds or ventilators) since the vast majority of cases do not require critical care, ∼90% of Chilean cases. When more data become available, these assumptions can be updated. Finally, we assume when beds become free at overwhelmed hospitals, new admissions are not associated with a patient's potential outcome.
To estimate the impact of interventions on deaths, we calculate infection fatality rates with and without interventions and the number of estimated deaths averted, as the difference in our estimates of cumulative deaths with and without interventions.
Illustrative scenarios and sensitivity analyses
To illustrate the model, we estimated the impact of implementing three intervention strategies in three regions of Chile with the most detected cases through April 6, 2020: Región Metropolitana (RM), an urban region with the largest population including the country’s capital Santiago, and Araucanía and Ñuble, two of the least dense urban regions in Chile, but which had experienced rapid growth in late March and were reporting treatment capacity was already strained. We implemented the following three intervention strategies (Table 1) in each region, beginning April 1: Strategy 1) Closure of schools and universities and telework for eight months; Strategy 2) Case isolation, home quarantine, social distancing of individuals >70 years, and telework for eight months; and Strategy 3) Lockdown for two months (six months shorter duration than the other strategies because the social and economic costs of this suppression strategy are not considered sustainable for the long-term). We chose these strategies because they are in use to some degree in all three regions (Ministry of Health, 2020b, Ministry of Health, 2020c) (Supplementary Material 2).
We conducted two sensitivity analyses to evaluate the effectiveness of varying the implementation of mitigation strategies. First, we assessed the influence of shortening the duration (by two, four, and six months) of mitigation strategies, which successfully reduced our healthcare demand estimates to within the range of treatment capacity. This analysis was chosen since policymakers may be pressured to lift mitigation strategies as early as possible due to their social disruption and economic costs. Next, we evaluated the impact of combining the Lockdown strategy with all other strategies, so that when the Lockdown strategy ends, another mitigation strategy begins and lasts six months. This analysis is intended to address the potential for the outbreak to rebound in the absence of intervention after a lockdown is lifted (Ferguson et al., 2020, Kissler et al., 2020).
Results
Infections and deaths without intervention
We estimate in the absence of any interventions, 5,682,168 to 6,592,016 infections would occur throughout the epidemic period modeled (5/5–12/31/20) in RM, 766,015 to 889,054 infections in Araucanía, and 384,509 to 446,285 infections in Ñuble (Figure 1 and Supplementary Material S2). These projected counts reflect the possibility that 80% to 93% of the population in these regions may be infected in the absence of any control measures or changes in individual behaviors. Under this scenario, the number of deaths is projected to be between 106,558 to 125,373 in RM (1.9% IFR), 13,860 to 16,378 deaths in Araucanía (1.8% IFR), and 7,247 to 8,520 deaths in Ñuble (1.9% IFR).
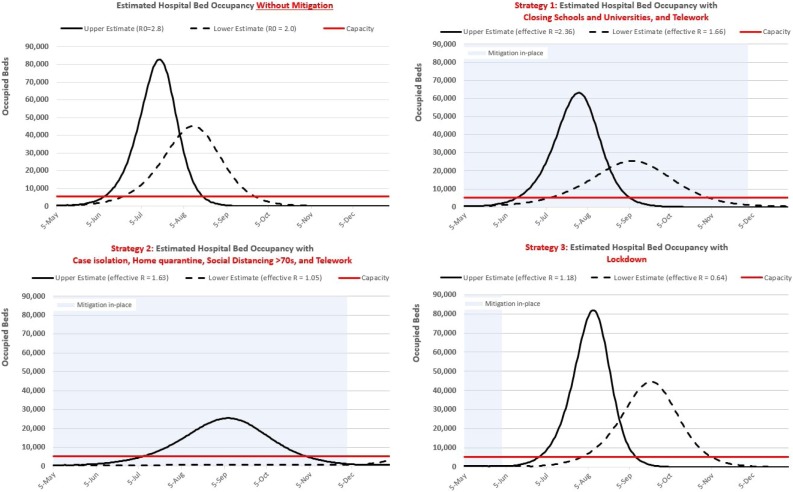
Figure 1.
Projected occupancy demands and capacity for hospital (non-ICU) beds in Región Metropolitana with and without intervention.
Notes. Solid curves: projections using the high estimate for the reproduction number. Dashed curves: projections using the low estimate for the reproduction number. Table 1 contains all reproduction numbers. Horizontal red line: Hospital bed capacity. Blue shaded region: interventions in place.
Hospital resource demands with and without interventions
Without intervening to control the outbreak, demands for all three of the healthcare resources evaluated by our model are projected to exceed capacity sometime in June in RM (Figure 1 and Supplementary Material S2), and peak sometime between the end of July and mid-August. Araucanía and Ñuble are projected to exceed capacity in July and peak sometime in August or September (approximately one month after RM on both metrics). The degree to which demand is projected to exceed supply differs by region. In RM, peak demand across all resources is six to 18 times the projected maximum available supplies. The situation is similar in Araucanía and Ñuble for hospital beds and ventilators but is more dire for ICU beds: in both regions, the unmitigated peak ICU bed demand is between 13 and 47 times greater than the supply.
Among the two mitigation strategies we evaluated (versus the suppression-type Lockdown strategy), Strategy 2 reduced the healthcare system's burden the most. In RM, compared to the no intervention scenario, this strategy reduced peak hospital bed occupancy demands by a range of 16,024 to 57,225 (35.4-69.2%), ICU bed occupancy between 2,945 to 8,270 beds (44.8-69.0%), and the number of ventilators needed by 1,572 to 4,237 (47.1-69.3%). This strategy would also push the peak demand for healthcare resources back between seven and 27 weeks, affording policymakers more time to plan or acquire more capacity. Larger percent reductions but similar delays in the peak demand were observed for Araucanía and Ñuble (Supplementary Material S2).
Our results suggest that this strategy can ease the demands for healthcare to levels below projected capacity constraints when the effectiveness of this strategy is at the higher end of our assumed range (i.e., reductions in R0 approach 47.7%) (Table 1, Figure 1).
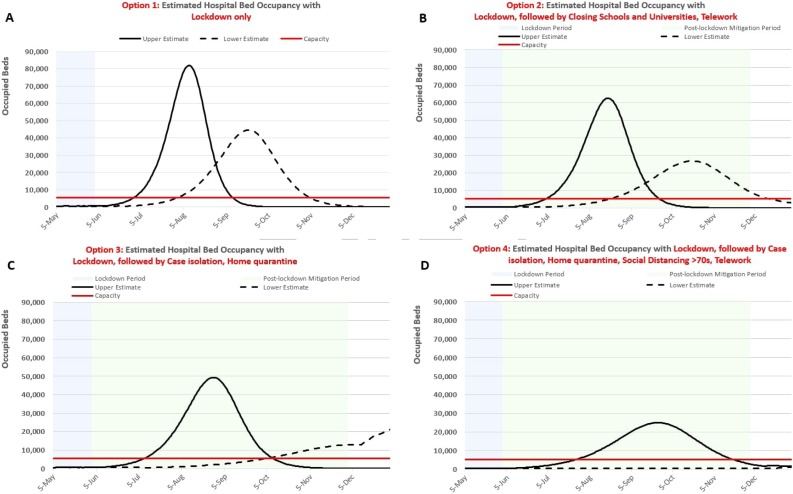
For policymakers willing to consider more restrictive measures, our results for the Lockdown strategy suggest that it is an incredibly effective strategy, even for a short duration. The pandemic is quickly subdued and remains so for the duration of the lockdown period in all three regions, with the numbers of cases in treatment remaining relatively flat at levels well below treatment capacity. However, once the lockdown is lifted, the number of infected individuals begins to rise again, resulting in demand curves similar in size to the no-intervention scenario but peaking later, sometime between mid- August and late September (Figure 3, panel A).
Figure 3.
Sensitivity Analysis: Effects of a 2 month Lockdown Suppression Strategy alone (A) and followed by various mitigation strategies for 6 months on Hospital Bed Occupancy Demands: Closing Schools and Universities + Telework (B), Case Isolation + Household Quarantine (C), and Case isolation, Household Quarantine, Social Distancing of >70 years of age, and Telework (D).
Notes: Solid and dashed curves reflect uncertainty in the effectiveness of intervention strategies during both the Lockdown period and Post-lockdown intervention period per Table 1.
Deaths averted with intervention
Based on the projected capacity to treat COVID patients in each region, the deaths resulting from patients being unable to obtain healthcare were 53,515 to 63,836 in RM, 7,130 to 8,567 in Araucanía, and 3,657 to 4,354 in Ñuble. With Strategy 2, the estimated number of deaths averted in RM ranged from 39,006 to 79,233 (36.6-63.2%), between 4,885 and 12,622 in Araucanía (35.2-77.1%), and 2,018 to 6,742 in Ñuble (27.8–79.1%) (Table 4). Lockdown eliminates between 99.8% and 99.9% of deaths in all three regions during the lockdown period, but deaths rise afterward with the subsequent rebound of transmission.
Table 4.
Deaths averted by each intervention strategy and region (compared to deaths without intervention) between May 5, 2020 and December 31, 2020.
| Intervention strategya | Metropolitana | Araucanía | Ñuble |
|---|---|---|---|
| Strategy 1: School closures, telework | 7,612–20,725 (7.1–16.5%) |
1,019–2,707 (7.4–16.5%) |
518–1,288 (7.1–15.1%) |
| Strategy 2: Case isolation, home quarantine, social distancing>70, telework | 39,006–79,233 (36.6–63.2%) |
4,885–12,622 (35.2–77.1%) |
2,018–6,742 (27.8–79.1%) |
| Strategy 3: Lockdownb | 106,381–125,140 (99.8–99.8%) |
13,855–16,372 (99.9–99.9%) |
7,706–8,274 (99.9–99.9%) |
Sensitivity analyses
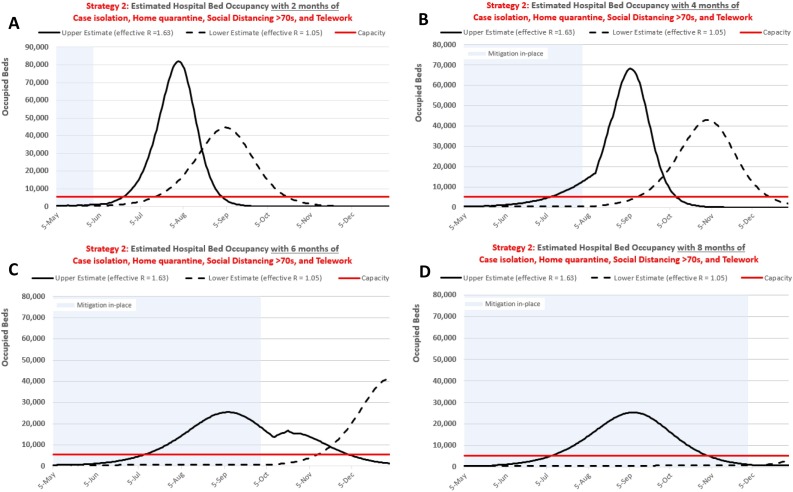
Figure 2 depicts the effects of shortening the duration of intervention Strategy 2 on hospital bed occupancy demands in RM over two (A), four (B), and six (C) months of implementation versus our initial eight-month (D) duration. Similar to our baseline results for Strategy 3 (Lockdown), these results show that the effectiveness of interventions depends upon the length of time they overlap the epidemic period. Specifically, if too many susceptible individuals remain (i.e., insufficient herd immunity) at the time interventions are lifted, transmission will return. Even when interventions are less effective (R is higher), if the intervention remains in place past peak demand, the resulting outbreak may be smaller than when the same strategy is more effective (R is lower) but lifted before peak demand (Figure 2, Panel C).
Table 3.
Model inputs by region for all illustrative scenarios.
| Region |
||||
|---|---|---|---|---|
| Metropolitana | Araucanía | Ñuble | Source | |
| Populationa | 7,112,808 | 957,224 | 480,609 | Instituto Nacional de Estadísticas (2017) |
| COVID-19 reported casesb | ||||
| Cumulative | 20,590 | 1,907 | 1,107 | Ministry of Health (2020a) |
| 2 weeks through 05/04/20 | 12,487 | 364 | 133 | Ministry of Health (2020a) |
| R0 | 2.0–2.8 | 2.0–2.8 | 2.0–2.8 | Riou et al. (2020); Li et al. (2020) |
| Intervention strategy | ||||
| School closures, telework | 4/1–12/1/20 | 4/1–12/1/20 | 4/1–12/1/20 | Assumed |
| Case isolation, home quarantine, social distancing>70, telework | 4/1–12/1/20 | 4/1–12/1/20 | 4/1–12/1/20 | Assumed |
| Lockdown | 4/1–6/1/20 | 4/1–6/1/20 | 4/1–6/1/20 | Assumed |
| Disease severity | ||||
| Infected who are hospitalizedc (%) | 4.5% | 4.8% | 5.1% | Verity et al. (2020) |
| Hospitalized, admitted to ICUc (%) | 11.4% | 12.2% | 12.7% | Verity et al. (2020) |
| Infection Fatality ratec (%) | 0.8% | 0.9% | 0.9% | Verity et al. (2020) |
| ICU patients needing ventilator (%) | 63.2% | 63.2% | 63.2% | ICNARC (2020) |
| Healthcare resourcesd | ||||
| Hospital (non-ICU) beds | 18,522 | 2,671 | 1,010 | Latorre and Sandoval (2020) |
| In-use by Non-COVID Patients (%) | 71% | 71% | 71% | OECD et al. (2019) |
| In-use by COVID Patients (%)e | 3% | 3% | 3% | Ministry of Health (2020a) |
| Critical Care Beds | 2,326 | 215 | 60 | Latorre and Sandoval (2020) |
| In-use by Non-COVID Patients (%) | 71% | 71% | 71% | OECD et al. (2019) |
| In-use by COVID Patients (%)e | 14% | 14% | 14% | Ministry of Health (2020a) |
| Ventilators | 867 | 80 | 22 | Latorre and Sandoval (2020) |
| In-use by Non-COVID Patients (%)f | 40% | 40% | 40% | Wunsch et al. (2013) |
| In-use by COVID Patients (%)g | 19% | 19% | 19% | Assumed |
Population distributed by age groups are shown in the Supplementary Material S2, based on INE’s Housing and Population Census 2017 Instituto Nacional de Estadísticas (2017).
Scaled counts to account for assumed 40% under-reporting in reported cases (based on 60% reported by Pan et al., 2020 minus 20% to account for improvements in case-detection in Chile since the outbreak’s start).
Estimates differ by region due to age structure of the populations (Supplementary Material S2).
All beds available in the healthcare system, from public and private hospitals, are now part of the “Sistema Integrado COVID-19" under the centralized administration of the Ministry of Health. An intensive care bed (ICU) consists of a cot with a monitor, healthcare professionals, and medication to treat patients. Some have a mechanical ventilator. There are an estimated 1,847 mechanical ventilators; 850 currently available and 997 were acquired in January 2020. Latorre and Sandoval (2020) We assumed the distribution of mechanical ventilators was proportional to the number of critical beds in each region: Metropolitana, 47.0%; Araucanía, 4.3%; Ñuble, 1.2%. (Supplementary Material S2)
Based on the reported number hospitalized in "basic beds" (1,216) and in "critical care beds" (699) in all of Chile by the Ministry of Health as of May 4, 2020, out of the total existing beds nationally in March 2020 plus anticipated beds being added to expand pandemic treatment capacity: 41,706 and 4,954, respectively. Latorre and Sandoval (2020).
Availability of mechanical ventilators was based on a three-year study of 97 ICUs in the US. (Wunsch et al., 2013).
Calculated by applying the % ICU patients needing ventilation (Table 2) to the number of COVID patients in critical care beds (see note e) and dividing the result by the total ventilators in Chile (see note f).
Figure 2.
Sensitivity analysis: Effects of the duration of intervention Strategy 2 (case isolation, home quarantine, social distancing of population >70 years of age, and telework) on hospital bed occupancy demands during the COVID-19 epidemic in Región Metropolitana when maintained for two (A), four (B), six (C), and eight (D) months (and initiated on April 1, 2020).
Notes: Solid and dashed curves reflect uncertainty in the effectiveness of intervention strategies (Table 1).
Figure 3 illustrates the results for combining a Lockdown suppression strategy with subsequent mitigation strategies in RM. The benefit of this approach is an additional one to two months delay in peak demand timing beyond delays afforded by each of the mitigation strategies on their own. This approach, however, has no effect on the amount of demand (i.e., peak demand is similar to the mitigation strategy without lockdown).
Discussion
In the absence of immunization, our illustrative results suggest the number of severely ill patients could overwhelm treatment capacity as early as late May to mid-June in all three regions of Chile we evaluated. Our projections also suggest that with immediate aggressive action to implement several combinations of interventions, the current number of hospital beds and critical care beds may be sufficient. In specific circumstances, regional authorities may find it easier to augment their current capacity (e.g., ventilators in Ñuble) along with some mitigation strategies to meet demand versus strictly burdening society with disruptive mitigations. However, policymakers should be aware that our results indicate that more effective intervention strategies at temporarily suppressing transmission can also result in more massive epidemics upon lifting the strategies, compared to less effective, longer-lasting strategies (in the absence of vaccine and changes in individual behavior). As such, it may be necessary to keep societal-wide interventions in place, or intermittently start and stop them again based on active monitoring of cases counts and treatment capacity, or until a vaccine, or a treatment that can be administered outside of the hospital setting are available.
While our projections are reasonable estimates for how the pandemic may play out given our current understanding of SARS-CoV-2, they should not be considered as forecasts of what will occur. This is due to the uncertainty in our knowledge of an outbreak that is still unfolding, the application of experiences of other countries to Chile (such as case severity, resource use by non-COVID patients, intervention effectiveness, compliance over time), simplifying assumptions (such as homogenous mixing), and case surveillance uncertainty. We assumed homogenous mixing to make implementing our model in a spreadsheet more tractable, but as a result, do not reflect potentially significant variations in contact patterns stemming from population social and spatial structures or behavioral differences that can affect disease dynamics. Because obtaining accurate data regarding contact patterns during an ongoing outbreak is challenging, and because these patterns may evolve along with the outbreak, we chose to focus on producing the simplest useful model. To address case surveillance uncertainty, users can scale upwards or downwards their case count inputs occurring over the prior two weeks based on perceived underreporting or overreporting and examine the influence on outputs (as we did in our illustrative scenario). Similarly, all assumptions and sources are explicitly presented in the tool, and all can be readily modified by users to reflect their interests, and as new information comes to light. Therefore, users should consider that the value of this tool is its ability to support the evaluation of relative differences in results associated with "what-if" scenarios.
Our COVID model has other limitations worth noting. Our estimates of beds and ventilators needed and the number of deaths averted also depends on associated resources not modeled here. Such resources include trained staff (respiratory therapists, nurses, and physicians) for the successful clinical management of hospitalized and ventilated patients and ancillary supplies associated with a bed or ventilator (e.g., electric circuits, oxygen, etc.). Furthermore, these resources may be impacted by the pandemic itself, e.g. staff absenteeism due to illnesses (Wu and McGoogan, 2020) and supply-chain disruptions in personal protective equipment (PPE) for healthcare personnel may further exacerbate the situation. The effects of seasonality on the transmission dynamics of COVID19 remains unclear, but the transmission of similar respiratory illnesses (e.g., influenza, syncytial virus) peaks in the wintertime (Lipsitch and Viboud, 2009, Shaman and Kohn, 2009). If COVID19 exhibits similar seasonality, or patients with these other illnesses place additional demands on the healthcare system, there may be even fewer resources available to treat COVID19 patients at the epidemic’s peak. Finally, we do not differentiate between specialized pediatric and non-pediatric resources. While this is justifiable because the current pandemic does not appear to pose a high enough risk to children to overwhelm pediatric healthcare capacity (Riou et al., 2020, Verity et al., 2020, Wu et al., 2020), users of the tool should take note of this meaningful difference when inputting resource amounts.
Conclusions
Our model allows decision-makers to examine the impacts of the current COVID-19 pandemic in their jurisdictions and evaluate the effects of various social-distancing mitigation strategies and augmenting treatment capacity on morbidity and mortality. Our illustrative scenario demonstrates the need for policymakers to take immediate and aggressive actions, and if they do so, substantial morbidity and mortality may be averted. As more data become available (e.g., new treatments or healthcare capacity is augmented) and the pandemic evolves (e.g., COVID case counts), our tool permits rapid updating of results applicable for making decisions.
Declaration of interest
All authors report no conflicts of interest.
Funding
Eduardo A. Undurraga was supported in part by The Millennium Science Initiative of the Ministry of Economy, Development, and Tourism, Government of Chile, grant “Millennium Nucleus for Collaborative Research in Antimicrobial Resistance.” This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical Approval
No ethical approvals sought. All data used were publicly accessible.
Acknowledgments
The authors thank Shannon Self-Brown for feedback on the manuscript and Marcelo Arenas and Rafael Araos for thoughtful comments and suggestions about the tool's applicability in the Chilean context.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.05.043.
Appendix A. Supplementary data
The following are Supplementary data to this article:
This is the modeling tool. It contains all data and calculations used in the analyses.
This file contains further details on the methods and additional analyses and results.
References
- Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Yang J., Wang W., Wang X., Zhou J., Chen Z. Case fatality risk of novel coronavirus diseases 2019 in China. medRxiv. 2020 [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N., Laydon D., Nedjati Gilani G., Imai N., Ainslie K., Baguelin M. 2020. Report 9: impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. [Google Scholar]
- Flaxman S., Mishra S., Gandy A. Imperial College COVID-19 Response Team; 2020. Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries; p. 30. [Google Scholar]
- Henderson M. 2020. Covid act now.https://www.covidactnow.org/ [Google Scholar]
- ICNARC . Intensive Care National Audit & Research Centre; London: 2020. ICNARC report on COVID-19 in critical care. March 27, 2020. [Google Scholar]
- Instituto Nacional de Estadísticas . 2017. Estimaciones y proyecciones de la población de Chile 1992-2050. Available from: https://www.censo2017.cl/. [Accessed 2 April 2020] [Google Scholar]
- Kissler S.M., Tedijanto C., Lipsitch M., Grad Y. Social distancing strategies for curbing the COVID-19 epidemic. medRxiv. 2020 [Google Scholar]
- Latorre R., Sandoval G. La Tercera; Santiago, Chile: 2020. El mapa actualizado de las camas de hospitales en Chile. [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discovery. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton N.M., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A.R., S-m Jung. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9(2):538. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M., Viboud C. Influenza seasonality: lifting the fog. Proc Natl Acad Sci. 2009;106(10):3645–3646. doi: 10.1073/pnas.0900933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M.I., Patel A., Ajao A., Nystrom S.V., Koonin L.M. Estimates of the demand for mechanical ventilation in the United States during an influenza pandemic. Clin Infect Dis. 2015;60(suppl_1):S52–S57. doi: 10.1093/cid/civ089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health . 2020. Cifras Oficiales COVID-19. Available from: https://www.gob.cl/coronavirus/cifrasoficiales/. [Accessed 2 April 2020] [Google Scholar]
- Ministry of Health . In: Dispone medidas sanitarias que indica por brote de COVID-19. Norms 1143498, 1143591, 1746958. Ministry of Health Chile, editor. Biblioteca del Congreso Nacional de Chile; Santiago: 2020. [Google Scholar]
- Ministry of Health . 2020. Dispone régimen especial de cumplimiento de jornada laboral y flexibilidad horaria por brote de coronavirus (COVID-19). Norm 1143629.https://www.leychile.cl/N?i=1143629&f=2020-03-20&p= Available from: [Google Scholar]
- Nature . 2020. First vaccine clinical trials begin in the United States. Available from: https://www.nature.com/articles/d41586-020-00154-w. [Accessed 17 March 2020] [DOI] [PubMed] [Google Scholar]
- OECD . OECD Publishing; Paris: 2019. Health at a glance 2019: OECD indicators. [Google Scholar]
- Wang C., Pan A., Liu L., Guo H., Hao X., Wang Q. Association of Public Health Interventions With the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA. 2020;323(19):1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn Healthcare . 2020. COVID-19 hospital impact model for epidemics (CHIME) Available from: https://penn-chime.phl.io/ [Accessed 3 March 20] [Google Scholar]
- Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4) doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou J., Hauser A., Counotte M.J., Althaus C.L. Adjusted age-specific case fatality ratio during the COVID-19 epidemic in Hubei, China, January and February 2020. medRxiv. 2020 [Google Scholar]
- Shaman J., Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A. 2009;106(9):3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020:1–9. doi: 10.1016/S1473-3099(20)30243-7. (published online March 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P.G., Whittaker C., Watson O., Baguelin M., Ainslie K., Bhatia S. Imperial College of London; 2020. The Global Impact of COVID-19 and Strategies for Mitigation and Suppression. On behalf of the imperial college covid-19 response team. [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem L., Hoang T.V., Funk S., Coletti P., Beutels P., Hens N. SOCRATES: an online tool leveraging a social contact data sharing initiative to assess mitigation strategies for COVID-19. medRxiv. 2020;2020 doi: 10.1186/s13104-020-05136-9. 03.03.20030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Coronavirus disease (COVID-19) outbreak. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Accessed 17 March 2020] [Google Scholar]
- Wu J.T., Leung K., Bushman M., Kishore N., Niehus R., de Salazar P.M. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat Med. 2020;26:506–510. doi: 10.1038/s41591-020-0822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wunsch H., Wagner J., Herlim M., Chong D., Kramer A., Halpern S.D. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med. 2013;41(12) doi: 10.1097/CCM.0b013e318298a139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., J Xia, Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C., Deng Y., Hu W., Sun J., Lin Q., Zhou F. Estimation of the Time-Varying Reproduction Number of COVID-19 Outbreak in China. Int J Hyg Environ Health. 2020;228 doi: 10.1016/j.ijheh.2020.113555. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104364. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yao W., Wang Y., Long C., Fu X. Wuhan and Hubei COVID-19 mortality analysis reveals the critical role of timely supply of medical resources. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This is the modeling tool. It contains all data and calculations used in the analyses.
This file contains further details on the methods and additional analyses and results.