Abstract
Objective:
Few studies have examined the effectiveness of shared decision making (SDM) in clinical practice. This study evaluated the impact of SDM on quality of life and symptom control in children with asthma.
Methods:
We conducted a prospective 3-year study in six community-based practices serving a low-income patient population. Practices received training on SDM using an evidence-based toolkit. Patients aged 2 to 17 with a diagnosis of asthma were identified from scheduling and billing data. At approximate 6-month intervals, patients completed a survey consisting of the Mini Pediatric Asthma Quality of Life Questionnaire (range 1–7) and the control domain of the Pediatric Asthma Therapy Assessment Questionnaire (range 0–7). We used propensity scores to match 46 children receiving SDM to 46 children receiving usual care with decision support. Included children had completed a baseline survey and at least one follow-up survey. Random coefficient models incorporated repeated measures to assess the effect of SDM on asthma quality of life and asthma control.
Results:
The sample was primarily of non-White patients (94.6%) with Medicaid insurance (92.4%). Receipt of SDM using an evidence-based toolkit was associated with higher asthma quality of life (mean difference 0.9; 95% confidence interval [CI] 0.4–1.4) and fewer asthma control problems (mean difference −0.9; 95% CI −1.6– −0.2) compared to usual care with decision support.
Conclusions:
Implementation of SDM within clinical practices using a standardized toolkit is associated with improved asthma quality of life and asthma control for low-income children with asthma when compared to usual care with decision support.
Keywords: practice-based research, Medicaid, mini asthma quality of life questionnaire (AQLQ), asthma therapy assessment questionnaire (ATAQ), propensity score
Introduction
Asthma is the most common childhood chronic disease, affecting 8.3% of all children in the U.S. and over 11% of children living in poverty (1). Children with uncontrolled asthma experience notable negative effects on their daily functioning including missed school days, activity limitations, and sleeplessness. The economic burden of childhood asthma is also significant, with asthma related doctors’ visits, hospitalizations and medications contributing to over $10 billion in annual costs (2). Recommendations for improving outcomes for patients with asthma include strategies to increase quality of life, control symptoms and reduce exacerbations requiring emergency care (3). Guidelines also recommend that clinicians consider patients’ treatment goals as a means to promote active participation and improve asthma management (3).
Quality of life, which encompasses physical function, social function and mental health, is important for gathering a full view of the impact of the disease on patient wellbeing and provides a critical complement to clinical measures of disease state (3). Poor quality of life among patients can signal a need to adjust therapy or provide education to prevent morbidity and reduce impairment (4, 5). Prior research has investigated factors associated with quality of life in patients with asthma and approaches to improve quality of life. Clinical studies have shown that lung function, number of asthma symptoms, level of asthma control and obesity influence asthma quality of life (6–10). Low coping and problem solving skills and depression have also been associated with worse quality of life in patients with asthma (11, 12). Approaches for improving quality of life include: pharmacotherapy, exercise programs, psychoeducational interventions (including coping strategies), and asthma action plans (11, 13–18). Methods to engage patients and physicians in treatment discussions have also shown some benefit (3, 19–21).
Shared decision making (SDM) advances the concept of patient-provider communication by involving patients and physicians as equal partners in developing a treatment plan that considers patient-centered goals and preferences (22, 23). A randomized controlled trial showed that SDM was associated with improved quality of life compared to usual care alone in adults (21). Cross-sectional studies have also shown that higher levels of SDM are associated with better symptom control and satisfaction among children with asthma (24, 25). Longitudinal studies in practice-based settings can add to the available evidence about the effectiveness of SDM for improving asthma outcomes and provide support for recommendations to increase the use of SDM in the management of chronic diseases (3–5, 26–28). This study adds to the literature on SDM and asthma by examining the impact of SDM in an outpatient practice-based setting and its effect on asthma quality of life and asthma control among low-income children with asthma. We hypothesized that patients involved in SDM through the use of a health coach and toolkit together would experience greater improvements in quality of life and control compared to patients involved in usual care with decision support alone.
Methods
Setting
This study took place within the Mecklenburg Area Partnership for Primary Care Research (MAPPR), a mature practice-based research network based in Charlotte, North Carolina. The MAPPR network includes outpatient clinics, hospitals, and urgent care centers with a robust network of physicians, researchers and community members (29, 30). Six primary care practices were enrolled in the study. Details of the SDM intervention have been published (28, 31). Briefly, starting in April 2011, providers and staff received training on how to use an evidence-based asthma SDM toolkit, with step-by-step instructions including an assessment of asthma control, a review of treatment goals and medication preferences, and education on asthma medications, inhaler technique, and triggers (https://asthma.carolinashealthcare.org). The “rollout” at each practice took approximately 12 weeks to complete (28). A family medicine provider who was also part of the research team facilitated and tailored the implementation of the SDM toolkit to the individual needs of each practice. The rollout typically consisted of hour-long weekly sessions with practice providers and staff. Topics included asthma health maintenance, population management, explanation of SDM and motivational interviewing, logistics of scheduling asthma visits, patient recruitment, and toolkit training. Practice staff varied but typically included a provider champion, practice manager, nurse manager, registration supervisor, interpreter if applicable, and health coaches. Health coaches included pharmacists, nurses, or patient educators who were interested in taking a more active role in asthma care.
During a specialized asthma visit, the patient (and caregiver) met with a health coach to utilize the toolkit to discuss goals and preferences prior to meeting with their provider to negotiate around a treatment plan. The toolkit was tailored to three age groups: 2 to 4 years, 5 to 11 years and 12 years and older. For ages 2 to 11, the health coach engaged both the parent and child in the discussion of goals and preferences for treatment. There were no reported instances of difficulties reaching consensus between parent and child during these discussions. For ages 12 and older, the child was primarily involved in the discussion of goals and preferences. All patients in the intervention completed at least one SDM visit. Following the initial SDM visit, additional visits were scheduled based on need. Adoption of the SDM intervention was reinforced with monthly meetings with representatives from participating practices and one refresher training at the end of year one (31). Usual care for patients with asthma incorporated clinical decision support tools comprised of computerized alerts and reminders for providers to review and document standard appropriate care measures such as daytime and nighttime symptoms, use of controller medication and administration of the flu vaccine annually in the electronic medical record (EMR), consistent with the chronic care model for quality improvement (32). The Carolinas HealthCare System Institutional Review Board approved this study.
Data Collection and Participants
Data for this study were obtained from surveys and from hospital and clinic billing records. Surveys combined two validated measures of asthma symptomology, the Mini Pediatric Asthma Quality of Life Questionnaire (MPAQLQ) (33, 34) and the control domain of the Pediatric Asthma Therapy Assessment Questionnaire (PATAQ) (24). Participants completed surveys either in-person during their SDM visit; via a mailed form; through an online web tool; or via phone with a member of the research team. Follow up surveys were mailed out at 6 month intervals to participants completing an initial survey. Non-respondents to any follow up survey were considered lost to follow up and were not included in subsequent surveys. Data collection began in March 2011 and continued through September 2013. Participants were incentivized with a $10 gift card for each completed survey. Survey responses were merged with clinic visit records to determine visit dates coinciding with survey completion.
A total of 664 children aged 2 to 17, 121 in the intervention group and 543 in usual care with decision support, were invited to complete the survey about their asthma symptoms (Figure 1). Children in the SDM toolkit intervention were identified prospectively through the practices’ scheduling systems. Clinical teams selected patients based on having poorly controlled asthma in the prior 18 months, which was defined as (i) prior asthma exacerbations resulting in an inpatient stay or emergency department visit or (ii) outpatient oral steroid use. Patients receiving usual care were identified from hospital and clinic billing records using criteria to ascertain an active asthma diagnosis: asthma diagnosis as defined by International Classification of Disease, 9th revision (ICD-9-CM) codes for asthma (493.xx); a history of asthma as determined by a minimum of two office visits with an asthma diagnosis or one office visit and one hospital or emergency department visit with an asthma diagnosis in the 18 month period prior to the intervention start date; and at least one visit to a participating primary care practice between April 2011 and September 2013 (index date was determined to be the first visit during this period). The survey response rate was 96.7% for participants in the SDM intervention (117/121) and 20.4% (111/543) for participants receiving usual care. We had a higher response rate in the SDM group due to 89% of surveys being completed in person during the SDM visit, while 80% of the usual care group completed surveys via mail and had a response rate that was comparable to other mailed surveys (35). The average number of surveys per participant was 3. Patients in the intervention were excluded from analysis if they did not complete a survey (n=4), were determined to have exposure to the intervention prior to their baseline survey (n=4), or did not complete a follow-up survey (n=58). Patients in the intervention who completed both a baseline and a follow-up survey were eligible for analysis (n=55). Usual care patients were excluded from the analytic sample for the following reasons: (i) they turned age 18 before first survey (n=1); or (ii) first survey was completed after their index date (n=18); or (iii) no follow up survey completed (n=46). Therefore, 46 patients remained for analysis.
Figure 1.
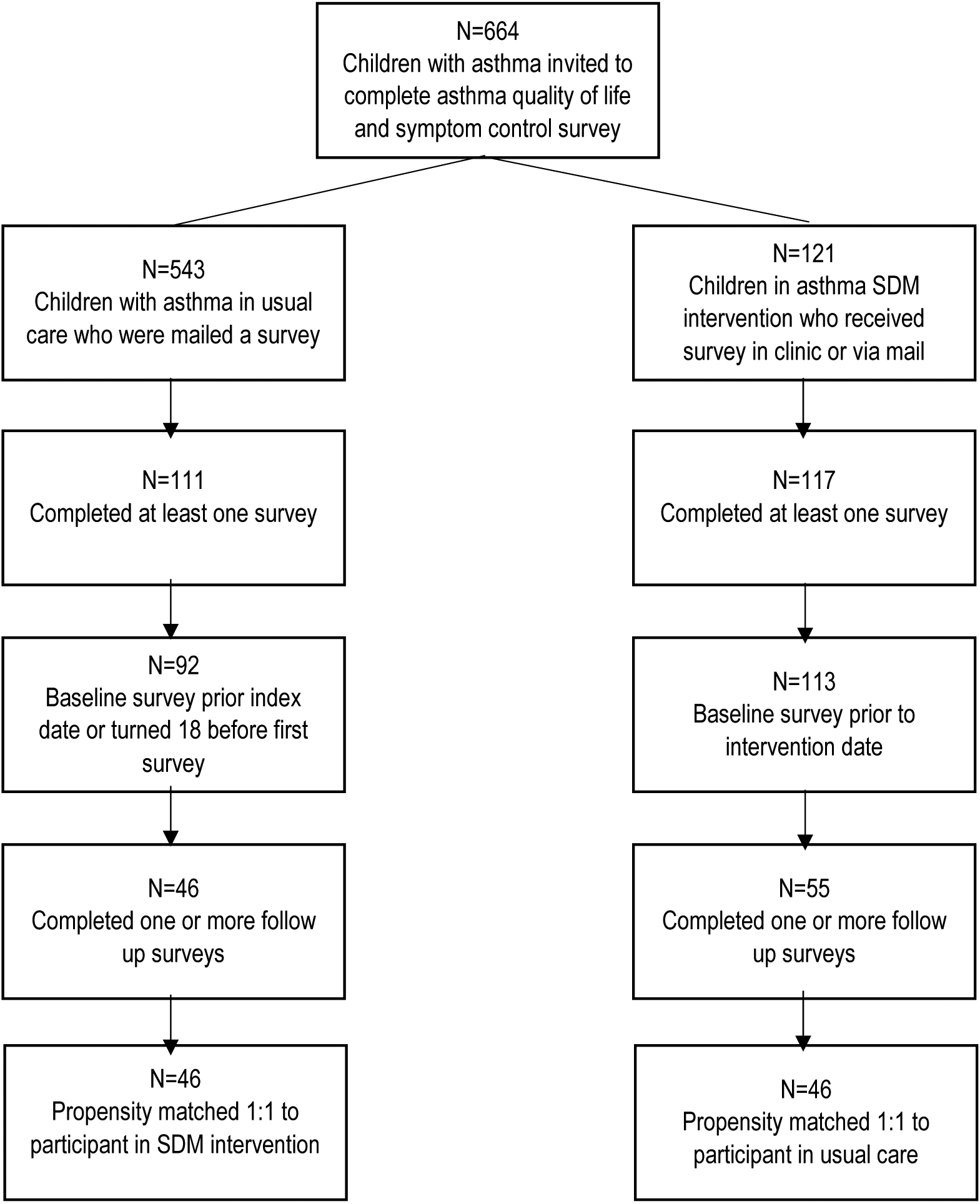
Diagram of study sample selection
*SDM=shared decision making
Propensity scores were used to match patients receiving the intervention to patients receiving usual care with decision support. Using propensity score matching reduced the bias associated with non-randomization of subjects by modeling the probability of being selected to the treatment group and matching intervention participants to controls based on that probability (36). For this analysis, we used an established propensity matching technique based on the nearest neighbor algorithm (37). Patients were matched on age, race/ethnicity, gender, insurance category, baseline asthma quality of life score, baseline asthma control score and the Charlson comorbidity index. Propensity scores were calculated using logistic regression and matching was without replacement. After matching, 46 pairs of children remained for analysis, thus 100% of patients in the usual care group were matched to a patient receiving the SDM toolkit intervention (Figure 1).
Measures
The primary outcome measures were asthma-related quality of life, which was measured using the MPAQLQ, and asthma control, which was measured using the control domain of the PATAQ. The MPAQLQ includes three domains that measure the frequency or level of bother experienced in the past week related to asthma symptoms (coughing, wheezing, chest tightness, breathlessness, fatigue and sleeplessness), activities (physical activities, being with animals, playing with family and friends) and emotions (frustration, fear, irritability, isolation), with Cronbach’s alpha values of 0.94, 0.92 and 0.88 respectively for the symptoms, emotions and activities subscales and 0.97 for the overall scale (33, 34). Scores range from 1 to 7 with higher score indicating better quality of life. We were missing data on one of four questions in the emotions domain of the MPAQLQ (“How often did you feel frightened or worried because of your asthma?”). Remaining questions in the emotions domain assessed how often children (a) felt frustrated because of their asthma, (b) felt irritable (cranky/grouchy) because of their asthma, and (c) felt different or left out because of their asthma. The MPAQLQ had the following Cronbach’s alpha values in our sample: 0.854 for the overall scale, and 0.842, 0.558 and 0.546 for the symptoms, emotions and activities subscales respectively. We examined average changes in quality of life scores from the MPAQLQ. The asthma control domain of the PATAQ captures patient experience in the past 4 weeks regarding wheezing, night awakenings, inhaler use, missed school or activities, and self-assessment of asthma control, and has a Cronbach’s alpha of 0.75 (24). The instrument includes seven questions with a score range of 0 to 7; higher score indicated more control problems (24). The PATAQ had a Cronbach’s alpha of 0.607 in our sample. We examined the average change in the asthma control score from the PATAQ.
Additional measures included patient demographics (age, gender, race/ethnicity, insurance status, and comorbid conditions) and comorbid conditions, which were obtained from hospital and clinic billing data. Race was categorized as Caucasian, African American, Hispanic/Latino or Other. Insurance was classified as Private or Medicaid/Other. The Charlson comorbidity index, a measure of comorbid disease burden based on ICD-9-CM codes (38, 39), was calculated for each patient, using their billing data from the 12 months prior to their index date. Consistent with prior research among patients with asthma, asthma was excluded from the list of diagnoses used to calculate the Charlson comorbidity index (40).
Analysis
Descriptive statistics, including means and percentages were computed for study participants. Sample characteristics were compared between intervention and control groups using standardized differences for comparison of matched samples (41). We compared quality of life scores from the MPAQLQ and asthma control scores from the PATAQ between patients in the SDM intervention and patients in usual care using random coefficient models. These models accounted for variations in number of surveys completed and time between baseline and follow up surveys and incorporated repeated measures over the study period. Initial models examined interactions between months from intervention and study group to determine whether the effect of time from intervention on quality of life scores varied by study group. Interactions were found to be non-significant and were excluded from final models. Final multivariate models were adjusted for variables that remained unbalanced after propensity score matching (Charlson comorbidity index) and for the number of months between the intervention date and survey completion date.
Results
The study sample included 92 children with an average age of 9.1 years (Table 1). The sample was largely Medicaid and non-White. The average baseline quality of life score from the MPAQLQ was 4.9 (on a scale from 1 to 7) and the average baseline number of control problems from the PATAQ was 5.3 (on a scale of 0 to 7). The matched sample did not differ with respect to age, gender, race, insurance or baseline quality of life scores or baseline number of asthma control problems. Comparison of baseline asthma exacerbations between the two groups also revealed no significant differences. Compared to children receiving usual care with decision support, children in the SDM intervention had a higher illness burden as measured by the Charlson comorbidity index.
Table 1.
Characteristics of matched sample of children with asthmaa
| Total (n=92) | Usual Care (n=46) | SDM Toolkit (n=46) | Standardized differenceb | |
|---|---|---|---|---|
| Age, y (mean, SD) | 9.1 (4.2) | 9.2 (3.7) | 9.1 (4.8) | 0.03 |
| Patient sex, n(%) | 0.00 | |||
| Male | 52 (56.5) | 26 (56.5) | 26 (56.5) | |
| Female | 40 (43.5) | 20 (43.5) | 20 (43.5) | |
| Race/Ethnicity, n(%) | 0.11 | |||
| Caucasian | 5 (5.4) | 4 (8.7) | 1 (2.2) | |
| African American | 60 (65.2) | 32 (69.6) | 28 (60.9) | |
| Hispanic/Latino | 24 (26.1) | 9 (19.6) | 15 (32.6) | |
| Other | 3 (3.3) | 1 (2.2) | 2 (4.4) | |
| Insurance, n(%) | 0.08 | |||
| Medicaid/Otherc | 85 (92.4) | 42 (91.3) | 43 (93.5) | |
| Private | 7 (7.6) | 4 (8.7) | 3 (6.5) | |
| Charlson comorbidity index, mean (SD) | 0.8 (0.4) | 0.7 (0.5) | 0.9 (0.3) | 0.64 |
| Asthma exacerbations in 12 months prior intervention, mean (SD) | 0.8 (1.2) | 0.7 (1.0) | 0.9 (1.3) | 0.17 |
| Baseline quality of life score from the MPAQLQ (range 1–7), mean (SD) | 4.9 (1.2) | 4.8 (1.3) | 5.0 (1.0) | 0.13 |
| Activities score | 4.9 (1.4) | 4.8 (1.6) | 5.0 (1.3) | 0.17 |
| Emotions score | 5.3 (1.3) | 5.4 (1.3) | 5.3 (1.3) | 0.08 |
| Symptoms score | 4.7 (1.4) | 4.6 (1.5) | 4.8 (1.3) | 0.16 |
| Baseline # of asthma control problems from the PATAQ (range 0–7), n(%) | 0.18 | |||
| 0 | 14 (15.2) | 9 (19.6) | 5 (10.9) | |
| 1 | 11 (12.0) | 3 (6.5) | 8 (17.4) | |
| 2 | 10 (10.9) | 6 (13.0) | 4 (8.7) | |
| 3 | 21 (22.8) | 7 (15.2) | 14 (30.4) | |
| 4 | 21 (22.8) | 13 (28.3) | 8 (17.4) | |
| 5 or more | 15 (16.3) | 8 (17.4) | 7 (15.2) |
Usual care and SDM toolkit participants were matched on baseline quality of life score, baseline asthma control, age, gender, insurance status, race/ethnicity, and the Charlson comorbidity index.
Standardized difference is difference in means or proportions divided by standard error; imbalance is defined by values greater than 0.20 (i.e. small effect size based on Cohen’s d).
Other includes other public insurance and uninsured
SDM, shared decision making; SD, standard deviation, MPAQLQ, Mini Pediatric Quality of Life Questionnaire (scores range from 1 to 7 with higher scores indicating better quality of life); PATAQ, Pediatric Asthma Therapy Assessment Questionnaire
Results of random coefficient models comparing quality of life and asthma control scores between patients receiving SDM toolkit intervention and matched patients in usual care are shown in Table 2. Model results are reported as mean differences and 95% confidence intervals (CI). The SDM toolkit intervention was associated with higher quality of life scores when compared to usual care (mean difference 0.9; 95% CI 0.4–1.4). Additional models assessing the impact of the SDM toolkit interventions on individual quality of life domains (activities, emotions, symptoms) revealed similar results (Table 3). Similarly, models examining differences in asthma control showed lower asthma control problems for children in the SDM toolkit intervention compared to usual care (mean difference −0.9; 95% CI −1.6– −0.2). Illness burden, as measured by the Charlson comorbity index, was negatively associated with asthma quality of life, however, illness burden was not significantly associated with asthma control. Months from intervention to survey was not significant in any of the models.
Table 2.
Repeated measures model comparing quality of life and asthma control scores between patients receiving SDM toolkit intervention and matched patients in usual care (n=92)a
| Variables in model | QOL Scoreb | Asthma Control Scorec | ||
|---|---|---|---|---|
| Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Treatment | ||||
| Usual Care | Reference | Reference | ||
| SDM Toolkit | 0.9 (0.4–1.4) | < 0.001 | −0.9 (−1.6– −0.2) | 0.010 |
| Charlson comorbidity index | −0.7 (−1.3–−0.1) | 0.020 | 0.6 (−0.2– 1.4) | 0.160 |
| Months from intervention | 0.0 (0.0–0.0) | 0.980 | 0.0 (−0.1– 0.1) | 0.750 |
Usual care and SDM toolkit participants were matched on baseline quality of life score, baseline asthma control, age, gender, insurance status, race/ethnicity, and the Charlson comorbidity index. Models were adjusted for variables that were unbalanced after matching.
Quality of life score from the Mini Pediatric Quality of Life Questionnaire (MPAQLQ) ranges from 1 to 7; higher score indicates better quality of life
Asthma control domain score from the Pediatric Asthma Therapy Questionnaire (PATAQ) ranges from 0 to 7; higher score indicates more control problems
QOL, quality of life; SDM, shared decision making
Table 3.
Repeated measures model comparing quality of life domain scores between patients receiving SDM toolkit intervention and matched patients in usual care (n=92)a
| Variables in model | Activities Score | Emotions Score | Symptoms Score | |||
|---|---|---|---|---|---|---|
| Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | Mean difference (95% CI) | p-value | |
| Treatment | ||||||
| Usual Care | Reference | Reference | ||||
| SDM Toolkit | 1.0 (0.5–1.6) | < 0.001 | 1.0 (0.5–1.6) | < 0.001 | 0.7 (0.2–1.3) | 0.009 |
| Charlson comorbidity index | −0.9 (−1.6–−0.3) | 0.006 | −0.8 (−1.4–−0.1) | 0.030 | −0.7 (−1.3–0.0) | 0.049 |
| Months from intervention | 0.0 (−0.1–0.1) | 0.940 | 0.0 (−0.1–0.0) | 0.910 | 0.0 (−0.1–0.1) | 0.940 |
Usual care and SDM toolkit participants were matched on baseline quality of life score, baseline asthma control, age, gender, insurance status, race/ethnicity, and the Charlson comorbidity index. Models were adjusted for variables that were unbalanced after matching. Quality of life domain scores from the Mini Pediatric Quality of Life Questionnaire (MPAQLQ) range from 1 to 7; higher score indicates better quality of life.
Discussion
We compared quality of life over a 3-year period among patients exposed to SDM in six primary care clinics that all had access to asthma clinical decision support tools including computerized alerts and reminders. Our study hypothesis was confirmed with our primary finding that children in a guided SDM intervention that included a health coach using an evidenced-based toolkit achieved notable improvements in asthma-specific quality of life and asthma control compared to children receiving usual care with decision support. This finding suggests that, consistent with the chronic care model, provider focused interventions such as decision support may be enhanced by the addition of patient-focused interventions like SDM (32). This study adds to the evidence regarding the benefit of SDM for low-income children with asthma by prospectively examining the impact of SDM on asthma quality of life and control in a practice-based setting.
Our finding of an association between SDM and improved quality of life for children is consistent with research among adults. A randomized trial of SDM in an adult sample found that SDM was associated with higher mean asthma quality of life scores at one-year post-randomization (21). However, a prior cross-sectional study in a cohort of 160 children aged 8 to 17 with asthma did not find significant correlation between parents’ satisfaction with SDM and a measure of asthma-specific quality of life (25). The study authors did find evidence of an indirect effect of SDM on asthma quality of life (25). The difference between our finding and that of the latter study could be due to differences in study design (cross-sectional vs. prospective) or measures used for asthma quality of life (MPAQLQ vs. Patient Reported Outcomes Measurement Information System). Additional longitudinal studies with larger samples can further clarify the impact of SDM on asthma quality of life in children.
Our finding of an association between SDM and better asthma control also follows prior research. Gandhi et al. (25) used structural equation models in a cross-sectional study of children with asthma and found that greater parental satisfaction with SDM was associated with a lower likelihood of poorly controlled asthma as measured by the asthma control questionnaire. SDM for children with asthma has also been associated with fewer exacerbations of asthma that result in hospitalizations, emergency department visits or oral steroid prescriptions (28). Among adults, SDM has been associated with greater odds of well-controlled asthma and decreased use of rescue inhaler medications (21). Improved medication adherence and greater self-management are benefits of SDM that may help improve asthma symptom control (42, 43).
SDM among children with asthma does not happen as often as recommended by national guidelines. A study of five suburban/rural clinics in North Carolina found that less than 10% of pediatric asthma visits involved SDM with caregivers or patients (44). Moreover, a systematic review of the literature regarding SDM in pediatrics found that while SDM overall is increasing, few interventions involve children in the decision-making process (45). While the extent of conversations between clinicians and pediatric patients will necessarily vary by age, intentionally seeking input from children about their preferences and concerns is encouraged because it helps build confidence in self-management of the disease (43). Differences in opinions between parents and children regarding goals and asthma quality of life is discussed by Guyatt et al (46), who state that clinicians can gain complementary information from questioning children and parents for children age less than 11, while for over age 11 parents provide little additional information. Clinicians can seek input regarding preferences for treatment from children of all ages, including preschool children, by tailoring their discussion to match their cognitive and social development (45, 47, 48). Using this approach, children’s participation can include receiving information about treatment and illness and having the opportunity to ask questions, sharing in decisions and goal-setting or autonomous decision making based on their capacity and preferences (47, 48). The SDM toolkit used in this study incorporated images and simpler words to facilitate such conversations with children under 12. Our finding that the use of an evidence-based SDM toolkit improved asthma quality of life and asthma control when compared to usual care with decision support gives further support to recommendations that standardized tools provide an effective approach for implementing SDM in clinical practice (49).
Limitations
The observational study design, implemented in the real-world setting of primary care practices seeking to improve asthma care, did not allow for random assignment of patients to intervention or control group. To reduce potential selection bias, we employed propensity score matching, a method that has been shown to reduce the impact of selection bias in observational studies like ours (28, 50). We also lacked data on clinical measures of disease severity, which would provide a better measure of disease burden. However, statistical models included adjustments for the Charlson comorbidity index, a measure of comorbid disease burden previously associated with quality of life among patients with asthma (51). While patients in the intervention were referred by a clinician, patients in the control group were identified through algorithms to identify patients with active asthma. Still, we achieved balance on several important characteristics between the groups including baseline number of asthma exacerbations, which is a proxy for asthma control.
We were also unable to distinguish between pediatric surveys that were completed by children or by parental proxy for mail and online surveys. The MPAQLQ was validated for self-report by children as young as 7 years old and we expect that parents completed surveys for children younger than 7 years (n=26 or 28% of our sample). Notwithstanding, previous research suggests strong concordance between parent and child responses on the PAQLQ (52) and completion by one or the other should be non-differential between the study groups. Reliability as measured by Cronbach’s alpha was lower in our sample than in previous published studies for the emotions and activities domains of the MPAQLQ and for the control domain of the PATAQ. Our smaller sample size and the fewer number of items in the MPAQLQ subscales compared to the overall scale may have contributed to this difference (53, 54). Prior studies with larger samples were able to achieve very good reliability and validity statistics suggesting the usefulness of both scales in clinical practice (24, 33, 34). We had a 20.4% survey response rate in our control group and respondents were more likely to identify as African American or Hispanic compared to non-respondents. However, respondents did not differ from non-respondents regarding age, gender, insurance status, baseline number of exacerbations or comorbidity burden. Finally, patients in this study were selected from practices that serve a mostly low-income patient population in a largely urban setting and results may not be generalizable to practice settings with a different patient population.
Conclusion
Children with asthma are likely to benefit from a SDM approach to care that considers their preferences, provides recommended education and solicits their participation and the participation of their caregivers in developing a care plan. Findings from this study suggest that high-risk minority patients with Medicaid insurance can achieve substantial improvement in quality of life and asthma control using this approach. Decision aids such as the asthma SDM toolkit used in this study can help providers effectively implement SDM into their practice. Future research can better elucidate the impact of SDM strategies on medication adherence and other measures of asthma control and disease severity among children with asthma and provide a better understanding of how children can benefit from an SDM approach to asthma care.
Acknowledgments
All phases of this study were supported by the Agency for Healthcare Research and Quality Grant Number 1R18HS019946-01.
The authors would like to acknowledge Mark Steuerwald for his assistance with data collection.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics. 2016;137(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang J, Gary Chan KC, Huang H, Sullivan SD. Trends in cost and outcomes among adult and pediatric patients with asthma: 2000–2009. Ann Allergy Asthma Immunol. 2013;111(6):516–22. [DOI] [PubMed] [Google Scholar]
- 3.National Asthma Education and Prevention Program Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. [Google Scholar]
- 4.Yawn BP, Yawn RA. Measuring asthma quality in primary care: can we develop better measures? Respir Med. 2006;100(1):26–33. [DOI] [PubMed] [Google Scholar]
- 5.Halterman JS, McConnochie KM, Conn KM, Yoos HL, Kaczorowski JM, Holzhauer RJ, et al. A potential pitfall in provider assessments of the quality of asthma control. Ambul Pediatr. 2003;3(2):102–5. [DOI] [PubMed] [Google Scholar]
- 6.Indinnimeo L, Chiarotti F, De Vittori V, Baldini L, De Castro G, Zicari AM, et al. Risk factors affecting quality of life in a group of Italian children with asthma. Int J Immunopathol Pharmacol. 2014;27(2):235–44. [DOI] [PubMed] [Google Scholar]
- 7.Fedele DA, Janicke DM, Lim CS, Abu-Hasan M. An examination of comorbid asthma and obesity: assessing differences in physical activity, sleep duration, health-related quality of life and parental distress. J Asthma. 2014;51(3):275–81. [DOI] [PubMed] [Google Scholar]
- 8.Mohanan S, Tapp H, McWilliams A, Dulin M. Obesity and asthma: Pathophysiology and implications for diagnosis and management in primary care. Exp Biol Med (Maywood). 2014;239(11):1531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vietri J, Burslem K, Su J. Poor Asthma control among US workers: health-related quality of life, work impairment, and health care use. J Occup Environ Med. 2014;56(4):425–30. [DOI] [PubMed] [Google Scholar]
- 10.Amaral LM, Moratelli L, Palma PV, Leite IC. The quality of life of Brazilian adolescents with asthma: associated clinical and sociodemographic factors. J Asthma. 2014;51(6):660–6. [DOI] [PubMed] [Google Scholar]
- 11.McCormick SP, Nezu CM, Nezu AM, Sherman M, Davey A, Collins BN. Coping and social problem solving correlates of asthma control and quality of life. Chron Respir Dis. 2014;11(1):15–21. [DOI] [PubMed] [Google Scholar]
- 12.Ross JA, Yang Y, Song PX, Clark NM, Baptist AP. Quality of life, health care utilization, and control in older adults with asthma. J Allergy Clin Immunol Pract. 2013;1(2):157–62. [DOI] [PubMed] [Google Scholar]
- 13.Sharma A, Adiga S, Chogtu B, Mohapatra AK, Magazine R. Comparing the efficacy and influence on the quality of life of three classes of drugs used in bronchial asthma - a prospective study. J Clin Diagn Res. 2014;8(9):HC13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vieira T, de Oliveira JF, da Graca Castel-Branco M. Short and long-term quality of life and asthma control with omalizumab therapy in a real life setting in Portugal. Allergol Immunopathol (Madr). 2014;42(1):3–10. [DOI] [PubMed] [Google Scholar]
- 15.Sodhi C, Singh S, Bery A. Assessment of the quality of life in patients with bronchial asthma, before and after yoga: a randomised trial. Iran J Allergy Asthma Immunol. 2014;13(1):55–60. [PubMed] [Google Scholar]
- 16.Latorre-Roman PA, Navarro-Martinez AV, Garcia-Pinillos F. The effectiveness of an indoor intermittent training program for improving lung function, physical capacity, body composition and quality of life in children with asthma. J Asthma. 2014;51(5):544–51. [DOI] [PubMed] [Google Scholar]
- 17.Pinnock H. Supported self-management for asthma. Breathe (Sheff). 2015;11(2):98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JR, Mildenhall S, Noble MJ, Shepstone L, Koutantji M, Mugford M, et al. The Coping with Asthma Study: a randomised controlled trial of a home based, nurse led psychoeducational intervention for adults at risk of adverse asthma outcomes. Thorax. 2005;60(12):1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small M, Vickers A, Anderson P, Kay S. The patient-physician partnership in asthma: real-world observations associated with clinical and patient-reported outcomes. Adv Ther. 2010;27(9):591–9. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter DM, Ayala GX, Williams DM, Yeatts KB, Davis S, Sleath B. The relationship between patient-provider communication and quality of life for children with asthma and their caregivers. J Asthma. 2013;50(7):791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681–92. [DOI] [PubMed] [Google Scholar]
- 23.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651–61. [DOI] [PubMed] [Google Scholar]
- 24.Skinner EA, Diette GB, Algatt-Bergstrom PJ, Nguyen TT, Clark RD, Markson LE, et al. The Asthma Therapy Assessment Questionnaire (ATAQ) for children and adolescents. Dis Manag. 2004;7(4):305–13. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi PK, Kenzik KM, Thompson LA, DeWalt DA, Revicki DA, Shenkman EA, et al. Exploring factors influencing asthma control and asthma-specific health-related quality of life among children. Respir Res. 2013;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219–26. [DOI] [PubMed] [Google Scholar]
- 27.Rivera-Spoljaric K, Halley M, Wilson SR. Shared clinician-patient decision-making about treatment of pediatric asthma: what do we know and how can we use it? Curr Opin Allergy Clin Immunol. 2014;14(2):161–7. [DOI] [PubMed] [Google Scholar]
- 28.Tapp H, Shade L, Mahabaleshwarkar R, Taylor YJ, Ludden T, Dulin MF. Results from a pragmatic prospective cohort study: Shared decision making improves outcomes for children with asthma. J Asthma. 2016:1–11. [DOI] [PubMed] [Google Scholar]
- 29.Dulin MF, Ludden TM, Tapp H, Smith HA, de Hernandez BU, Blackwell J, et al. Geographic Information Systems (GIS) demonstrating primary care needs for a transitioning hispanic community. J Am Board Fam Med. 2010;23(1):109–20. [DOI] [PubMed] [Google Scholar]
- 30.Tapp H, Hebert L, Dulin M. Comparative effectiveness of asthma interventions within a practice based research network. BMC Health Serv Res. 2011;11:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tapp H, Kuhn L, Alkhazraji T, Steuerwald M, Ludden T, Wilson S, et al. Adapting community based participatory research (CBPR) methods to the implementation of an asthma shared decision making intervention in ambulatory practices. J Asthma. 2014;51(4):380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20(6):64–78. [DOI] [PubMed] [Google Scholar]
- 33.Wing A, Upton J, Svensson K, Weller P, Fletcher M, Walker S. The standardized and mini versions of the PAQLQ are valid, reliable, and responsive measurement tools. J Clin Epidemiol. 2012;65(6):643–50. [DOI] [PubMed] [Google Scholar]
- 34.Mahabaleshwarkar R, Taylor YJ, Tapp H, Dulin MF. Psychometric Properties of the Mini Pediatric Asthma Quality of Life Questionnaire in a US Sample. Pediatr Allergy Immunol Pulmonol. 2016;29(3):137–42. [DOI] [PubMed] [Google Scholar]
- 35.Goolsby Hunter A, Brenneman S. Response Rates In Direct-To-Patient Surveys. Value Health. 18(3):A33–A4. [Google Scholar]
- 36.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 37.Rassen J, Doherty M, Huang W, Schneeweiss S. Pharmacoepidemiology Toolbox Boston, MA [Available from: http://www.drugepi.org/dope-downloads/.
- 38.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 39.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–9; discussion 81–90. [DOI] [PubMed] [Google Scholar]
- 40.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129(5):1229–35. [DOI] [PubMed] [Google Scholar]
- 41.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klok T, Kaptein AA, Brand PL. Non-adherence in children with asthma reviewed: the need for improvement of asthma care and medical education. Pediatr Allergy Immunol. 2015. [DOI] [PubMed] [Google Scholar]
- 43.Butz AM, Walker JM, Pulsifer M, Winkelstein M. Shared decision making in school age children with asthma. Pediatr Nurs. 2007;33(2):111–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Sleath BL, Carpenter DM, Sayner R, Ayala GX, Williams D, Davis S, et al. Child and caregiver involvement and shared decision-making during asthma pediatric visits. J Asthma. 2011;48(10):1022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyatt KD, List B, Brinkman WB, Prutsky Lopez G, Asi N, Erwin P, et al. Shared Decision Making in Pediatrics: A Systematic Review and Meta-analysis. Acad Pediatr. 2015;15(6):573–83. [DOI] [PubMed] [Google Scholar]
- 46.Guyatt GH, Juniper EF, Griffith LE, Feeny DH, Ferrie PJ. Children and adult perceptions of childhood asthma. Pediatrics. 1997;99(2):165–8. [DOI] [PubMed] [Google Scholar]
- 47.McCabe MA. Involving children and adolescents in medical decision making: developmental and clinical considerations. J Pediatr Psychol. 1996;21(4):505–16. [DOI] [PubMed] [Google Scholar]
- 48.Lipstein EA, Brinkman WB, Fiks AG, Hendrix KS, Kryworuchko J, Miller VA, et al. An emerging field of research: challenges in pediatric decision making. Med Decis Making. 2015;35(3):403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuppermann M, Sawaya GF. Shared decision-making: easy to evoke, challenging to implement. JAMA Intern Med. 2015;175(2):167–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mancuso CA, Peterson MG, Charlson ME. Effects of depressive symptoms on health-related quality of life in asthma patients. J Gen Intern Med. 2000;15(5):301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ungar WJ, Boydell K, Dell S, Feldman BM, Marshall D, Willan A, et al. A parent-child dyad approach to the assessment of health status and health-related quality of life in children with asthma. Pharmacoeconomics. 2012;30(8):697–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonett DG. Sample Size Requirements for Testing and Estimating Coefficient Alpha. J Educ Behav Stat. 2002;27(4):335–40. [Google Scholar]


