Abstract
Purpose:
Exposure to increasing amounts of artificial light during the night may contribute to the high prevalence of reported sleep dysfunction. Release of the sleep hormone melatonin is mediated by the intrinsically photosensitive retinal ganglion cells (ipRGCs). This study sought to investigate whether melatonin level and sleep quality can be modulated by decreasing night-time input to the ipRGCs.
Methods:
Subjects (ages 17–42, n = 21) wore short wavelength-blocking glasses prior to bedtime for 2 weeks. The ipRGC-mediated post illumination pupil response was measured before and after the experimental period. Stimulation was presented with a ganzfeld stimulator, including one-second and five-seconds of long and short wavelength light, and the pupil was imaged with an infrared camera. Pupil diameter was measured before, during and for 60 s following stimulation, and the six-second and 30 s post illumination pupil response and area under the curve following light offset were determined. Subjects wore an actigraph device for objective measurements of activity, light exposure, and sleep. Saliva samples were collected to assess melatonin content. The Pittsburgh Sleep Quality Index (PSQI) was administered to assess subjective sleep quality.
Results:
Subjects wore the blue-blocking glasses 3:57 ± 1:03 h each night. After the experimental period, the pupil showed a slower redilation phase, resulting in a significantly increased 30 s post illumination pupil response to one-second short wavelength light, and decreased area under the curve for one and five-second short wavelength light, when measured at the same time of day as baseline. Night time melatonin increased from 16.1 ± 7.5 pg mL−1 to 25.5 ± 10.7 pg mL−1 (P < 0.01). Objectively measured sleep duration increased 24 min, from 408.7 ± 44.9 to 431.5 ± 42.9 min (P < 0.001). Mean PSQI score improved from 5.6 ± 2.9 to 3.0 ± 2.2.
Conclusions:
The use of short wavelength-blocking glasses at night increased subjectively measured sleep quality and objectively measured melatonin levels and sleep duration, presumably as a result of decreased night-time stimulation of ipRGCs. Alterations in the ipRGC-driven pupil response suggest a shift in circadian phase. Results suggest that minimising short wavelength light following sunset may help in regulating sleep patterns.
Keywords: actigraphy, intrinsically photosensitive retinal ganglion cells, melanopsin, melatonin, sleep
Introduction
Intrinsically photosensitive retinal ganglion cells (ipRGCs) in the inner retina are photosensitive and directly stimulated through activation of the photopigment melanopsin. Melanopsin stimulation through this intrinsic pathway is most sensitive to short wavelength light, with a peak sensitivity of ~482 nm.1,2 The ipRGCs are also stimulated synaptically through the rod/cone pathway (the extrinsic pathway).3 ipRGCs are primarily involved in non-image forming processes such as circadian rhythm entrainment and pupil size regulation,4,5 and can be considered irradiance detectors. They are linked to various aspects of circadian rhythm via the retinohypothalamic tract.2,6,7 In humans, two subtypes of ipRGCs with distinct morphology and axonal projections have thus far been identified,2,8,9 with a recent study reporting four subtypes (M1–M4).10 One subset of ipRGC axons projects to the suprachiasmatic nucleus, in a pathway that ultimately leads to the pineal gland to control melatonin release.11 Melatonin is a hormone released in dim light and involved in the physiological control of sleep.12
Intrinsic, melanopsin-driven ipRGC activity can be measured through the post illumination pupil response (PIPR).5,13 With high intensity short wavelength light, a rod/cone and ipRCG driven pupil constriction ensues, which continues for up to 3 min following light offset.2,14 Known circuits suggest that pupil dynamics following light offset are mediated by sustained firing of ipRGCs.2,5,15
Light is a potent cue for entraining the circadian system, and has been shown to affect a wide variety of physiologic functions, including potential roles in cardiovascular, metabolic, endocrine, and neurologic systems.16-22 Light exposure during the night can lead to chronodisruption, or impaired physiological, behavioural and biochemical rhythms.23 While other cues for synchronisation of the circadian clock include feeding and physical activity, light alone is sufficient to synchronise circadian processes.24
Evening exposure to short wavelength light prior to bedtime may disrupt sleep wake cycles through ipRGCinduced melatonin suppression,25,26 contributing to the high frequency of reported incidence of sleep dysfunction, shown to affect up to 40% of the population.27,28 Software has recently been introduced which aims to reduce blue light exposure from electronic devices at night;29 however, computers and handheld devices represent only a portion of the artificial light in the environment. Exposure to high intensity short wavelength light increases sleep latency, as measured by EEG.30,31 Additionally, night-time exposure to backlit computer screens attenuates salivary melatonin levels.32 Subjective improvements in both sleep quality and mood,33 as well as a decrease in LED-induced night-time melatonin suppression,34 have been demonstrated in subjects wearing blue-blocking glasses at night-time for 2 weeks. Short-wavelength blocking glasses have also been shown to prevent melatonin suppression from bright light during simulated shift work at night.35
This study sought to investigate whether previously observed improvements in sleep following evening wear of blue blocking glasses is mediated through the ipRGCs. The PIPR was utilised as an indirect measure of ipRGC activity to understand its contribution to systemic melatonin and sleep patterns. Based on evidence from previous studies, we hypothesised that attenuating night-time blue light stimulation would result in increased melatonin and improved sleep, potentially complemented with an increase in the PIPR.
Methods
Subjects
Twenty-two subjects, ages 17–42, were recruited to participate in this study. All lab visits occurred between 9:00 am to 11:30 am to minimise circadian influences on the PIPR.36 Visual acuity was measured with habitual correction and an anterior eye exam using slit lamp biomicroscopy was performed to confirm suitability for dilation. All subjects had a visual acuity of 20/25 (0.1 logMAR, Snellen 6/7.5) or better. Exclusion criteria included ocular pathology (including cataracts), prescription or over-the-counter medications known to affect sleep or the pupil, sleep aids such as melatonin, and shift work or travel across time zones during the previous month. Methods were approved by the University of Houston institutional review board and carried out in accordance with relevant guidelines. The research followed the tenets of the Declaration of Helsinki. Informed consent was obtained after explaining the nature of the study to subjects.
The experimental protocol is shown in Figure 1a. As described in detail below, melatonin level and the PIPR were measured before and after 2 weeks of wearing short wavelength-blocking glasses before bedtime. In addition, light exposure, activity and sleep were objectively and continuously monitored before and during the experimental period.
Figure 1.
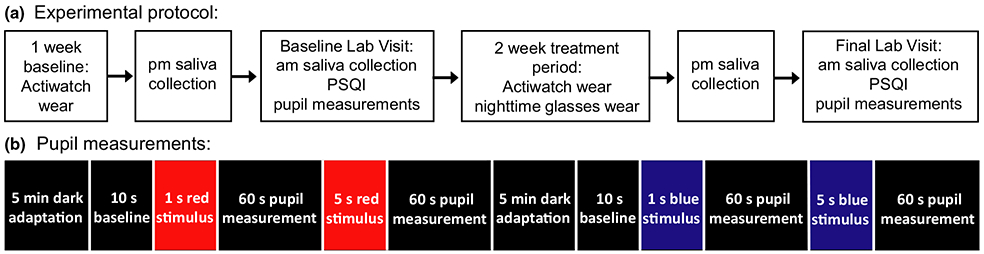
(a) Flow chart of the three week experimental protocol (b) For pupillometry, subjects dark adapted for 5 min. Baseline pupil diameter was recorded for 10 s, then a 1 s long wavelength (red) stimulus was presented followed by 60 s recording, and a 5 s long wavelength stimulus was presented followed by 60 s recording. Subjects dark adapted again, and the protocol was repeated with a short wavelength (blue) stimulus.
Objective light exposure, activity and sleep monitoring
Subjects wore an actigraph device (Actiwatch Spectrum, http://www.usa.philips.com) for 1 week prior to the first lab visit and during the two-week experimental period for objective and continuous measurements of activity, light exposure, sleep quality, and sleep duration. The Actiwatch Spectrum is a wrist worn actigraph device that measures ambient light exposure and activity continuously at 32 Hz, and was set to average over one minute epochs. The light sensor consists of a photodiode that measures the illuminance of broad band light in units of lux (range 0.1–200 000 lux). Additionally, three colour sensitive diodes measure the irradiance of red (600–700 nm), green (500–600 nm) and blue (400–500 nm) spectral components. The battery life and memory allowed for continuous wear over the entire 3 week study period. Subjects were asked to not remove the device for the entire experimental period, and compliance was monitored by an off-wrist sensor in the device.
Melatonin analysis
A saliva sample was collected the night before each lab visit, just before the subject’s habitual bedtime, and the morning of the lab visit, for subsequent melatonin analysis;37 samples were collected within 15 min of each other for baseline and final time points. Prior to collection, subjects were instructed not to eat, drink any liquids other than water, or brush their teeth within an hour of collection. In addition, subjects were asked to abstain from alcohol and nicotine 12 h prior to collection. Subjects collected approximately 1 mL of saliva, and the vial was immediately placed in the freezer. For night-time collections that were done at the subject’s home, the sample was brought into the lab in a provided thermos and insulated bag. Samples were stored at −20°C for subsequent analysis using a melatonin ELISA kit (Salimetrics, https://www.salimetrics.com). All samples were run in duplicate.
Subjective sleep quality
The Pittsburgh Sleep Quality Index questionnaire (PSQI) was administered as a subjective measure of sleep quality. PSQI scores distinguish ‘good’ vs ‘poor’ sleepers based on seven different sleep components, with lower scores indicating better sleep quality.38 A score of 5 or greater indicates poor sleep quality. Subjects answered the questionnaire at baseline, with respect to their habitual sleep over the last month (as the survey indicates), and after 2 weeks, with the latter survey being answered with respect to the two-week experimental period.
Pupillometry
The ipRGC-driven PIPR was measured at the baseline lab visit and repeated at the two-week follow up visit. The left eye was dilated with 2.5% phenylephrine and 1% tropicamide, and a frame mounted binocular eye tracker with infrared illumination (ViewPoint EyeTracker, http://www.arringtonresearch.com) was used to record the pupil size of the right eye at 60 Hz. The IR LED light source has a lambda max of 943 nm with a half-max width of 46 nm (Ocean Optics Spectrometer, https://oceanoptics.com, spectrum provided in the Appendix S1). Pupil diameter was calibrated for each subject by capturing an image of a 5 mm printed black circle approximately placed at the cornea plane. Following calibration, subjects dark adapted for five minutes (<0.1 lux), then placed their head in a chinrest with an LED-driven Ganzfeld system (Color Burst, Espion, Diagnosys LLC, http://diagnosysllc.com) centred in front of the left eye at 10 mm, providing full field stimulation. The subject viewed a red fixation point with the right eye during measurements, which was on the wall 10 feet (~3 m) away.
Stimuli presented to the left eye consisted of 1 and 5 s pulses of either long wavelength (red) or short wavelength (blue) light (Figure 1b). Long wavelength stimuli were 651 nm with a half-max width of 25 nm (Spectroradiometer CS1W, http://sensing.konicaminolta.us, spectral output provided in Appendix S1), set to 33.3 cd m2, and with a measured corneal irradiance of 5.58 × 1013 photons cm2 s1 (Power Meter, https://www.newport.com). Short wavelength stimuli were 456 nm (half-max width of 20 nm), set to 16.67 cd m2, with a measured corneal irradiance of 5.85 × 1013 photons cm2 s1. At this irradiance, with full field Ganzfeld stimulation at a distance of 10 mm, the intensity was above the melanopsin threshold,2,39 as indicated by reduced pupil diameter measured at 6 and 30 s following short wavelength stimulus offset compared to long wavelength stimuli. Excitation for each photoreceptor class is estimated using the toolbox provided by Lucas et al., (provided in the Appendix S1), which shows that the differential melanopsin activation between short and long wavelengths is large.40
Stimulus parameters included two durations, 1 and 5 s. Previous studies have used a range of stimulus durations from 4 ms to 30 s,14,41 and while it has been concluded that a 1 s stimulus is sufficient to stimulate the melanopsin pathway, the 5 s stimulation was included here to understand if PIPR metrics would be different for longer stimulations, as the ipRGCs have been shown to continue firing for the duration of a stimulus,5 while rod and cone activity decays over time.42 The order of stimulus presentation was consistent from baseline to follow up so that direct comparisons could be made. Following dark adaptation, the baseline pupil size was recorded in the dark for 10 s. A long wavelength 1 s pulse was presented to the left eye, and the pupil diameter of the right eye was measured for 60 s. Then a long wavelength 5 s pulse was presented to the left eye and the response of the right eye was again measured for 60 s. The subject dark adapted for 5 min, and the protocol was repeated with short wavelength light. Previous evidence suggests that prior long-wavelength light exposure enhances short-wavelength induced pupil constriction.43 Therefore, both long wavelength stimuli were presented, followed by a dark adaptation period, and then blue stimuli were presented, similar to previously published experiments.44 One measure was recorded for each stimulus duration, as the melanopsin response can persist for up to 3 min,14 and attempts were made to avoid potentiation of the response.45 This portion of the protocol took 16 min, including the two 5-min adaptation periods. Because of the short length, subject fatigue was not a concern.
Experimental period
Short wavelength-blocking glasses (Ultraspec 2000, https://www.uvex.com/en) were dispensed for the subjects to wear in the evenings for 2 weeks. The transmission spectrum of the glasses was confirmed, showing that the lenses absorb approximately 99% of light shorter than 540 nm, and transmit approximately 90% longer than 540 nm (Humphrey Lens Analyzer, https://www.zeiss.com, transmission spectrum provided in the Appendix S1). All experiments took place in the summer and autumn, in which sunset occurred prior to 8:00 pm (Earth System Research Laboratory, https://www.esrl.noaa.gov/gmd). Subjects were asked to wear the glasses from 8:00 pm until the lights were turned off in their home for bedtime, unless they habitually went to bed prior to 11:00 pm, in which case they were asked to put them on 3 h before bedtime. Therefore, all subjects wore the glasses for at least 3 h, although some wore them longer based on their habitual bedtime. In addition, subjects were asked to wear the glasses if they awoke during the night (any time before sunrise), before using any electronic devices or turning on a light. Subjects were advised to set an alarm reminding them to wear the glasses each night, and compliance was monitored by subject report.
Data analysis
Pupil data were analysed off line using custom written software (MATLAB, https://www.mathworks.com). Pupil diameter measurements were sampled at 60 Hz. The raw data included extreme, fast excursions of pupil diameter due to blinks, when the video frame of the ViewPoint system was not able to capture the pupil. The samples that the ViewPoint system identified as poor quality were removed. This strategy did not remove all extreme excursions, so additional filtering was applied. Samples were removed if they were less than 1 mm, if the rate of change was outside one standard deviation from the mean, or if the recorded pupil aspect ratio was outside one standard deviation from the mean. This strategy left very few excursions in the data.
Three metrics were used to evaluate the PIPR, which have been used in previous studies, and are useful in describing various parameters of the PIPR. Metrics included the 6 s PIPR, 30 s PIPR, and area under the curve (AUC). The PIPR 6 s after stimulus offset is commonly used in the literature to describe pupil redilation and takes into account inter-subject variability and age related effects in baseline pupil size, as values are normalised to each subject’s baseline pupil diameter.41,46 The 30 s PIPR provides further information on duration of ipRGC activity, as the PIPR can be sustained up to 83–180 s depending on stimulus duration.14 We found that the pupil diameter oscillated during redilation following short wavelength stimulation, and the 6 s PIPR value could fall on an oscillation, increasing the noise inherent in PIPR measurements. Therefore, the AUC was also calculated,47 which captures the influence of these ‘melanopsin oscillatory responses (mORs),’ or hippus, in pupil diameter seen during the redilation phase. Pupil metrics were calculated as follows (Table 1, Figure 2):
Table 1.
Pupil metrics. Key. s: seconds; PIPR: post illumination pupil response; AUC: area under the curve.
| Metric | Calculation |
|---|---|
| Baseline pupil diameter | Average pupil diameter 10 s prior to light stimulation |
| Maximum constriction | Minimum pupil diameter during light stimulation |
| 6 s PIPR | Pupil diameter averaged over 6–7 s after stimulus offset, relative to the baseline pupil diameter |
| 30 s PIPR | Pupil diameter averaged over 30–31 s after stimulus offset, relative to the baseline pupil diameter |
| AUC | Trapezoidal sum of the interpolated normalised trace for 20 s after light offset |
Figure 2.
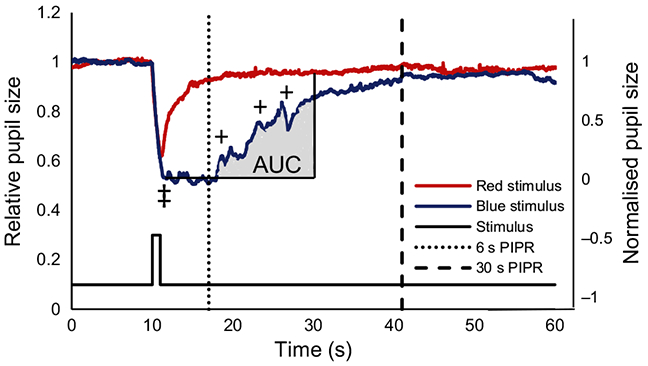
Pupil diameter of the right eye during a 1 s long wavelength stimulus (red trace) presented to the left eye, overlaid with the pupil diameter during a 1 s short wavelength stimulus (blue trace) for one representative subject. ‡ indicates the maximum pupil constriction, dotted and dashed lines show where the 6 s and 30 s PIPR (post illumination pupil response) are measured, + indicates the melanopsin oscillatory responses. The area under the curve (AUC) for the blue stimulus is shaded grey, and is calculated with respect to the normalised pupil size.
6 and 30 s PIPR
Pupil diameter was normalised to baseline, setting the average baseline pupil diameter during the 10 s prior to stimulation to 1. Relative pupil diameter was averaged over 6 to 7 s after stimulus offset and 30–31 s after stimulus offset for the retained samples following filtering.
Area under the curve (AUC)
Area under the curve was computed as the trapezoidal approximation of the integral of the interpolated, normalised trace for 20 s following light offset. The units are normalised pupil diameter times seconds.
For each of these metrics, a lower number indicates slower redilation following light offset and hence, increased melanopsin-driven ipRGC activity.
Data from the Actiwatch were downloaded and analysed with Actiware software (Actiware 6.0.4, http://www.usa.philips.com), which calculated mean daytime activity (counts per minute, CPM) and sleep latency, duration, and efficiency for each day. Values were averaged separately for the baseline week and for the two-week experimental period. Ambient illumination values greater than 1000 lux were classified as outdoors, as in previously published studies,48-50 and light exposure analyses were performed using log average daily illumination.51
Statistical analyses were performed with R (R Core Team 2105, https://www.r-project.org). For PIPR metrics, analyses were performed on normalised values. Shapiro–Wilk test showed that melatonin levels, PIPR metrics and log light exposure values were normally distributed and therefore, were analysed with simple linear regressions. Outlier analyses for melatonin levels and light exposure were performed with a modified Thompson tau test. Melatonin levels, pupil diameters, and actigraph parameters from baseline and the experimental period were analysed with paired two-tailed t-tests. P values less than 0.05 were considered statistically significant. Values are expressed as mean ± standard deviation.
Results
Of the 22 subjects, one subject was excluded due to noncompliance wearing the Actiwatch and the glasses. Actiwatch data indicated that the remaining subjects did not remove the watch during the experimental period, and hence, 21 were included in analyses. Subjects consisted of 10 females and 11 males, with an average age of 26.7 ± 7.8 years (range 17.4–39.7 years). Mean wear time of the glasses was 3:57 ± 1:03 h per night.
Actigraphy, melatonin and sleep analysis
During the baseline week, subjects spent an average of 93.6 ± 44.7 min outdoors per day, which was not significantly different from the two-week experimental period, 93.1 ± 43.0 min outdoors (P = 0.93). Subjects received a similar amount of daily light exposure during the baseline week and experimental period (1.4 × 106 lux per day, P = 0.83). For salivary melatonin analysis, three subjects did not provide sufficient saliva volume for night-time analysis, and one subject for morning analysis. One subject was determined to be an outlier using the modified Thompson tau test based on very low light exposure and excluded in regression analyses. For the remaining subjects, baseline morning melatonin, but not night-time melatonin, was statistically significantly associated with time spent outdoors, total daily cumulative broad band light exposure (Figure 3), blue light exposure, and red light exposure (P < 0.05 for all, see Table 2 for statistics).
Figure 3.
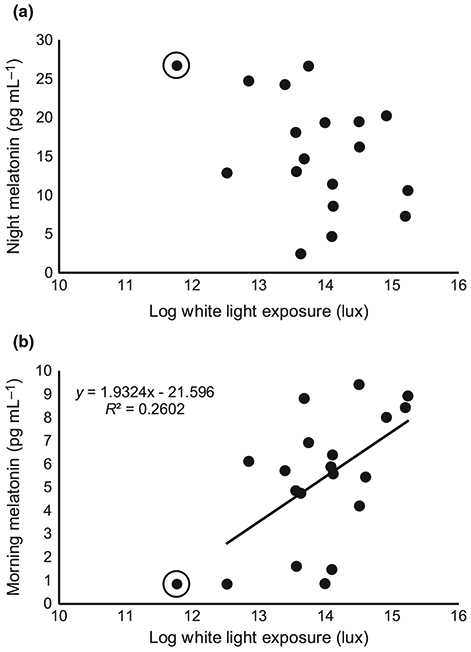
For the baseline week, (a) night melatonin was not significantly associated with total daily log white light exposure (P = 0.32). (b) Morning melatonin levels were statistically significantly associated with total daily log white light exposure during the baseline week (P < 0.05). Circled points are outliers and not included in regression analyses.
Table 2.
Linear regression statistics for morning melatonin and objectively measured time outdoors, and total daily broadband light exposure, and short and long wavelength light exposure.
| R2 | df | F | P-value | |
|---|---|---|---|---|
| Time outdoors | 0.25 | 19 | 5.85 | 0.02 |
| Broad band light exposure | 0.26 | 19 | 8.97 | 0.008 |
| Short wavelength (blue) light exposure | 0.34 | 19 | 9.46 | 0.007 |
| Long wavelength (red) light exposure | 0.30 | 19 | 7.89 | 0.01 |
Night-time melatonin at baseline was 16.1 ± 7.5 pg mL−1 (Figure 4). After the two-week experimental period, night-time melatonin statistically significantly increased 58% to 25.5 ± 10.7 pg mL−1 (effect size r = −0.44, t19 = −3.95, P = 0.0005). Morning melatonin decreased from 5.3 ± 2.8 pg mL−1 to 4.5 ± 2.6 pg mL−1 (not significantly different, P = 0.16).
Figure 4.
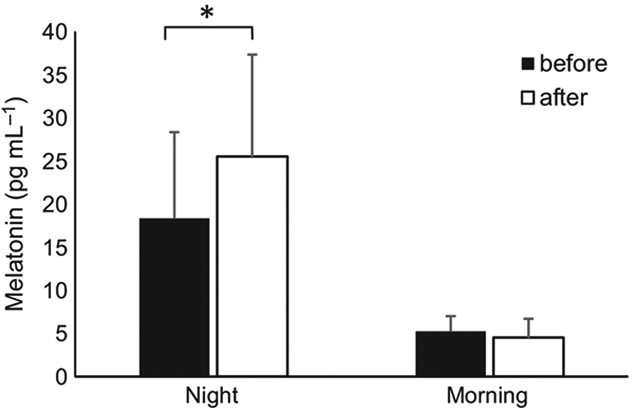
Melatonin levels measured via salivary assay at night and in the morning before (solid bars) and after (open bars) wearing blue blocking glasses at night-time for 2 weeks. * indicates P < 0.05
PSQI scores decreased (i.e. improved) or remained the same for all subjects after the experimental period, with an average score of 5.6 ± 2.9 at baseline, and a score of 3.0 ± 2.2 after wearing blue blocking glasses (effect size r = 0.43, t20 = 7.23, P < 0.0005). Objectively measured sleep duration statistically significantly increased by 24 min, from 408.7 ± 44.9 min to 431.5 ± 42.9 min (effect size r = −0.25, t20 = −3.77, P = 0.001). Average baseline time of sleep was 12:24 am ± 1:04, and during the experimental period was 11:57 pm ± 1:03, which was statistically significantly earlier by 27 min (effect size r = 0.22, t19 = 3.33, P = 0.004). Average morning wake time was similar between baseline, 7:18 am ± 0:38, and during the experimental period, 7:12 am ± 0:44 (P = 0.34). Sleep efficiency was not significantly different between baseline, 83.0 ± 9.2%, and the experimental period, 84.0 ± 6.1% (P = 0.37). Sleep latency was not significantly different between baseline, 12.4 ± 8.7 min, and the experimental period, 16.3 ± 10.6 min (P = 0.12). Mean daily activity in counts per minute was 260 ± 64 during the baseline week, and 266 ± 65 during the experimental period (P = 0.42).
Pupil analysis
Resting pupil diameter at baseline following dark adaptation was 6.01 ± 0.86 mm. After the experimental period, resting dark adapted pupil diameter increased to 6.18 ± 1.1 mm (not significantly different, P = 0.65). Baseline resting pupil diameter was statistically significantly correlated with age (R2 = 0.34, F20 = 9.85, P = 0.005). Minimum pupil diameter during 1 and 5 s short wavelength light stimulations at baseline were 2.87 ± 0.6 mm and 2.18 ± 0.39 mm, respectively. This increased, but was not statistically different, following the experimental period to 3.05 ± 0.76 mm and 2.32 ± 0.69 mm (P = 0.41 and 0.28).
Several parameters of the pupil response showed statistically significant changes following the experimental period (Table 3). Figure 5 shows the mean pupil traces for all subjects to 1 s short wavelength stimuli before and after the experimental period, illustrating a delayed redilation period following short wavelength stimulus offset after the experimental period. The 30 s PIPR to a 1 s short wavelength stimulus increased and the AUC for 1 and 5 s short wavelength stimuli decreased, suggesting increased ipRGC activity (Figure 6). Observed increases in the 6 s PIPR following the experimental period did not reach statistical significance. There were no significant differences in any pupil metrics to long wavelength light before and after the experimental period.
Table 3.
Statistical analysis for changes in pupil metrics to a 1 and 5 s short wavelength stimulus before and after the experimental period.
| Stimulus | PIPR metric | Effect size r | df | t stat | P |
|---|---|---|---|---|---|
| 1 s | 6 s PIPR | 0.22 | 19 | 1.68 | 0.06 |
| 30 s PIPR | 0.31 | 19 | 3.17 | *0.005 | |
| AUC | 0.34 | 19 | 3.68 | *0.002 | |
| 5 s | 6 s PIPR | 0.09 | 20 | −0.08 | 0.53 |
| 30 s PIPR | −0.04 | 20 | −0.94 | 0.82 | |
| AUC | 0.23 | 20 | 2.51 | *0.02 |
indicates P < 0.05. Key: PIPR: post illumination pupil response; AUC: area under the curve.
Figure 5.

Mean normalised pupil diameter for all subjects (n = 21) during a 1 s long wavelength and a 1 s short wavelength stimulus at baseline (dark red and blue, respectively) and after wearing blue blocking glasses at night-time for 2 weeks (light red and blue, respectively). 95% confidence intervals are shown in light grey for mean baseline pupil diameter and dark grey for two-week mean pupil diameter.
Figure 6.
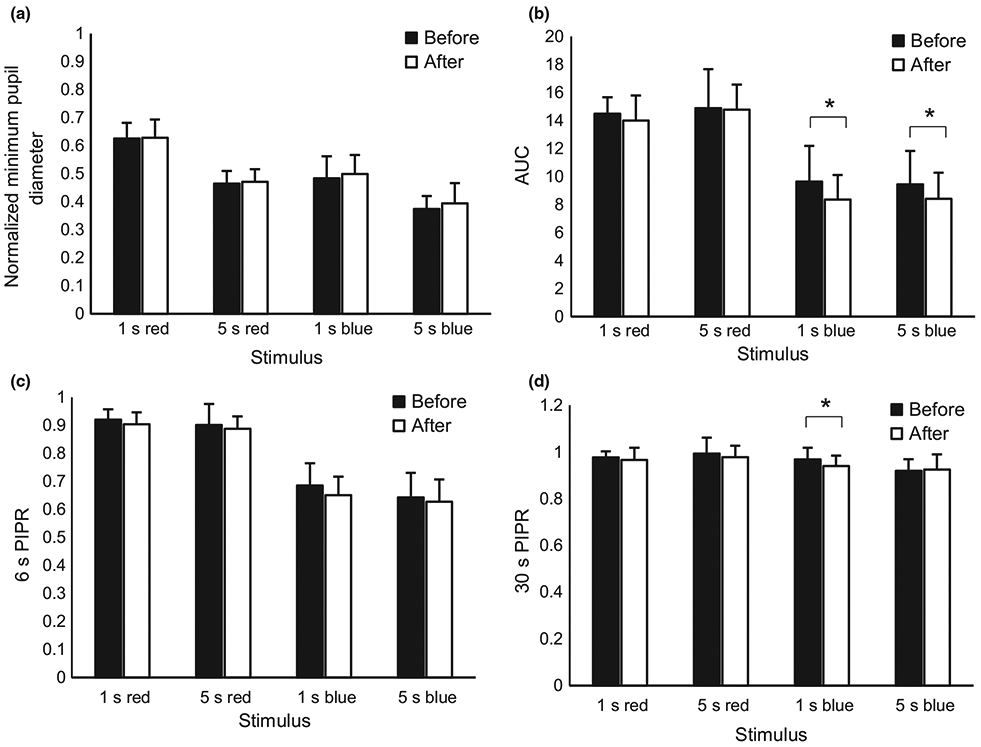
Post illumination pupil response (PIPR) metrics to 1 s and 5 s long wavelength (red) and short wavelength (blue) stimuli before (solid bars) and after (open bars) wearing blue blocking glasses at night-time for 2 weeks. (a) normalised minimum pupil diameter, (b) area under the curve (AUC), (c) 6 s PIPR, and (d) 30 s PIPR. * indicates P < 0.05.
Discussion
This study demonstrates that the PIPR, which is driven primarily by direct activation of melanopsin in the ipRGCs, is subject to modulation by attenuating short wavelength input at night. The ipRGC-driven pupil response has been shown to undergo circadian variations.36 Zele, et al., showed that the PIPR increases throughout the morning, with increased redilation dynamics towards the peak at 14:58 hours.36 Our results suggest that attenuating short wavelength light approximately four hours prior to subjects’ habitual bedtime, induced either an advance in the phase of circadian rhythm, or an increase in sensitivity of the ipRGCs, so that some metrics of the PIPR, measured at the same time of day as baseline measures, increased following the experimental period compared to the baseline period. Based on observed changes in sleep parameters and increase in night-time melatonin, we speculate that circadian phase was advanced. The blue blocking glasses likely prevented the circadian delay that has been shown to occur with exposure to light in the evening.52
Blocking artificial short wavelength light at night-time resulted in a statistically significant subjective improvement in sleep quality and an objective increase in sleep duration of 24 min per night. Subjects went to sleep 27 min earlier during the experimental period compared to baseline. Additionally, the PSQI score improved from poor sleep quality to good sleep quality. While no subjects had diagnosed sleep abnormalities at baseline, the mean PSQI score of 5.6 indicates that some subjects did have poor quality sleep at baseline, which improved following the experimental period. Melatonin levels, as measured through a salivary assay, statistically significantly increased 58% at night-time following approximately 4 h of blue blocking glasses wear (from approximately sunset to bedtime) for 2 weeks. Altered pupil dynamics following the experimental period suggest that demonstrated improvements in sleep and increases in melatonin are potentially mediated by the ipRGCs.
We showed statistically significant changes in the 30 s PIPR as well as the AUC following 2 weeks of blocking short wavelength light at night-time. An increase in the 6 s PIPR was observed, but the change did not reach statistical significance. Some studies have suggested that the 6 s PIPR is the most sensitive metric, at least in terms of intra- and interindividual variation.14 Perhaps with a larger sample size, observed changes in the 6 s PIPR would have reached statistical significance. The increase in 30 s PIPR and decrease in AUC suggest that the firing of ipRGCs following light offset is potentially sustained longer following decreased night-time input. Alternatively, there could be modifications in signal gain of the pupil control pathway. The change in measured activity could be due to a shift in the diurnal variation previously demonstrated in the PIPR.36 In a similar study in which subjects with delayed sleep phase disorder wore blue light-blocking glasses from 9:00 pm to bedtime, dim light melatonin onset was shown to advance by 78 min.53 While the previous study did not measure pupil responses, circadian shifts in melatonin suggest that the ipRGCs may also be undergoing circadian changes, as the ipRGC pathway ultimately leads to the pineal gland to control the release of melatonin. The increase in PIPR seen here suggests that a clinically significant change was induced in the ipRGCs as seen by the subsequent downstream increase in night-time melatonin and increase in objectively measured sleep duration.
Previous studies have evaluated circadian photoentrainment and sleep following implantation of blue-blocking intraocular lenses (IOLs).54,55 The authors reported that patients with blue-blocking IOLs showed no differences in any ipRGC-driven pupil responses, sleep-specific actigraph measures, melatonin onset or PSQI scores compared to subjects with neutral IOLs after 1 year. The authors did find that peak melatonin concentration was 50% lower in the blue-blocking IOL group compared to the neutral IOL group. The study design of the IOL studies is very different than that of the current study, in that IOL subjects were viewing blue blocking conditions at all times, i.e. the subjects never had the opportunity to view full intensity blue light in the environment. The number of hours of light and dark were not altered, whereas here, blue-blocking lenses were only used at a particular time of the day, which increased the number of hours that blue light was not available, while not eliminating blue light at all hours of the day. By increasing the hours without blue light stimulation, ‘darkness’ was effectively shifted earlier in the evening, resulting in alterations to night-time melatonin concentration, increases in sleep duration and an increase in the PIPR as measured in the morning.
Here, the Actiwatch Spectrum was employed to measure subjects’ habitual sleep and light exposure patterns over a 3 week period. The Actiwatch Spectrum is ideal because in addition to continuous activity and sleep measures, ambient illumination is recorded in terms of broad band light exposure and spectral composition. Modern sensor technology now allows these types of sleep studies to be carried out in subjects’ homes,56,57 in some cases, replacing the need for overnight stays in sleep labs.32,58,59 The Actiwatch has been shown to be similar to polysomnography, the gold standard for monitoring sleep,60 and has been actively used in sleep related studies.61,62 Specifically, the Actiwatch-derived sleep duration was demonstrated to be strongly correlated to that derived from polysomnography.60 Actigraphy has been shown to be a reliable and cost-effective method to obtain continuous measurements of sleep and activity.57 However, home monitoring does present some limitations. Subjects were instructed to go about their daily routine, without a specification of when they should go to bed or wake up, or how to set their environmental illumination, which may have introduced variability to the data that could have been controlled within a sleep facility. We also utilised the Actiwatch for measuring light exposure.50 In order to measure light exposure, the device must be unobstructed by clothing. If the data indicated that the device was obstructed, the data were not included in the analysis. Additionally, the device is located at wrist level as opposed to eye level, which could potentially results in a discrepancy between measured and actual light exposure. However, the correlation between light exposure measured at eye level versus the wrist has been reported to be 0.76.63
Intrinsic stimulation of ipRGCs is most sensitive to short wavelength light with a peak at ~482 nm, although the spectral sensitivity spans a wider range.1 The ipRGCs also receive input from rod and cone photoreceptors.3,64,65 The majority of direct input to the ipRGCs was blocked via short wavelength absorbing glasses; however, the ipRGCs continued to receive synaptic (extrinsic) stimulation from long wavelength light via the rod and cone pathway. Unless subjects are in complete darkness, it is not possible to block all input to the ipRGCs. However, input from the rod-cone circuitry does not activate melanopsin, and these extrinsic responses have different temporal properties than intrinsic signals,66 suggesting that demonstrated changes in dynamics were a function of successfully reducing melanopsin-driven ipRGC signalling at night-time.
In this study, a control group with clear lenses was not utilised, potentially introducing a placebo effect. The blue blocking glasses used here (Uvex) are yellow tinted, and therefore, it would have been difficult to blind subjects as to which condition they were in. As opposed to many narrow spectrum blue blocking lenses that appear clear, the Uvex lenses block 99% of blue light, and the goal of this experiment was to decrease short wavelength stimulation to the highest degree possible. To minimise a placebo effect, we sought to use objective measures of sleep, melatonin and the pupil at baseline for each subject as a control. Additionally, subjects were not made aware of the changes we expected to observe in sleep following glasses wear. The increase in night-time melatonin and increased sleep duration afforded from the Uvex glasses was robust, and it is possible that these increases would remain if lenses that decrease, rather than block, blue light are utilised. Therefore, to expand on the results found here, a randomised controlled experiment could be performed in the future in which clear lenses are utilised and subjects are blinded to whether the lenses filter blue light or not.
A potential limitation for the use of orange tinted glasses before bedtime as a therapeutic method to improve sleep is the yellow tinted percept induced by the lenses. Subjects reported that they adapted to the yellow tint after wearing the glasses for about 10 minutes. However, the tint may cause concern during some activities such as night driving. Additionally, some individuals might be self-conscious about wearing the lenses outside of the home. These limitations could be combatted in the future through the development of clear blue blocking lenses that can block close to 100% of the short wavelength light. Moreover, it may not be necessary to wear the glasses every night to appreciate beneficial effects.
In conclusion, attenuating input to the ipRGCs via short wavelength-blocking glasses at night is a practical method to increase endogenous melatonin before sleep, improve sleep duration and help regulate circadian rhythm by combatting the abundance of night-time blue light exposure, while allowing the continued use of artificial light and electronic devices after sunset. Evidence suggests that these demonstrated improvements in sleep are mediated by melanopsin-driven ipRGC activity.
Supplementary Material
Acknowledgements
This work was supported by NIH NEI P30 EY007551 and NIH T35 EY07088. Special thanks to Edwin Ostrin for statistical support and to David Calkins for helpful comments on the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Spectral properties of stimuli and transmission spectrum of blue blocking glasses.
References
- 1.Berson DM, Dunn FA & Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002; 295: 1070–1073. [DOI] [PubMed] [Google Scholar]
- 2.Dacey DM, Liao HW, Peterson BB et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 2005; 433: 749–754. [DOI] [PubMed] [Google Scholar]
- 3.Guler AD, Ecker JL, Lall GS et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature 2008; 453: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruby NF, Brennan TJ, Xie X et al. Role of melanopsin in circadian responses to light. Science 2002; 298: 2211–2213. [DOI] [PubMed] [Google Scholar]
- 5.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW & Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res 2007; 47: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hattar S, Kumar M, Park A et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol 2006; 497: 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc 1983; 42: 2783–2789. [PubMed] [Google Scholar]
- 8.Liao HW, Ren X, Peterson BB et al. Melanopsin-expressing ganglion cells on macaque and human retinas form two morphologically distinct populations. J Comp Neurol 2016; 524: 2845–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasir-Ahmad S, Lee SC, Martin PR & Grunert U. Melanopsin-expressing ganglion cells in human retina: morphology, distribution, and synaptic connections. J Comp Neurol 2017. doi: 10.1002/cne.24176. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Hannibal J, Christiansen AT, Heegaard S, Fahrenkrug J & Kiilgaard JF. Melanopsin expressing human retinal ganglion cells: subtypes, distribution, and intraretinal connectivity. J Comp Neurol 2017; 525(8): 1934–1961. [DOI] [PubMed] [Google Scholar]
- 11.Gooley JJ, Lu J, Fischer D & Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci 2003; 23: 7093–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardeland R, Pandi-Perumal SR & Cardinali DP. Melatonin. Int J Biochem Cell Biol 2006; 38: 313–316. [DOI] [PubMed] [Google Scholar]
- 13.Young RS & Kimura E. Pupillary correlates of light-evoked melanopsin activity in humans. Vision Res 2008; 48: 862–871. [DOI] [PubMed] [Google Scholar]
- 14.Adhikari P, Zele AJ & Feigl B. The post-illumination pupil response (PIPR). Invest Ophthalmol Vis Sci 2015; 56: 3838–3849. [DOI] [PubMed] [Google Scholar]
- 15.Hartwick AT, Bramley JR, Yu J et al. Light-evoked calcium responses of isolated melanopsin-expressing retinal ganglion cells. J Neurosci 2007; 27: 13468–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buijs RM, Chun SJ, Niijima A, Romijn HJ & Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol 2001; 431: 405–423. [DOI] [PubMed] [Google Scholar]
- 17.Kalsbeek A, Fliers E, Franke AN, Wortel J & Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 2000; 141: 3832–3841. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Shlomo R Chronodisruption, cell cycle checkpoints and DNA repair. Indian J Exp Biol 2014; 52: 399–403. [PubMed] [Google Scholar]
- 19.Reid KJ & Zee PC. Circadian rhythm sleep disorders. Handb Clin Neurol 2011; 99: 963–977. [DOI] [PubMed] [Google Scholar]
- 20.Monteleone P, Martiadis V & Maj M. Circadian rhythms and treatment implications in depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1569–1574. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz JR & Roth T. Shift work sleep disorder: burden of illness and approaches to management. Drugs 2006; 66: 2357–2370. [DOI] [PubMed] [Google Scholar]
- 22.Erren TC, Gross JV & Meyer-Rochow VB. Light, clocks, mood, and cancer: consolidation and novel tests of latitude and instability hypotheses. Chronobiol Int 2011; 28: 471–473; author reply p 473. [DOI] [PubMed] [Google Scholar]
- 23.Erren TC & Reiter RJ. Light Hygiene: time to make preventive use of insights–old and new–into the nexus of the drug light, melatonin, clocks, chronodisruption and public health. Med Hypotheses 2009; 73: 537–541. [DOI] [PubMed] [Google Scholar]
- 24.Van Someren EJ & Riemersma-Van Der Lek RF. Live to the rhythm, slave to the rhythm. Sleep Med Rev 2007; 11: 465–484. [DOI] [PubMed] [Google Scholar]
- 25.Vartanian GV, Li BY, Chervenak AP et al. Melatonin suppression by light in humans is more sensitive than previously reported. J Biol Rhythms 2015; 30:351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brainard GC, Hanifin JP, Warfield B et al. Short-wavelength enrichment of polychromatic light enhances human melatonin suppression potency. J Pineal Res 2015; 58: 352–361. [DOI] [PubMed] [Google Scholar]
- 27.Mai E & Buysse DJ. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Medicine Clinics 2008; 3: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mottram AR & Svenson JE. Rhythm disturbances. Emerg Med Clin North Am 2011; 29: 729–746, vi. [DOI] [PubMed] [Google Scholar]
- 29.Heath M, Sutherland C, Bartel K et al. Does one hour of bright or short-wavelength filtered tablet screenlight have a meaningful effect on adolescents’ pre-bedtime alertness, sleep, and daytime functioning? Chronobiol Int 2014; 31: 496–505. [DOI] [PubMed] [Google Scholar]
- 30.Cajochen C, Dijk DJ & Borbely AA. Dynamics of EEG slowwave activity and core body temperature in human sleep after exposure to bright light. Sleep 1992; 15: 337–343. [PubMed] [Google Scholar]
- 31.Munch M, Kobialka S, Steiner R, Oelhafen P, Wirz-Justice A & Cajochen C. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Physiol Regul Integr Comp Physiol 2006; 290: R1421–R1428. [DOI] [PubMed] [Google Scholar]
- 32.Cajochen C, Frey S, Anders D et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol 2011; 110: 1432–1438. [DOI] [PubMed] [Google Scholar]
- 33.Burkhart K & Phelps JR. Amber lenses to block blue light and improve sleep: a randomized trial. Chronobiol Int 2009; 26: 1602–1612. [DOI] [PubMed] [Google Scholar]
- 34.van der Lely S, Frey S, Garbazza C et al. Blue blocker glasses as a countermeasure for alerting effects of evening light-emitting diode screen exposure in male teenagers. J Adolesc Health 2015; 56: 113–119. [DOI] [PubMed] [Google Scholar]
- 35.Kayumov L, Casper RF, Hawa RJ et al. Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work. J Clin Endocrinol Metab 2005; 90: 2755–2761. [DOI] [PubMed] [Google Scholar]
- 36.Zele AJ, Feigl B, Smith SS & Markwell EL. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS ONE 2011; 6: e17860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voultsios A, Kennaway DJ & Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms 1997; 12: 457–466. [DOI] [PubMed] [Google Scholar]
- 38.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR & Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 39.Barrionuevo PA, Nicandro N, McAnany JJ, Zele AJ, Gamlin P & Cao D. Assessing rod, cone, and melanopsin contributions to human pupil flicker responses. Invest Ophthalmol Vis Sci 2014; 55:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucas RJ, Peirson SN, Berson DM et al. Measuring and using light in the melanopsin age. Trends Neurosci 2014; 37: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH & Hood DC. Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci 2011; 52: 6624–6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perlman I & Normann RA. Light adaptation and sensitivity controlling mechanisms in vertebrate photoreceptors. Prog Retin Eye Res 1998; 17: 523–563. [DOI] [PubMed] [Google Scholar]
- 43.Mure LS, Rieux C, Hattar S & Cooper HM. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms 2007; 22: 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munch M, Leon L, Crippa SV & Kawasaki A. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Invest Ophthalmol Vis Sci 2012; 53: 4546–4555. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, Tu DC, Denner D, Shane T, Fitzgerald CM & Van Gelder RN. Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Invest Ophthalmol Vis Sci 2007; 48: 1268–1275. [DOI] [PubMed] [Google Scholar]
- 46.Adhikari P, Pearson CA, Anderson AM, Zele AJ & Feigl B. Effect of age and refractive error on the melanopsin mediated Post-Illumination Pupil Response (PIPR). Sci Rep 2015; 5:17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herbst K, Sander B, Lund-Andersen H et al. Intrinsically photosensitive retinal ganglion cell function in relation to age: a pupillometric study in humans with special reference to the age-related optic properties of the lens. BMC Ophthalmol 2012; 12:4. doi: 10.1186/1471-2415-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Read SA, Collins MJ & Vincent SJ. Light exposure and physical activity in myopic and emmetropic children. Optom Vis Sci 2014;91:330–341. [DOI] [PubMed] [Google Scholar]
- 49.Dharani R, Lee CF, Theng ZX et al. Comparison of measurements of time outdoors and light levels as risk factors for myopia in young Singapore children. Eye (Lond) 2012; 26: 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostrin LA. Objectively measured light exposure in emmetropic and myopic adults. Optom Vis Sci 2017; 94: 229–238. [DOI] [PubMed] [Google Scholar]
- 51.Read SA, Collins MJ & Vincent SJ. Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci 2015; 56: 6779–6787. [DOI] [PubMed] [Google Scholar]
- 52.Khalsa SB, Jewett ME, Cajochen C & Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol 2003; 549: 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esaki Y, Kitajima T, Ito Y et al. Wearing blue light-blocking glasses in the evening advances circadian rhythms in the patients with delayed sleep phase disorder: an open-label trial. Chronobiol Int 2016; 33: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 54.Brondsted AE, Haargaard B, Sander B, Lund-Andersen H, Jennum P & Kessel L. The effect of blue-blocking and neutral intraocular lenses on circadian photoentrainment and sleep one year after cataract surgery. Acta Ophthalmol 2016. doi: 10.1111/aos.13323. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 55.Brondsted AE, Sander B, Haargaard B et al. The effect of cataract surgery on circadian photoentrainment: a randomized trial of blue-blocking versus neutral intraocular lenses. Ophthalmology 2015; 122: 2115–2124. [DOI] [PubMed] [Google Scholar]
- 56.Lichstein KL, Stone KC, Donaldson J et al. Actigraphy validation with insomnia. Sleep 2006; 29: 232–239. [PubMed] [Google Scholar]
- 57.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W & Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003; 26: 342–392. [DOI] [PubMed] [Google Scholar]
- 58.Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA & Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep 2014; 37: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West KE, Jablonski MR, Warfield B et al. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J Appl Physiol 2011; 110: 619–626. [DOI] [PubMed] [Google Scholar]
- 60.Mantua J, Gravel N & Spencer RM. Reliability of sleep measures from four personal health monitoring devices compared to research-based actigraphy and polysomnography. Sensors 2016; 16: pii: E646. doi: 10.3390/s16050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamasaki A, Kawasaki Y, Takeda K et al. The relationships among sleep efficiency, pulmonary functions, and quality of life in patients with asthma. Int J Gen Med 2014; 7: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuo HH, Chiu MJ, Liao WC & Hwang SL. Quality of sleep and related factors during chemotherapy in patients with stage I/II breast cancer. J Formos Med Assoc 2006; 105: 64–69. [DOI] [PubMed] [Google Scholar]
- 63.Okudaira N, Kripke DF & Webster JB. Naturalistic studies of human light exposure. Am J Physiol 1983; 245: R613–R615. [DOI] [PubMed] [Google Scholar]
- 64.Jasser SA, Hanifin JP, Rollag MD & Brainard GC. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms 2006; 21: 394–404. [DOI] [PubMed] [Google Scholar]
- 65.Jusuf PR, Lee SC, Hannibal J & Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci 2007; 26: 2906–2921. [DOI] [PubMed] [Google Scholar]
- 66.Wong KY, Dunn FA, Graham DM & Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol 2007; 582: 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


