Abstract
Phospholipase A2 (PLA2) enzymes play a major role in many diseases including the inflammatory cascade and specific potent small molecule inhibitors could be useful in studying their physiological role as well as for the development of drugs. In order to discover novel small molecule inhibitor platforms for members of the PLA2 superfamily of enzymes, we have applied computational approaches to determine the binding mode of potent inhibitors specific for particular PLA2s to the screening of chemical libraries. This has including the U.S. National Institutes of Health (NIH) National Cancer Institute (NCI) Diversity Set V and the ChemBridge commercial compound libraries. We have then subjected identified inhibitor structures to recently developed lipidomics based screening assays to determine the XI(50) and specificity of the identified compounds for specific PLA2s. Herein we review this approach and report the identity of initial hits for both the Group IVA cytosolic PLA2 and the Group VIA Ca2+-independent PLA2 that are worthy of further structural modification to develop novel platforms for inhibitor development.
Keywords: Phospholipase A2, virtual screening, enzymatic assays, dose-response inhibition, hit compounds
1. Introduction
Assaying the enzymatic activity of phospholipase A2 (PLA2) is a challenging task because the superfamily of PLA2s include water-soluble enzymes acting on aggregated water-insoluble phospholipids (Dennis et al., 2011; Mouchlis et al., 2015). Thus, these enzymes must initially associate with the surface of the membrane to extract and bind their phospholipid substrate. Traditional PLA2 assays have employed radio-labeled phospholipids that contain 3H- or 14C-labeled fatty acids at the sn-2 position of the phospholipid. Such phospholipids are challenging to synthesize, expensive, and very limited in terms of commercial availability (Yang et al., 1999).
The “surface dilution kinetics model” was developed and successfully employed by our laboratory to explain the action of PLA2 enzymes on phospholipid/detergent mixed micelles (Carman et al., 1995; Roberts et al., 1977). The success of the surface dilution approach to explain kinetics of PLA2 enzymes in mixed micelles, the stability of the micelle structure in the presence of various phospholipids and/or inhibitors, and the high efficiency in preparing mixed micelles makes mixed micelles an extremely suitable and attractive physical form of substrate to employ in a PLA2 assay (Ribeiro and Dennis, 1975).
Lipidomics-based liquid chromatographic/mass spectrometric (LC/MS) approaches have proven to be very powerful in establishing novel high-throughput assays for PLA2 enzymes (Mouchlis et al., 2018). These assays enabled us to perform detailed studies to define headgroup and sn-2 acyl-chain specificity on a wide variety of phospholipid substrates with great success (Mouchlis et al., 2018; Mouchlis and Dennis, 2019). We have used these assays for the three most well-studied human PLA2 enzymes including cytosolic (cPLA2), calcium-independent (iPLA2), and secreted (sPLA2) enzymes (Vasquez et al., 2018). In addition, dose-response inhibition studies on commercially available inhibitors allowed us to validate the application of these assays in identifying novel PLA2 inhibitors (Mouchlis et al., 2019).
The PLA2 superfamily contains 16 groups of structurally and functionally diverse enzymes (Dennis et al., 2011). The six main types of PLA2 enzymes include the secreted (sPLA2), cytosolic (cPLA2), calcium-independent (iPLA2), platelet-activating factor acetylhydrolase (PAF-AH), also known as lipoprotein-associated PLA2 (Lp-PLA2), lysosomal PLA2 (LPLA2), and adipose-PLA2 (AdPLA). Our recent studies have focused on three human recombinant enzymes, namely the Group IVA cytosolic (cPLA2), Group VIA calcium-independent (iPLA2), and Group V secreted (sPLA2), which are all water-soluble, membrane-associated enzymes with distinct structures and biological functions (Mouchlis and Dennis, 2016, 2019). The structure of each enzyme contains a unique active site where the substrate binds and an interfacial surface that mediates association with cellular membranes (Mouchlis et al., 2015). Virtual screening using computational chemistry is a useful tool to identify new hit compounds for druggable targets (Mouchlis et al., 2011; Mouchlis et al., 2020). This manuscript discusses the application of a newly developed lipidomics-based high-throughput assay in combination with computational approaches to screen compound libraries for the identification of novel PLA2 inhibitors.
2. Lipidomics-based PLA2 assay
Advances in lipidomics enabled the identification and classification of numerus lipid metabolites using state-of-the-art LC/MS methods (Quehenberger et al., 2010). As part of our continuous efforts to advance the lipidomics field, we have developed a high-throughput assay that allowed as to define substrate specificity for cPLA2, iPLA2 and sPLA2 (Mouchlis et al., 2018). A combination of hydrophilic interaction chromatography (HILIC), reversed-phase chromatography (C18), and multiple reaction monitoring (MRM), allowed quantification of a variety of lysophospholipid and free fatty acid products (Figure 1) (Mouchlis et al., 2019). Lysophospholipids and free fatty acids were detected using both positive and negative ion mode, respectively (Mouchlis et al., 2018). Dose-response inhibition experiments on pyrrophenone which is a specific pyrrolidine cPLA2 inhibitor (Seno et al., 2001), OTFP which is a specific fluoroketone iPLA2 inhibitor (Mouchlis et al., 2016b), and Ly315920 which is a specific indole sPLA2 inhibitor (Snyder et al., 1999) were employed to validate the applicability of the assay in identifying PLA2 inhibitors.
Figure 1.

Identification of lysophospholipid and free fatty acid products: (A) for lysophospholipids using a HILIC column, and (B) for free fatty acids using a C18 reversed-phase column (from reference Mouchlis et al., 2019).
3. Virtual Screening
Molecular docking and molecular dynamics are powerful computational techniques for understanding the interactions of an inhibitor with the active site of an enzyme. Normally, molecular dynamics simulations allow full flexibility for both the inhibitor and the enzyme, but such flexibility is time-consuming in terms of computational time especially when a large number of inhibitors is to be studied. Therefore, while ligand flexibility is well accounted for in molecular docking techniques, in the current application, the enzyme is not allowed to move during the calculations. The relaxed complex scheme combines the advantages of docking calculations with dynamic structural information provided by molecular dynamics simulations (Amaro et al., 2008). In particular, the enzyme-inhibitor complex is subjected to molecular dynamics simulations followed by clustering analysis to various selected conformations of the enzyme for molecular docking (Figure 2).
Figure 2.

Clustering analysis for (A) cPLA2 and (B) iPLA2. Five conformations of each enzyme are depicted in various colors.
The U.S. National Institutes of Health (NIH) National Cancer Institute (NCI) Diversity Set V and the ChemBridge commercial compound libraries were virtually screened against the five “selected” conformations of each enzyme using GLIDE (Friesner et al., 2004). From each of the two compound libraries, eighty compounds were selected for in vitro screening based on the following criteria: (i) the docking score, (ii) the ability of the compound to form hydrogen-bonding with the oxyanion hole (Gly/Gly) of each enzyme, and (iii) the frequency that a compound appeared as top-ranked among the five conformations of each enzyme. Based on the above criteria 80 compounds were selected from the NCI Diversity Set V and 160 compounds from the ChemBridge compound libraries for each enzyme. Each compound was dissolved in DMSO at a concentration of 5 mM and tested for its inhibitory activity against cPLA2 and iPLA2.
4. In vitro screening and inhibitor binding interactions
Group specific assays were employed to determine the activity of human recombinant Group IVA cytosolic (cPLA2) and Group VIA calcium-independent (iPLA2) phospholipases A2 in a mixed micelle 96 well-plate assay, as previously described (Mouchlis et al., 2019; Mouchlis et al., 2018). The strategy is schematically summarized in Figure 3. The substrate for each enzyme consisted of 100 μM phospholipid, 400 μM C12E8 surfactant, and 2.5 μM 17:0 LPC internal standard. For cPLA2, the total phospholipid concentration (100 μM) consisted of 97 μM phospholipid substrate and 3 μM PI(4,5)P2 which enhances the activity of the enzyme. A specific buffer was prepared to achieve optimum activity for each enzyme. The buffer for cPLA2 contained 100 mM HEPES pH 7.5, 90 μM CaCl2, and 2 mM DTT (Mouchlis et al., 2016a). For iPLA2, the buffer consisted of 100 mM HEPES pH 7.5, 2 mM ATP, and 4 mM DTT Mouchlis et al., 2016b). The enzymatic reaction was performed in a 96 well-plate using a Benchmark Scientific H5000-H MultiTherm heating shaker for 30 min at 40 °C. Each reaction was quenched with 120 μL of methanol/acetonitrile (80/20, v/v), and the samples were analyzed using the HPLC-MS system. A blank in which enzyme was omitted, was also included for each experiment to determine the product of non-enzymatic hydrolysis and to detect any changes in the intensity of 17:0 LPC. Dose-response inhibition curves were generated using GraphPad Prism 5.0 and the non-linear regression by plotting percentage of inhibition vs log (mole fraction) to calculate the reported XI(50) values and their associated error.
Figure 3.
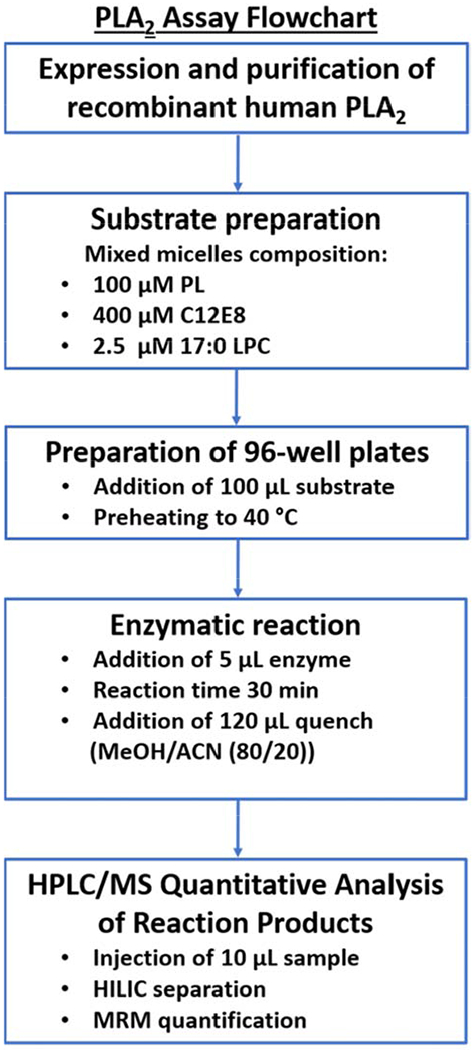
Schematic representation of PLA2 assay (PLA2, phospholipase A2; PL, phospholipid; C12E8, surfactant; LPC, lysophospholipid; MeOH, methanol; ACN, acetonitrile; HPLC/MS, high-performance liquid chromatography/mass spectrometry; HILIC, hydrophilic interaction chromatography; MRM, Multiple reaction monitoring).
Table 1 summarizes the structure, percent inhibition at a 5 mM concentration, and the XI(50) values of six novel hit compounds. Compounds 1, 3, and 4 showed better inhibitory activity toward iPLA2 and they exhibited lower inhibitor activity towards cPLA2. Compound 2 showed good activity toward cPLA2 while its activity toward iPLA2 was lower. Compound 5 showed low activity toward both enzymes. Compound 6 exhibited good activity toward both enzymes. The binding mode of compound 2 in the active site of cPLA2 revealed hydrogen-bonding with the oxyanion hole of Gly197/Gly198 and pi-pi stacking of the aromatic rings with Phe199, Trp232, Phe295, and Phe683. The amide group participates in hydrogen-bonding with Asn555 (Figure 4A). The binding mode of compound 3 in the active site of iPLA2 revealed hydrogen bonding with the oxyanion hole of Gly486/Gly487 and a halogen bond of the chlorine atom with the backbone amide of Met544. The aromatic rings participate in pi-pi stacking with Tyr643, Phe644, Phe722, and Tyr541 (Figure 4B).
Table 1.
XI(50) values of cPLA2 and iPLA2 inhibitors.
| cPLA2 | iPLA2 | |||||
|---|---|---|---|---|---|---|
| No | Code | Structure | % Inh. | XI(50) | % Inh. | XI(50) |
| 1 | 643029 (Div. Set V) |  |
94 | 1.5 ± 1 | 99 | 0.05 ± 0.01 |
| 2 | 6353053 (ChemBridge) |  |
98 | 0.05 ± 0.05 | 100 | 0.1 ± 0.1 |
| 3 | 6937786 (ChemBridge) |  |
98 | 0.3 ± 0.1 | 100 | 0.020 ± 0.008 |
| 4 | 20192 (Div. Set V) |  |
97 | 0.2 ± 0.2 | 100 | 0.040 ± 0.005 |
| 5 | 204262 (Div. Set V) | 94 | 0.5 ± 0.2 | 84 | 0.10 ± 0.08 | |
| 6 | 46492 (Div. Set V) |  |
96 | 0.03 ± 0.02 | 100 | 0.020 ± 0.005 |
Figure 4.
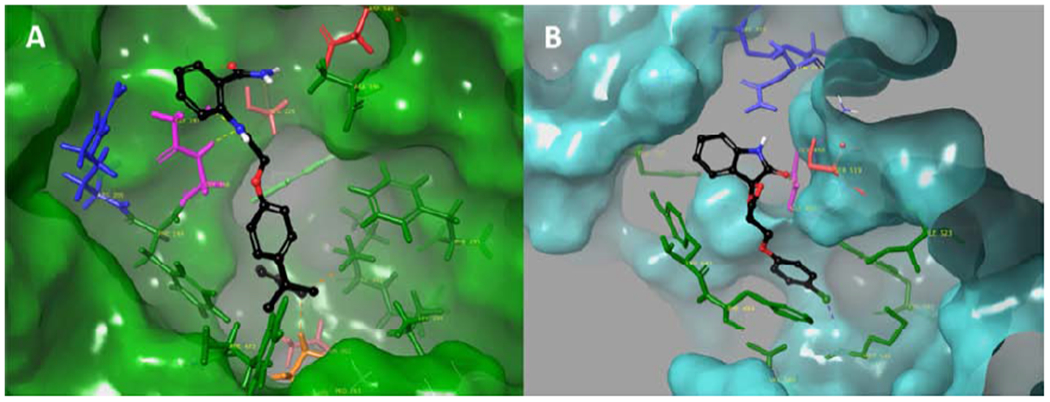
Binding mode of PLA2 inhibitors. (A) compound 2 in the active site of cPLA2 and (B) compound 3 in the active site of iPLA2.
5. Conclusion
In this manuscript, we have summarized our new lipidomics-based high-throughput assay approach for PLA2 enzymes and explored the use of this assay to identify novel chemical platforms from which potent and selective inhibitors can be developed for members of this enzyme superfamily. The relaxed complex scheme was employed to virtually screen two compound libraries, the NCI Diversity Set V and the ChemBridge collection. Based on the docking score, the ability of the compound to form hydrogen-bonding with the oxyanion hole (Gly/Gly) of each enzyme, and the frequency that a compound appeared as top-ranked among the five conformations of each enzyme, 160 compounds from each library were selected for in vitro screening against cPLA2 and iPLA2. New hit compounds were identified which are now candidates for further hit-to-lead optimization.
Acknowledgment
We wish to thank the NIH grant GM20501-44 for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CM and RLH carried out the computational selection and assays. VM and EAD designed and supervised the study and drafted the manuscripts.
References
- Amaro R, Baron R, McCammon J, 2008. An improved relaxed complex scheme for receptor flexibility in computer-aided drug design. J. Comput.-Aided Mol. Des. 22, 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman G, Deems R, Dennis E, 1995. Lipid signaling enzymes and surface dilution kinetics. J. Biol. Chem. 270, 18711–18714. [DOI] [PubMed] [Google Scholar]
- Dennis E, Cao J, Hsu Y-H, Magrioti V, Kokotos G, 2011. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111, 6130–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS, 2004. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47, 1739–1749. [DOI] [PubMed] [Google Scholar]
- Mouchlis VD, Armando A, Dennis EA, 2019. Substrate-Specific Inhibition Constants for Phospholipase A2 Acting on Unique Phospholipid Substrates in Mixed Micelles and Membranes Using Lipidomics. J. Med. Chem. 62, 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchlis VD, Barbayianni E, Mavromoustakos TM, Kokotos G, 2011. The application of rational design on phospholipase A2 inhibitors. Curr. Med. Chem. 18, 2566–2582. [DOI] [PubMed] [Google Scholar]
- Mouchlis VD, Bucher D, McCammon JA, Dennis EA, 2015. Membranes serve as allosteric activators of phospholipase A2, enabling it to extract, bind, and hydrolyze phospholipid substrates. PNAS 112, E516–E525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchlis VD, Chen Y, McCammon JA, Dennis EA, 2018. Membrane Allostery and Unique Hydrophobic Sites Promote Enzyme Substrate Specificity. J. Am. Chem. Soc. 140, 3285–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchlis VD, Dennis EA, 2016. Membrane and inhibitor interactions of intracellular phospholipases A2. Adv. Biol. Regul. 61, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchlis VD, Dennis EA, 2019. Phospholipase A2 catalysis and lipid mediator lipidomics. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchlis VD, Limnios D, Kokotou MG, Barbayianni E, Kokotos G, McCammon JA, Dennis EA, 2016a. Development of potent and selective inhibitors for group VIA calcium-independent phospholipase A2 guided by molecular dynamics and structure-activity relationships. J. Med. Chem. 59, 4403–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchlis VD, Melagraki G, Zacharia LC, Afantitis A, 2020. Computer-Aided Drug Design of β-Secretase, γ-Secretase and Anti-Tau Inhibitors for the Discovery of Novel Alzheimer’s Therapeutics. Int. J. Mol. Sci. 21, 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchlis VD, Morisseau C, Hammock BD, Li S, McCammon JA, Dennis EA, 2016b. Computer-aided drug design guided by hydrogen/deuterium exchange mass spectrometry: A powerful combination for the development of potent and selective inhibitors of Group VIA calcium-independent phospholipase A2. Bioorg. Med. Chem. 24, 4801–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CRH, Guan ZQ, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA, 2010. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 51, 3299–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AA, Dennis EA, 1975. PMR relaxation times of micelles of the nonionic surfactant Triton X-100 and mixed micelles with phospholipids. Chem. Phys. Lipids 14, 193–199. [DOI] [PubMed] [Google Scholar]
- Roberts MF, Deems RA, Dennis EA, 1977. Dual role of interfacial phospholipid in phospholipase A2 catalysis. PNAS 74, 1950–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno K, Okuno T, Nishi K, Murakami Y, Yamada K, Nakamoto S, Ono T, 2001. Pyrrolidine inhibitors of human cytosolic phospholipase A2. Part 2: synthesis of potent and crystallized 4-triphenylmethylthio derivative ‘pyrrophenone’. Bioorg. Med. Chem. Lett. 11, 587–590. [DOI] [PubMed] [Google Scholar]
- Snyder DW, Bach NJ, Dillard RD, Draheim SE, Carlson DG, Fox N, Roehm NW, Armstrong CT, Chang CH, Hartley LW, 1999. Pharmacology of LY315920/S-5920,[[3-(Aminooxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl] oxy] acetate, a potent and selective secretory phospholipase A2 inhibitor: a new class of anti-inflammatory drugs, SPI. J. Pharmacol. Exp. Ther. 288, 1117–1124. [PubMed] [Google Scholar]
- Vasquez AM, Mouchlis VD, Dennis EA, 2018. Review of four major distinct types of human phospholipase A2. Adv. Biol. Regul. 67, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Mosior M, Johnson C, Chen Y, Dennis E, 1999. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal. Biochem. 269, 278–288. [DOI] [PubMed] [Google Scholar]


