Abstract
The objective of this commentary was to analyze the causes and outcomes of gut microbiome dysbiosis in preterm infants who are born at very-low-birth-weight (VLBW). The intrauterine development of VLBW infants is interrupted abruptly with preterm birth and followed by extrauterine, health-threatening conditions and sequelae. These infants develop intestinal microbial dysbiosis characterized by low diversity, overall reduction in beneficial and/or commensal bacteria, and enrichment of opportunistic pathogens of the Gammaproteobacteria class. The origin of VLBW infant dysbiosis is not well understood and is likely the result of a combination of immaturity and medical care. We propose that these factors interact to produce inflammation in the gut, which further perpetuates dysbiosis. Understanding the sources of dysbiosis could result in interventions to reduce gut inflammation, decrease enteric pathology, and improve health outcomes for these vulnerable infants.
Keywords: prematurity, gut microbiome, dysbiosis, health
Precis
The authors describe gut microbiome dysbiosis in preterm infants, related influencing factors, and potential for inflammatory outcomes.
Very-low-birth-weight (VLBW) infants weigh less than 1500 gm, and most of these infants will also be very low gestational age (preterm). These infants are physiologically immature and susceptible to infections and side effects from medical interventions. The births of VLBW preterm infants frequently occur after their mothers are hospitalized for treatments of pregnancy-related conditions, illnesses, or early onset of labor (Backhed et al., 2015; Jiang, Mishu, Lu, & Yin, 2018). They are vulnerable to maternal illnesses, medications, and physiological factors that may lead to adverse pre and postnatal consequences.
At birth, VLBW infants are immediately separated from their parents for stabilization and are placed into incubators to regulate their body temperatures. These infants often require invasive, life-sustaining medical interventions such as intubation for mechanical ventilation, central and peripheral line placements for intravenous fluids and parenteral nutrition, blood transfusions, needle sticks for blood samples, and surgeries. In addition to these invasive procedures, many infants are exposed to antibiotics and other medications. These infants are cared for in the NICU environment for months and are at risk for acute and chronic illnesses such as respiratory distress, sepsis, and chronic lung disease. The cumulative and prolonged exposures to illnesses, surgeries, pain, stress, and separation from their parents during early life in the NICU may result in toxic stress phenomena, defined as strong and prolonged stress without the buffer of a supportive and protective adult relationship (Shonkoff & Garner, 2012). These circumstances, along with the developmental immaturity of the gut and immune system, contribute to the onset of microbial dysbiosis that includes overgrowth of pathogenic bacteria concurrent with the suppressed growth of beneficial and/or commensal bacteria in the intestines (Groer, Duffy, et al., 2014; Groer, Gregory, Louis-Jacques, Thibeau, & Walker, 2015).
In this commentary, we explore the major known factors that affect the developing microbiome in preterm neonates. The purpose is to describe characteristics of the VLBW microbiome and review factors that most likely contribute to VLBW dysbiosis, including prenatal influences, infant feeding, the NICU environment, immaturity, antibiotics, iron, and stress. We also propose a potential model of bacteria-host interactions to explain these interrelationships.
The VLBW Microbiome
Bacterial genetic material is often found in the umbilical cord, placenta, and meconium of healthy term infants, which implies that microbial exposure within the uterine environment is possible (Guzzardi et al., 2019; Tapiainen et al., 2018). However, in the absence of infection, evidence of live bacterial translocation is lacking (Perez-Munoz, Arrieta, Ramer-Tait, & Walter, 2017). Findings from other studies and the ability to rear germ free animals argue against intrauterine translocation (Aagaard et al., 2014; Blaser & Dominguez-Bello, 2016; Lauder et al., 2016; Leiby et al., 2018; Moles et al., 2013; Perez-Munoz et al., 2017; Walker, Clemente, Peter, & Loos, 2017). Microbial products alone or through their influence on the maternal immune system might influence fetal development (Guzzardi et al., 2019). It is clear that the human intestinal tract undergoes significant bacterial colonization during and after birth from exposures to maternal vaginal and enteric organisms (the bacterial baptism; Backhed et al., 2015).
Distinct patterns of succession become apparent soon after birth (Lan, Kriete, & Rosen, 2013). For example, the term infant’s colon is first colonized by facultative anaerobes and then replaced by obligate anaerobes (Weng & Walker, 2013). The first meconium stools contain bacteria that may play an early role in priming the immature neonatal immune system and are dominated by Firmicutes, Proteobacteria (especially Escherichia and Klebsiella), and Bacteroidetes (Chu et al., 2017; Tapiainen et al., 2018). Specifically, the early gut of the vaginally born, term infant is dominated by vertically transmitted Lactobacilli, followed by facultative anaerobes such as those from genera Enterococcus, Enterobacteria, Streptococcus, and Staphylococcus. Within days to weeks these are replaced by anaerobes, commonly Bifidobacterium, Bacteroides, and the Clostridia (Dogra et al., 2015). These succession patterns are influenced by many factors, including mode of birth, amount of human milk consumed by the infant, gestational age, antibiotic therapy and other medications, and exposure to bacteria from caregivers and surfaces in the environment (Groer et al., 2015). Microbial succession patterns in the gut of term infants is well understood, but less is known about these ecological changes in preterm infants. Studies suggest that these changes are delayed in the preterm infant gut, which results in extended periods where Proteobacteria dominate in the first months after birth (Hill et al., 2017; La Rosa et al., 2014; Moles et al., 2015; Underwood & Sohn, 2017; Valentine, Chu, Stewart, & Aagaard, 2018; Yee et al., 2019).
The term dysbiosis is often used to describe any type of microbial anomaly associated with a disease state. In the absence of a better mechanistic understanding of dysbiosis (Byndloss, Pernitzsch, & Baumler, 2018), the term is most often used to describe abnormal abundances of distinct bacteria and low overall diversity. Among VLBW preterm infants, dysbiosis during the NICU stay is characterized by lower alpha diversity (bacterial diversity within a sample) and a greater proportion of Gammaproteobacteria compared to healthy term infants. Gammaproteobacteria (Phylum Proteobacteria) are a diverse class of facultative anaerobes with potentially pathogenic members, some of which cause health care associated infections such as Escherichia coli, Klebsiella spp., Serratia spp., Enterobacter spp. and others. Many of these are related to common NICU morbidities (Patel et al., 2016). An elevated proportion of Gammaproteobacteria in the gut is often associated with delayed colonization of the anti-inflammatory commensal bacteria Lactobacillus and Bifidobacterium; colonization of the gut by these organisms may protect against the growth of potential pathogens (Hakansson & Molin, 2011).
Perinatal Influences on Dysbiosis
The incidence of preterm birth in the United States is high compared to the incidence in other industrialized counties and even compared to some developing countries. The 2018 U.S. preterm birth rate was estimated to be 10.02%; the greatest incidence (14.13%) occurred in non-Hispanic Black women (Martin, Hamilton, & Osterman, 2019). Known reasons for preterm birth include preeclampsia and intrauterine growth restriction, short cervix, periodontal disease, infections (bacterial and viral), vascular disease, Black race, and extreme thinness (Frey & Klebanoff, 2016).
About 37% of pregnant women receive antibiotics during pregnancy, and 33% receive antibiotics in the intrapartum period for conditions such as Group B Streptococcus colonization, urinary tract infections, premature rupture of the membranes, or cesarean birth (Stokholm et al., 2013). These conditions shape the infant gut microbiome (Stearns et al., 2017). When faced with the potential for a preterm birth, mothers are often given antenatal steroids to mature the infant respiratory function (Sackey & Tagoe, 2018). In comparison to healthy term infants, VLBW infants have greater exposure to altered maternal health, immune perturbations related to genetic factors, or an inflammatory intrauterine environment (Zijlmans, Korpela, Riksen-Walraven, de Vos, & de Weerth, 2015). Early or prolonged ruptured membranes, frequent cervical examinations, and procedures such as cerclage may influence early labor and affect preterm infants. Maternal pregnancy stress has been associated with specific Proteobacteria genera (Escherichia, Enterobacter, Serratia) in the infant gut, which suggests possible early developmental and intergenerational effects on the microbiome (Zijlmans et al., 2015)
In addition, 44% to 64% of preterm infants (Stoll et al., 2015) are born by cesarean compared to 32% of term infants (Martin et al., 2019). Cesarean birth results in an early abnormal colonization of the gut by skin-associated microorganisms, such as Staphylococci (Dominguez-Bello et al., 2010). The developing gut microbiome of healthy term neonates begins to resemble the mother’s microbiome within 6 weeks (Chu et al., 2017). However, the preterm infant gut microbiome appears less resistant to external perturbations.
Enteral Feeding Practices
The lack of microbial maturity and dysbiosis in preterm infants is likely multifactorial but is almost certainly influenced by the introduction of enteral feeding. Nasogastric tubes are used to initiate feeding, and this continues for weeks or months until the infant is able to feed by mouth. With enteral feeding, small volumes are advanced slowly to reach nutritional goals over the first week or two. Therefore, until full enteral nutrition is achieved, VLBW infants also depend on parenteral nutrition. Interruptions of this feeding advancement due to intolerance or illnesses often prolongs the time until these infants reach full enteral feeds, which can affect the development of their gut microbiome (Salas et al., 2017).
Preterm infants who are VLBW are fed initially with their mother’s own milk (MOM), pasteurized donor human milk, or with preterm formula when human milk is not available. To ensure adequate nutrition for growth, human milk is fortified with bovine-based or human milk-based fortifiers (HMF). The cost of human milk-based fortifier remains high, so many NICUs in the United States still rely heavily on bovine-based fortifiers. When MOM is unavailable, infants are transitioned to preterm formula by 34 to 36 weeks corrected gestational age. The microbiomes of term infants fed formula are significantly different than those fed with MOM (Poroyko et al., 2011). Thus, it is not surprising if early exposure to bovine products in VLBW infants affects the successional development of the gut microbiome.
The amount of human milk an infant receives is potentially one of the most significant and modifiable factors that affects the microbiome. Human milk is a source of live bacteria as well as oligosaccharides that provide metabolic substrates to commensal bacteria such as Bifidobacterium and Lactobacillus (Sela & Mills, 2010). Infants require commensal microbes and bioactive components in breast milk for optimal nutritional, developmental, defensive, and physiologic processes that can affect the gut bacterial flora (Neu, 2007).
The most frequently cultured bacteria in human milk include Staphylococcus, Streptococcus, Lactotoccus, Weissella, Enterococcus, Propionibacterium, Lactobacillus, and Bifidobacterium. Human milk is also a source of Gammaproteobacteria, which can represent about 30% of the milk microbiome (Togo et al., 2019). The composition of these bacteria can vary greatly among mothers and is affected by factors such as diet and body mass index (Williams et al., 2017). However, less is known about the milk microbiome of mothers of preterm infants. In a study of 39 Canadian women, Murphy et al. (2017) reported that the dominant phyla in human milk regardless of gestational age were Proteobacteria and Firmicutes; Actinobacteria and Bacteroidetes were present at lower levels with no distinct clustering of microbiota related to prematurity.
The origin of milk-associated bacteria is likely the gastrointestinal tract, as studies have demonstrated that oral administration of live bacteria (i.e., probiotics) to a lactating woman results in the appearance of those microbes in her milk (Arroyo et al., 2010). In addition, it is known that while not all gut microbes are found in the milk, nearly all milk microbes are found in the maternal gut (Pannaraj et al., 2017). How these bacteria translocate from the gut to the milk ducts remains unclear, as are the selective processes involved. Viable gut bacteria may translocate to the breasts (i.e., enteromammary pathway) via the lymphatic system (McGuire & McGuire, 2017). Another important source of milk microbes is contamination with environmental microbes by handling the breasts during nursing and in the pumping process; additional studies are required to evaluate the effects of these microbes on dysbiosis in the infant gut.
There is also a substantial prebiotic component to human milk, and more than 100 human milk oligosaccharides (HMOs) are known to supply essential carbon for commensal microbes (Chen, 2015). The HMO composition of milk from mothers who give birth preterm is not well characterized and how these HMOs affect the composition of the preterm gut microbiome is not yet known. However, among term infants, changes in HMOs over time correlate to changes in the gut microbiome (Moossavi et al., 2019). A greater variation in HMOs has been reported in milk from women who give birth preterm (De Leoz et al., 2012). Understanding the type and variation of HMOs in the breastmilk of term and preterm infants’ mothers is warranted since these glycans play essential roles in shaping and protecting the newborn’s developing microbiome.
As discussed above, VLBW infants are often fed MOM, pasteurized donor milk, and preterm infant formula in varying proportions. Formula is prepared to be nutritionally complete for the infant but lacks the beneficial microbes and the prebiotic HMOs. The effect of Holder pasteurization on HMO composition remains unclear (Daniels et al., 2017), although some HMOs are likely lost (Peila et al., 2016). Donor milk in the NICU is usually pooled milk from mothers of term infants, which can be nutritionally and immunologically different from the MOM of women who give birth preterm (Gidrewicz & Fenton, 2014). The milk of mothers who give birth at term gestation has less protein and less concentration of critical cytokines and secretory immunoglobulin A (sIgA) than the breastmilk of women who give birth preterm (Groer, Luciano, et al., 2014). sIgA can shape colonization of the infant gut by facilitating commensals while decreasing the relative abundance of virulent bacteria (Gregory & Walker, 2013). After about 30 weeks gestation, the breastmilk of women who give birth preterm has higher sIgA than milk of those who give birth at term or pooled, pasteurized donor milk (Demers-Mathieu et al., 2019)
The influence of MOM on preterm infant gut dysbiosis remains unclear. Our group found that the percent of MOM feeding volume had a small but significant effect on microbial compositional dynamics, with greater diversity and dynamics correlated with a higher percent of human milk (Yee et al., 2019). In another cohort of preterm infants, exclusive human milk feeding did not demonstrate the expected enrichment of Bifidobacteria as observed in term infants. This was possibly due to the altered nutritional and immune composition of preterm human milk (Butcher et al., 2018) and the prolonged dominance of Proteobacteria in the preterm infant’s gut. A final consideration is that preterm MOM most often requires fortifiers (HMF) and bovine-based versions are most commonly used. Because these fortifiers may affect oxidative changes in the gut, which in turn can shape inflammatory and dysbiotic processes, it is essential that the risk and benefits of fortifiers be more thoroughly investigated (Cai et al., 2019; Friel et al., 2011; Reuter, Gupta, Chaturvedi, & Aggarwal, 2010)
Immaturity
Prematurity on its own is an important factor that shapes gut microbiomes independent of commonly ascribed factors such as breastfeeding and antibiotics; thus, the observed dysbiosis may be in part developmentally programmed. Dahl et al (2018) observed that preterm infants (N=160) had significantly reduced alpha diversity than term infants, and the major factors accounting for this variance included gestational age and number of postnatal NICU days. Preterm infants born vaginally and breast-fed with no exposure to antibiotics (i.e., limited disruption to the predicted microbial succession) had fewer Firmicutes and more Proteobacteria than term infants. This suggests that biological immaturity alone may disrupt microbial colonization and succession.
The onset of necrotizing enterocolitis (NEC) is another example of the importance of gut developmental maturation. Necrotizing enterocolitis is a catastrophic necrosis of the bowel that occurs at a higher rate in VLBW infants, often requires resection of the bowel, and has a high mortality rate among preterm infants. Regardless of their gestational age at birth, preterm infants are most susceptible to developing NEC at around 31 weeks corrected gestational age (Neu & Pammi, 2017). Human milk is known to protect infants from developing NEC (Cacho, Parker, & Neu, 2017). Before the onset of NEC, the gut microbiome reveals less diversity, a greater proportion of Gammaproteobacteria, and a lower proportion of other species (Wang et al., 2009), which suggests that dysbiosis precedes the development of NEC (Neu & Pammi, 2017).
In addition to developmental or anatomical immaturity, immaturity of immune function in VLBW infants may disrupt the ability of the gut to distinguish between pathogenic and commensal bacteria and contribute to dysbiosis. The intestinal epithelium in VLBW infants produces less viscous mucus and is a weaker barrier to microbial penetration (McElroy & Weitkamp, 2011). The sIgA repertoire is less mature and less able to react with and coat virulent microbes (Rogosch et al., 2012). Peristalsis is also less effective in the premature gastrointestinal tract, and this too can affect the onset and/or development of a dysbiotic state (Quigley, 2011). An increased proportion of microorganisms that promote inflammation and decreased commensal growth, combined with impaired and immature innate immune functions, likely contributes to dysbiosis, inflammation, and increased permeability in the gut (Hackam, Good, & Sodhi, 2013; Hunter & De Plaen, 2014).
NICU Environment
The physical environment of the NICU has diverse microbes that may influence the onset or development of dysbiosis and occasionally pathogenic infection (Hartz, Bradshaw, & Brandon, 2015). Despite rigorous cleaning practices in the NICU, the hospital environment contains a diverse microbiota shaped by its human occupants (Gilbert & Stephens, 2018; Lax et al., 2017). NICU rooms are populated by a distinct microbiota compared to other hospital units, and most surfaces are colonized with microbes derived from the human gut; most originate from the infants, and many harbor antibiotic resistance genes (Brooks et al., 2014; Brooks et al., 2018). Thus, preterm infants with microbial dysbiosis may be transferring some of these microbes to the environment, creating a self-inoculation cycle. Differences among term infant gut microbiomes depend upon the hospital of delivery and the year of measurement (Taft et al., 2014) with dominant components of the fecal microbiota changing from year to year. To date, there is no solid correlation between environmental microbes of the NICU and their effects on infant microbiomes or NICU-associated co-morbidities.
Antibiotics
The use of broad-spectrum antibiotics is a common NICU practice. Eighty-five percent of extremely low birth weight infants (<1,000 g) receive at least one course, and often many more courses of antibiotics (Ting et al., 2016). Sepsis can be difficult to diagnose in VLBW infants and it is a major cause of mortality. Antibiotics are given empirically before 72 hours after birth for suspected early onset sepsis while waiting for confirmatory blood cultures, and 60% of these neonates receive antibiotics for 48–72 hours (Zea-Vera & Ochoa, 2015). The effects of antibiotics on the adult gut microbiome are well known and include both transient and long-term consequences and may select for antibiotic resistance. Intestinal inflammation and treatment with antibiotics increase epithelial oxygenation in the colon (Litvak, Byndloss, & Bäumler, 2018), disrupting anaerobic conditions. These dysbiotic changes then favor an overgrowth of facultative anaerobes, such as Gammaproteobacteria (Litvak, Byndloss, Tsolis, & Baumler, 2017).
The use of prophylactic probiotics in the NICU remains controversial but most evidence maintains that probiotic supplementation is safe and may reduce NICU length of stay (Hu, Zhang, Zhang, Shakya, & Li, 2017). Colonization by the opportunistic yeast, Candida sp., may also be reduced by probiotics (Hu et al., 2017). Authors of a Cochrane review concluded that probiotic administration can prevent severe NEC and all-cause mortality in preterm infants in the NICU (Samuels, van de Graaf, de Jonge, Reiss, & Vermeulen, 2017).
Enteral Iron
A routine NICU practice is the administration of enteral iron supplementation (EIS). All VLBW infants are born with low iron stores and are at increased risk for iron deficiency anemia, due to inadequate maternal iron transfer, high iron demand from rapid body growth and erythropoiesis, and multiple blood draws. With a consistently low enteral absorption rate in preterm infants, 60% to 75% of iron from EIS is not absorbed and therefore is available to distal intestinal bacteria (Rao & Georgieff, 2009). Iron is a cofactor in the growth of Gammaproteobacteria (Hedrich, Schlomann, & Johnson, 2011); in vitro experiments find that iron potentiates the proliferation and virulence of various pathogens, including many Proteobacteria (Kortman, Boleij, Swinkels, & Tjalsma, 2012). In a randomized double-blinded study of anemic children in Cote d’Ivoire, home iron supplementation increased pathogenic Proteobacteria, such as Shigella, Salmonella, and enteroinvasive Escherichia coli and reduced beneficial bacteria, such as Lactobacilli and Bifidobacteria (Zimmermann et al., 2010). Iron supplementation was also shown to increase intestinal dysbiosis and mucosal inflammation in a population of Kenyan infants (Jaeggi et al., 2015; Krebs et al., 2013; Tang et al., 2017; Zimmermann et al., 2010). We find that VLBW infants who received a higher dose of enteral iron supplementation harbor a higher proportion of Proteobacteria in their stool (Ho et al., 2020). These results indicate that enteral iron therapy could contribute to preterm infant gut dysbiosis and inflammation.
Stress
Lifesaving interventions performed in the NICU are potentially extraordinarily stressful and traumatic experiences for VLBW infants (D’Agata et al., 2016). Of additional concern, toxic stress, leading to an abnormal stress response, is associated with lack of nurturance or support of a parent or caregiver (Franke, 2014). How these early life experiences shape physiology and neurodevelopment continues to be studied in these infants (D’Agata, Roberts, et al., 2019; D’Agata et al., 2017). D’Agata and colleagues developed an algorithm to count the types, amounts, and severities of stressful events experienced by NICU preterm infants every day for six weeks (D’Agata, Wu, et al., 2019). Events like diaper changes were classified as moderate stressors, while severe stressors included airway suctioning. It was found that stress scores calculated during the preceding two weeks before the stool was collected were positively correlated with the proportion of the Gammaproteobacteria genera, Proteus and Veillonella, in the infants’ gut microbiome. Additional research is needed to further understand the relationship of early life NICU stressors and development, as well as supportive interventions for infants and families.
Discussion
There are important differences (Figure 1) between the inflamed and non-inflamed gut, and the distinct cellular and biochemical differences in bacteria-host interactions are important contributors to dysbiosis in VLBW infants. We suggest that these factors scaffold and perpetuate onto each other in a way that negatively affects the composition of the microbiome, with gut inflammation at the center of these perturbations. The dominance of Gammaproteobacteria, which affects colonization by obligate anaerobes, is the common abnormality seen in dysbiosis in these infants. This overgrowth of facultative anaerobes in the immature gut may produce intestinal disruption associated with inflammation, reduced gut barrier function, increased leakiness, and local effects and systemic effects in other organs. These effects are then amplified by standard NICU care practices such as infant feeding, enteral iron supplementation, antibiotic use, and stressful interventions.
Figure 1:
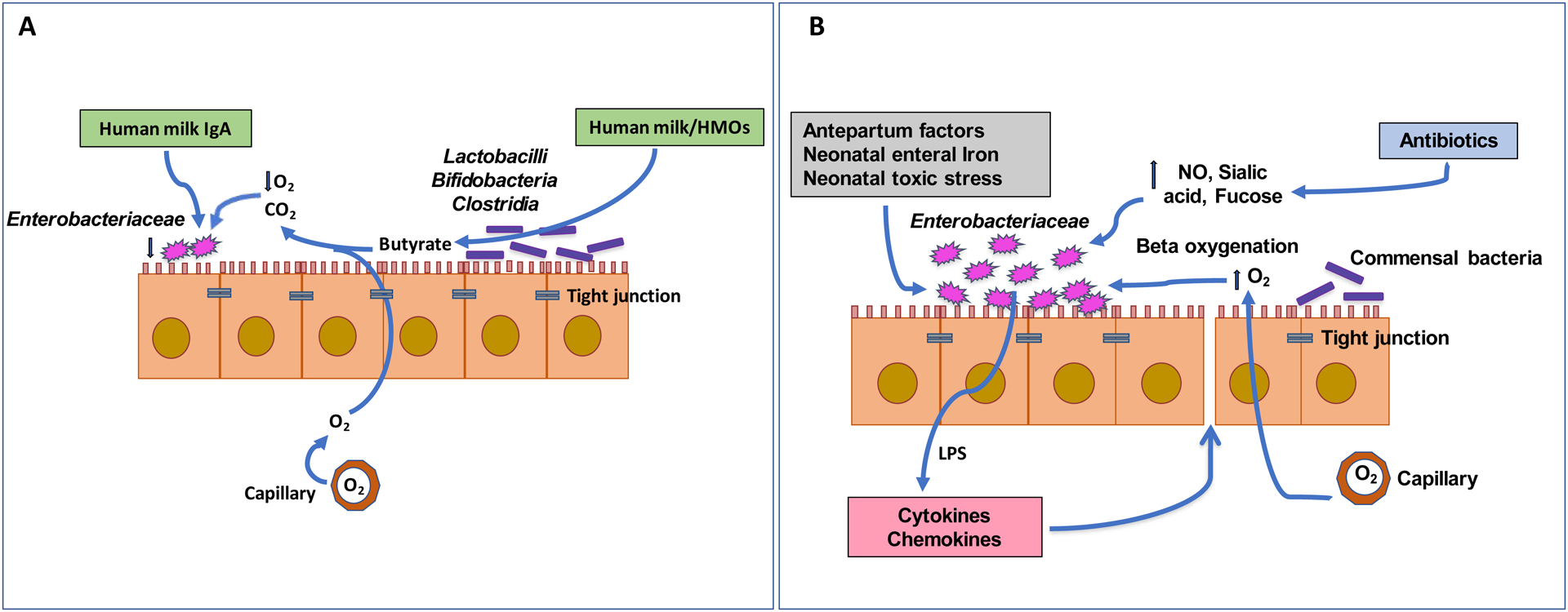
A non-inflamed (A) and inflamed (B) infant guts. A. In the non-inflamed infant gut, butyrate produced by commensal bacteria decreases oxygen levels in the gut lumen, lowering abundance of Enterobacteriaceae. Human milk promotes the growth of commensal bacteria via HMOs, while IgA in milk reduces Enterobacteriaceae growth. B. In the inflamed infant gut, oxygen in the gut lumen facilitates the growth of Enterobacteriaceae, which produces LPS and promotes inflammation. Antepartum factors, antibiotics, neonatal toxic stress, and neonatal enteral iron can also exacerbate the inflammatory milieu.
Since dysbiosis in preterm infants is often shaped by inflammation, we propose that dysbiosis may lead to inflammation as a response by the innate immune system to the endotoxin produced by Gammaproteobacteria. Inflammation progresses through migration of blood cells that can act to cause vasodilation, increased vascular and gut permeability, tissue damage, and ultimately even tissue death (Kamada, Seo, Chen, & Nunez, 2013; Underwood, 2014). The outcome of inflammation in VLBW infants can lead to severe gastrointestinal disease such as NEC, or could affect gut membrane integrity and health through various linked pathways with both short and long-term consequences (R. M. Patel & Denning, 2015).
Interfacing with the epithelium is the gut-associated lymphoid tissue (GALT). During early life, as the GALT is maturing, it is educated to respond to pathogens and tolerate commensals through a complex interplay of dendritic cell sampling and presentation, T and B cell activation, and sIgA production (Ahluwalia, Magnusson, & Ohman, 2017). Not only is this process delayed by the immaturity of the infant, but it is likely delayed further by dysbiosis; a lack of specific sIgA production facilitates an overgrowth of Gammaproteobacteria (Rizzatti, Lopetuso, Gibiino, Binda, & Gasbarrini, 2017). Thus, without the antigen-specific activities of T and B cells, for example, inflammation may increase in response to endotoxin produced by these bacteria.
The use of antibiotics in VLBW infants in the NICU can lead to a reduction in the synthesis of microbially-mediated butyrate, a short-chain fatty acid produced by commensal bacteria and a key nutrient for the developing colonocytes. Thus, antibiotics could be delaying maturation of the gut, and creating metabolic conditions that favor oxygen translocation from gut arteries to the gut lumen, upregulation of nitric oxide, and increase nitrate release. All these favor the growth of facultative anaerobes (Rivera-Chavez, Lopez, & Baumler, 2017; Rizzatti et al., 2017). The inflamed gut is a metabolic environment that increases dysbiosis in preterm infants by favoring overgrowth of facultative anaerobes. In addition, excess iron from enteral supplementation also promotes Gammaproteobacteria overgrowth and increases inflammation. Proinflammatory factors like nitrate produced by the epithelium can promote the growth of Gammaproteobacteria and are opposed by butyrate. Gammaproteobacteria produce acetate and ethanol, which are proinflammatory. Added to these insults, are the treatments, stressors and traumas associated with life in the NICU, the perturbation by antibiotics, the influences of maternal factors, and the ingestion of formula that favors potential pathogens. In addition, the enteric microorganisms in the NICU environment readily colonize the infant gut.
Conclusions
Contributing factors to gut dysbiosis in VLBW infants include maternal health, perinatal events, immaturity, enteral feeding, medications, and the NICU environment. Moving forward, we should consider how best to interrupt the resulting inflammatory cycle as we design strategies to improve the health and outcomes of these vulnerable infants. Reducing preterm deliveries is an obvious first choice, as the U.S. has unacceptably high rates of preterm births compared to other developed and developing nations. Better prenatal care for all women is a priority. Ensuring MOM as the sole source of food for VLBW preterm infants, reducing stress by clustered care and increasing skin-to-skin care, using appropriate probiotics along with more judicious use of antibiotics and iron should be considered. We should encourage the development of new algorithms for sepsis evaluation, and more scrupulous hygiene of all personnel equipment and visitors as these are all potentially important interventions that can reduce the immediate and long-term health threats associated with preterm dysbiosis.
Acknowledgement
The research that led to this critical commentary was funded by the National Institutes of Health: R01-NR015546.
Samia Valeria Ozorio Dutra acknowledges support from the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) for graduate education.
Author Bios
M.W. Groer, PhD, RN, Gordon Keller Professor, College of Nursing, University of South Florida, Tampa, FL.
E.M. Miller, PhD, is an associate professor, Department of Anthropology, University of South Florida, Tampa, Florida.
A. D’Agata, PhD, RN, is an assistant professor, School of Nursing, University of Rhode Island, Providence, RI.
T.T. Ho, MD, Assistant Professor, Pediatrics, Morsani College of Medicine, University of South Florida, Tampa, Florida.
S.V. Dutra, PhD, RN, Post-doctoral scholar, University of South Florida College of Nursing, Tampa, Florida
J. Y. Yoo, PhD, Post-doctoral scholar, University of South Florida College of Nursing, Tampa, Florida
A. L. Yee, PhD, Medical Student, Pritzer School of Medicine University of Chicago, Chicago, Illinois
J.A. Gilbert, PhD, Professor, Department of Pediatrics and Scripps Institution of Oceanography, UC San Diego School of Medicine
L.J. Dishaw, Ph.D., Associate Professor, Pediatrics, Morsani College of Medicine, University of South Florida, Tampa, Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no conflicts of interest or relevant financial relationships.
Contributor Information
Maureen E. Groer, College of Nursing, University of South Florida, Tampa, FL..
Elizabeth M. Miller, Department of Anthropology, University of South Florida, Tampa, Florida..
Amy D’Agata, School of Nursing, University of Rhode Island, Providence, RI..
Thao T.B. Ho, Pediatrics, Morsani College of Medicine, University of South Florida, Tampa, Florida..
Alyson L. Yee, Pritzer School of Medicine University of Chicago, Chicago, Illinois.
Jack A. Gilbert, Department of Pediatrics and Scripps Institution of Oceanography, UC San Diego School of Medicine.
Larry J. Dishaw, Pediatrics, Morsani College of Medicine, University of South Florida, Tampa, Florida..
References
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, & Versalovic J (2014). The placenta harbors a unique microbiome. Science Translational Medicine, 6(237), 237ra265. doi: 10.1126/scitranslmed.3008599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia B, Magnusson MK, & Ohman L (2017). Mucosal immune system of the gastrointestinal tract: Maintaining balance between the good and the bad. Scandinavian Journal of Gastroenterology, 52(11), 1185–1193. doi: 10.1080/00365521.2017.1349173 [DOI] [PubMed] [Google Scholar]
- Arroyo R, Martin V, Maldonado A, Jimenez E, Fernandez L, & Rodriguez JM (2010). Treatment of infectious mastitis during lactation: Antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clinical Infectious Diseases, 50(12), 1551–1558. doi: 10.1086/652763 [DOI] [PubMed] [Google Scholar]
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, … Wang J (2015). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host & Microbe, 17(5), 690–703. doi: 10.1016/j.chom.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Blaser MJ, & Dominguez-Bello MG (2016). The human microbiome before birth. Cell Host & Microbe, 20(5), 558–560. doi: 10.1016/j.chom.2016.10.014 [DOI] [PubMed] [Google Scholar]
- Brooks B, Firek BA, Miller CS, Sharon I, Thomas BC, Baker R, … Banfield JF (2014). Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome, 2(1), 1. doi: 10.1186/2049-2618-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B, Olm MR, Firek BA, Baker R, Geller-McGrath D, Reimer SR, … Banfield JF (2018). The developing premature infant gut microbiome is a major factor shaping the microbiome of neonatal intensive care unit rooms. Microbiome, 6(1), 112. doi: 10.1186/s40168-018-0493-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher J, Unger S, Li J, Bando N, Romain G, Francis J, … O’Connor DL (2018). Independent of birth mode or gestational age, very-low-birth-weight infants fed their mothers’ milk rapidly develop personalized microbiotas low in bifidobacterium. Journal of Nutrition, 148(3), 326–335. doi: 10.1093/jn/nxx071 [DOI] [PubMed] [Google Scholar]
- Byndloss MX, Pernitzsch SR, & Baumler AJ (2018). Healthy hosts rule within: Ecological forces shaping the gut microbiota. Mucosal Immunology, 11(5), 1299–1305. doi: 10.1038/s41385-018-0010-y [DOI] [PubMed] [Google Scholar]
- Cacho NT, Parker LA, & Neu J (2017). Necrotizing enterocolitis and human milk feeding: A systematic review. Clinics in Perinatology, 44(1), 49–67. doi: 10.1016/j.clp.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Cai C, Zhang Z, Morales M, Wang Y, Khafipour E, & Friel J (2019). Feeding practice influences gut microbiome composition in very low birth weight preterm infants and the association with oxidative stress: A prospective cohort study. Free Radical Biology and Medicine. doi: 10.1016/j.freeradbiomed.2019.02.032 [DOI] [PubMed] [Google Scholar]
- Chen X (2015). Human Milk Oligosaccharides (HMOS): Structure, function, and enzyme-catalyzed synthesis. Advances in Carbohydrate Chemistry and Biochemistry, 72, 113–190. doi: 10.1016/bs.accb.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, & Aagaard KM (2017). Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nature Medicine, 23, 314. doi: 10.1038/nm.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agata AL, Roberts MB, Ashmeade T, Dutra SVO, Kane B, & Groer MW (2019). Novel method of measuring chronic stress for preterm infants: Skin cortisol. Psychoneuroendocrinology, 102, 204–211. doi: 10.1016/j.psyneuen.2018.12.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agata AL, Walsh S, Vittner D, Cong X, McGrath JM, & Young EE (2017). FKBP5 genotype and early life stress exposure predict neurobehavioral outcomes for preterm infants. Developmental Psychobiology, 59(3), 410–418. doi: 10.1002/dev.21507 [DOI] [PubMed] [Google Scholar]
- D’Agata AL, Wu J, Welandawe MKV, Dutra SVO, Kane B, & Groer MW (2019). Effects of early life NICU stress on the developing gut microbiome. Developmental Psychobiology. doi: 10.1002/dev.21826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agata AL, Young EE, Cong X, Grasso DJ, McGrath JM, & Forsythe PL (2016). Infant medical trauma in the neonatal intensive care unit (IMTN). Advances in Neonatal Care, 16(4), 289–297. [DOI] [PubMed] [Google Scholar]
- Dahl C, Stigum H, Valeur J, Iszatt N, Lenters V, Peddada S, … Eggesbo M (2018). Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. International Journal of Epidemiology, 47(5), 1658–1669. doi: 10.1093/ije/dyy064 [DOI] [PubMed] [Google Scholar]
- Daniels B, Coutsoudis A, Autran C, Amundson Mansen K, Israel-Ballard K, & Bode L (2017). The effect of simulated flash heating pasteurisation and Holder pasteurisation on human milk oligosaccharides. Paediatrics and International Child Health, 37(3), 204–209. doi: 10.1080/20469047.2017.1293869 [DOI] [PubMed] [Google Scholar]
- De Leoz ML, Gaerlan SC, Strum JS, Dimapasoc LM, Mirmiran M, Tancredi DJ, … Underwood MA (2012). Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. Journal of Proteome Research, 11(9), 4662–4672. doi: 10.1021/pr3004979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers-Mathieu V, Huston RK, Markell AM, McCulley EA, Martin RL, Spooner M, & Dallas DC (2019). Differences in maternal immunoglobulins within mother’s own breast milk and donor breast milk and across digestion in preterm infants. Nutrients, 11(4). doi: 10.3390/nu11040920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Bruck WM, Berger B, … Holbrook JD (2015). Rate of establishing the gut microbiota in infancy has consequences for future health. Gut Microbes, 6(5), 321–325. doi: 10.1080/19490976.2015.1078051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, & Knight R (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences of the United States of America, 107(26), 11971–11975. doi: 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke HA (2014). Toxic stress: Effects, prevention and treatment. Children (Basel), 1(3), 390–402. doi: 10.3390/children1030390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey HA, & Klebanoff MA (2016). The epidemiology, etiology, and costs of preterm birth. Seminars in Fetal and Neonatal Medicine, 21(2), 68–73. doi: 10.1016/j.siny.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Friel JK, Diehl-Jones B, Cockell KA, Chiu A, Rabanni R, Davies SS, & Roberts LJ 2nd. (2011). Evidence of oxidative stress in relation to feeding type during early life in premature infants. Pediatric Research, 69(2), 160–164. doi: 10.1203/PDR.0b013e3182042a07 [DOI] [PubMed] [Google Scholar]
- Gidrewicz DA, & Fenton TR (2014). A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatrics, 14, 216. doi: 10.1186/1471-2431-14-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JA, & Stephens B (2018). Microbiology of the built environment. Nature Reviews: Microbiology, 16(11), 661–670. doi: 10.1038/s41579-018-0065-5 [DOI] [PubMed] [Google Scholar]
- Gregory KE, & Walker WA (2013). Immunologic factors in human milk and disease prevention in the preterm infant. Current Pediatrics Reports, 1(4). doi: 10.1007/s40124-013-0028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer MW, Duffy A, Morse S, Kane B, Zaritt J, Roberts S, & Ashmeade T (2014). Cytokines, chemokines, and growth factors in banked human donor milk for preterm infants. Journal of Human Lactation, 30(3), 317–323. doi: 10.1177/0890334414527795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer MW, Gregory KE, Louis-Jacques A, Thibeau S, & Walker WA (2015). The very low birth weight infant microbiome and childhood health. Birth Defects Research C Embryo Today, 105(4), 252–264. doi: 10.1002/bdrc.21115 [DOI] [PubMed] [Google Scholar]
- Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, & Gilbert JA (2014). Development of the preterm infant gut microbiome: A research priority. Microbiome, 2, 38. doi: 10.1186/2049-2618-2-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzardi MA, Ait Ali L, D’Aurizio R, Rizzo F, Saggese P, Sanguinetti E, … Iozzo P (2019). Fetal cardiac growth is associated with in utero gut colonization. Nutrition, Metabolism, and Cardiovascular Diseases, 29(2), 170–176. doi: 10.1016/j.numecd.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Good M, & Sodhi CP (2013). Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Seminars in Pediatric Surgery, 22(2), 76–82. doi: 10.1053/j.sempedsurg.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson A, & Molin G (2011). Gut microbiota and inflammation. Nutrients, 3(6), 637–682. doi: 10.3390/nu3060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz LE, Bradshaw W, & Brandon DH (2015). Potential NICU environmental influences on the neonate’s microbiome: A systematic review. Advances in Neonatal Care, 15(5), 324–335. doi: 10.1097/ANC.0000000000000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich S, Schlomann M, & Johnson DB (2011). The iron-oxidizing proteobacteria. Microbiology, 157(Pt 6), 1551–1564. doi: 10.1099/mic.0.045344-0 [DOI] [PubMed] [Google Scholar]
- Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, … Stanton C (2017). Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome, 5(1), 4. doi: 10.1186/s40168-016-0213-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TTB, Kumar A, Louis-Jacques AF, Dishaw LJ, Yee AL, & Groer MW (2020). The development of intestinal dysbiosis in anemic preterm infants. Journal of Perinatology. doi: 10.1038/s41372-020-0599-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Zhang GQ, Zhang Q, Shakya S, & Li ZY (2017). Probiotics prevent candida colonization and invasive fungal sepsis in preterm neonates: A systematic review and meta-analysis of randomized controlled trials. Pediatrics and Neonatology, 58(2), 103–110. doi: 10.1016/j.pedneo.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Hunter CJ, & De Plaen IG (2014). Inflammatory signaling in NEC: Role of NF-kappaB, cytokines and other inflammatory mediators. Pathophysiology, 21(1), 55–65. doi: 10.1016/j.pathophys.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, … Zimmermann MB (2015). Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut, 64(5), 731–742. doi: 10.1136/gutjnl-2014-307720 [DOI] [PubMed] [Google Scholar]
- Jiang M, Mishu MM, Lu D, & Yin X (2018). A case control study of risk factors and neonatal outcomes of preterm birth. Taiwanese Journal of Obstetrics & Gynecology, 57(6), 814–818. doi: 10.1016/j.tjog.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Kamada N, Seo SU, Chen GY, & Nunez G (2013). Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews: Immunology, 13(5), 321–335. doi: 10.1038/nri3430 [DOI] [PubMed] [Google Scholar]
- Kortman GA, Boleij A, Swinkels DW, & Tjalsma H (2012). Iron availability increases the pathogenic potential of Salmonella typhimurium and other enteric pathogens at the intestinal epithelial interface. PloS One, 7(1), e29968. doi: 10.1371/journal.pone.0029968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs NF, Sherlock LG, Westcott J, Culbertson D, Hambidge KM, Feazel LM, … Frank DN (2013). Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. Journal of Pediatrics, 163(2), 416–423. doi: 10.1016/j.jpeds.2013.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, … Tarr PI (2014). Patterned progression of bacterial populations in the premature infant gut. Proceedings of the National Academy of Sciences of the United States of America. doi: 10.1073/pnas.1409497111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Kriete A, & Rosen GL (2013). Selecting age-related functional characteristics in the human gut microbiome. Microbiome, 1(1), 2. doi: 10.1186/2049-2618-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder AP, Roche AM, Sherrill-Mix S, Bailey A, Laughlin AL, Bittinger K, … Bushman FD (2016). Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome, 4(1), 29. doi: 10.1186/s40168-016-0172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax S, Sangwan N, Smith D, Larsen P, Handley KM, Richardson M, … Gilbert JA (2017). Bacterial colonization and succession in a newly opened hospital. Science Translational Medicine, 9(391). doi: 10.1126/scitranslmed.aah6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiby JS, McCormick K, Sherrill-Mix S, Clarke EL, Kessler LR, Taylor LJ, … Bushman FD (2018). Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome, 6(1), 196. doi: 10.1186/s40168-018-0575-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak Y, Byndloss MX, & Bäumler AJ (2018). Colonocyte metabolism shapes the gut microbiota. Science, 362(6418), eaat9076. doi: 10.1126/science.aat9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak Y, Byndloss MX, Tsolis RM, & Baumler AJ (2017). Dysbiotic proteobacteria expansion: A microbial signature of epithelial dysfunction. Current Opinion in Microbiology, 39, 1–6. doi: 10.1016/j.mib.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, & Osterman MJK (2019). Births in the United States, 2018. National Center for Health Statistics Data Brief(346), 1–8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31442195 [PubMed] [Google Scholar]
- McElroy SJ, & Weitkamp JH (2011). Innate immunity in the small intestine of the preterm infant. Neoreviews, 12(9), e517–e526. doi: 10.1542/neo.12-9-e517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire MK, & McGuire MA (2017). Got bacteria? The astounding, yet not-so-surprising, microbiome of human milk. Current Opinion in Biotechnology, 44, 63–68. doi: 10.1016/j.copbio.2016.11.013 [DOI] [PubMed] [Google Scholar]
- Moles L, Gomez M, Heilig H, Bustos G, Fuentes S, de Vos W, … Jimenez E (2013). Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PloS One, 8(6), e66986. doi: 10.1371/journal.pone.0066986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles L, Gomez M, Jimenez E, Fernandez L, Bustos G, Chaves F, … Del Campo R (2015). Preterm infant gut colonization in the neonatal ICU and complete restoration 2 years later. Clinical Microbiology and Infection. doi: 10.1016/j.cmi.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Moossavi S, Atakora F, Miliku K, Sepehri S, Robertson B, Duan QL, … Moraes TJ (2019). Integrated analysis of human milk microbiota with oligosaccharides and fatty acids in the CHILD cohort. Frontiers in nutrition, 6, 58. doi: 10.3389/fnut.2019.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Curley D, O’Callaghan TF, O’Shea C-A, Dempsey EM, O’Toole PW, … Stanton C (2017). The composition of human milk and infant faecal microbiota over the first three months of life: A pilot study. Scientific Reports, 7, 40597. doi: 10.1038/srep40597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J (2007). Perinatal and neonatal manipulation of the intestinal microbiome: A note of caution. Nutrition Reviews, 65(6 Pt 1), 282–285. [DOI] [PubMed] [Google Scholar]
- Neu J, & Pammi M (2017). Pathogenesis of NEC: Impact of an altered intestinal microbiome. Seminars in Perinatology, 41(1), 29–35. doi: 10.1053/j.semperi.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, … Aldrovandi GM (2017). Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr, 171(7), 647–654. doi: 10.1001/jamapediatrics.2017.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AL, Mutlu EA, Sun Y, Koenig L, Green S, Jakubowicz A, … Chen AL (2016). Longitudinal survey of microbiota in hospitalized preterm very low birth weight infants. Journal of Pediatric Gastroenterology and Nutrition, 62(2), 292. doi: 10.1097/MPG.0000000000000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RM, & Denning PW (2015). Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatric Research, 78(3), 232–238. doi: 10.1038/pr.2015.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peila C, Moro GE, Bertino E, Cavallarin L, Giribaldi M, Giuliani F, … Coscia A (2016). The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: A review. Nutrients, 8(8). doi: 10.3390/nu8080477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, & Walter J (2017). A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome, 5(1), 48. doi: 10.1186/s40168-017-0268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poroyko V, Morowitz M, Bell T, Ulanov A, Wang M, Donovan S, … Liu DC (2011). Diet creates metabolic niches in the “immature gut” that shape microbial communities. Nutricion Hospitalaria, 26(6), 1283–1295. doi: 10.1590/s0212-16112011000600015 [DOI] [PubMed] [Google Scholar]
- Quigley EM (2011). Microflora modulation of motility. Journal of Neurogastroenterology and Motility, 17(2), 140–147. doi: 10.5056/jnm.2011.17.2.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, & Georgieff MK (2009). Iron therapy for preterm infants. Clinics in Perinatology, 36(1), 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, & Aggarwal BB (2010). Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biology and Medicine, 49(11), 1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chavez F, Lopez CA, & Baumler AJ (2017). Oxygen as a driver of gut dysbiosis. Free Radical Biology and Medicine, 105, 93–101. doi: 10.1016/j.freeradbiomed.2016.09.022 [DOI] [PubMed] [Google Scholar]
- Rizzatti G, Lopetuso LR, Gibiino G, Binda C, & Gasbarrini A (2017). Proteobacteria: A common factor in human diseases. BioMed Research International 2017, 9351507. doi: 10.1155/2017/9351507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch T, Kerzel S, Hoss K, Hoersch G, Zemlin C, Heckmann M, … Zemlin M (2012). IgA response in preterm neonates shows little evidence of antigen-driven selection. Journal of Immunology, 189(11), 5449–5456. doi: 10.4049/jimmunol.1103347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackey AH, & Tagoe LG (2018). Audit of antenatal steroid use in mothers of preterms admitted to a neonatal intensive care unit in Ghana. Ghana Medical Journal, 52(1), 3–7. doi: 10.4314/gmj.v52i1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas AA, Kabani N, Travers CP, Phillips V, Ambalavanan N, & Carlo WA (2017). Short versus extended duration of trophic feeding to reduce time to achieve full enteral feeding in extremely preterm infants: An observational study. Neonatology, 112(3), 211–216. doi: 10.1159/000472247 [DOI] [PubMed] [Google Scholar]
- Samuels N, van de Graaf RA, de Jonge RC, Reiss IK, & Vermeulen MJ (2017). Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatrics, 17(1), 105. doi: 10.1186/s12887-017-0847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela DA, & Mills DA (2010). Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends in Microbiology, 18(7), 298–307. doi: 10.1016/j.tim.2010.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, & Garner AS (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–246. doi: 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- Stearns JC, Simioni J, Gunn E, McDonald H, Holloway AC, Thabane L, … Hutton EK (2017). Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants. Scientific Reports, 7. doi: 10.1038/s41598-017-16606-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokholm J, Schjorring S, Pedersen L, Bischoff AL, Folsgaard N, Carson CG, … Bisgaard H (2013). Prevalence and predictors of antibiotic administration during pregnancy and birth. PloS One, 8(12), e82932. doi: 10.1371/journal.pone.0082932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, … Wyckoff M (2015). Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA, 314(10), 1039–1051. doi: 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft DH, Ambalavanan N, Schibler KR, Yu Z, Newburg DS, Ward DV, & Morrow AL (2014). Intestinal microbiota of preterm infants differ over time and between hospitals. Microbiome, 2, 36. doi: 10.1186/2049-2618-2-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Frank DN, Hendricks AE, Ir D, Esamai F, Liechty E, … Krebs NF (2017). Iron in micronutrient powder promotes an unfavorable gut microbiota in Kenyan infants. Nutrients, 9(7), 776. doi: 10.3390/nu9070776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiainen T, Paalanne N, Tejesvi MV, Koivusaari P, Korpela K, Pokka T, … Renko M (2018). Maternal influence on the fetal microbiome in a population-based study of the first-pass meconium. Pediatric Research, 84(3), 371–379. doi: 10.1038/pr.2018.29 [DOI] [PubMed] [Google Scholar]
- Ting JY, Synnes A, Roberts A, Deshpandey A, Dow K, Yoon EW, … Shah PS (2016). Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr, 170(12), 1181–1187. doi: 10.1001/jamapediatrics.2016.2132 [DOI] [PubMed] [Google Scholar]
- Togo A, Dufour JC, Lagier JC, Dubourg G, Raoult D, & Million M (2019). Repertoire of human breast and milk microbiota: A systematic review. Future Microbiology. doi: 10.2217/fmb-2018-0317 [DOI] [PubMed] [Google Scholar]
- Underwood MA (2014). Intestinal dysbiosis: Novel mechanisms by which gut microbes trigger and prevent disease. Preventive Medicine, 65, 133–137. doi: 10.1016/j.ypmed.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Underwood MA, & Sohn K (2017). The microbiota of the extremely preterm infant. Clinics in Perinatology, 44(2), 407–427. doi: 10.1016/j.clp.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine G, Chu DM, Stewart CJ, & Aagaard KM (2018). Relationships between perinatal interventions, maternal-infant microbiomes, and neonatal outcomes. Clinics in Perinatology, 45(2), 339–355. doi: 10.1016/j.clp.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Walker RW, Clemente JC, Peter I, & Loos RJF (2017). The prenatal gut microbiome: Are we colonized with bacteria in utero? Pediatric Obesity, 12 Suppl 1, 3–17. doi: 10.1111/ijpo.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, … Claud EC (2009). 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. The International Society for Microbial Ecology journal 3(8), 944–954. doi: 10.1038/ismej.2009.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M, & Walker W (2013). The role of gut microbiota in programming the immune phenotype. Journal of Developmental Origins of Health and Disease, 4(3), 203–214. doi: 10.1017/S2040174412000712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JE, Carrothers JM, Lackey KA, Beatty NF, York MA, Brooker SL, … McGuire MA (2017). Human milk microbial community structure is relatively stable and related to variations in macronutrient and micronutrient intakes in healthy lactating women. The Journal of nutrition, 147(9), 1739–1748. doi: 10.3945/jn.117.248864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee AL, Miller E, Dishaw LJ, Gordon JM, Ji M, Dutra S, … Groer M (2019). Longitudinal microbiome composition and stability correlate with increased weight and length of very-low-birth-weight infants. MSystems, 4(1), e00229–00218. doi: 10.1128/mSystems.00229-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zea-Vera A, & Ochoa TJ (2015). Challenges in the diagnosis and management of neonatal sepsis. Journal of Tropical Pediatrics, 61(1), 1–13. doi: 10.1093/tropej/fmu079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, & de Weerth C (2015). Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology, 53, 233–245. doi: 10.1016/j.psyneuen.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Chassard C, Rohner F, N’Goran EK, Nindjin C, Dostal A, … Hurrell RF (2010). The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Cote d’Ivoire. The American journal of clinical nutrition, 92(6), 1406–1415. doi: 10.3945/ajcn.110.004564 [DOI] [PubMed] [Google Scholar]


