Abstract
Endogenous pain inhibition is less efficient in chronic pain patients. Diffuse noxious inhibitory control (DNIC), a form of endogenous pain inhibition, is compromised in women and older people, making them more vulnerable to chronic pain. However, the underlying mechanisms remain unclear. Here, we used a capsaicin-induced DNIC test and resting state functional MRI to investigate the impact of aging and sex on endogenous pain inhibition and associated brain circuitries in healthy rats. We found that DNIC was less efficient in young females compared to young males. DNIC response was lost in old rats of both sexes, but the brain networks engaged during DNIC differed in a sex-dependent manner. Young males had the most efficient analgesia with the strongest connectivity between anterior cingulate cortex (ACC) and periaqueductal gray (PAG). The reduced efficiency of DNIC in young females appeared to be driven by a widespread brain connectivity. Old males showed increased connectivity between PAG, raphe nuclei, pontine reticular nucleus and hippocampus, which may not be dependent on connections to ACC, while old females showed increased connectivity between ACC, PAG and more limbic regions. These findings suggest that distinct brain circuitries including the limbic system may contribute to higher susceptibility to pain modulatory deficits in the elderly population, and sex may be a risk factor for developing age-related chronic pain.
1. Introduction
Chronic pain prevalence increases sharply with age and impacts physical and cognitive function, ultimately decreasing quality of life [17]. Risk factors for chronic pain in older people include female sex, psychosocial comorbidities, increased pain facilitation and diminished descending pain inhibitory capacity [29]. Without any clinical history of age-related neurological disorders, aging itself contributes to changes in brain structure and function, which affect pain processing [5].
Accordingly, animal studies show that aging is associated with increased pain sensitivity, reduced analgesic effect of morphine and higher risk for developing chronic pain [3; 26]. Potential mechanisms for these phenomena are that advancing age results in enhanced nociceptor excitability [24] and a decline in endogenous inhibitory control of mu and delta opioid receptors in the spinal cord [4]. To our knowledge, one basic science study investigated supraspinal pain mechanisms related to natural aging and found that molecular changes in amygdala lead to higher pain-related behaviors in old male mice following inflammatory pain [40]. These available data suggest an apparent dysregulation of pain processing and pain modulation with aging at all levels of neuraxis.
We have previously demonstrated that endogenous pain inhibition and brain networks are modulated in a sex-dependent manner in rats [7]. Our paradigm assessed the diffuse noxious inhibitory control (DNIC) or the pain-inhibits-pain phenomenon, which has been extensively reported in animals and humans under healthy and chronic pain conditions [35; 36]. However, our study innovatively led to the characterization of a model for associating brain regions with behavioral responses under a DNIC paradigm that is regulated by sex. Compared to females, males show increased connectivity between anterior cingulate cortex (ACC) and periaqueductal gray (PAG), and enhanced analgesic response induced by DNIC, which indicate a stronger descending pain inhibition in the presence of testosterone. In contrast, compared to males, females had increased ACC connectivity with hippocampal and thalamic regions, which was associated with reduced efficiency of pain modulation. These findings suggest that ACC plays a key role in descending modulatory pathways that affect pain response.
While we documented sex differences in pain inhibition and brain networks in healthy rats, there is a lack of understanding of age-related effects on endogenous pain modulation and brain function. In this study, we combined DNIC behavioral testing and functional magnetic resonance imaging (fMRI) to assess age- and sex-dependent alterations in endogenous pain inhibition. DNIC responses and whole brain connectivity to ACC and PAG were assessed in young (3–6 months) and aged (20–24 months) male and female Fischer 344 rats. Our findings show that young males have the most efficient endogenous pain inhibition due to the strongest connectivity between ACC and PAG, while connections to the limbic system and distinct brain regions may contribute to higher susceptibility to pain modulatory deficits in young females and the elderly population. We further propose a model to point to the possibility of targeting specific brain regions in age- and sex-dependent painful conditions.
2. Methods
2.1. Animals
Fischer-344 rats consisting of young male (YM; 3–6 months old – 250–290 g), young female (YF; 3–6 months old – 160–175 g), old male (OM; 20–24 months old – 405–455 g) and old female (OF; 20–24 months old – 230–250 g) rats were obtained from the National Institute on Aging. Although the exact relationship between age of rats and age of humans is unknown, we approximate that young rats in our study would be comparable to 18-year-old humans, and old rats comparable to 60-year-old humans [42]. Animals were housed in a temperature-controlled room under a 12:12 light-dark cycle with access to food and water ad libitum. Rats in the same experimental groups were housed together in groups of two or three. Male and female rats were housed together with similarly aged cage mates of the same sex in the same colony room and each experimental group was tested at a different time. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and under a University of Maryland-approved Institutional Animal Care and Use Committee protocol. For DNIC behavioral assay, capsaicin was administered to YM, YF, OM and OF rats. A control injection of phosphate-buffered saline (PBS) was made to age and sex matched rats. In an fMRI study, we investigated DNIC effects after capsaicin injection in separate groups of YM, YF, OM and OF rats. All rats were randomly assigned to experimental and control groups (n=5).
2.2. Drug Preparation and Administration
Capsaicin (Millipore Sigma, St. Louis, MO, USA) was dissolved in ethanol (20%), Tween 20 (7%) and PBS (93%). For behavioral studies, capsaicin (0.3% in 100 μL) was administered once intradermally in the left forepaw using a 27-gauge needle. The same volume of vehicle was injected in the same manner in control animals. For fMRI studies, the same concentration of capsaicin was administered in the left hindpaw as described previously.
2.3. Behavioral Assay and statistical analysis
The model of DNIC in this study was adapted from our previous study in rats [7]. Hindpaw withdrawal latencies to noxious thermal stimulation, a test stimulus, were measured before and 15, 30, 45, 60, 90 and 120 min following the administration of capsaicin, a conditioning stimulus, into the left forepaw. Hindpaw withdrawal latencies to a thermal nociceptive stimulus were assessed according to the methods described in a previous study (Hargreaves et al., 1988). Rats were allowed to habituate to the experimental room for 30 min per day for three consecutive days. Rats were placed on an elevated glass surface and allowed to acclimate for 10–20 min. A radiant heat source was directed to the plantar surface of the hindpaw from underneath the glass floor. A motion detector halted both lamp and timer when the paw was withdrawn. The voltage of the bulb was adjusted to result in an average paw withdrawal latency of 10–12 s in naive animals. A 20 s cutoff was used to prevent tissue damage. Three trials (with an inter-trial interval of at least 5 min) were determined for each hindpaw and the average of the trials was used as the mean thermal paw withdrawal latency. The increase in hindpaw withdrawal latency after capsaicin treatment in the forepaw was used as the measure of DNIC. In order to assess the overall magnitude of drug-induced changes in DNIC over time, area under the curve (AUC) was calculated for the normalized data for each rat using the trapezoid rule.
Results were analyzed using the statistical analysis software package SigmaPlot. Two-Way Repeated Measures ANOVA with Holm-Sidak method for correction of multiple comparisons were performed to determine significant treatment and time effects for each age and sex group. Two-way ANOVA was used to compare AUC across different age and sex groups treated with either capsaicin or vehicle. Differences were considered statistically significant at p < .05 and the data were presented as mean ± standard error of the mean (S.E.M.). The investigators conducting the behavioral study were blinded to the experimental groups and drug administration, i.e., animals who received either capsaicin or vehicle.
2.4. rsfMRI data acquisition
Data were acquired using a Bruker BioSpec 70/30USR Avance III 7-Tesla scanner (Bruker Biospin MRI GmbH, Germany) and a 40-mm circular polarized volume coil. During scanning, rats were anesthetized at a constant mixture of 1.5% isoflurane in oxygen-enriched air and respiration and heart rate were monitored with a small animal monitoring and gating system and software (SA Instruments, Inc., Stony Brook, NY, USA). T2-weighted images were obtained using a 2D RARE (400×400 matrix, 22 coronal 1mm slice thickness, in plane resolution 100 μm, TR 2000 ms, TE 28 ms). rsfMRI scans were acquired using an echo planar imaging (EPI) sequence (TR 1500 ms, TE 24 ms, 128×128 matrix, in plane resolution 0.40 × 0.40 × 1 mm, 22 coronal slices, 620 volumes per scan). The anatomical and the first rsfMRI scans were performed for each rat as baseline (prior to capsaicin injection, 15 min). Rats subsequently received an injection of capsaicin (0.3% in 100 μL) into the left hindpaw and three rsfMRI scans were acquired for 15 min each (Fig. 1). The investigators analyzing the MRI data were blinded to the experimental groups. Investigators running the MRI sessions were not blinded to the experimental groups due to visible sex and weight differences between animals.
Figure 1.
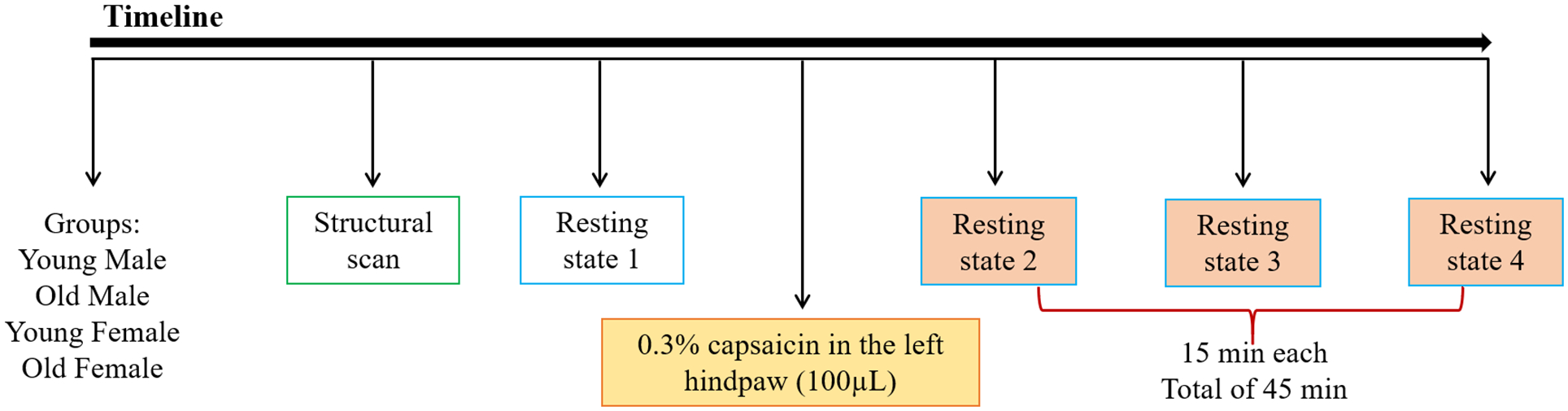
Schematic illustrating the fMRI experimental design. All groups underwent structural scan (green box), 1 resting state scan prior capsaicin injection (blue box – baseline 15 min), capsaicin injection and 3 resting state scans of 15 min each (orange filled boxes - post-capsaicin 15, 30 and 45 min). The total time of resting state scans after capsaicin injection was 45 min.
2.5. rsfMRI preprocessing, statistical analysis and data availability
All preprocessing and analyses were performed in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). We used seed-based analysis to assess how functional connectivity (FC) between right anterior cingulate cortex (R ACC) or periaqueductal gray (PAG) and the whole brain varies after capsaicin injection between the different groups. The contralateral side to capsaicin injection was used. We selected ACC and PAG as regions of interest based upon prior literature showing changes in connectivity and neuronal activation of these areas during descending pain inhibition, which was also modulated by sex [7; 30]. Anatomical locations were chosen according to Paxinos and Watson atlas (2004). We first created a study-specific template by coregistering and averaging the T2-weighted images across animals and interpolating to voxel size of isotropic 0.5 mm. Preprocessing steps included slice timing correction (number of slices: 22, reference slice: 11), realignment and motion correction (separation: 0.57, smoothing - FWHM: 0.76, interpolation for reslicing: 4th Degree B-Spline), within-subject registration (separation: 1 0.5, histogram smoothing: 1.7 1.7), normalization to the study-specific template (source imaging smoothing: 1.52), normalization of functional images (0.5 isotropic voxel, interpolation: trilinear), bandpass filtering (0.009 to 0.2 Hz), and smoothing at 1mm FWHM. We then extracted time series data from the regions of interest (ROIs) and regressed these time-courses with the signal at each voxel across the whole brain to reveal FC patterns for each animal. Six motion parameters were included as regressors of no interest. To assess the effects of capsaicin on resting state connectivity, the first-level β-contrast images representing ACC and PAG FC for each of the four time-points (baseline resting state and three resting state scans post-capsaicin) were entered as dependent variables in the general linear model for each rat within each group. Second level analyses were done according to the following three designs: 1. Flexible factorial design to show age differences in ACC FC to the whole brain with 2 Group (young and old) × 4 Time (baseline and three resting state scans of 15 min each), with Group and Time specified as fixed factors. Comparisons were done between baseline and resting state 4 (from 30 to 45 min). 2. Flexible factorial design to show sex differences in ACC FC to the whole brain with 2 Group (male and female) × 4 Time (baseline and three resting state scans of 15 min each), with Group and Time specified as fixed factors. Comparisons were done between baseline and resting state 4. 3. Finally, two flexible factorial designs to examine the full age-by-sex interaction (YM, YF, OM and OF) in ACC and PAG FC to the whole brain during DNIC comparing baseline versus resting state 4. Because of the small sample size and exploratory nature of the study, second level maps used cluster-forming (voxel level) thresholds at p < .05, .01, .005 and .001. Significant clusters for each threshold were reported. Analyses described in 1 and 2 were also done in the entire 45 min of fMRI data after capsaicin injection, and in resting state 2 (0 to 15 min post-capsaicin) and resting state 3 (15 to 30 min post-capsaicin) separately. Results are reported in the supplemental material. For visualization, we extracted and plotted the average beta values ± S.E.M. from all significant clusters at p < .05 for each animal and time point. We note that the cluster-forming threshold of p < .05 is overly liberal but we present these results as exploratory. All data including code, ROIs, and the template brain are available upon reasonable request.
3. Results
3.1. DNIC impaired in young females and lost in old rats.
Our previous study showed a sex-dependent modulation of the analgesia produced by capsaicin-induced DNIC, which we assessed with mechanical sensitivity testing in rats [7]. In the current study, DNIC was induced by capsaicin injection into the forepaw, as the conditioning stimulus, but thermal sensitivity was assessed on the hindpaw, as a test stimulus, to determine age- and sex-related differences. Capsaicin, but not vehicle, resulted in significant increases in hindpaw withdrawal latencies in young male rats (Fig. 2A; treatment effect F=179.7, p < 0.001; time effect F=47.7, p < 0.001). The increase in latency was observed as early as 15 minutes post capsaicin treatment. The significant increase was maintained at the cut-off limit of 20 seconds for 60 minutes before it gradually declined to the baseline level by 120 minutes. The vehicle administration in the forepaw in another group of young male rats did not alter the paw withdrawal latencies at any of the time points we observed. The capsaicin administration in young female rats also induced an immediate increase in hindpaw withdrawal latencies (Fig. 2B. treatment effect F=21.06, p < 0.01; time effect F=21.38, p < 0.001). Although the increase in latency was significant up to first 45 minutes, unlike in young male rats, the capsaicin-induced latency began to decline within 30 minutes and reached the near baseline in 90 minutes. The vehicle administration in the forepaw in another group of young female rats did not alter the paw withdrawal latencies.
Figure 2.
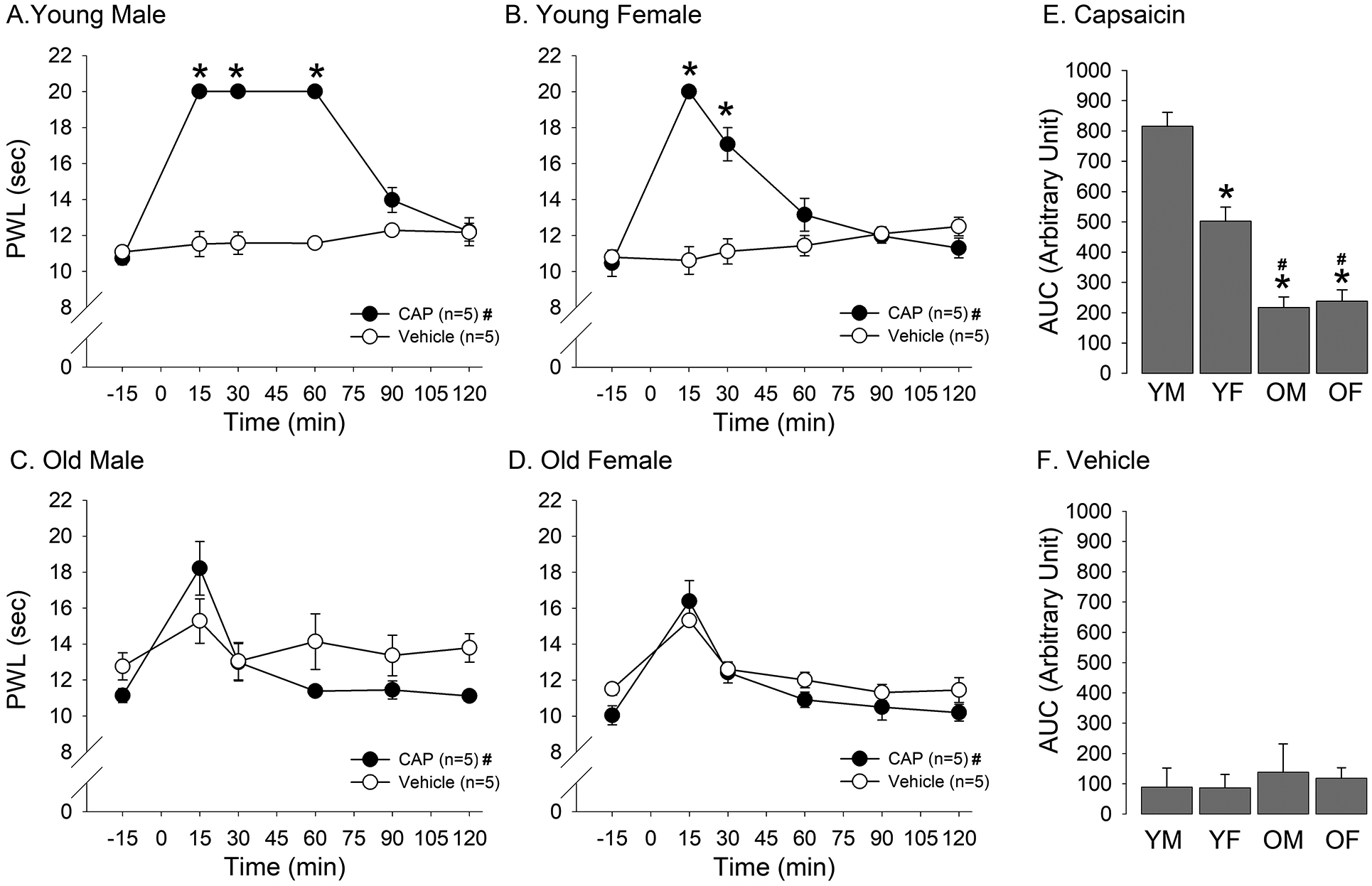
Age- and sex-related differences in DNIC. Changes in hindpaw thermal withdrawal latencies to capsaicin and vehicle treatment in forepaw of young male (A), young female (B), old male (C), and old female (D) rats were plotted against time. * denotes significant main effect between treatments, and # denotes significant time effect. Bar graphs shown in (E) and (F) compare AUC as the measure of overall magnitude of DNIC between YM, YF, OM, and OF rats treated with capsaicin and vehicle, respectively. * denotes significant difference compared to YM rats, and # denotes significant difference compared to YF rats.
In old male rats, there was no significant difference in withdrawal latencies between capsaicin and vehicle treated rats (Fig. 2C; F=1.96, p > 0.05). There was a significant main effect for time (F=7.65, p < 0.001), which was primarily contributed by the increase in latency at the 15 min time point. Similarly, no significant difference in withdrawal latencies between capsaicin and vehicle treated old female rats was observed (Fig. 2D; F=1.95, p > 0.05). Both vehicle and capsaicin tended to increase the withdrawal latency at 15 minutes post injection, a time point at which significant differences was detected (F=7.6, p < 0.01).
In order to compare the extent of DNIC across the two-hour time span, we compared AUC obtained from each age and sex groups. The two-way ANOVA revealed significant sex (F=108.7, p < 0.001) and age (Fig 2E; F=12.5, p < 0.01) effects. Post-hoc multiple group comparisons revealed that AUC obtained from young males treated with capsaicin was significantly greater than AUC from young females, old males and old females under the same condition. AUC from young females was significantly greater than AUC from old males and old females. There was no difference in AUC between old males and old females. There was no significant age or sex effect between any groups treated with the vehicle. (Fig. 2F; F=0.42, p > 0.05, F=0.03, p > 0.05, respectively). These findings are consistent with our previous study showing that young males have a more efficient DNIC response compared to young females. The data also supported our hypothesis that DNIC is lost in old animals. Based on these observations, we hypothesized that brain connectivity related to DNIC response would be modulated in an age- and sex-dependent manner.
3.2. Age- and sex-dependent changes in FC of ACC after DNIC induction.
The age and sex effects on DNIC response were more robust 30 min after capsaicin injection, which suggest the use of capsaicin as a reliable conditioning stimulus at later time points. Therefore, we decided to explore the age and sex effects on ACC FC between 30 to 45 min (resting state 4) after DNIC induction. For age-dependent effects, young rats had increased right (R) ACC FC with anterior pretectal nucleus (APT), retrosplenial cortex (RS), deep mesencephalic nucleus (DpMe) and tegmental nucleus (Tg) compared to old rats (Table 1 - Fig. 3A). Old animals had increased R ACC FC with hippocampus, raphe nuclei (Rn), pontine nuclei (Pn) and PAG relative to the young group (Table 1 - Fig. 3B). For sex-dependent effects, males had increased R ACC FC with hippocampus, PAG and DpMe compared to females (Table 1 - Fig. 3C). Females had strong R ACC FC with RS, Pn, locus coeruleus (LC), paragigantocellular reticular nucleus (PGi) and gigantocellular reticular nucleus (Gi) (Table 1 - Fig. 3D), relative to males. Since we also performed the full factorial analysis (supplemental material), stronger age- and sex-dependent effects observed during the entire fMRI acquisition appear to be driven by resting state 4 (30 to 45 min post-capsaicin). This phenomenon may occur due to the decrease of the initial nociceptive effects of capsaicin over time, which may suggest the use of capsaicin as a reliable conditioning stimulus at later time points. Thus, results from the entire post-capsaicin fMRI acquisition, resting state 2 (0 to 15 min post-capsaicin) and resting state 3 (15 to 30 min post-capsaicin) are reported in the supplemental material to avoid massive amount of data. Consequently, in order to investigate R ACC FC related to DNIC in a homogeneous sample of rats at later time point, we decided to further stratify the groups by age and sex.
Table 1.
Summary of the analysis approach and outcomes.
| Analysis | Objective | Outcomes |
|---|---|---|
| 1. Flexible factorial design with 2 groups (young and old) × 4 time points (baseline and three resting state scans). Comparisons between baseline and resting state 4. | To show age differences in ACC FC with the whole brain during DNIC. | Young group had increased ACC FC with the following network compared to old group (Fig. 3A): APT, RS, DpMe and Tg. Old group had increased ACC FC with the following network compared to young group (Fig. 3B): PAG, hippocampus, Rn and Pn. |
| 2. Flexible factorial design with 2 groups (male and female) × 4 time points (baseline and three resting state scans). Comparisons between baseline and resting state 4. | To show sex differences in ACC FC with the whole brain during DNIC. | Males had increased ACC FC with the following network compared to females (Fig. 3C): PAG, hippocampus and DpMe. Females had increased ACC FC with the following network compared to males (Fig. 3D): RS, Pn, LC, PGi and Gi. |
| 3. Flexible factorial design with 4 groups (YM, YF, OM and OF) and comparisons between baseline and resting state 4 for ACC FC. | To examine the full age-by-sex interaction in ACC FC with the whole brain during DNIC. |
Effects of age in sex-matched groups YF had increased ACC FC with the following network compared to OF (Fig. 4A): RS, DpMe, Tg, Pt and cerebellum. OF had increased ACC FC with the following network compared to YF (Fig. 4B): PAG, hippocampus, Rn, Pn, and Sub. No differences between YM and OM. Effects of sex in age-matched groups YM had increased ACC FC with the following network compared to YF (Fig. 4C): PAG, hippocampus and DpMe. YF had increased ACC FC with the following network compared to YM (Fig. 4D): RS, Pn, LC, PGi, cerebellum and Pt. No differences between OM and OF. |
| 4. Flexible factorial design with 4 groups (YM, YF, OM and OF) and comparisons between baseline and resting state 4 for PAG FC. | To examine the full age-by-sex interaction in PAG FC with the whole brain in old males during DNIC. |
Effects of age in sex-matched groups OM had increased PAG FC with the following network compared to YM (Fig. 5A): Hippocampus, APT and RS. Effects of sex in age-matched groups OM had increased PAG FC with the following network compared to OF (Fig. 5B): Hippocampus, RS, Rn, Rt, Pn and LC. |
Abbreviations: ACC: anterior cingulate cortex, APT: anterior pretectal nucleus, DpMe: deep mesencephalic nucleus, FC: functional connectivity, Gi: gigantocellular reticular nucleus, LC: locus coeruleus, OF: old female, OM: old male, PAG: periaqueductal gray, PGi: paragigantocellular nucleus, Pn: pontine nuclei, Pt: parietal association cortex, Rn: raphe nucleus, RS: retrosplenial cortex, Rt: pontine reticular nucleus, Sub: subiculum, Tg: tegmental area, YF: young female and YM: young male.
Figure 3.
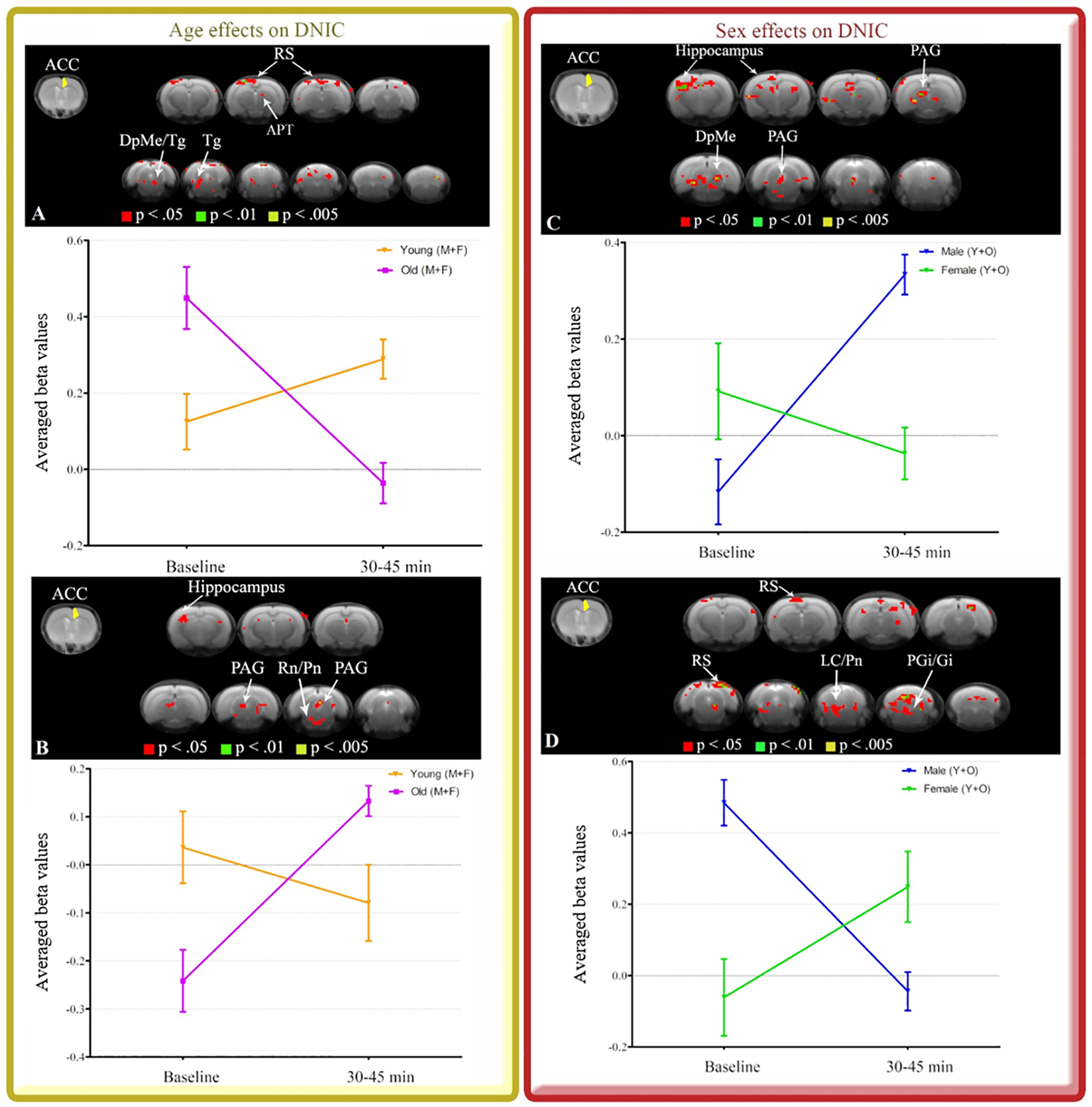
R ACC seed (yellow) and maps indicating connectivity to the whole brain during 30 to 45 min after DNIC induction. Effects of age (A and B) and sex (C and D) are illustrated. Brain images show cluster-forming thresholds at p < .05, .01 and .005. Plots show extracted beta values from all significant clusters thresholded at p < .05 for each animal and time point (average ± S.E.M.). DpMe: deep mesencephalic nucleus, Gi: gigantocellular reticular nucleus, LC: locus coeruleus, PAG: periaqueductal gray, PGi: paragigantocellular nucleus, Pn: pontine nuclei, APT: anterior pretectal nucleus, Rn: raphe nuclei, RS: retrosplenial cortex, Tg: tegmental nucleus.
3.3. FC of ACC in groups stratified by age and sex after DNIC induction.
To advance our previous observations, we further stratified the animal samples by age and sex and investigated specific patterns of ACC connectivity during DNIC. For sex-matched groups showing effects of age, young females had increased R ACC FC with RS, DpMe, Tg, and additional connections to parietal association cortex (Pt) and cerebellum compared to old females (Table 1 - Fig. 4A). Old females had increased R ACC FC with hippocampus, Rn, Pn, PAG, and additional connections to subiculum (Sub) relative to young females (Table 1 - Fig. 4B). R ACC FC did not differ between young males and old males. For age-matched groups showing effects of sex, young males had increased R ACC FC with hippocampus, PAG and DpMe compared to young females (Table 1 - Fig. 4C). Young females had increased R ACC FC with RS, Pn, LC, PGi, cerebellum (Table 1 - Fig. 4D), and additional connections to Pt relative to young males. R ACC FC did not differ between old males and old females. These results support the importance of age- and sex-matched groups to investigate connectivity of endogenous pain modulatory systems.
Figure 4.
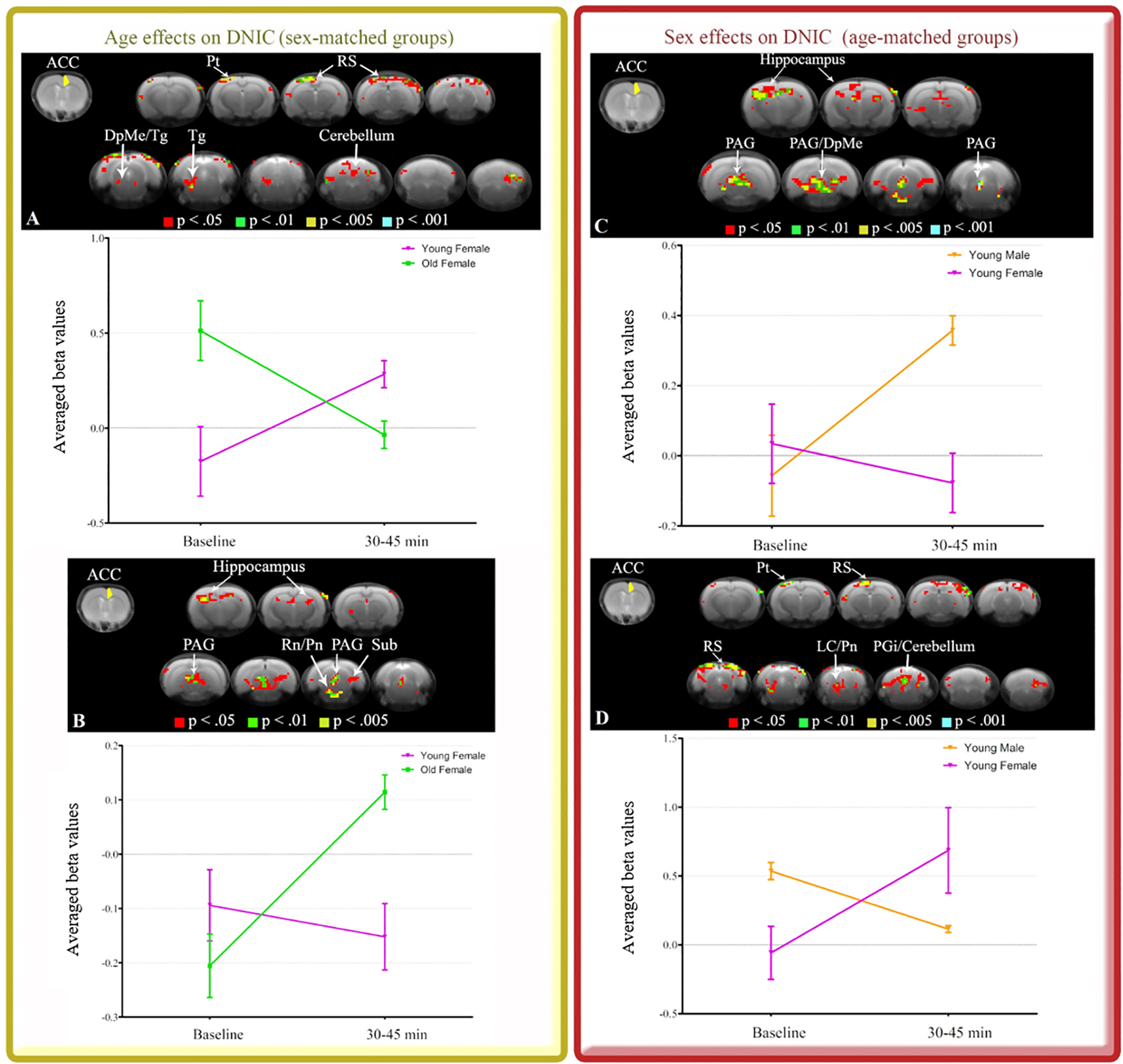
R ACC seed (yellow) and maps indicating unique patterns of R ACC connectivity during 30 to 45 min after DNIC induction. Sex-matched groups showing effects of age (A and B) and age-matched groups showing effects of sex (C and D) are illustrated. Brain images show cluster-forming thresholds at p < .05, .01, .005 and .001. Plots show extracted beta values from all significant clusters thresholded at p < .05 for each animal and time point (average ± S.E.M.). DpMe: deep mesencephalic nucleus, LC: locus coeruleus, PAG: periaqueductal gray, PGi: paragigantocellular nucleus, Pn: pontine nuclei, Pt: parietal association cortex, Rn: raphe nuclei, RS: retrosplenial cortex, Sub: subiculum, Tg: tegmental nucleus.
3.4. FC of PAG in groups stratified by age and sex after DNIC induction.
There was an increased ACC FC with PAG in the old group relative to young group with male and female rats combined. However, this effect was not seen in sex-matched groups when old males were compared to young males. Therefore, we decided to investigate the PAG connectivity to the whole brain in old males. We hypothesized that old males would distinctively recruit PAG connections during DNIC response, which may not be ACC-dependent. For sex-matched groups showing effects of age, old males had increased PAG FC with hippocampus, anterior pretectal nucleus (APT) and RS compared to young males (Table 1 - Fig. 5A). For age-matched groups showing effects of sex, old males had increased PAG FC with hippocampus, RS, Rn, pontine reticular nucleus (Rt), Pn and LC (Table 1 - Fig. 5B), relative to old females. Thus, considering that PAG connectivity in old males differs from both young male and old female groups, this appears to be a unique mechanism in old males. This effect may be driven, at least in part, by the deficits observed in DNIC responses of old males compared to young males. It is worth noting that even though the group differences in FC were larger at baseline, the opposite direction of the FC effects after DNIC may indicate a differential engagement of brain networks in each group, e.g. old males had increased FC while young male and old female had decreased FC during DNIC.
Figure 5.
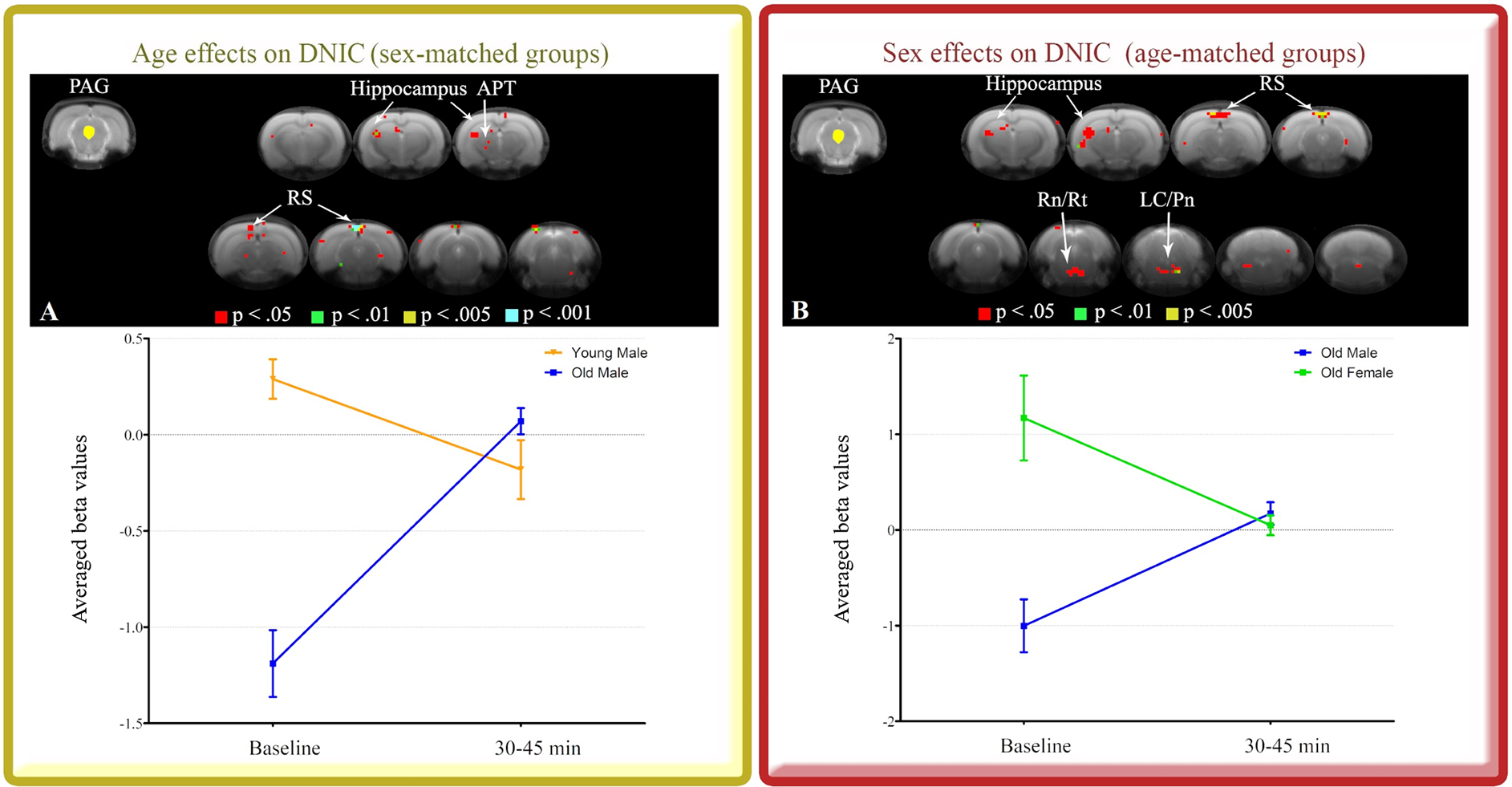
PAG seed (yellow) and maps indicating unique patterns of PAG connectivity during 30 to 45 min after DNIC induction. Sex-matched groups showing effects of age (A) and age-matched groups showing effects of sex (B) are illustrated. Brain images show cluster-forming thresholds at p < .05, .01, .005 and .001. Plots show extracted beta values from all significant clusters thresholded at p < .05 for each animal and time point (average ± S.E.M.). APT: anterior pretectal nucleus, LC: locus coeruleus, PAG: periaqueductal gray, Pn: pontine nuclei, Rn: raphe nuclei, RS: retrosplenial cortex, Rt: pontine reticular nucleus.
4. Discussion
Human studies have shown that the age-related decline in endogenous analgesia is due to reduced efficacy of descending inhibitory systems [25; 29; 47]. Consistent with these findings, we show that endogenous pain inhibition is lost in old rats and altered connectivity of brain regions associated with DNIC efficiency (Fig. 6). Although the analgesic effects of DNIC are lost in both old males and females, the brain networks engaged following the conditioning stimulus differ in a sex-dependent manner suggesting that sex may be a risk factor for developing age-related chronic pain associated with compromised DNIC.
Figure 6.
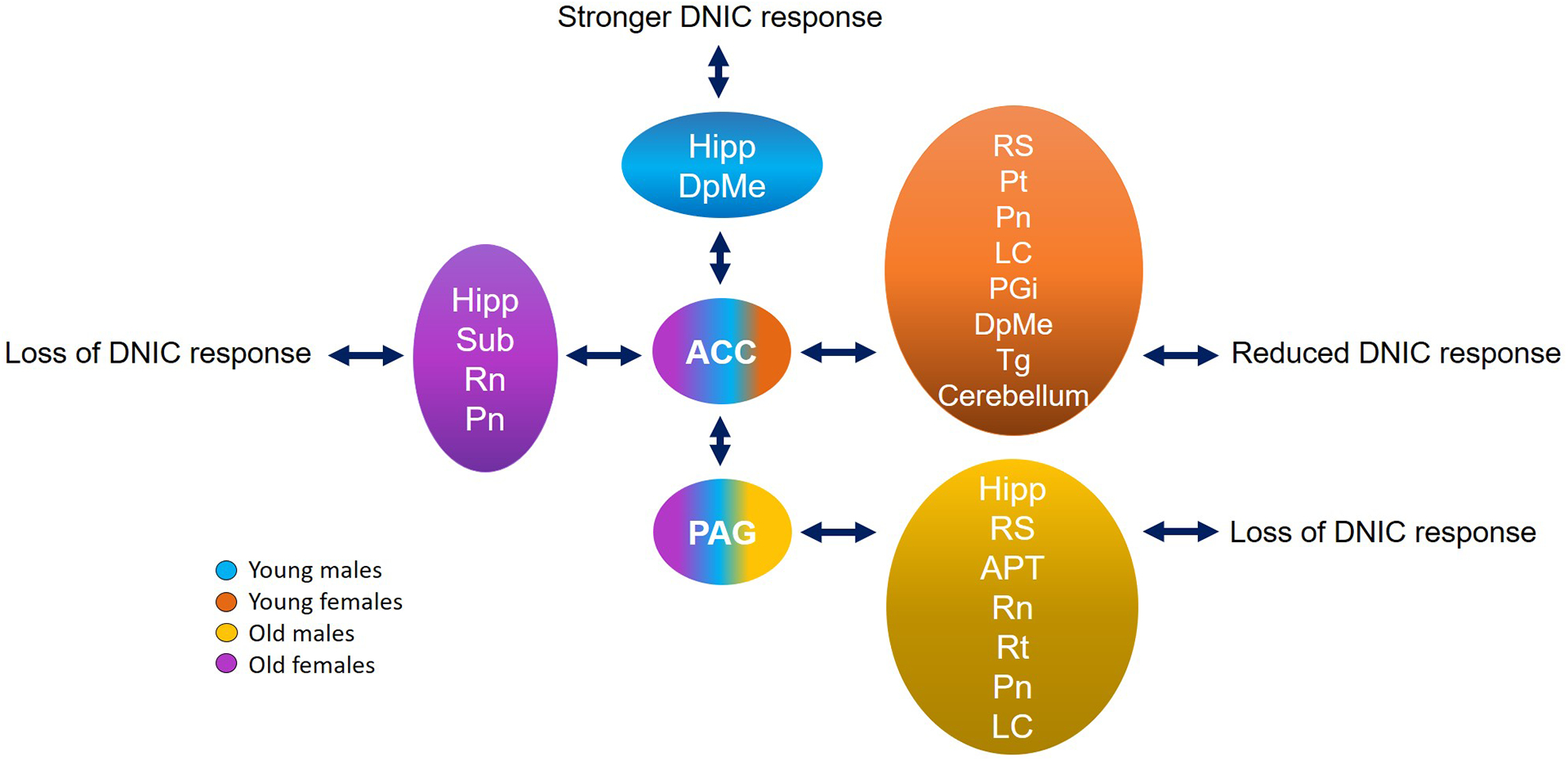
Model of potential pathways related to age and sex differences in DNIC. Group comparisons show brain regions that had increased connectivity during endogenous pain inhibition in rats stratified by age and sex. ACC and PAG were the regions of interest (seeds) and arrows show the increased connectivity between them and several brain areas. Young males had the most efficient analgesia with the strongest connectivity between ACC and PAG (blue). The reduced efficiency of DNIC in young females appeared to be driven by a widespread brain connectivity that was not dependent on PAG (orange). Although both old groups showed behavioral loss of DNIC response, old males had increased connectivity between PAG and several brain regions that did not include ACC (yellow); and old females had increased connectivity between ACC, PAG and more limbic regions (purple). ACC: anterior cingulate cortex, APT: anterior pretectal nucleus, DpMe: deep mesencephalic nucleus, Hipp: hippocampus, LC: locus coeruleus, PGi: paragigantocellular nucleus, PAG: periaqueductal gray, Pn: pontine nuclei, Pt: parietal association cortex, Rn: raphe nuclei, RS: retrosplenial cortex, Rt: pontine reticular nucleus, Sub: subiculum, Tg: tegmental nucleus.
We showed that in old rats DNIC induction via capsaicin as the conditioning stimulus did not affect paw withdrawal latencies, suggesting inefficiency of descending inhibitory systems. Capsaicin is known to produce ongoing and dose-dependent pain [43]. However, it is worth noting that the dose of capsaicin we used induces similar nocifensive responses and c-Fos activation in the spinal cord of female and male rats regardless of age [37]. Furthermore, capsaicin-induced pain decays exponentially after 15 min [15]. We assessed the same dose of capsaicin as a reliable conditioning stimulus for 120 min of behavioral testing and 45 min of fMRI acquisition. Capsaicin-induced analgesia from young rats was seen as early as 15 min and remained for at least 45 min, which likely represents DNIC. Therefore, the effects of capsaicin on central processing through descending pain modulation appears to last longer than its effects on primary afferent nociceptors, which ceases in a relatively short period [43].
The ACC lies in a unique position in the brain, with connections to the limbic system and top-down influences on the PAG to gate pain modulation [44]. In this study, the fMRI data from 30 to 45 min post-capsaicin injection captured several age- and sex-related changes in ACC connectivity, which had stronger effects and exclusion of potential false positive voxels compared to findings from earlier time points. A possible explanation for these findings is that the reduction of the initial nociceptive effects of capsaicin over time could, to some extent, justify the use of capsaicin as a reliable conditioning stimulus at later time points [43]. Regardless of sex, we found that age is a crucial factor to change the connectivity of descending pain pathways that directly influence the endogenous ability to inhibit pain. The deficits in pain inhibition seen in old rats were associated with a brain network containing regions rich in serotonergic neurons, such as ACC, raphe nuclei PAG and limbic areas. The blockade of serotonergic receptors in the raphe nuclei modulates the serotonin release in hippocampus and PAG, which are involved in emotional responses including anxiety [31]. The prevalence of anxiety symptoms is higher in the elderly population than in younger populations [50]. Thus, the increased connectivity between the limbic system and descending pain pathways may be associated not only with the pain inhibitory deficits, but also with the increased affective component of pain and susceptibility to comorbid mood disorders in older people.
In contrast, young rats engaged a network with heterogeneous neurochemical and functional properties during pain modulation [9; 28; 39; 45; 46]. We found that ACC and RS are the main cortical structures modulating the brain network observed in young rats. According to previous studies, RS can activate opioid and serotonergic terminals in APT that indirectly modulate responses to noxious input in the spinal cord through DpMe connections [12; 28; 39; 45; 46]. Cholinergic neurons from the Tg project to ACC and may be involved in pain modulation [9; 38; 41]. Indeed, we observed increased connectivity between ACC, RS, APT, Tg and DpMe in young rats. Brain networks were also modulated by sex when we combined young and old rat: males strongly engaged PAG during DNIC, while females recruit a widespread network that includes ACC and RS as main cortical regions and connections to PGi, LC and Pn. Therefore, our findings suggest that DNIC response can engage heterogeneous brain circuits to promote pain inhibition depending on age and sex.
We advance our observations by demonstrating specific patterns of brain connectivity comparing age- and sex-matched groups. The matching procedure in research can slightly diminish the statistical power of the results [11]. However, studies with stratifications according to age and sex have elucidated how these variables can directly influence pain outcomes and mechanisms [1; 25]. We showed that old females have a unique pattern of brain connectivity during DNIC, which engages the limbic system in order to modulate the serotonergic descending pathway [14; 16 ; 32]. On the other hand, young females robustly engage multiple brain regions to achieve pain inhibition, such as noradrenergic connections between LC and ACC, RS, Pt and PGi [22], Pn connections to the cerebellum which are mostly glutamate-dependent [27], and GABAergic projections from DpMe to Tg [38; 41]. Although, the increased connectivity between PAG and ACC appears to drive the most efficient pain inhibitory control seen in young males [7], we also found that young males had increased connectivity to a limbic region (hippocampus). A point to consider is that hippocampus is divided in different regions, which have been shown to exert opposite roles in pain modulation [49]. Regarding aging, DNIC responses were lost in old males and females, but the brain networks engaged during DNIC differed in a sex-dependent manner. Old males recruited brain connections to PAG that are not ACC-dependent, while old females engaged ACC, PAG and limbic regions. DNIC has been described as one of many components of the descending pain modulatory system, which also includes pathways associated with placebo and stress-induced analgesia [18–20; 34]. We defined our behavioral and brain network outcomes as DNIC based on the widely used description of DNIC as the phenomenon of “pain inhibits pain.” Descending pain pathways exert a bidirectional pain modulation, i.e., inhibiting and facilitating pain [33]. Even though DNIC responses were lost in both old groups, the sex differences in brain connectivity may show distinct circuities that can similarly facilitate pain. Therefore, the balance between descending inhibitory and facilitatory pathways may influence DNIC responses and this balance may be strongly modulated by sex and age [34]. We acknowledge that there were group differences in brain connectivity at baseline while the behavioral responses were mostly comparable. However, the fact that each group differently engaged brain regions during the same DNIC paradigm may suggest that baseline or resting state networks work in the same manner.
We propose a model of how potential pathways are related to age and sex differences in DNIC (Fig. 6). Even though both old groups have shown behavioral loss of DNIC response, old females strongly have the participation of the limbic system, ACC and PAG connections; and old males engage the limbic system and a specific PAG circuitry that are not dependent on ACC connections. The higher DNIC response observed in young males is related to increased connectivity between ACC and PAG, while young females have reduced DNIC responses associated with widespread connectivity that is not dependent on PAG modulation. This model also suggests potential areas for circuit manipulations to experimentally demonstrate causal relationships between brain processing and DNIC response. A potential confound of animal neuroimaging studies is the use of anesthesia, which may also be influenced by age and sex. Compared to young adult rats under long exposure (≥2 hours) to isoflurane, aged rats had altered stress responses, acetylcholine release and hippocampal neurogenesis [10; 13]. In contrast, no differences were observed in the effects of isoflurane anesthesia on behavioral responses between females and males [23]. Here, we used relatively low doses and short durations of isoflurane exposure because the brain activity and resting state networks are generally preserved and highly reproducible between animals, compared to alpha-chloralose and awake fMRI [6; 21; 48]. The BOLD signal changes under isoflurane are also similar to those observed under medetomidine anesthesia [48].
From a clinical perspective, brain mechanisms of pain inhibition are of considerable interest. Increased understanding of pain inhibitory pathways as they relate to sex and age factors can highlight potential avenues for both pharmacological and nonpharmacological interventions. Therapeutic strategies to manage chronic pain in young and elderly population are still mostly reliant on opioids, and older patients are more likely to experience cognitive impairment and fall injuries when exposed to opioids [2; 8]. It is therefore feasible to speculate that if the normal aging and sex can induce maladaptive pain modulation and changes in brain circuitries, they may contribute to the low efficiency of treatment approaches to many pain syndromes and increased prevalence of chronic pain within women and older populations, which warrant further investigation.
In summary, our study provides insight into the impact of aging and sex on brain circuitries involved in pain modulation and contributes to a mechanistic understanding of the impairment in endogenous pain inhibition seen in older patients. Our findings suggest that the limbic system and a widespread brain connectivity appear to enhance susceptibility to pain modulatory deficits in the elderly population, and sex may be a risk factor for developing age-related chronic pain.
Supplementary Material
Acknowledgments:
This study was supported by NIH-NIA grant AG053783 (JYR) and NIH-NIDCR grant DE0027808 (JYR). All authors declare that they have no conflicts of interest.
References
- [1].Borsook D, Youssef AM, Simons L, Elman I, Eccleston C. When pain gets stuck: the evolution of pain chronification and treatment resistance. Pain 2018;159(12):2421–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effects. Clin Interv Aging 2008;3(2):273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Crisp T, Giles JR, Cruce WL, McBurney DL, Stuesse SL. The effects of aging on thermal hyperalgesia and tactile-evoked allodynia using two models of peripheral mononeuropathy in the rat. Neurosci Lett 2003;339(2):103–106. [DOI] [PubMed] [Google Scholar]
- [4].Crisp T, Stafinsky JL, Hoskins DL, Dayal B, Chinrock KM, Uram M. Effects of aging on spinal opioid-induced antinociception. Neurobiol Aging 1994;15(2):169–174. [DOI] [PubMed] [Google Scholar]
- [5].Cruz-Almeida Y, Fillingim RB, Riley JL 3rd, Woods AJ, Porges E, Cohen R, Cole J. Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain 2019;160(5):1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Da Silva JT, Seminowicz DA. Neuroimaging of pain in animal models: a review of recent literature. Pain Rep 2019;4(4):e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Da Silva JT, Zhang Y, Asgar J, Ro JY, Seminowicz DA. Diffuse noxious inhibitory controls and brain networks are modulated in a testosterone-dependent manner in Sprague Dawley rats. Behav Brain Res 2018;349:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Daoust R, Paquet J, Moore L, Émond M, Gosselin S, Lavigne G, Choinière M, Boulanger A, Mac-Thiong JM, Chauny JM. Recent opioid use and fall-related injury among older patients with trauma. Cmaj 2018;190(16):E500–E506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Oliveira RC, de Oliveira R, Falconi-Sobrinho LL, Biagioni AF, Almada RC, Dos Anjos-Garcia T, Bazaglia-de-Sousa G, Khan AU, Coimbra NC. Neurotoxic lesions of the pedunculopontine tegmental nucleus impair the elaboration of postictal antinociception. Physiol Behav 2018;194:162–169. [DOI] [PubMed] [Google Scholar]
- [10].Erasso DM, Camporesi EM, Mangar D, Saporta S. Effects of isoflurane or propofol on postnatal hippocampal neurogenesis in young and aged rats. Brain Res 2013;1530:1–12. [DOI] [PubMed] [Google Scholar]
- [11].Faresjö T, Faresjö A. To match or not to match in epidemiological studies--same outcome but less power. Int J Environ Res Public Health 2010;7(1):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].González-Hernández T, Barroso-Chinea P, Pérez de la Cruz MA, Valera P, Dopico JG, Rodríguez M. Response of GABAergic cells in the deep mesencephalic nucleus to dopaminergic cell degeneration: an electrophysiological and in situ hybridization study. Neuroscience 2002;113(2):311–321. [DOI] [PubMed] [Google Scholar]
- [13].Jansson A, Olin K, Yoshitake T, Hagman B, Herrington MK, Kehr J, Permert J. Effects of isoflurane on prefrontal acetylcholine release and hypothalamic Fos response in young adult and aged rats. Exp Neurol 2004;190(2):535–543. [DOI] [PubMed] [Google Scholar]
- [14].Kim M, Kwak S, Yoon YB, Kwak YB, Kim T, Cho KIK, Lee TY, Kwon JS. Functional connectivity of the raphe nucleus as a predictor of the response to selective serotonin reuptake inhibitors in obsessive-compulsive disorder. Neuropsychopharmacology 2019;12(10):019–0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kinnman E, Nygards EB, Hansson P. Effects of dextromethorphan in clinical doses on capsaicin-induced ongoing pain and mechanical hypersensitivity. J Pain Symptom Manage 1997;14(4):195–201. [DOI] [PubMed] [Google Scholar]
- [16].Kong J, Wolcott E, Wang Z, Jorgenson K, Harvey WF, Tao J, Rones R, Wang C. Altered resting state functional connectivity of the cognitive control network in fibromyalgia and the modulation effect of mind-body intervention. Brain Imaging Behav 2019;13(2):482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Larsson C, Hansson EE, Sundquist K, Jakobsson U. Chronic pain in older adults: prevalence, incidence, and risk factors. Scand J Rheumatol 2017;46(4):317–325. [DOI] [PubMed] [Google Scholar]
- [18].Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 1979;6(3):283–304. [DOI] [PubMed] [Google Scholar]
- [19].Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 1978;2(8091):654–657. [DOI] [PubMed] [Google Scholar]
- [20].Lewis JW, Cannon JT, Stapleton JM, Liebeskind JC. Stress activates endogenous pain-inhibitory systems: opioid and non-opioid mechanisms. Proc West Pharmacol Soc 1980;23:85–88. [PubMed] [Google Scholar]
- [21].Liang Z, King J, Zhang N. Intrinsic organization of the anesthetized brain. J Neurosci 2012;32(30):10183–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 2016;338:93–113. [DOI] [PubMed] [Google Scholar]
- [23].Mansouri MT, Fidler JA, Meng QC, Eckenhoff RG, Garcia PS. Sex effects on behavioral markers of emergence from propofol and isoflurane anesthesia in rats. Behav Brain Res 2019;367:59–67. [DOI] [PubMed] [Google Scholar]
- [24].Mis MA, Rogers MF, Jeffries AR, Wilbrey AL, Chen L, Yang Y, Dib-Hajj S, Waxman SG, Stevens EB, Randall AD. Differential aging-related changes in neurophysiology and gene expression in IB4-positive and IB4-negative nociceptive neurons. Aging Cell 2018;25(10):12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Monroe TB, Fillingim RB, Bruehl SP, Rogers BP, Dietrich MS, Gore JC, Atalla SW, Cowan RL. Sex Differences in Brain Regions Modulating Pain Among Older Adults: A Cross-Sectional Resting State Functional Connectivity Study. Pain Med 2018;19(9):1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morgan D, Mitzelfelt JD, Koerper LM, Carter CS. Effects of morphine on thermal sensitivity in adult and aged rats. J Gerontol A Biol Sci Med Sci 2012;67(7):705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Res Rev 2010;65(1):14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Murray PD, Masri R, Keller A. Abnormal anterior pretectal nucleus activity contributes to central pain syndrome. J Neurophysiol 2010;103(6):3044–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Naugle KM, Cruz-Almeida Y, Fillingim RB, Riley JL 3rd. Loss of Temporal Inhibition of Nociceptive Information Is Associated With Aging and Bodily Pain. J Pain 2017;18(12):1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 2015;35(18):7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nunes-de-Souza V, Nunes-de-Souza R, Rodgers RJ, Canto-de-Souza A. Blockade of 5-HT2 receptors in the periaqueductal grey matter (PAG) abolishes the anxiolytic-like effect of 5-HT1A receptor antagonism in the median raphe nucleus in mice. Behavioural Brain Research 2011;225(2):547–553. [DOI] [PubMed] [Google Scholar]
- [32].Nunes-de-Souza V, Nunes-de-Souza R, Rodgers RJ, Canto-de-Souza A. Blockade of 5-HT(2) receptors in the periaqueductal grey matter (PAG) abolishes the anxiolytic-like effect of 5-HT(1A) receptor antagonism in the median raphe nucleus in mice. Behav Brain Res 2011;225(2):547–553. [DOI] [PubMed] [Google Scholar]
- [33].Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014;8(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Patel R, Dickenson AH. A study of cortical and brainstem mechanisms of diffuse noxious inhibitory controls in anaesthetised normal and neuropathic rats. Eur J Neurosci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Petersen KK, McPhee ME, Hoegh MS, Graven-Nielsen T. Assessment of conditioned pain modulation in healthy participants and patients with chronic pain: manifestations and implications for pain progression. Curr Opin Support Palliat Care 2019;13(2):99–106. [DOI] [PubMed] [Google Scholar]
- [36].Phelps CE, Navratilova E, Dickenson AH, Porreca F, Bannister K. Kappa opioid signaling in the right central amygdala causes hind paw specific loss of diffuse noxious inhibitory controls in experimental neuropathic pain. Pain 2019;160(7):1614–1621. [DOI] [PubMed] [Google Scholar]
- [37].Ro JY, Zhang Y, Tricou C, Yang D, da Silva JT, Zhang R. Age and Sex Differences in Acute and Osteoarthritis-Like Pain Responses in Rats. J Gerontol A Biol Sci Med Sci 2019;14(5550010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rodriguez M, Abdala P, Barroso-Chinea P, Gonzalez-Hernandez T. The deep mesencephalic nucleus as an output center of basal ganglia: morphological and electrophysiological similarities with the substantia nigra. J Comp Neurol 2001;438(1):12–31. [DOI] [PubMed] [Google Scholar]
- [39].Rossaneis AC, Reis GM, Prado WA. Stimulation of the occipital or retrosplenial cortex reduces incision pain in rats. Pharmacology Biochemistry and Behavior 2011;100(2):220–227. [DOI] [PubMed] [Google Scholar]
- [40].Sadler KE, Gartland NM, Cavanaugh JE, Kolber BJ. Central amygdala activation of extracellular signal-regulated kinase 1 and age-dependent changes in inflammatory pain sensitivity in mice. Neurobiol Aging 2017;56:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sapin E, Lapray D, Bérod A, Goutagny R, Léger L, Ravassard P, Clément O, Hanriot L, Fort P, Luppi P-H. Localization of the Brainstem GABAergic Neurons Controlling Paradoxical (REM) Sleep. PLoS One 2009;4(1):e4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sengupta P The Laboratory Rat: Relating Its Age With Human’s. Int J Prev Med 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- [43].Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain 1989;38(1):99–107. [DOI] [PubMed] [Google Scholar]
- [44].Stevens FL, Hurley RA, Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci 2011;23(2):121–125. [DOI] [PubMed] [Google Scholar]
- [45].Villarreal CF, Del Bel EA, Prado WA. Involvement of the anterior pretectal nucleus in the control of persistent pain: a behavioral and c-Fos expression study in the rat. Pain 2003;103(1–2):163–174. [DOI] [PubMed] [Google Scholar]
- [46].Wang XM, Yuan B, Hou ZL. Role of the deep mesencephalic nucleus in the antinociception induced by stimulation of the anterior pretectal nucleus in rats. Brain Res 1992;577(2):321–325. [DOI] [PubMed] [Google Scholar]
- [47].Washington LL, Gibson SJ, Helme RD. Age-related differences in the endogenous analgesic response to repeated cold water immersion in human volunteers. Pain 2000;89(1):89–96. [DOI] [PubMed] [Google Scholar]
- [48].Williams KA, Magnuson M, Majeed W, LaConte SM, Peltier SJ, Hu X, Keilholz SD. Comparison of alpha-chloralose, medetomidine and isoflurane anesthesia for functional connectivity mapping in the rat. Magn Reson Imaging 2010;28(7):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xuhong W, Ren W, Centeno MV, Procissi D, Xu T, Jabakhanji R, Martina M, Radulovic J, Surmeier DJ, Liu XG, Apkarian AV. Dorsal Hippocampal Activation Suppresses Neuropathic Pain Behaviors: Chronic pain as extinction-resistant pain-related memory traces. bioRxiv 2018:292094. [Google Scholar]
- [50].Yohannes AM, Baldwin RC, Connolly MJ. Prevalence of depression and anxiety symptoms in elderly patients admitted in post-acute intermediate care. Int J Geriatr Psychiatry 2008;23(11):1141–1147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


